Intervenciones basadas en mensajes de texto y aplicaciones de teléfonos móviles para el abandono del hábito de fumar
Resumen
Antecedentes
El apoyo para dejar de fumar a través de teléfonos móviles (mCessation) ofrece la oportunidad de brindar apoyo conductual a las personas que no pueden o no quieren recibir apoyo presencial. Además, mCessation se puede automatizar y, por lo tanto, ofrecerse de forma asequible incluso en contextos de bajos recursos. Esta es una actualización de una revisión Cochrane publicada por primera vez en 2006 y actualizada previamente en 2009 y 2012.
Objetivos
Determinar si las intervenciones basadas en teléfonos móviles para el abandono del hábito de fumar aumentan la tasa de abandono del hábito de fumar en las personas que fuman.
Métodos de búsqueda
Para esta actualización, se realizaron búsquedas en el Registro Especializado del Grupo Cochrane de Adicción al Tabaco, junto con clinicaltrials.gov y la ICTRP. La fecha de las búsquedas más recientes fue el 29 de octubre 2018.
Criterios de selección
Los participantes eran fumadores de cualquier edad. Las intervenciones elegibles fueron las que probaron cualquier tipo de programa basado predominantemente en teléfonos móviles (como mensajes de texto o aplicaciones para teléfonos inteligentes) para el abandono del hábito de fumar. Se incluyeron los ensayos controlados aleatorizados con resultados de abandono del hábito de fumar que informaron al menos seis meses de seguimiento.
Obtención y análisis de los datos
Se utilizaron los procedimientos metodológicos estándar descritos en el Manual Cochrane de Revisiones Sistemáticas de Intervenciones (Cochrane Handbook for Systematic Reviews of Interventions). Se realizaron comprobaciones por duplicado de la elegibilidad de los estudios y de la extracción de los datos. Con el uso del método de efectos aleatorios de Mantel‐Haenszel se realizaron metanálisis de las medidas más rigurosas de abstinencia a los seis meses de seguimiento o más, agrupando los estudios con intervenciones y comparadores similares para calcular los cocientes de riesgos (CR) y sus correspondientes intervalos de confianza (IC) del 95%. Se realizaron análisis que incluyeron todos los casos asignados al azar (con los abandonos considerados como todavía fumadores) y los completos solamente.
Resultados principales
Esta revisión incluye 26 estudios (33 849 participantes). En general, se consideró que 13 estudios presentaron un riesgo de sesgo bajo; tres, un riesgo alto; y el resto, un riesgo incierto. Los contextos y los procedimientos de reclutamiento variaron entre los estudios, pero la mayoría de los estudios se realizaron en países de ingresos altos. Hubo evidencia de certeza moderada, limitada por la inconsistencia, de que las intervenciones automatizadas de mensajería de texto fueron más efectivas que el apoyo mínimo para el abandono del hábito de fumar (CR 1,54; IC del 95%: 1,19 a 2,00; I2 = 71%; 13 estudios, 14 133 participantes). También hubo evidencia de certeza moderada, limitada por la imprecisión, de que los mensajes de texto agregados a otras intervenciones para el abandono del hábito de fumar fueron más efectivos que las otras intervenciones para el abandono del hábito de fumar solas (CR 1,59; IC del 95%: 1,09 a 2,33; I2 = 0%, 4 estudios, 997 participantes). Dos estudios que compararon la mensajería de texto con otras intervenciones para el abandono del hábito de fumar y tres estudios que compararon la mensajería de alta y baja intensidad no mostraron diferencias significativas entre los grupos (CR 0,92; IC del 95%: 0,61 a 1,40; I2 = 27%; 2 estudios, 2238 participantes y CR 1,00; IC del 95%: 0,95 a 1,06; I2 = 0%; 3 estudios, 12 985 participantes, respectivamente), pero los intervalos de confianza fueron amplios en la primera comparación. Cinco estudios compararon una aplicación para el abandono del hábito de fumar con un apoyo de menor intensidad para el abandono del hábito de fumar (ya sea un apoyo mínimo con aplicaciones de teléfonos móviles de menor intensidad o sin aplicaciones de teléfonos móviles). La evidencia se agrupó y se consideró que era de certeza muy baja debido a la inconsistencia y la imprecisión grave. No hubo evidencia de que las aplicaciones de teléfonos inteligentes mejoraran la probabilidad de dejar de fumar (CR 1,00; IC del 95%: 0,66 a 1,52; I2 = 59%; 5 estudios, 3079 participantes). Otras aplicaciones de teléfonos inteligentes probadas diferían de las aplicaciones incluidas en el análisis, ya que dos utilizaron el manejo de contingencias y una combinó mensajería de texto con una aplicación, por lo que no se agruparon. El uso de los datos de casos completos en lugar de utilizar datos de todos los participantes asignados al azar no alteró significativamente los resultados.
Conclusiones de los autores
Existe evidencia de certeza moderada de que las intervenciones automatizadas para el abandono del hábito de fumar basadas en mensajes de texto producen tasas de abandono mayores que el apoyo mínimo para el abandono del hábito de fumar. Existe evidencia de certeza moderada del efecto beneficioso de las intervenciones de mensajes de texto, además de otro apoyo para el abandono del hábito de fumar, en comparación con el apoyo solo para el abandono del hábito de fumar. La evidencia que compara las aplicaciones de teléfonos inteligentes con un apoyo menos intensivo fue de certeza muy baja y se necesitan más ensayos controlados aleatorizados para probar estas intervenciones.
PICO
Resumen en términos sencillos
¿Los programas implementados mediante teléfonos móviles pueden ayudar a las personas a abandonar el hábito de fumar?
Antecedentes
Fumar tabaco es una causa principal de muerte evitable. Los teléfonos móviles se pueden utilizar para ayudar a las personas que desean dejar de fumar. Esta revisión se ha centrado en los programas que utilizan mensajes de texto o aplicaciones de teléfonos inteligentes con este objetivo.
Fecha de la búsqueda
En octubre 2018 se realizaron búsquedas de estudios publicados y no publicados.
