Mobile phone‐based interventions for smoking cessation
Referencias
References to studies included in this review
References to studies excluded from this review
References to ongoing studies
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | RCT in US | |
| Participants | 503 participants aged ≥ 18 years recruited via online advertisements when Internet searching for 'quitting smoking'. 34% men, mean age of 35.7 years, and mean FTND score of 5.3. Eligibility criteria included smoking ≥ 5 cigarettes/day, having an e‐mail address, a mobile phone number with an unlimited SMS plan, an interest in quitting smoking in the next month and not pregnant | |
| Interventions | Automated bidirectional text messages, personalisation and interactive components, automated unidirectional emails and an Internet portal ‐ has been revised since pilot study Text2Quit: a 6‐month SMS programme with the first 3 months offering both outgoing messages about quitting smoking and on‐demand help using keywords. Outgoing messages peaked in the period just prior to and following the quit date. Participants received 5 messages on their quit date and approximately 2/day in the week after the quit date. Frequency declined in the subsequent weeks to approximately 3/week for the next 2 months and then < 1/week for the remaining portion of the outgoing phase. After the outgoing messages stopped, participants could still text at any time for help through keywords ‐ to reset a quit date (DATE), get help with a craving through a tip or a trivia game (CRAVE); get a summary of their quitting statistics (STATS) and to indicate that they had smoked (SMOKED). The SMS were supplemented by a personalised Internet portal (text2quit.com) and e‐mails Control: sent an Internet link to Smokefree.gov, a leading website with quitting smoking information run by the National Cancer Institute. Later, once the website began to offer an SMS programme, a guidebook on quitting smoking was offered via an Internet link that led participants to a document containing similar advice and information as Smokefree.gov | |
| Outcomes | Primary outcome: biochemically confirmed repeated point prevalence abstinence, defined as a self report of no smoking in the past 30 days on the 3‐ and 6‐month surveys and a cotinine level ≤ 15 ng/mL at 6 months Secondary outcomes: 7‐ and 30‐day abstinence at 1‐, 3‐, and 6‐month follow‐up and biochemically confirmed abstinence at the 6‐month follow‐up | |
| Notes | Enrolment procedures were modified after a group of participants was discovered to be fraudulent and disqualified | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised via computer system |
| Allocation concealment (selection bias) | Low risk | Recruited and randomised online |
| Blinding (performance bias and detection bias) | Low risk | Participants completed questionnaires online |
| Incomplete outcome data (attrition bias) | Low risk | 52 in control and 70 in intervention group lost to follow‐up at 6 months but ITT analysis presented |
| Other bias | Unclear risk | 13 participants from the control group (5%) indicated on their 3‐month survey that they had used a texting programme for smoking cessation since enrolling in the study Saliva was collected by mail for participants reporting abstinence at 6 months. There was a low response rate (64.7%) among participants eligible for providing a saliva sample for biochemical verification although this did not differ across the groups. Of those participants who provided a sample, 21 (24.4%) had high levels of cotinine and were coded as smokers in analyses |
| Methods | Pilot RCT in US | |
| Participants | Adults aged ≥ 18 years of age current daily smokers recruited online and eligible if interested in quitting smoking in the next 30 days, with a mobile phone with SMS text messaging capability, and using SMS text messaging at least once monthly. 43% men, mean age 30.7 years (range 18‐52 years), and mean FTND of 4.9 (moderate level of dependence on nicotine) | |
| Interventions | All participants received a single individual 30‐minute smoking cessation counselling session TXT‐2‐Quit: an 8‐week programme with 1‐4 text messages/day (depending on quit stage). Smoking cessation messages were tailored to the participant's stage of smoking cessation, with specialised messages provided on‐demand based on user requests for additional support, and an optional peer‐to‐peer social support network Control: an 8‐week programme of daily non‐smoking related text messages | |
| Outcomes | Primary outcome: 7‐day point‐prevalence abstinence using a 2 (treatment groups) × 3 (time points) repeated measures design across 3 time points: 8 weeks, 3 months and 6 months; showed a significant main effect for treatment group (P value = 0.02) with higher odds of quitting in the intervention group compared with the control group (odds ratio 4.52, 95% CI 1.24 to 16.53). Although there was no individual time point difference between groups at 6 months (20% with intervention programme vs. 3.6% with control programme; odds ratio 6.75, 95% CI 0.76 to 60.15) it was likely to have been affected by reduced statistical power. Secondary outcome: 24‐hour point prevalence abstinence at 8 weeks, 3 months and 6 months | |
| Notes | Designed as a small study to develop and provide initial testing of the system During the 6 months' follow‐up, there was a significant improvement in Mood and Physical Symptoms Scale (MPSS) mood symptoms of nicotine withdrawal (P value = 0.03) among the TXT‐2‐Quit participants as compared with the control participants | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Simple randomisation via computerised random number generator |
| Allocation concealment (selection bias) | Low risk | Assignments in a sealed envelope delivered after completion of the baseline data collection |
| Blinding (performance bias and detection bias) | Low risk | Participants completed questionnaires online. Research assistants and counsellors were blind to allocation |
| Incomplete outcome data (attrition bias) | Low risk | 2 participants in control group appeared to be missing at 6 months; however, ITT analysis presented |
| Other bias | Unclear risk | Self report and not biochemically verified |
| Methods | RCT in Australia | |
