Parenteral anticoagulation in ambulatory patients with cancer
Información
- DOI:
- https://doi.org/10.1002/14651858.CD006652.pub4Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 10 diciembre 2014see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Cáncer ginecológico, neurooncología y otros cánceres
- Copyright:
-
- Copyright © 2015 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
EAA: protocol development, searching for trials, screening, data extraction, data analysis, manuscript drafting, review co‐ordination. LK: searching for trials, screening, full text retrieval, data extraction, data analysis, manuscript drafting. RM: data extraction. MB: screening. VY: full text retrieval, data extraction. FFvD: data extraction, data analysis. SK: data extraction, data analysis. SM: data extraction, data analysis. HOD: statistical analysis, methodological advice, data extraction, manuscript drafting. AB: statistical analysis. HJS: protocol development, searching for trials, data analysis, methodological expertise.
Sources of support
Internal sources
-
No sources of support supplied
External sources
-
Research Grants, Other.
H Schünemann: no personal payments from for‐profit sponsors. Research grants and honoraria were received into research accounts or received by a research group that he belongs to from AstraZeneca, Amgen, Chiesi Foundation, Lily, Pfizer, Roche and UnitedBioSource for development or consulting regarding quality of life instruments for chronic respiratory diseases and as lecture fees related to the methodology of evidence‐based practice guideline development and research methodology. Institutions or organizations that he is affiliated with likely receive funding from for‐profit sponsors that are supporting infrastructure and research that may serve his work.
-
National Institute for Health Research Cochrane Review Incentive Scheme 2013Updating systematic reviews on anticoagulation in patients with cancer, UK.
Declarations of interest
HJS: no personal payments from for‐profit sponsors related to the subject matter in the past three years. EAA is an executive committee member of the ACCP Antithrombotic Therapy Guidelines.All other co‐authors declare no conflicts of interests.
Acknowledgements
We thank Ms. Ann Grifasi for her administrative support. We thank Dr. Loprinzi and Dr. Paul Novotny of the Mayo Clinic for supplying additional data relating to the study Sideras 2006. We also thank Dr. Lebeau, Dr. Altinbas and Dr. Pelzer for supplying additional data. We thank Sameer K Gunukula, Saskia Kuipers and Heather Dickinson for their contributions to previous versions of this manuscript.
The National Institute for Health Research (NIHR) is the largest single funder of the Cochrane Gynaecological Cancer Group.
The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the NIHR, NHS or the Department of Health
Version history
| Published | Title | Stage | Authors | Version |
| 2017 Sep 11 | Parenteral anticoagulation in ambulatory patients with cancer | Review | Elie A Akl, Lara A Kahale, Maram B Hakoum, Charbel F Matar, Francesca Sperati, Maddalena Barba, Victor ED Yosuico, Irene Terrenato, Anneliese Synnot, Holger Schünemann | |
| 2014 Dec 10 | Parenteral anticoagulation in ambulatory patients with cancer | Review | Elie A Akl, Lara A Kahale, Rami A Ballout, Maddalena Barba, Victor E D Yosuico, Frederiek F van Doormaal, Saskia Middeldorp, Andrew Bryant, Holger Schünemann | |
| 2011 Apr 13 | Parenteral anticoagulation in patients with cancer who have no therapeutic or prophylactic indication for anticoagulation | Review | Elie A Akl, Sameer Gunukula, Maddalena Barba, Victor E D Yosuico, Frederiek F van Doormaal, Saskia Kuipers, Saskia Middeldorp, Heather O Dickinson, Andrew Bryant, Holger Schünemann | |
| 2011 Jan 19 | Parenteral anticoagulation in patients with cancer who have no therapeutic or prophylactic indication for anticoagulation | Review | Elie A Akl, Sameer Gunukula, Maddalena Barba, Victor E D Yosuico, Frederiek F van Doormaal, Saskia Kuipers, Saskia Middeldorp, Heather O Dickinson, Andrew Bryant, Holger Schünemann | |
| 2007 Jul 18 | Parenteral anticoagulation for prolonging survival in patients with cancer who have no other indication for anticoagulation | Review | Elie A Akl, Frederiek F van Doormaal, Maddalena Barba, Ganesh Kamath, Seo Young Kim, Saskia Kuipers, Saskia Middeldorp, Victor E D Yosuico, Heather O Dickinson, Holger Schünemann | |
Differences between protocol and review
None.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
- Anticoagulants [*administration & dosage, adverse effects];
- Cause of Death;
- Hemorrhage [chemically induced, epidemiology];
- Heparin [*administration & dosage, adverse effects];
- Heparin, Low‐Molecular‐Weight [administration & dosage];
- Neoplasms [*mortality];
- Quality of Life;
- Randomized Controlled Trials as Topic;
- Survival Analysis;
- Time Factors;
- Venous Thromboembolism [epidemiology, *prevention & control];
- Warfarin [administration & dosage];
Medical Subject Headings Check Words
Humans;
PICO