Características de los estudios
Se incluyeron 26 estudios controlados aleatorizados (con más de 33 000 personas) que compararon las tasas de abandono del hábito de fumar en personas que recibieron mensajes de texto o utilizaron aplicaciones de teléfonos inteligentes para ayudarles en el abandono con personas que no recibieron estos programas. Fueron de interés los estudios que midieron el hábito de fumar durante seis meses o más.
Resultados clave
Se encontró que los programas de mensajes de texto pueden ser efectivos para ayudar a las personas a dejar de fumar, con un aumento de las tasas de abandono del hábito de entre un 50% a un 60%. Este fue el caso cuando se compararon con ayuda mínima o se probaron como complemento de otras formas de ayuda para dejar de fumar. No hubo evidencia suficiente para determinar el efecto de las aplicaciones para teléfonos inteligentes.
Calidad y completitud de la evidencia
La mayoría de los estudios fueron de calidad alta, aunque tres estudios tuvieron tasas de abandono altas. Se tiene una confianza moderada en los resultados de las intervenciones de mensajería de texto, pero hubo algunos aspectos con diferencias inexplicables entre los hallazgos de los estudios, y para algunas comparaciones no hubo muchos datos. Hay poca confianza en los resultados relacionados con las aplicaciones para teléfonos inteligentes, y se necesitan más estudios en este campo.
Conclusiones de los autores
Summary of findings
| Text messaging compared to minimal support for smoking cessation | ||||||
| Patient or population: people who smoke | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
| Risk with minimal SC support | Risk with text messaging | |||||
| Long‐term abstinence (all randomised) Measured with self‐report and biochemical validation at 6 to 12 months | Study population | RR 1.54 | 14,133 | ⊕⊕⊕⊝ | ||
| 6 per 100 | 9 per 100 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| aDowngraded one level due to inconsistency: substantial unexplained heterogeneity (I2 = 71%). | ||||||
| Text messaging in addition to other smoking cessation support compared to other smoking cessation support alone for smoking cessation | ||||||
| Patient or population: people who smoke | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
| Risk with other SC support alone | Risk with text messaging + other SC support | |||||
| Long‐term abstinence (all randomised) Measured as self‐reported and biochemical validation at 6 to 12 months | Study population | RR 1.59 | 997 | ⊕⊕⊕⊝ | ||
| 8 per 100 | 12 per 100 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| aDowngraded one level due to imprecision: fewer than 300 events overall. | ||||||
| Smartphone app compared to lower‐intensity support for smoking cessation | ||||||
| Patient or population: people who smoke Intervention: smartphone app | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
| Risk with lower intensity SC support | Risk with Smartphone app | |||||
| Long‐term abstinence (all randomised) Measured with self‐report and biochemical validation at 6 months | Study population | RR 1.00 | 3079 | ⊕⊝⊝⊝ | ||
| 8 per 100 | 8 per 100 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| aDowngraded one level due to inconsistency: considerable unexplained statistical heterogeneity (I2 = 59%). | ||||||
Antecedentes
Descripción de la afección
El tabaco sigue siendo uno de los factores de riesgo más importantes para los problemas de salud en todo el mundo (IHME 2018). Muchos países buscan opciones sostenibles para la prestación de apoyo para dejar de fumar a gran escala.
Descripción de la intervención
"mHealth" describe el uso de las tecnologías de comunicaciones móviles y de los teléfonos móviles para apoyar la atención sanitaria. Esta revisión está específicamente interesada en el uso de la mensajería de texto y las aplicaciones de los teléfonos inteligentes (apps) para apoyar el abandono del hábito de fumar.
De qué manera podría funcionar la intervención
Los efectos beneficiosos de las intervenciones de apoyo para dejar de fumar basadas en teléfonos móviles (mCessation) son: la facilidad de uso en cualquier lugar y en cualquier momento; la administración coste‐efectiva y la adaptabilidad a grandes poblaciones, independientemente de su ubicación; la capacidad de adaptar los mensajes a las características clave del usuario (como la edad, el sexo, el origen étnico); la capacidad de enviar mensajes urgentes con un dispositivo "siempre encendido"; el suministro de contenido que puede distraer al usuario de los deseos; y la capacidad de vincular al usuario con otras personas para recibir apoyo social.
Un beneficio clave del uso de los teléfonos móviles para los programas de salud es su amplia aceptación en áreas en que los servicios de salud no son fácilmente accesibles o utilizados. En 2018, el número de abonados a la telefonía móvil en todo el mundo superó los 8 000 000 000 y el mundo en desarrollo tiene ahora más abonados a la telefonía móvil que población (penetración de la población del 102%; UIT 2018). Existe evidencia que indica que las personas de los grupos socioeconómicos con menos recursos pueden preferir las intervenciones mCessation debido a la mayor sensación de control asociada con la capacidad de decidir cuándo y dónde acceden a los mensajes y la percepción de apoyo las 24 horas del día (Boland 2017). Centrar los esfuerzos en mCessation en las poblaciones más necesitadas podría ayudar a abordar las desigualdades en materia de salud que se derivan del elevado consumo de tabaco y de la falta de servicios accesibles de promoción y prevención de salud en contextos de bajos recursos a escala mundial.
Además, la investigación inicial indica que el uso de mensajes de texto para dejar de fumar es coste‐efectivo. Guerriero 2013 encontró que el coste del apoyo basado en mensajes de texto era de 278 GBP por persona que dejó de fumar. Cuando se incluyeron los costes futuros de los servicios de salud ahorrados (como resultado del abandono del hábito de fumar), con 0,5 años de vida ajustados en función de la calidad (AVAC) obtenidos por cada persona que dejaba de fumar, se consideró que el apoyo basado en los textos significó un ahorro de costes.
Por qué es importante realizar esta revisión
Los teléfonos inteligentes (teléfonos móviles con sistema operativo informático) se convierten rápidamente en el ordenador preferido, o al menos el más accesible, en muchos países. Según la International Telecommunications Union, solo el 36,3% de los países de ingresos bajos y medios tiene un ordenador en el domicilio, pero el 61% tiene suscripciones de banda ancha móvil (lo que permite a los teléfonos móviles acceder a Internet; UIT 2018). Por lo tanto, fue importante actualizar esta revisión para incluir los estudios sobre la efectividad de las aplicaciones para teléfonos inteligentes, así como las intervenciones de mensajes de texto, para el abandono del hábito de fumar.