| Participants | 3530 participants in total (control = 422; onQ = 756; QuitCoach = 809; both = 785; participant choice = 758). 60% female, mean age 42.1 years, and 87.4% were currently smoking a mean of 16.9 cigarettes/day | |
| Interventions | onQ programme: provides a stream of SMS messages to the person that mixes snippets of advice on strategy and motivational messages. The user can interact with it by indicating their stage of quitting so that appropriate stage‐specific messages are sent, and once quit can also call up messages in crisis situations QuitCoach: a personalised, automated tailored cessation programme delivered via the Internet. It generates letters of advice based on answers to an assessment questionnaire, including suggestions about strategy and motivational messages. It also provides further untailored supplementary resources Control: brief information on Internet‐ and phone‐based assistance available in Australia | |
| Outcomes | Self reported 6‐month sustained abstinence at 7‐month follow‐up Intention‐to‐quit analysis and sensitivity analysis around treatment of missing data | |
| Notes | Only onQ and control arms used in analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerised random number generator embedded within the baseline survey |
| Allocation concealment (selection bias) | Low risk | Not a typical RCT as participants were enrolled in a study described to them as being about "the effectiveness of Internet and telephone‐based resources in helping smokers quit", and were only then randomised to a condition that they were offered with no obligation to use |
| Blinding (performance bias and detection bias) | Low risk | Not a typical RCT as participants were enrolled in a study described to them as being about "the effectiveness of Internet and telephone‐based resources in helping smokers quit", and were only then randomised to a condition that they were offered with no obligation to use |
| Incomplete outcome data (attrition bias) | Low risk | Loss to follow‐up 475 (13% total) with similar numbers across groups (control = 66, onQ = 89, QuitCoach = 104, both = 121, participant choice = 95); 2 excluded as reported to have died at 7‐month follow‐up |
| Other bias | Unclear risk | Nothing else described |
| Methods | RCT in Australia | |
| Participants | Participants recruited via advertisements in traditional and social media in Tasmania, Australia. 49% male, mean age 42.1 years and mean FTND of 4.8, and with a high motivation to quit (≥ 75 on 100‐point scale) Eligibility criteria included: daily smokers of > 10 cigarettes/day for past 3 years | |
| Interventions | Intervention: Self‐help Quit booklet plus 4 or 5 randomly timed text messages/day containing quit smoking advice and encouragement tailored to participants' current quit status (preparing to quit, first week of the quit attempt, second week of attempt etc.). Participants could request additional text messages Control: Self‐help Quit booklet containing tips for quitting and cognitive and behavioural coping mechanisms Study visit days: ‐11 (enrolment/randomisation), ‐7 (commence study group), 0 (QD), day 7, day 28, and day 180 post quit | |
| Outcomes | Primary outcome: 7‐day point prevalence abstinence verified by expired CO Secondary outcomes: 1‐month abstinence, cigarette consumption by time‐line follow back, mean time to first lapse | |
| Notes | Not published as yet | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding (performance bias and detection bias) | Unclear risk | No information |
| Incomplete outcome data (attrition bias) | Low risk | 20 in control and 22 in intervention did not commence study. ITT analysis presented in this meta‐analysis |
| Other bias | Unclear risk | No information |
| Methods | Pilot RCT in UK | |
| Participants | 200 participants aged ≥ 16 years; smoking daily and interested in quitting; current owner of mobile phone. 63% men, median age 36 years, median 20 cigarettes/day, 7% FTND dependence score > 5 | |
| Interventions | 6‐month programme delivered solely over mobile phone based on programme in Rodgers 2005 but messages adapted for UK population. Participant nominates QD and receives regular personalised text messages with advice, support and distraction, with a countdown to QD, intensive 4 weeks of 5 or 6 messages/day then maintenance phase of 1 message/2 weeks. Messages selected from database matched to participant characteristics. Free month of text messaging from QD. Optional Quit Buddy, and Text Crave (messages on demand). Interactive polls and quizzes | |
| Outcomes | Primary outcome: point prevalence abstinence (no smoking in past 7 days) at 6 weeks post randomisation (approximates 4 weeks post‐QD) | |
| Notes | Pilot study ‐ full trial is Free 2011 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Central computerised randomisation |
| Allocation concealment (selection bias) | Low risk | Concealed until after assignment |
| Blinding (performance bias and detection bias) | Low risk | Single blind (participants not blinded) |
| Incomplete outcome data (attrition bias) | Low risk | Lost to follow‐up: 4 (control) and 1 (intervention) at 4 weeks (98% follow‐up); 8 (control) and 8 (intervention) at 6 months (92% follow‐up) |
| Other bias | Unclear risk | None described |
| Methods | RCT in UK | |
| Participants | 5800 participants aged ≥ 16 years, willing to make an attempt to quit smoking in the next month and owned a mobile phone. 45% women, mean age of 37 years, 89% white and 25% students/unemployed. 60% of participants had an FTND dependence score of ≤ 5 | |
| Interventions | 6‐month programme: delivered solely over mobile phone based on programme in Rodgers 2005. Participants asked to set a QD within 2 weeks of randomisation. They received 5 text messages/day for the first 5 weeks and then 3/week for the next 26 weeks. Intervention included motivational messages and behaviour‐change techniques. The programme was also personalised with an algorithm based on demographic and other information gathered at baseline, such as smoker's concerns about weight gain after quitting. The core programme consisted of 186 messages and the personalised messages were selected from a database of 713 messages. For instance, by texting the word "lapse", participants received a series of 3 text messages that encouraged them to continue with their quit attempt. Participants could also request the mobile phone number of another trial participant so that they could text each other for support. Participants in the intervention group using pay‐as‐you‐go mobile phone schemes were given a £20 top‐up voucher to provide sufficient credit to participate in the intervention Control: fortnightly, simple, short, text messages related to the importance of trial participation | |