Study flow diagram.
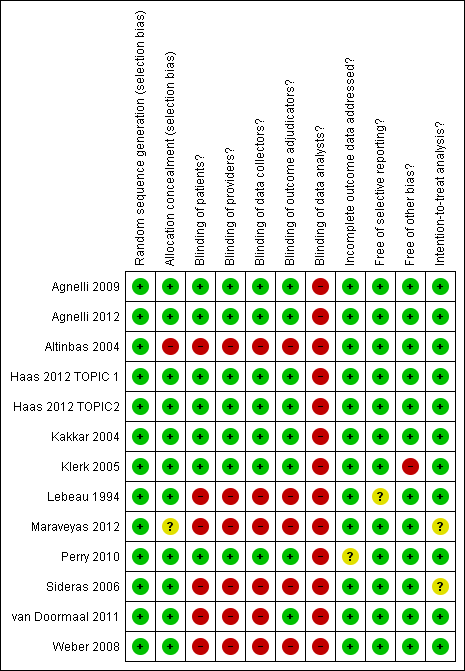
'Risk of bias' summary ‐ mortality at 12 months: review authors' judgements about each methodological quality item for each included study that evaluated mortality at 12 months

Funnel plot of comparison: 1 Heparin versus placebo, outcome: 1.1 Mortality at 12 months.

'Risk of bias' summary ‐ venous thromboembolism: review authors' judgements about each methodological quality item for each included study that evaluated venous thromboembolism

'Risk of bias' summary ‐ major bleeding: review authors' judgements about each methodological quality item for each included study that evaluated major bleeding

'Risk of bias' summary ‐ minor bleeding: review authors' judgements about each methodological quality item for each included study that evaluated minor bleeding

Comparison 1 Heparin versus placebo, Outcome 1 Mortality at 12 months.
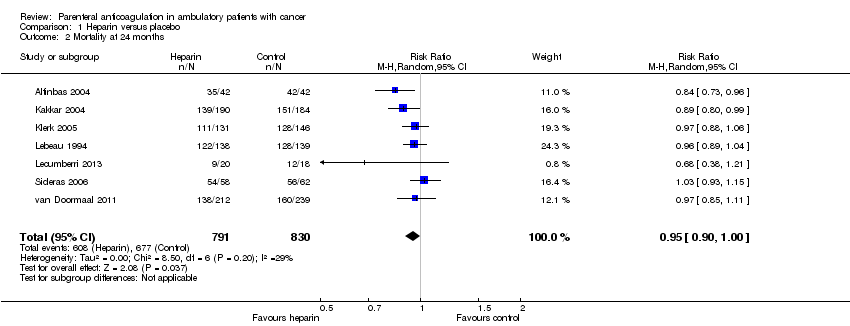
Comparison 1 Heparin versus placebo, Outcome 2 Mortality at 24 months.
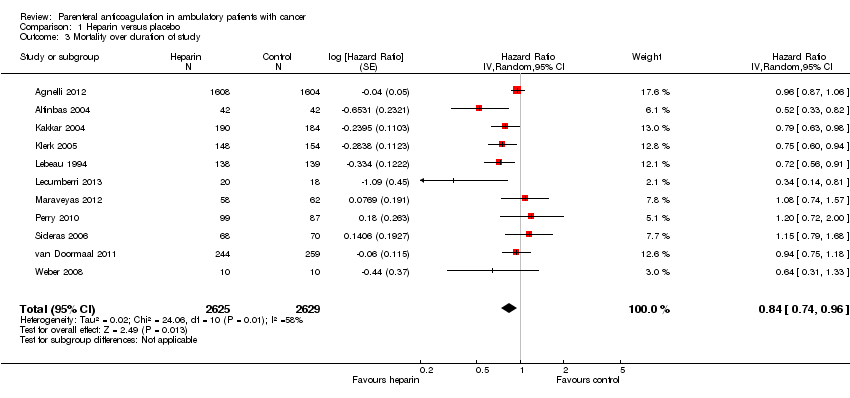
Comparison 1 Heparin versus placebo, Outcome 3 Mortality over duration of study.