Objetivos
Determinar si las intervenciones basadas en teléfonos móviles para el abandono del hábito de fumar aumentan la tasa de abandono del hábito de fumar en las personas que fuman.
Métodos
Criterios de inclusión de estudios para esta revisión
Tipos de estudios
Ensayos aleatorizados o cuasialeatorizados. Fueron elegibles para inclusión los ensayos asignados al azar por grupos.
Tipos de participantes
Personas que fumaban en el momento de la inscripción en el estudio.
Tipos de intervenciones
Se incluyeron los estudios que examinaron cualquier intervención que se pudiera considerar predominantemente un programa basado en teléfonos móviles (como la mensajería de texto o las aplicaciones para teléfonos inteligentes) para el abandono del hábito de fumar. Se excluyeron las intervenciones en que los teléfonos móviles se consideraron como complemento de los programas predominantemente personales o por Internet, como recordar a los participantes los turnos, o donde no se pudo separar los efectos de los diversos componentes de un programa multifacético. También se excluyeron las intervenciones que se podían realizar mediante cualquier tipo de teléfono, como el asesoramiento telefónico. No se excluyeron estudios sobre la base del comparador, sino que en los análisis se agruparon los estudios por comparadores.
Tipos de medida de resultado
La medida de resultado primaria fue la abstinencia del hábito de fumar durante el seguimiento más largo y al menos seis meses desde el inicio del estudio. Cuando hubo múltiples medidas disponibles, se prefirió la abstinencia sostenida a la prevalencia puntual de la abstinencia y los resultados bioquímicamente validados al autoinforme.
No existe un riesgo evidente de eventos adversos de las intervenciones de mensajería de texto o de las aplicaciones para teléfonos inteligentes, por lo que no se ha incluido como resultado en esta revisión.
Métodos de búsqueda para la identificación de los estudios
For the present update of the review, we searched the Cochrane Tobacco Addiction Group's Specialised Register on 29 October 2018 using the terms 'mobile phone', 'cell phone', 'txt', 'pxt', 'sms', or 'mms' in the title, abstract or keyword fields. The Specialised Register includes reports of possible controlled trials of smoking cessation interventions identified from sensitive searches of databases. At the time of the search, the Register included the following results of searches
-
Cochrane Central Register of Controlled trials (CENTRAL; 2018, Issue 1)
-
MEDLINE (via Ovid, to 26 October 2018)
-
Embase (via Ovid, to 28 October 2018)
-
PsycINFO (via Ovid; to 22 October 2018)
See the Cochrane Tobacco Addiction Group website for full search strategies and a list of other resources searched. We also searched the World Health Organization International Clinical Trials Registry Platform (WHO ICTRP; apps.who.int/trialsearch/) and ClinicalTrials.gov trials registers for ongoing or recently completed studies. We searched through the reference lists of identified studies for any additional eligible studies and attempted to contact the authors of ongoing studies.
We placed no restrictions on publication language or date.
Obtención y análisis de los datos
Selección de los estudios
The Cochrane Tobacco Addiction Group's Information Specialist ran the searches and provided the results. Two review authors (YG, HM) independently pre‐screened the titles and abstracts of records identified in duplicate to exclude reports that had no relevance to the topic and to provide a list of potentially relevant citations. A third reviewer (CB) resolved any differences in initial screening. Two review authors (from RW, YG, CB, RD) independently reviewed full‐text manuscripts in duplicate for the final eligibility screen.
We resolved any disagreements by discussion or by obtaining further information through contacting study authors. We recorded reasons for exclusion of studies in the Characteristics of excluded studies table. We contacted authors of unpublished, registered studies, which could potentially have been completed, to determine ongoing status or to request unpublished data.
Extracción y manejo de los datos
We extracted the following methodological details from the included study reports and presented them in the Characteristics of included studies table. Two review authors (from RW, YG, RD, CB, HM) independently extracted data using the standardised Covidence data extraction form. A third review author provided a review of the quality assessment and a consensus check.
-
Funding source
-
Authors' declarations of interest
-
Country and context of the study
-
Study design
-
Number of participants
-
Age and other relevant recorded characteristics of study participants
-
Inclusion criteria
-
Exclusion criteria
-
Intervention details
-
Control details
-
Definition of abstinence outcome
-
Smoking cessation rates at six months (self‐reported abstinence or biochemically verified abstinence, or both)
-
Smoking cessation rates at final follow‐up (if follow‐up greater than six months and where these data were available)
Evaluación del riesgo de sesgo de los estudios incluidos
Two review authors (from RW, YG, RD, CB, HM) independently assessed the risk of bias for included studies, based on the guidance of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2017), and the Cochrane Tobacco Addiction Group. For each study, we assessed the following domains.
-
Random sequence generation
-
Allocation concealment
-
Blinding of outcome assessment
-
Incomplete outcome data
-
Other sources of bias
Specific 'Risk of bias' guidance developed by the Cochrane Tobacco Addiction Group to assess smoking cessation studies states that performance bias (relating to the blinding of participants and providers) should not be assessed for behavioural interventions, as it is impossible to blind people to these types of interventions. We graded detection bias as low where there was biochemical verification of abstinence, or where abstinence was self‐reported with no difference in face‐to‐face contact between control and intervention arms. We considered bias due to incomplete outcome as low risk where numbers lost to follow‐up were clearly reported for each group, the overall loss was not greater than 50%, and the difference between groups was not greater than 20%, or sensitivity analysis showed that the direction of effect was not sensitive to different imputation methods for loss to follow‐up.
Each review author recorded information in study reports relevant to each relevant domain and then judged each domain as either at low, high, or unclear risk of bias. We resolved disagreements through discussion with a third review author.
Medidas del efecto del tratamiento
We recorded the information below.