| Outcomes | No more than 5 cigarettes smoked since the start of the abstinence period at 6 months of follow‐up, self reported and verified by postal salivary cotinine testing or a CO test in person | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | An independent telephone randomisation system |
| Allocation concealment (selection bias) | Low risk | Concealed until after assignment |
| Blinding (performance bias and detection bias) | Low risk | Single blind (participants not blinded) |
| Incomplete outcome data (attrition bias) | Low risk | 176 intervention and 92 control lost to follow‐up (< 5% total) |
| Other bias | Unclear risk | None described |
| Methods | RCT in US | |
| Participants | 474 participants aged ≥ 18 years recruited from an HIV clinic in a low‐income multi‐ethnic urban population in Texas, USA. 70% men, mean age 44.8 years, mean FTND 5.8. Inclusion criteria: HIV‐positive, current smoker (≥ 5 cigarettes/day and expired CO ≥ 7 ppm), willing to set a QD within 7 days, and ability to speak English or Spanish | |
| Interventions | Participants in the mobile phone intervention group received the usual care components plus a mobile phone‐delivered counselling intervention over 3 months and access to a supportive hotline. They were provided with a pre‐paid mobile phone on which a series of 11 proactive counselling sessions were conducted. The phone calls spanned a 3‐month period but were front loaded such that the frequency of the calls was highest near the time of scheduled quit attempt. Counselling session content was primarily drawn from a cognitive‐behavioural foundation emphasising problem solving and skills training techniques Control: participants completed an audio computer‐assisted self interview, then received provider advice to quit smoking. Usual care was provided with targeted written smoking cessation materials (i.e. a "tip sheet" designed to address concerns of HIV‐positive smokers) and instructions on how to obtain nicotine‐replacement therapy in the form of nicotine patches at the clinic | |
| Outcomes | Primary outcome: self reported repeated measures 7‐day point prevalence at 3, 6 and 12 months Secondary outcomes: 3, 6 and 12 months' smoking abstinence (24 hours, 7 days and 30 days), CO verified quitting, number of quit attempts, length of abstinence (in days), use of nicotine‐replacement therapy, use of other cessation treatments and exposure to other forms of tobacco. Other smoking‐related measures included the FTND, the Reasons for Quitting scale (intrinsic and extrinsic quit motivation) and the 9‐item quitting self efficacy scale. Depressive symptoms: Center for Epidemiologic Studies Depression (CES‐D), State Trait Anxiety Inventory. Quality of life: Medical Outcomes Study HIV Health Survey (MOS‐HIV). Alcohol use: Alcohol Use Disorders Identification Test. A single item was used to assess illicit drug | |
| Notes | Expanded programme based on Vidrine 2006 Varied from the other interventions in using pre‐paid provided mobile phones to provide counselling instead of an SMS intervention. Many smokers were excluded (40%) due to not meeting 5 cigarettes/day and CO ≥ 7 ppm). Low absolute quit rates may be due to high nicotine dependence, high rate of alcohol and drug use, and the substantial burden of mental illness amongst participants | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised ‐ method not stated |
| Allocation concealment (selection bias) | Low risk | Allocated after baseline data collected |
| Blinding (performance bias and detection bias) | Low risk | Risk was minimised with the following measures:
|
| Incomplete outcome data (attrition bias) | Low risk | 51 in control and 61 in intervention did not complete 6‐month follow‐up but ITT analysis presented |
| Other bias | Low risk | Expired CO to validate smoking status. Use of a single HIV clinic, a county site with a large patient population |
| Methods | Cluster RCT in Switzerland | |
| Participants | 755 daily or occasional smokers (≥ 4 cigarettes in the preceding month and ≥ 1 cigarette during the preceding week) recruited at 24 vocational schools (178 classes). 48% male, mean age 18.2 years (SD 2.3) | |
| Interventions | SMS‐COACH: a 3‐month programme including a weekly SMS text message assessment of smoking‐related target behaviour, 2 weekly text messages tailored to baseline data and responses to the SMS text message assessments, and an optional further integrated QD preparation and relapse prevention SMS programme. Participants who did not use the integrated programme for QD preparation and relapse prevention received a total of 37 text messages (1 welcome message, 11 assessment messages, 24 tailored feedback messages, 1 goodbye message). Participants, who used the QD preparation and relapse‐prevention programme for the whole period from 1 week before the scheduled quit date until 3 weeks afterwards, received an additional 42 text messages Control: all students in participating classes were invited to participate in an online health screening survey during a regular school lesson reserved for health education. The control group did not receive anything else | |
| Outcomes | Primary outcome: 7‐day point prevalence smoking abstinence at 6 months Secondary outcomes: 4‐week point prevalence smoking abstinence, the number of cigarettes smoked/day, stage of change and number of attempts to quit smoking | |
| Notes | The study did not reach the target sample size of 910 participants due to smaller class size than expected and time restrictions. Nicotine dependence was not calculated but number of cigarettes smoked/day used as an indicator and outcome variable | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Cluster randomisation with class as the unit of randomisation, stratified by school to control for heterogeneity between schools. Block randomisation with computer‐generated randomly permuted blocks of 4 cases |