Comparison 1 Heparin versus placebo, Outcome 4 Symptomatic VTE.

Comparison 1 Heparin versus placebo, Outcome 5 Symptomatic DVT.
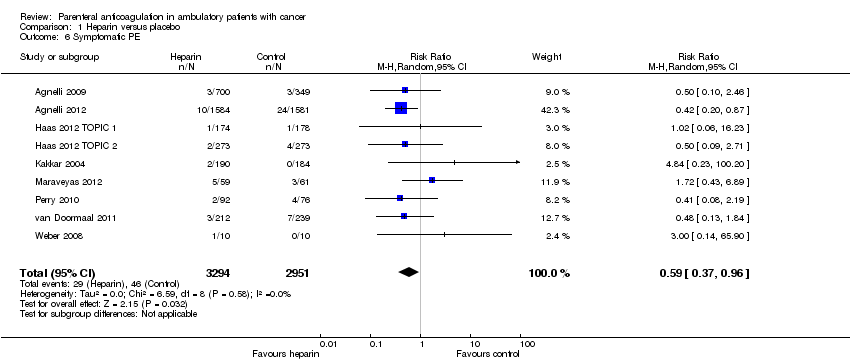
Comparison 1 Heparin versus placebo, Outcome 6 Symptomatic PE.

Comparison 1 Heparin versus placebo, Outcome 7 Major bleeding.

Comparison 1 Heparin versus placebo, Outcome 8 Minor bleeding.