-
Smoking cessation rates at six months or longer using the most stringent measure available
-
Biochemically verified abstinence, where available
We calculated risk ratios (RR) and 95% confidence intervals (CI) for the smoking cessation outcome for each included study. We calculated outcomes on an intention‐to‐treat basis, including all participants randomised to a trial arm and assuming that participants lost to follow‐up had continued to smoke or relapsed.
Manejo de los datos faltantes
If we found any important study characteristics or outcome data to be missing, we followed up with study authors where possible.
Evaluación de la heterogeneidad
In order to assess whether it was appropriate to pool studies and conduct meta‐analyses we assessed the characteristics of included studies to identify any clinical or methodological heterogeneity. Where we deemed studies homogeneous enough to be combined meaningfully, we conducted a meta‐analysis, and we assessed statistical heterogeneity using the I2 statistic; we deemed an I2 value greater than 50% to indicate substantial heterogeneity (Higgins 2003).
Evaluación de los sesgos de notificación
We planned to use funnel plots to assess reporting bias for any comparisons where we identified and analysed abstinence rates from at least 10 studies. Only the 'text messaging versus minimal smoking cessation support' comparison met this criteria in this review; therefore a funnel plot was generated for this comparison only. Funnel plots illustrate the relationship between the effect estimates from individual studies against their size or precision. The greater the degree of asymmetry, the greater the risk of reporting bias.
Síntesis de los datos
We conducted meta‐analyses of the included studies, using the Mantel‐Haenszel random‐effects method to pool RRs and 95% CIs calculated for the smoking abstinence outcome, across the following comparisons.
-
Text messaging versus minimal smoking cessation support (including standard self‐help materials, as is standard practice in the Cochrane Tobacco Addiction Group)
-
Text messaging in addition to another form of smoking cessation support
-
Text messaging versus other smoking cessation support
-
Higher‐ versus lower‐frequency text messaging
-
Smartphone app versus less intensive smoking cessation support
Where studies had multiple intervention arms relevant to a single meta‐analysis, we split control arm data to avoid double‐counting.
Análisis de subgrupos e investigación de la heterogeneidad
We carried out the following subgroup analyses.
-
We split the 'smartphone app versus less intensive smoking cessation support' comparison into two subgroups to reflect the different comparators used across studies; either minimal non‐app smoking cessation support (e.g. self‐help materials, information on existing stop‐smoking services) or a less intensive smartphone app.
Análisis de sensibilidad
We conducted the following sensitivity analyses.
-
We calculated pooled RRs and 95% CIs for all analyses using complete case data to calculate quit rates. People may drop out of studies for reasons other than still smoking, and these reasons may differ between groups. For example, people who successfully stop smoking may withdraw from receiving an intervention if the text messages remind them of smoking. Therefore, this analysis tests whether assuming that all people lost to follow‐up are smoking (as in our primary analyses of all participants randomised) is potentially biasing our results.
-
Removing any studies judged to be at high risk of bias from all comparisons
-
Removing the only cluster‐RCT (Haug 2013), as information was not available to adjust for any potential clustering effect
-
Removing the two studies carried out in a pregnant (Abroms 2017), or postnatal population (Yu 2017), as these populations differ substantially from those recruited in the other studies.
'Summary of findings' tables
Following standard Cochrane methods (Schünemann 2017), we created a 'Summary of findings' table for the primary outcome (smoking abstinence), for the following comparisons.
-
Text messaging versus minimal smoking cessation support
-
Text messaging in addition to other smoking cessation support
-
Smartphone app versus less intensive smoking cessation support
Also following standard Cochrane methodology (Schünemann 2017), we used the five GRADE considerations (risk of bias, inconsistency, imprecision, indirectness and publication bias) to assess the certainty of the body of evidence for the abstinence outcome for each comparison, and to draw conclusions about the certainty of evidence within the text of the review.
Results
Description of studies
See Characteristics of included studies; Characteristics of excluded studies; and Characteristics of ongoing studies for further details.
Results of the search
The previous version of this review (Whittaker 2016), included 12 studies (Abroms 2014; Bock 2013; Borland 2013; Ferguson 2015; Free 2009; Free 2011; Gritz 2013; Haug 2013; Naughton 2014; Rodgers 2005; Tseng 2017; Whittaker 2011). Gritz 2013 was excluded at this update, as their intervention (telephone counselling and help line) was significantly different from the other interventions included in this review. A telephone help line intervention does not need to be carried out using a mobile phone specifically. Therefore, 11 of the previously included studies were included at this update, as well as one previously 'ongoing' study that was changed to 'included' as the study is now complete and data was available (Danaher 2019).
For this update of our review, the new literature search identified 370 studies (Figure 1). Many were duplicates, or unrelated and were immediately excluded at the title and abstract screening phase. We screened the full‐text of 71 reports of 62 studies, excluding 16 studies, and leaving 14 new studies eligible for inclusion at this update (Abroms 2017; Alessi 2017; Augustson 2017; Baskerville 2018; BinDhim 2018; Chan 2015; Cobos‐Campos 2017; Garrison 2018; Herbec 2019; Liao 2018; Peiris 2019; Squiers 2017; Wilson 2016; Yu 2017). Data were supplied by the authors for two studies (Danaher 2019; Herbec 2019). Reasons for excluding studies included: intervention that was not predominantly a mobile phone programme; not a randomised controlled trial; relapse prevention only; or no abstinence outcome measured at ≥ 6 months follow‐up (see Characteristics of excluded studies table for further details).

Study flow diagram for this update
We also identified 32 ongoing studies at this update. When added to the previously identified ongoing studies there was a total of 34 ongoing studies (for further details see the Characteristics of ongoing studies table).
Included studies
Context and participants
The settings and recruitment methods, and therefore the participants, varied considerably across studies. Where previously this review had included only studies from a small range of high‐income countries, the new studies included in this update provided greater variation in settings, including China (Augustson 2017; Chan 2015; Liao 2018).