| Allocation concealment (selection bias) | Low risk | Students recruited prior to randomisation and informed after baseline |
| Blinding (performance bias and detection bias) | Low risk | Baseline and follow‐up assessors blinded to allocation |
| Incomplete outcome data (attrition bias) | Low risk | 111 in control and 85 in intervention were lost to follow‐up at 6 months. ITT analysis conducted |
| Other bias | Unclear risk | Self report outcomes |
| Methods | RCT in UK | |
| Participants | Participants aged 18‐75 years and current smokers (≥ 1 cigarette /day and smoked within previous 7 days) who were willing to quit within 14 days of randomisation, recruited in primary care. Primary care practices were those that had smoking cessation advisors (primary care nurses or healthcare assistants) providing level 2 cessation advice. Participants were self referred or referred by a health professional. 47% men, mean age 41.8 years (range 18‐75 years), able to read English and provide written informed consent, with a mobile phone and familiar with sending and receiving text messages and not enrolled in another formal smoking cessation study or other cessation programme | |
| Interventions | Intervention: usual care plus a tailored advice report and a 90‐day programme of tailored text messages generated by the iQuit system (number of messages sent each day varied from 0 to 2, mean/day over 90 days 1.2). The messages were designed to advise smokers on their quit attempt, provide information about the consequences of smoking and expectations for quitting, provide encouragement, boost self efficacy, maintain motivation to quit and remind smokers how to cope with difficult situations Control: 'usual care' consisted of routine 'level 2' smoking cessation advice delivered by smoking cessation adviser. This included a brief discussion about smoking habits and history, measurement of expired‐air CO, brief advice to quit, setting a QD within the next 14 days, options for pharmacotherapy, a prescription and arranging a follow‐up visit. Usually the opportunity for multiple follow‐up visits was offered | |
| Outcomes | Primary outcome: self reported 2‐week point prevalence abstinence at 8 weeks Secondary outcomes: CO‐verified abstinence at 4‐week for at least 2 weeks, assessed by a smoking cessation adviser (a CO reading assessed 25‐42 days from QD that was < 10 ppm), self reported 3‐month prolonged abstinence at 6 months, 6‐month prolonged abstinence at 6‐month follow‐up and a strict continuous abstinence measure using all outcome time points: CO‐validated 2‐week point prevalence abstinence at 4 weeks, 4‐week point prevalence abstinence at 8 weeks and 6‐month prolonged abstinence at 6 months | |
| Notes | Outcomes used in this meta‐analysis were self reported. Control programme was fairly intensive, i.e. smoking cessation advice provided in‐person | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised by online programme |
| Allocation concealment (selection bias) | Low risk | Randomised by online programme once baseline data collected |
| Blinding (performance bias and detection bias) | Low risk | 6‐month data collected by postal questionnaire or by researchers blinded to allocation |
| Incomplete outcome data (attrition bias) | High risk | 65 in control and 70 in intervention lost to follow‐up at 6 months but ITT analyses presented |
| Other bias | Unclear risk | Self reported outcomes included |
| Methods | RCT in New Zealand | |
| Participants | 1705 participants aged ≥ 16 years recruited by direct advertising, smoking daily, wanting to quit within the next month, and were able to send and receive text messages on their own mobile phone 58% female, median age 22 years, 20.8% Maori (indigenous population), smoked mean 15 cigarettes/day and mean FTND dependence score 5 | |
| Interventions | 6‐month programme delivered solely over mobile phone. Participant nominated QD and received regular personalised text messages with advice, support and distraction, with a countdown to QD, intensive 4 weeks of 5 or 6 messages/day then maintenance phase of 1 message/2 weeks. Messages selected from database matched to participant characteristics. Free month of text messaging from QD. Optional Quit Buddy and Text Crave (messages on demand). Interactive polls and quizzes | |
| Outcomes | Primary outcome: point prevalence abstinence (no smoking in past 7 days) at 6 weeks' post‐randomisation (approximates 4 weeks post‐QD). Verification with salivary cotinine in small number of quitters at 6 weeks | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Central computerised randomisation |
| Allocation concealment (selection bias) | Low risk | Concealed until after assignment |
| Blinding (performance bias and detection bias) | Low risk | Single blind (participants not blinded) |
| Incomplete outcome data (attrition bias) | High risk | Lost to follow‐up: 35 control (95.9%) and 46 intervention (94.6%) followed up at 6 weeks; but differential loss to follow‐up at 6 months (79% control vs. 69% intervention). Possibly due to incentive being offered to control group for follow‐up, may in turn have affected long‐term results of study (by underestimating effect) |
| Other bias | High risk | The authors suggested that some participants in the control group may have thought their incentive at follow‐up (month of free text messaging) depended on reporting quitting. This could account for an unexpected increase in control group participants reporting quitting from 6 weeks (109 participants) to 6 months (202 participants reporting no smoking in the past 7 days), which could have led to an underestimation of the effect of the intervention |
| Methods | 3‐arm RCT in US | |
| Participants | Participants aged ≥ 18 years recruited from large urban HIV clinics in the USA Inclusion criteria: current patient of the clinics, current or regular smoker (≥ 5 cigarettes/day), CO ≥ 8 ppm, willing to set a QD within the next 2 weeks, willing to use a mobile phone and able to read text messages, and eligible to take varenicline Exclusion criteria: alcohol dependence and active drug abuse, and conditions that would prevent the use of varenicline | |