Comparison 1 Heparin versus placebo, Outcome 9 Thrombocytopenia.
| Heparin compared to placebo for ambulatory patients with cancer who have no therapeutic or prophylactic indication for anticoagulation | ||||||
| Patient or population: Ambulatory patients with cancer who have no therapeutic or prophylactic indication for anticoagulation | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Heparin | |||||
| Mortality at 12 months | Study population | RR 0.97 | 7013 | ⊕⊕⊕⊝ | Moderate‐quality evidence owing to imprecision. A survival analysis based on data from 11 studies found a hazard ratio of 0.84 (95% CI 0.74 to 0.96); however heterogeneity in that analysis was relatively high (I2 = 58%) and subgroup analyses did not conclusively identify any subgroup effect | |
| 472 per 1000 | 458 per 1000 | |||||
| Symptomatic VTE | Study population | RR 0.56 | 6809 | ⊕⊕⊕⊕ | The data are combined for pulmonary embolism and symptomatic deep venous thrombosis | |
| 55 per 1000 | 31 per 1000 | |||||
| Major bleeding | Study population | RR 1.14 | 7363 | ⊕⊕⊕⊝ | Moderate‐quality evidence owing to imprecision | |
| 19 per 1000 | 21 per 1000 | |||||
| Minor bleeding | Study population | RR 1.32 | 6884 | ⊕⊕⊕⊝ | Moderate‐quality evidence owing to imprecision | |
| 30 per 1000 | 40 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Confidence interval includes values suggesting clinically significant benefit and values suggesting no effect. 2Confidence interval includes values suggesting clinically significant benefit and values suggesting harm. 3Confidence interval includes values suggesting clinically significant harm and values suggesting no effect. | ||||||
| Term | Definition |
| Adjuvant therapy | Assisting in the amelioration or cure of disease |
| Anticoagulation | The process of hindering the clotting of blood especially by treatment with an anticoagulant |
| Antithrombotic | Used against or tending to prevent thrombosis (clotting) |
| Bacteremia | The presence of bacteria in the blood |
| Central venous line | Synthetic tube that is inserted into a central (large) vein of a patient to provide temporary intravenous access for the administration of fluid, medication or nutrients |
| Coagulation | Clotting |
| Deep vein thrombosis (DVT) | A condition marked by the formation of a thrombus within a deep vein (as of the leg or pelvis) that may be asymptomatic or be accompanied by symptoms (such as swelling and pain) and that is potentially life‐threatening if dislodgment of the thrombus results in pulmonary embolism |
| Fibrin | A white insoluble fibrous protein formed from fibrinogen by the action of thrombin, especially in the clotting of blood |
| Fondaparinux | An anticoagulant medication |
| Hemostatic system | The system that shortens the clotting time of blood and stops bleeding |
| Heparin | An enzyme occurring especially in the liver and lungs that prolongs the clotting time of blood by preventing the formation of fibrin. Two forms of heparin that are used as anticoagulant medications are: unfractionated heparin (UFH) and low molecular weight heparins (LMWH) |
| Impedance plethysmography | A technique that measures the change in blood volume (venous blood volume as well as the pulsation of the arteries) for a specific body segment |
| Kappa statistics | A measure of degree of non‐random agreement between observers and/or measurements of a specific categorical variable |
| Metastasis | The spread of cancer cells from the initial or primary site of disease to another part of the body |
| Oncogene | A gene having the potential to cause a normal cell to become cancerous |
| Osteoporosis | A condition that especially affects older women and is characterized by a decrease in bone mass with decreased density and enlargement of bone spaces producing porosity and brittleness |
| Parenteral nutrition | The practice of feeding a patient intravenously, circumventing the gut |
| Pulmonary embolism (PE) | Embolism of a pulmonary artery or one of its branches that is produced by foreign matter and most often a blood clot originating in a vein of the leg or pelvis and that is marked by labored breathing, chest pain, fainting, rapid heart rate, cyanosis, shock and sometimes death |
| Stroma | The supporting framework of an organ typically consisting of connective tissue |
| Thrombin | A proteolytic enzyme formed from prothrombin that facilitates the clotting of blood by catalyzing conversion of fibrinogen to fibrin |
| Thrombocytopenia | Persistent decrease in the number of blood platelets that is often associated with hemorrhagic conditions |
| Thrombosis | The formation or presence of a blood clot within a blood vessel |
| Vitamin K antagonists | Anticoagulant medications that are used for anticoagulation. Warfarin is a vitamin K antagonist |
| Warfarin | An anticoagulant medication that is a vitamin K antagonist, which is used for anticoagulation |
| Ximelagatran | An anticoagulant medication |
| LMWH | Generic name | Prophylactic dose | Therapeutic dose |
| Lovenox | Enoxaparin | 40 mg once daily | 1 mg/kg twice daily |
| Fragmin | Dalteparin | 2500 to 5000 units once daily | 200 U/kg once daily or |
| Innohep, Logiparin | Tinzaparin, | 4500 units once daily | 90 U/kg twice daily |
| Fraxiparine | Nadroparin | 35 to 75 anti‐Xa international units/kg once daily | 175 anti‐Xa int. units/kg once daily |
| Certoparin | Sandoparin | 3000 anti‐Xa international units once daily | — |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality at 12 months Show forest plot | 13 | 7013 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.92, 1.01] |
| 2 Mortality at 24 months Show forest plot | 7 | 1621 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.90, 1.00] |
| 3 Mortality over duration of study Show forest plot | 11 | 5254 | Hazard Ratio (Random, 95% CI) | 0.84 [0.74, 0.96] |
| 4 Symptomatic VTE Show forest plot | 13 | 6809 | Risk Ratio (M‐H, Random, 95% CI) | 0.56 [0.43, 0.74] |
| 5 Symptomatic DVT Show forest plot | 9 | 6209 | Risk Ratio (M‐H, Random, 95% CI) | 0.49 [0.28, 0.86] |
| 6 Symptomatic PE Show forest plot | 9 | 6245 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.37, 0.96] |
| 7 Major bleeding Show forest plot | 15 | 7305 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.63, 2.01] |
| 8 Minor bleeding Show forest plot | 13 | 6884 | Risk Ratio (M‐H, Random, 95% CI) | 1.32 [1.02, 1.71] |
| 9 Thrombocytopenia Show forest plot | 9 | 4890 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.26, 1.12] |