Bock 2013 (USA) found usual in‐person recruitment methods slow and shifted to online recruitment methods during the study. Baskerville 2018 (Canada), Borland 2013 (Australia), Danaher 2019 (USA), Garrison 2018 (USA), Squiers 2017 (USA), Herbec 2019 (UK), and Abroms 2014 (USA) also used online recruitment via Internet advertisements. In Abroms 2014 this initially led to some fraudulent participants who were discovered and disqualified, and extra procedures were put in place to prevent this from happening again. Free 2009 and Free 2011 recruited via advertisements at UK primary care centres, smoking cessation clinics, pharmacies, newspapers, websites, bus billboards and on the radio in the UK, and Liao 2018 used similar advertising methods in China. Rodgers 2005 also used direct advertising via websites, email, and posters at tertiary institutions across New Zealand. Similarly Whittaker 2011 (New Zealand) used a wide range of advertising media, including Māori‐specific media, and targeted young people. Alessi 2017 recruited through email, flyers, and print advertisements and Ferguson 2015 (Australia) used advertisements in papers, radio, and Facebook. Abroms 2017 was embedded in the Text4Baby text message (three messages a week) health information programme for pregnant women in the USA. Women who had smoked at least one puff in the past two weeks were eligible to also receive Quit4Baby text messages (between one to eight messages a day) to support smoking cessation. Augustson 2017 recruited through Nokia Life Tools, a service providing more than 100 million users with tools pre‐installed on their Nokia mobile phones, in urban and rural areas of China’s Zhejiang, Heilongjiang and Shaanxi provinces. BinDhim 2018 recruited through the Apple App Store in several countries (Australia, Singapore, UK, USA). Participants were advised that by downloading the app they would be participating in a study. Chan 2015 recruited through a Quit and Win competition in Hong Kong that was promoted in shopping malls and other public areas. Wilson 2016 mailed letters to potential participants in the US Veterans Administration health system. Naughton 2014 was set in primary care practices in the UK with trained smoking cessation advisors providing smoking cessation advice; Cobos‐Campos 2017 in two health clinics in Spain with health advice provided by a doctor or nurse; and Tseng 2017 in large urban HIV clinics. Haug 2013 recruited in vocational schools and differed from the other studies by allowing the inclusion of occasional smokers (at least four cigarettes in the past month or at least one in the preceding week). Peiris 2019 (Australia) recruited via an Aboriginal Community Controlled Health Service, a regional community event, and the New South Wales Government telephone coaching service. Yu 2017 recruited in maternal‐child health centres in China after asking mothers about household second‐hand smoke exposure. The intervention included messages on both the harms of second‐hand smoke (to the mother and her husband) and additional messages to the husband to encourage quitting.
Four studies deliberately targeted young adults (Baskerville 2018 in Canada, Haug 2013 in Switzerland; Squiers 2017 in USA; Whittaker 2011 in New Zealand). Most studies had similar proportions of men and women or slightly more women than men. The exceptions were Abroms 2017, as the intervention was targeted at pregnant women (100% women), Wilson 2016, which recruited 89% male veterans, and the studies in China, where the rates of smoking in women are low (Chan 2015 > 80% men, Liao 2018 94.6% men, Yu 2017 100% men).
Intervention programmes
Text messaging
All studies tested automated text messaging interventions. Eighteen of the included studies used text messaging (SMS) as a central component of the intervention (Abroms 2014; Abroms 2017; Augustson 2017; Bock 2013; Borland 2013; Chan 2015; Cobos‐Campos 2017; Ferguson 2015; Free 2009; Free 2011; Haug 2013; Liao 2018; Naughton 2014; Rodgers 2005; Tseng 2017; Squiers 2017; Whittaker 2011; Yu 2017). Whittaker 2011 sent text messages containing links to theoretically driven video messages from 'ordinary' role models coping with quitting. Several studies paired text messages with in‐person visits or assessments (Bock 2013; Cobos‐Campos 2017; Haug 2013; Naughton 2014).
The text message interventions varied in length from one week (Chan 2015), to five weeks (Ferguson 2015), six weeks (Augustson 2017; Yu 2017), eight weeks (Bock 2013;Squiers 2017), three months (Abroms 2017; Haug 2013; Naughton 2014; Tseng 2017), and six months (Abroms 2014; Cobos‐Campos 2017; Free 2009; Free 2011; Liao 2018; Rodgers 2005; Whittaker 2011), or were variable (Borland 2013).
Eight studies did not state that text messages were tailored to the individual (Abroms 2017; Augustson 2017; Chan 2015; Cobos‐Campos 2017; Liao 2018; Tseng 2017; Squiers 2017; Yu 2017). In other studies using text messages, the degree of individual tailoring varied:
-
Abroms 2014 tailored messages to include first name, quit date, top three reasons for quitting, money saved by quitting, and use of quit‐smoking medications;
-
Bock 2013 and Haug 2013 tailored messages to the stage of readiness to quit;
-
Borland 2013's programme could be interacted with by reporting changes in smoking behavior (e.g. a quit attempt, relapse), so that appropriate stage‐specific messages could be sent;
-
Ferguson 2015 tailored their intervention text messages to contain advice and encouragement tailored to participants' current quit status (preparing to quit, first week of the quit attempt, second week of attempt etc.)
-
Free 2009 and Free 2011 tailored the messages to information collected at baseline about the individual;
-
Naughton 2014 individually tailored messages using 24 items from the iQuit questionnaire and information on smoking status at three and seven weeks;
-
Rodgers 2005 matched participant characteristics to messages by keyword to create an individualised programme;
-
Whittaker 2011's participants selected the role model from whom they wished to receive messages.
A number of text messaging interventions included interactive components such as:
-
the ability to text for more support in the instance of cravings or lapses (Abroms 2014; Bock 2013; Free 2011; Liao 2018; Naughton 2014; Rodgers 2005);
-
an optional Quit Buddy in Rodgers 2005 and Free 2011;
-
a Quit support network in Bock 2013;
-
polls and quizzes (Rodgers 2005);
-
regular checking in on smoking status (Haug 2013).
Borland 2013 was the only study to include some degree of choice. Participants received offers of support via a personalised tailored Internet programme, a text message programme, both programmes, a choice of all three, or a minimal control. For the purposes of meta‐analyses, we compared the text message group with the control group.