| Interventions | Intervention: participants received standard care (see below) and 2 text messages/day for 12 weeks. 1 message reminding them to take their medication and 1 motivational message regarding cessation Control group: received standard care, which consisted of a self help information sheet, tailored to HIV‐positive smokers and an offer of varenicline for 12 weeks according to the standard dosage schedule. Participants needed to return to the clinic each 4 weeks to receive further medication. All participants were provided with a pre‐paid mobile phone ‐ the control group received phones to facilitate their ability to call the quit line and receive text message appointment reminders only A third arm received standard care, text messages, plus behavioural therapy delivered via 7 proactive mobile phone‐delivered counselling sessions over a 6‐week period. These combined cognitive behavioural therapy and motivational interviewing techniques | |
| Outcomes | Primary outcome: 7‐day point prevalence abstinence verified by CO < 8 ppm at 24 weeks, also measured at 1, 4, 8 and 12 weeks | |
| Notes | Unpublished ‐ we did not have the participant characteristics tables | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation schedule, stratified by people smoking 5‐10 and people smoking > 10 cigarettes/day |
| Allocation concealment (selection bias) | Low risk | After consent and baseline data collected, the research assistant called to receive the assignment |
| Blinding (performance bias and detection bias) | Unclear risk | Participants self complete questionnaires through audio computer‐assisted self interviewing |
| Incomplete outcome data (attrition bias) | Unclear risk | 21 participants in the control group, 19 in the text message group (and 24 in the text message + phone counselling group) did not complete 24‐week follow‐up visits. ITT analysis is used in this meta‐analysis |
| Other bias | Unclear risk | None described |
| Methods | RCT in New Zealand | |
| Participants | 226 participants aged ≥ 16 years recruited by advertising if they were current daily smokers ready to quit, and had a video message‐capable phone. Advertising particularly targeted young adults. 47% female, 24% Maori (indigenous population), mean age 27 years and appeared to be highly addicted due to the Hooked on Nicotine Checklist mean scores of 8 (SD 1.9) out of 10 | |
| Interventions | Intervention group: received an automated package of video and text messages over 6 months that was tailored to self selected quit date, role model and timing of messages. Video messages were video diary‐style from a selected 'ordinary' person going through a quit attempt in advance of the participant. Frequency of messages varied from 1/day in the lead up to QD, 2/day from QD for 4 weeks, then reducing to 1 every 2 days for 2 weeks and then 1 every 4 days for about 20 weeks until 6 months after randomisation. Extra messages were available on demand to beat cravings and address lapses. Additional website for intervention group participants to review video messages they had been sent (and rate them if desired), change their selected time periods and change (or add to) their selected role model. Control: also set a QD and received a general health video message sent to their phone every 2 weeks | |
| Outcomes | Self reported continuous abstinence ‐ no more than 5 cigarettes smoked since the start of the abstinence period at 6 months of follow‐up | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Central computerised randomisation |
| Allocation concealment (selection bias) | Low risk | Concealed until after assignment |
| Blinding (performance bias and detection bias) | Low risk | Single blind (participants not blinded) |
| Incomplete outcome data (attrition bias) | Low risk | 32% intervention and 22% control lost to follow‐up at 6 months |
| Other bias | Unclear risk | None described |
CI: confidence interval; CO: carbon monoxide; FTND: Fagerström Test of Nicotine Dependence; HIV: human immunodeficiency virus; ITT: intention to treat; ppm: parts per million; QD: quit day; RCT: randomised controlled trial; SD: standard deviation; SMS: short messaging service.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Abstract describing intervention to increase adherence to the use of nicotine replacement gum in people attempting to quit smoking. Duration 8 weeks | |
| Editorial comment on the Free 2011 trial | |
| Internet‐based telemonitoring programme for secondary prevention in cardiovascular disease with parameters sent by mobile phone | |
| Mobile phone intervention confounded with Internet intervention (previously included in Whittaker 2009) | |
| Mobile phone intervention confounded with Internet intervention (previously included in Whittaker 2009) | |
| Pilot study with 2‐month follow‐up only | |
| Follow‐up only to 3 months | |
| Focused on cardiac rehabilitation | |
| Focused on cardiac rehabilitation | |
| Not focused on delivery by mobile phone | |
| Not focused on delivery by mobile phone | |
| Non‐randomised feasibility study. Duration 12 weeks | |
| Mainly about acceptability, 3 months' follow‐up | |
| Protocol for a study on alcohol and smoking in adolescents | |
| 3 months' follow‐up | |
| Not focused on smoking cessation | |
| Not randomised. No control group. Feasibility study for the programme presented in Vidrine 2006 | |
| Not focused on delivery by mobile phone | |
| Randomised controlled trial with pregnant smokers, follow‐up to 3 months | |
| Not randomised. No control group | |
| Not randomised | |
| Not focused on delivery by mobile phone | |
| Gradual reduction in pregnant women | |
| Small non‐randomised study with only 6 weeks' follow‐up | |
| Follow‐up only 3 months | |
| Not focused on delivery by mobile phone | |
| Compared tailored with un‐tailored text messages ‐ no control group | |
| Not a trial | |
| Not focused on delivery by mobile phone | |
| Randomised trial of phone counselling with mobile phones, follow‐up only 3 months | |
| Follow‐up only 3 months | |
| Not focused on smoking cessation | |
| Pilot RCT, follow‐up only 3 months | |
| Pilot RCT, follow‐up only 3 months | |
| Single‐blind RCT, follow‐up 24 weeks, but with no details available on the randomisation method or the intervention content. Abstract only, unable to contact authors |
RCT: randomised controlled trial.
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Assessing the Effect of an Interactive Decision‐Aid Smartphone Smoking Cessation Application (app) on Quit Rates: a Double‐Blind Automated Randomised Control Trial Protocol |
| Methods | RCT |
| Participants | Daily smokers aged ≥ 18 years in Australia, Singapore, the UK and the US |
| Interventions | Smoking cessation app |
| Outcomes | Continuous abstinence at 1 and 6 months |
| Starting date | May 2014 |
| Contact information | Nasser F BinDhim; [email protected] |
| Notes |
| Trial name or title | MiQuit Trial: Tailored Text Messages for Pregnant Women |
| Methods | RCT |
| Participants | Smokers aged ≥ 16 years, pregnant, in UK |
| Interventions | MiQuit is an automated responsive text message support programme lasting 12 weeks |
| Outcomes | Continuous abstinence from 4 weeks after randomisation until follow‐up at the end of pregnancy |
| Starting date | February 2014 |
| Contact information | Tim Coleman, University of Nottingham |
| Notes |
| Trial name or title | Internet and Text Messaging Intervention in Norway |
| Methods | RCT |
| Participants | Current smokers aged ≥ 16 years |
| Interventions | Internet‐based smoking cessation versus Internet plus text messaging |
| Outcomes | Point prevalence abstinence at 1, 3, 6 and 12 months |
| Starting date | 2011 |
| Contact information | Professor Gram, Faculty of Health Sciences, Institute of Community Medicine, University of Tromsø, Norway, [email protected] |
| Notes |
| Trial name or title | Abstinence Reinforcement Therapy for Rural Veteran Smokers |
| Methods | RCT |
| Participants | Durham VA enrolees |
| Interventions | Cognitive behavioural telephone counselling, telemedicine clinic for access to nicotine‐replacement therapy, mobile contingency management |
| Outcomes | Quality‐adjusted life years at 12 months |
| Starting date | November 2013 |
| Contact information | Patrick Calhoun, Durham VA Medical Center, Durham NC |
| Notes |
| Trial name or title | Effectiveness of Messages to Mobile Phone in Smoke Cessation |
| Methods | RCT |
| Participants | Current smokers aged > 18 years in Spain |
| Interventions | Quit advice by a doctor and support messages to mobile phones |
| Outcomes | Continuous abstinence at 6 months |
| Starting date | December 2012 |
| Contact information | Raquel Cobos Campos, Basque Health Service |
| Notes |
| Trial name or title | Study of Mobile Phone Support for the DC Tobacco Quitline |
| Methods | RCT |
| Participants | Current smokers aged ≥ 18 years in US |
| Interventions | Internet‐based system using mobile phones to increase the quality, frequency and accessibility of support |
| Outcomes | Point prevalence abstinence at 3, 6 and 9 months |
| Starting date | July 2010 |
| Contact information | Thomas Kirchner, American Legacy Foundation |
| Notes |
| Trial name or title | Web and Mobile Smoking Cessation |
| Methods | RCT |
| Participants | Smokers aged ≥ 18 years in US |
| Interventions | Internet plus mobile compared with Internet only |
| Outcomes | Point prevalence 3 and 6 months |
| Starting date | April 2015 |
| Contact information | Brian Danaher [email protected] |
| Notes |
| Trial name or title | A Mindfulness Based Application for Smoking Cessation |
| Methods | RCT |
| Participants | Smokers aged ≥ 18 years in the US |
| Interventions | A mindfulness based smartphone app |
| Outcomes | Number of cigarettes smoked at 6 months |
| Starting date | September 2013 |
| Contact information | Jennifer Penberthy [email protected] |
| Notes |
| Trial name or title | A Randomised Controlled Trial to Test the Effect of a Smartphone Quit Smoking Intervention on Young Adult Smokers |
| Methods | RCT |
| Participants | Smokers aged 19‐29 years in Canada |
| Interventions | Crush The Crave smartphone application |
| Outcomes | Point prevalence (30 day) at 6 months |
| Starting date | January 2014 |
| Contact information | |
| Notes |
| Trial name or title | Use of Technological Advances to Prevent Smoking Relapse Among Smokers with PTSD |
| Methods | RCT |
| Participants | Smokers aged 18‐70 years in US |
| Interventions | Quit4ever combines counselling sessions, bupropion and nicotine‐replacement therapy, mobile contingency management and the smartphone application Stay Quit Coach |
| Outcomes | Point prevalence abstinence at 3 and 6 months |
| Starting date | December 2013 |
| Contact information | Jean Beckham, Duke University |
| Notes |
| Trial name or title | Korean Youth Smoking Cessation Study |
| Methods | RCT |
| Participants | Smokers aged 14‐19 years Korean/American youth in US |
| Interventions | Tailored interactive cognitive behavioural motivation enhancement therapy delivered through Internet and mobile phones |
| Outcomes | Point prevalence abstinence at 6 months |
| Starting date | June 2016 |
| Contact information | Steve Shoptaw [email protected] |
| Notes |
| Trial name or title | Mobile Mindfulness Training for Smoking Cessation |
| Methods | RCT |
| Participants | Smokers aged 18‐65 years in US |
| Interventions | Smartphone‐based training programme |
| Outcomes | Point prevalence abstinence at 6 months |
| Starting date | August 2015 |
| Contact information | Judson Brewer [email protected] |
| Notes |
| Trial name or title | Smartphone Application for Smoking Cessation |
| Methods | RCT |
| Participants | Smokers aged 18‐65 years in US |
| Interventions | 3‐week smartphone‐based training programme |
| Outcomes | Point prevalence at 6 months |
| Starting date | October 2014 |
| Contact information | Kathleen Garrison, Yale University |
| Notes |
| Trial name or title | Internet‐Based Medication Adherence Program for Nicotine Dependence Treatment |
| Methods | RCT |
| Participants | Smokers aged 18‐65 years |
| Interventions | My Mobile Advice Program via smartphone or Internet device |
| Outcomes | Point prevalence abstinence at 5 months |
| Starting date | October 2014 |
| Contact information | |
| Notes |
| Trial name or title | A Quit Smoking Study Using Smartphones |
| Methods | RCT |
| Participants | Smokers aged ≥ 18 years in the US |
| Interventions | Mobile games |
| Outcomes | Number of cigarettes/day |
| Starting date | October 2014 |
| Contact information | Tanya Schlam [email protected] |
| Notes |
| Trial name or title | Social Media Intervention for Young Adult Smokers |