Some of the included interventions were somewhat related to each other. The text messaging intervention in Rodgers 2005 was developed in New Zealand, and later adapted and tested in a UK pilot study (Free 2009), and then a large randomised controlled study (Free 2011). The intervention in Abroms 2017 was developed for pregnant women from the same group’s previous intervention for adult smokers in Abroms 2014. The Augustson 2017 intervention in China was adapted from the smoke‐free text programme that was evaluated in Squiers 2017. For further details of the messaging interventions across individual studies see the Characteristics of included studies table.
The control conditions used in the text message studies could be categorised into four groups.
-
Minimal smoking cessation support (13 studies): the control programmes across the studies in this category varied from no smoking cessation support (Haug 2013; Yu 2017), to non‐smoking‐related text messages sent two‐weekly (Free 2009; Free 2011; Rodgers 2005; Whittaker 2011), or weekly (Liao 2018), to written or Internet untailored materials (Abroms 2014; Chan 2015; Ferguson 2015), to links to smoking cessation support (Borland 2013; Rodgers 2005), or regular general health advice provided by a clinician (Cobos‐Campos 2017). Abroms 2017's control group participants received standard non‐smoking‐related Text4Baby text messages (three a week) without the extra smoking cessation‐related Quit4Baby text messages.
-
Another form of smoking cessation support (matched to support received by the intervention group, but without the text messaging intervention; four studies): support varied across studies and included a single session of smoking cessation counselling plus non‐smoking‐related text messages (Bock 2013); smoking cessation behavioural support and pharmacotherapy (Naughton 2014), and behavioural support and pharmacotherapy (Tseng 2017). Participants in the comparison arm of Yu 2017 received in‐person counselling and materials on establishing a smoke‐free home.
-
Another form of smoking cessation support (not matched in the intervention arm; two studies): an Internet‐based interactive smoking cessation programme (Borland 2013), and a five‐minute smoking cessation counselling session (Chan 2015).
-
Higher‐ versus lower‐frequency text messaging. Three studies examined the effect of higher‐ versus lower‐frequency text messages (Augustson 2017; Liao 2018; Squiers 2017). In Augustson 2017 this was comparing 91 messages over six weeks (three a day initially, followed by two a day, then one a day), with one text message a week for six weeks. In Liao 2018 this was three to five messages per day compared with three to five messages per week. Squiers 2017 compared smoking assessment and quit date messages only, with those messages plus motivational preparatory messages for two weeks prior to quitting, and with all of those messages plus six weeks of follow‐up post‐quit messages.
Smartphone apps
Five studies tested the effectiveness of smoking cessation smartphone apps alone (Baskerville 2018; BinDhim 2018; Garrison 2018; Herbec 2019; Peiris 2019). These apps varied considerably in intervention content and components. The app in Baskerville 2018 was described as comprehensive and evidence‐informed, including components such as a quit plan, contingency reinforcement, a link to an online Facebook community, supportive messages through the app, web‐based distraction, information and performance feedback, access to evidence‐based cessation services. BinDhim 2018 described their app as a decision aid (based on the Ottawa Decision Support Framework drawing from a number of psychological and behavioural theories) with additional support with push notifications, messages, diary and benefits tracker. The Garrison 2018 app training modules taught mindfulness for smoking cessation and how to work through cravings. Herbec 2019 included craving management tools within an app that supported smokers to be smoke free for 28 days.
The control conditions used in these smoking cessation app studies could be categorised into two groups:
-
minimal non‐app smoking cessation support that included: a printed self‐help guide (Baskerville 2018), and encouragement to access available smoking cessation services (Peiris 2019);
-
less intensive app support that included an app that provided only basic information. In BinDhim 2018 this included information only on quitting (no structured process or support). Garrison 2018 delivered experience sampling to query smoking, craving, and mindfulness in real time, and the control app in Herbec 2019 was designed to be a minimally credible intervention that resembled the intervention but without key intervention components.
Carbon monoxide (CO) monitoring and contingency management
Alessi 2017 and Wilson 2016 used mobile phone technology slightly differently to the above studies, by specifically using mobile phones to monitor the concentration of carbon monoxide in end‐expiratory air (CO levels). In Alessi 2017 interactive voice response calls would prompt the participant to conduct a CO test using a CO monitor. This was video recorded on the mobile phone and submitted using multimedia messaging. The CO result was provided via interactive voice response call. In the reinforcement arm of the trial, this was supplemented by negative CO test results (not smoking), which were rewarded with chances to win prizes. Therefore, the study had two arms that received mHealth CO monitoring as well as counselling and nicotine replacement therapy (NRT), with one of the arms also receiving rewards for smoking abstinence. Wilson 2016 combined cognitive behavioural telephone counselling and access to NRT with a mobile app for CO monitoring and contingency management in one study arm, and compared this to the same intervention without the CO monitoring and contingency management app. Participants provided CO readings twice a day by video through the app and received payment for abstinence in the intervention arm.
Smartphone app plus text messaging
Danaher 2019 tested an intervention that used both an integrated mobile web app and text messaging. Text messages were prompts and motivations to visit parts of the web programme as well as information, motivation and smoking questions (290 messages over six months). The control group received a PC‐based web intervention with interactive and multimedia features based on phases of quitting, the main difference to the intervention app being that it was not adapted for the small screen and did not include text messaging. Emails were sent as prompts if there were periods of inactivity.
Outcome
The included studies provided a range of abstinence outcome measures. Five studies (Cobos‐Campos 2017; Free 2009; Free 2011; Liao 2018; Peiris 2019), reported the strictest outcome as biochemically verified sustained/continuous abstinence, and Abroms 2014 and Alessi 2017 defined abstinence as biochemically confirmed repeat point prevalence at six months.
Seven additional studies reported self‐reported continuous abstinence at six months, without biochemical verification Baskerville 2018; BinDhim 2018; Borland 2013; Herbec 2019; Naughton 2014; Rodgers 2005; Whittaker 2011).