| Methods | RCT |
| Participants | Smokers aged 18‐25 years in the US |
| Interventions | 3 month Facebook intervention |
| Outcomes | Point prevalence abstinence at 6 and 12 months |
| Starting date | October 2014 |
| Contact information | Danielle A Ramo, UCSF |
| Notes |
| Trial name or title | Developing a Smartphone App With Mindfulness Training for Teen Smoking Cessation |
| Methods | RCT |
| Participants | Smokers aged 13‐19 years in US |
| Interventions | Smoking cessation treatment delivered through a smartphone app via mindfulness training |
| Outcomes | Point prevalence abstinence at 3 and 6 months |
| Starting date | September 2014 |
| Contact information | Lori Pbert, University of Massachusetts |
| Notes |
| Trial name or title | Smoking Response Inhibition Training |
| Methods | RCT |
| Participants | Smokers aged 18‐45 years in US |
| Interventions | Smoking‐specific response inhibition training programme in the context of a quit attempt. The task is based on a modified stop‐signal task |
| Outcomes | Smoking relapse at 6 months |
| Starting date | September 2014 |
| Contact information | Robert D Dvorak, North Dakota State University |
| Notes |
| Trial name or title | Harnessing the Power of Technology: MoMba for Postpartum Smoking |
| Methods | RCT |
| Participants | Smokers aged 18‐50 years in US |
| Interventions | MoMba Live Long Smartphone application |
| Outcomes | Point prevalence abstinence at 21 months |
| Starting date | February 2016 |
| Contact information | Ruth M Arnold, [email protected] |
| Notes |
| Trial name or title | Abstinence Reinforcement Therapy (ART) for Homeless Veteran Smokers |
| Methods | RCT |
| Participants | Smokers aged 18‐75 years |
| Interventions | Nicotine patches plus mobile contingency management (participants upload videos of themselves taking carbon monoxide readings. Any time a participant uploads a video that suggests abstinence, he/she is provided with a monetary reward) |
| Outcomes | Smoking abstinence at 6 months |
| Starting date | October 2014 |
| Contact information | Angela C Kirby, [email protected] |
| Notes |
| Trial name or title | Mobile Media‐Rich Interactive Guideline System (MMRIGS) Pilot Study |
| Methods | RCT |
| Participants | Smokers aged ≥ 18 years and older |
| Interventions | Brief advice to quit smoking (tailored video clips) and an 8‐week automated intervention (interactive text messages and graphical messages) for support in quitting smoking via smartphone |
| Outcomes | Smoking abstinence 3 months |
| Starting date | January 2015 |
| Contact information | Alex Prokhorov, M.D. Anderson Cancer Center |
| Notes |
| Trial name or title | Randomized Clinical Trial to Reduce Harm From Tobacco |
| Methods | RCT |
| Participants | Smokers aged ≥ 18 years |
| Interventions | eCigarettes, nicotine‐replacement therapy and other pharmacotherapy, incentives and a standard programme of support including text messaging |
| Outcomes | Verified abstinence at 6 months |
| Starting date | January 2015 |
| Contact information | Kathryn A Saulsgiver [email protected] |
| Notes |
| Trial name or title | Penn State TXT2Quit Study |
| Methods | RCT |
| Participants | Smokers aged ≥ 21 years |
| Interventions | Varenicline and motivational text messages |
| Outcomes | Point prevalence at 3 months |
| Starting date | January 2015 |
| Contact information | Jonathan Foulds, Milton S. Hershey Medical Center |
| Notes |
| Trial name or title | Efficacy of a Mobile Application in the Smoking Cessation Among Young People (TOBB_STOP) |
| Methods | RCT |
| Participants | Smokers aged 18‐30 years in Spain |
| Interventions | Mobile phone application for smartphone |
| Outcomes | Smoking cessation at 6 months |
| Starting date | January 2013 |
| Contact information | Empar Valdivieso López evaldivieso.tarte.ics%40gencat.cat |
| Notes |
| Trial name or title | Smoking Cessation for Low‐Income Adults |
| Methods | RCT |
| Participants | Smokers aged ≥ 18 years in US |
| Interventions | Standard care plus mobile phone‐delivered text/graphical messaging component plus 11 mobile phone‐delivered proactive counselling sessions |
| Outcomes | Smoking abstinence at 12 months |
| Starting date | June 2010 |
| Contact information | Alex Prokhorov, MD Anderson Cancer Center |
| Notes |
RCT: randomised controlled trial.
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 26‐week cessation outcomes all studies Show forest plot | 12 | 11885 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.67 [1.46, 1.90] |
| Analysis 1.1  Comparison 1 Mobile phone intervention versus control, Outcome 1 26‐week cessation outcomes all studies. | ||||
| 2 26‐week continuous abstinence Show forest plot | 8 | 10679 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.72 [1.50, 1.98] |
| Analysis 1.2  Comparison 1 Mobile phone intervention versus control, Outcome 2 26‐week continuous abstinence. | ||||
| 3 26‐week 7‐day point prevalence Show forest plot | 7 | 3888 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [1.03, 1.35] |
| Analysis 1.3  Comparison 1 Mobile phone intervention versus control, Outcome 3 26‐week 7‐day point prevalence. | ||||
| 4 Biochemically verified 26‐week abstinence Show forest plot | 6 | 7360 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.83 [1.54, 2.19] |
| Analysis 1.4  Comparison 1 Mobile phone intervention versus control, Outcome 4 Biochemically verified 26‐week abstinence. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 26‐week quitting outcomes Show forest plot | 7 | 9887 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.69 [1.46, 1.95] |
| Analysis 2.1  Comparison 2 Text messaging‐only interventions, Outcome 1 26‐week quitting outcomes. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Text message plus face‐to‐face interventions Show forest plot | 5 | 1995 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.54 [1.12, 2.11] |
| Analysis 3.1  Comparison 3 Text messaging plus face‐to‐face interventions, Outcome 1 Text message plus face‐to‐face interventions. | ||||

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Forest plot of comparison: 1 Mobile phone intervention v ersus control, outcome: 1.1 26‐week cessation outcomes all studies.

Forest plot of comparison: 1 Mobile phone intervention versus control; outcome: 1.4 26‐week biochemically verified cessation outcomes (six studies).
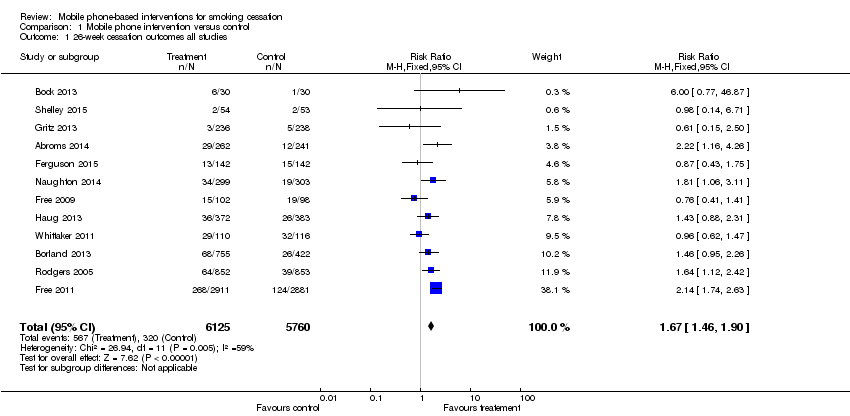
Comparison 1 Mobile phone intervention versus control, Outcome 1 26‐week cessation outcomes all studies.

Comparison 1 Mobile phone intervention versus control, Outcome 2 26‐week continuous abstinence.
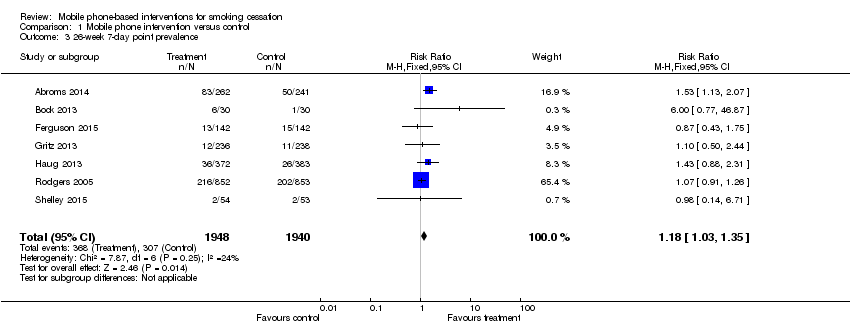
Comparison 1 Mobile phone intervention versus control, Outcome 3 26‐week 7‐day point prevalence.
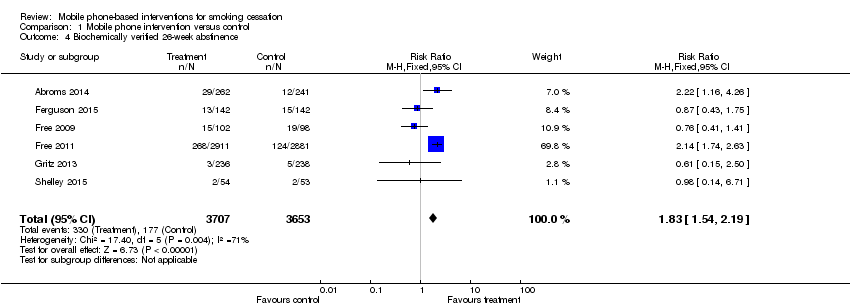
Comparison 1 Mobile phone intervention versus control, Outcome 4 Biochemically verified 26‐week abstinence.
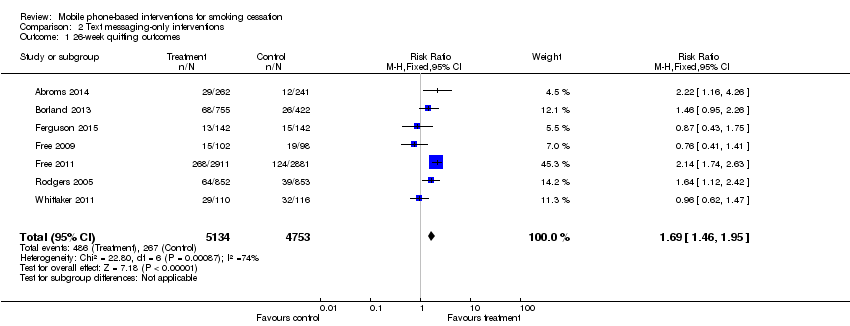
Comparison 2 Text messaging‐only interventions, Outcome 1 26‐week quitting outcomes.

Comparison 3 Text messaging plus face‐to‐face interventions, Outcome 1 Text message plus face‐to‐face interventions.
| Mobile phone‐based interventions for smoking cessation | ||||||
| Patient or population: people who smoke | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed quitters without intervention | Estimated quitters with mobile phone interventions | |||||
| 26‐week smoking cessation | Study population | RR 1.67 | 11,885 | ⊕⊕⊕⊝ | There was evidence of moderate heterogeneity across the included studies | |
| 56 per 1000 | 93 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 There was evidence of moderate heterogeneity. Sensitivity analyses around potential explanations for heterogeneity did not make substantial differences to the findings. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 26‐week cessation outcomes all studies Show forest plot | 12 | 11885 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.67 [1.46, 1.90] |
| 2 26‐week continuous abstinence Show forest plot | 8 | 10679 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.72 [1.50, 1.98] |
| 3 26‐week 7‐day point prevalence Show forest plot | 7 | 3888 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [1.03, 1.35] |
| 4 Biochemically verified 26‐week abstinence Show forest plot | 6 | 7360 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.83 [1.54, 2.19] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 26‐week quitting outcomes Show forest plot | 7 | 9887 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.69 [1.46, 1.95] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Text message plus face‐to‐face interventions Show forest plot | 5 | 1995 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.54 [1.12, 2.11] |