Two studies used self‐reported four‐week or 30‐day point prevalence abstinence at six‐month follow‐up (Abroms 2017; Haug 2013), three studies used self‐reported seven‐day point prevalence at six months (Augustson 2017; Bock 2013; Danaher 2019), one used self‐reported point prevalence abstinence at 32 weeks (Squiers 2017), one at 12 months (Yu 2017), and an additional four studies used six‐month biochemically verified measures of seven‐day point prevalence (Chan 2015; Ferguson 2015; Garrison 2018; Tseng 2017). Chan 2015 also provided biochemically verified seven‐day point prevalence abstinence rates at 12‐month follow‐up. Wilson 2016 reported six month follow‐up data in their trial registry entry; however they do not specify whether these rates were validated or not.
Risk of bias in included studies
The Characteristics of included studies table provides details of 'Risk of bias' judgements for each domain of each included study. Figure 2 illustrates judgements for each included study. Overall, we judged 13 studies to be at low risk of bias (judged at low risk for all domains), and three to be at high risk (judged to be at high risk in at least one domain). We judged the remaining studies to be at unclear risk (judged to be at unclear risk of bias for at least one domain, but with no judgements of high risk).

'Risk of bias' summary: review authors' judgements about each 'Risk of bias' item for each included study
Selection bias
The majority of studies (17 of 26) appeared to have adequate procedures for random sequence generation and allocation concealment, so we judged them to be at low risk of bias for these domians; however Alessi 2017; Augustson 2017; Chan 2015; Ferguson 2015; Garrison 2018; Haug 2013; Squiers 2017; Wilson 2016 and Yu 2017 did not provide sufficient description of either randomisation, concealment procedures, or both. Therefore, it is impossible to know whether the lack of information is due to actual bias or simply because it has not been reported, and we judged them to be at unclear risk of bias for at least random sequence generation or allocation concealment.
Detection bias
Blinding of participants is not possible in studies of behavioural interventions. In this case participants knew if they were receiving text messages or using an app. Therefore, we did not assess performance bias, and instead judged the likelihood of detection bias. We did not deem a study to be high risk for this domain where there was biochemical verification of abstinence, or where both arms received the same amount of face‐to‐face contact (or none).
In most cases, studies collected outcomes electronically and remotely (Abroms 2014; Augustson 2017; Baskerville 2018; BinDhim 2018; Bock 2013; Danaher 2019; Free 2009; Free 2011; Garrison 2018; Squiers 2017). Chan 2015; Herbec 2019; Liao 2018; Haug 2013 and Wilson 2016 all collected outcomes by phone, and Naughton 2014 by mailed questionnaire or in person. Cobos‐Campos 2017 collected outcomes in person in the clinic and this was not blinded, however this was mitigated by biochemical verification of quitting.
A number of the trials sought biochemical verification of long‐term abstinence with salivary cotinine (Abroms 2014; Free 2009; Free 2011), urinary cotinine (Liao 2018), or expired CO (Cobos‐Campos 2017; Garrison 2018). Chan 2015 assessed both CO and cotinine concentrations. Abroms 2017 biochemically validated their primary outcome at three months, but not at six months, and Rodgers 2005 validated abstinence at six weeks but not long‐term follow‐up. Similarly, Naughton 2014 used verification at four weeks only. Wilson 2016 stated that they planned to verify abstinence at all follow‐up points using salivary cotinine; however it is not stated whether the abstinence rates reported in their trial registry entry were the validated rates or not. However, as data was collected remotely this study was still deemed to be at low risk of bias for this domain. In fact, we deemed all studies to be at low risk of detection bias.
Attrition bias
We judged three studies to be at high risk of bias due to greater than 50% of participants lost to follow‐up at six months (Augustson 2017; Cobos‐Campos 2017; Herbec 2019). Several other studies had moderately high loss to follow‐up but the numbers were clearly reported. The difference between groups was not greater than 20%, and overall loss was not greater than 50%. Ferguson 2015 did not report loss to follow‐up and so we judged it to be at unclear risk of attrition bias.
Other
In Abroms 2014 there were some issues with fraudulent enrolment at the outset of the study, although this was corrected once detected. In Haug 2013, although clustering is adjusted for in this study's analysis the authors do not report the clustering effect, making it impossible to adjust for this in our analysis. Therefore, it is not clear how much the clustering adjustment influences the result from this study and our meta‐analyses. Rodgers 2005 suggested that some participants in their control group may have thought their incentive at follow‐up (a month of free text messaging) depended on reporting quitting. This could account for an unexpected increase in control group participants reporting quitting from six weeks (109 participants) to six months (202 participants reporting no smoking in the past seven days), which could have led to an underestimation of the effect of the intervention.
Effects of interventions
See: Summary of findings for the main comparison Text messaging compared to minimal support for smoking cessation; Summary of findings 2 Text messaging in addition to other smoking cessation support; Summary of findings 3 Smartphone app compared to lower‐intensity support for smoking cessation
Text messaging versus minimal smoking cessation support
We pooled those studies that compared a text messaging intervention with minimal smoking cessation support. This included 13 studies (Abroms 2014; Abroms 2017; Borland 2013; Chan 2015; Cobos‐Campos 2017; Ferguson 2015; Free 2009; Free 2011; Haug 2013; Liao 2018; Rodgers 2005; Whittaker 2011; Yu 2017). The analysis of all randomised participants, with those lost to follow‐up classified as smokers resulted in a RR of 1.54 (95% CI 1.19 to 2.00; I2 = 71%; 14,133 participants; Analysis 1.1) with minimal difference found in the result when we carried out a complete case analysis (RR 1.56, 95% CI 1.21 to 2.02; I2 = 72%; 11,969 participants; Analysis 1.2).
We conducted the following sensitivity analyses:
-
removing studies with very different populations from the main analysis (i.e. Analysis 1.1), pregnant women only in Abroms 2017 and postnatal families only in Yu 2017. This made very little difference to the overall result (RR 1.57, 95% CI 1.18 to 2.07; I2 = 68%; 13,408 participants);
-
removing the only cluster‐randomised trial, which we were unable to adjust for (Haug 2013). That again had minimal impact on the result (RR 1.57, 95% CI 1.19 to 2.07; I2 = 73%; 13,378 participants);
-
removing the only study judged to be at high risk of bias (Cobos‐Campos 2017), which again had minimal impact on the result (RR 1.49, 95% CI 1.13 to 1.96; I2 = 72%; 13,813 participants).
Text messaging versus other smoking cessation intervention
Only two studies (Borland 2013; Chan 2015; 2238 participants), compared text messaging with another smoking cessation intervention. When pooled these did not show a superior effect of either text message support to quit or the other forms of smoking cessation intervention in either an analysis including all randomised participants (RR 0.92, 95% CI 0.61 to 1.40; I2 = 27%; 2238 participants; Analysis 2.1) or a complete case analysis (RR 0.93, 95% CI 0.63 to 1.36; I2 = 20%; 1813 participants; Analysis 2.2).
Text messaging plus other smoking cessation support versus other smoking cessation support alone
Four studies (Bock 2013; Naughton 2014; Tseng 2017; Yu 2017; 997 participants), compared those who received both text messaging and another form of smoking cessation support with those only receiving the other form of smoking cessation support. The analysis of all randomised participants, assuming those lost to follow‐up were smoking, showed a benefit of adding the text messaging with RR of 1.59 (95% CI 1.09 to 2.33; I2 = 0%; 997 participants; Analysis 3.1). The result was comparable when we carried out a complete case analysis (RR 1.63; 1.12 to 2.37; I2 = 0%; 796 participants; Analysis 3.2).
We carried out a sensitivity analysis on Analysis 3.1 removing Yu 2017, as it had a substantially different population (postnatal families). The interpretation of the effect remained the same (RR 1.87, 95% CI 1.13 to 3.09; I2 = 0%; 769 participants).
High‐frequency versus low‐frequency text messaging
Three studies (Augustson 2017; Liao 2018; Squiers 2017; 12,985 participants), compared high‐frequency text messaging interventions with low‐frequency text messaging interventions. The pooled effect indicated no difference in cessation rates between groups in either the analysis of all participants randomised (RR 1.00, 95% CI 0.95 to 1.06; I2 = 0%; Analysis 4.1) or the complete case analysis (RR 1.04, 95% CI 1.00 to 1.09; I2 = 0%; 6798 participants; Analysis 4.2). A sensitivity analysis removing the one study judged to be at high risk of bias (Augustson 2017), led to no difference in the interpretation of the effect (RR 1.02, 95% CI 0.92 to 1.12; I2 = 0%; 4985 participants).
Smartphone app versus lower‐intensity smoking cessation support
We divided studies of smartphone apps according to the type of control. Two studies (Baskerville 2018; Peiris 2019; 1645 participants), compared a smartphone app with minimal non‐app smoking cessation support. There was no evidence of a favourable effect of smartphone apps in comparison with minimal non‐app smoking cessation support (RR 0.82, 95% CI 0.56 to 1.18; I2 = n/a as Peiris 2019 had no events; Analysis 5.1). Interpretation remained the same when we carried out a complete case analysis (RR 0.87, 95% CI 0.62 to 1.23; I2 = n/a; 771 participants; Analysis 5.2). Three studies (BinDhim 2018; Garrison 2018; Herbec 2019; 2175 participants), compared a smoking cessation smartphone app with a less intensive smoking cessation smartphone app. The analysis including all randomised participants resulted in an RR of 1.12 (95% CI 0.60 to 2.09; I2 = 68%; Analysis 5.1) with a very similar result in the complete case analysis (RR 1.18, 95% CI 0.67 to 2.09; I2 = 65%; 1003 participants). When we pooled all five studies, the resulting RR for all randomised participants was 1.00 (95% CI 0.66 to 1.52; I2 = 59%; 3079 participants; Analysis 5.1), providing no clear evidence of an increase in quit rates as a result of smart phone smoking cessation apps when compared to smoking cessation support of lower intensity. A sensitivity analysis removing the only study judged to be at high risk of bias (Herbec 2019), led to no difference in the interpretation of the effect (RR 1.10, 95% CI 0.60 to 2.00; I2 = 71%; 2654 participants).
Carbon monoxide monitoring + contingency management versus smoking cessation support
Neither of the studies that used mobile phones to monitor CO and provide contingency management provided evidence that these strategies were more effective than standard smoking cessation support.
Alessi 2017 compared messages prompting CO monitoring via video alone with the same CO monitoring plus reinforcement (with the chance to win prizes) for negative readings, and resulted in a RR of 0.88 (95% CI 0.35 to 2.21; 90 participants; Analysis 6.1).
Wilson 2016 compared CO monitoring and contingency management combined with smoking cessation telephone counselling and NRT, with the counselling and NRT alone, and resulted in an RR of 0.94 (95% CI 0.64 to 1.38; 310 participants; Analysis 6.1).
In both cases carrying out a complete case analysis resulted in a change in the direction of the effect estimate; however CIs still incorporated evidence of both considerable benefit and harm (Alessi 2017: RR 1.13, 95% CI 0.44 to 2.93; 81 participants; Wilson 2016: RR 1.06, 95% CI 0.74 to 1.54; 250 participants; Analysis 6.2).
Smartphone app + text messaging versus web‐based interventions
Danaher 2019 compared a smartphone app plus text messaging with a web‐based smoking cessation intervention and found evidence for a benefit of the app plus text messaging (RR 1.80, 95% CI 1.32 to 2.45; 1271 participants; Analysis 7.1). Complete case analysis resulted in a similar point estimate (RR 1.56, 95% CI 1.19 to 2.05; 463 participants; Analysis 7.2).
Discusión
Resumen de los resultados principales
Se encontraron 26 ensayos controlados aleatorizados de intervenciones para el abandono del hábito de fumar a través de teléfonos móviles que cumplieron con los criterios de inclusión.
Aunque las intervenciones de mensajería de texto tienden a ser muy similares en diseño y contenido, la elección del control varió considerablemente. En esta actualización, se separaron las comparaciones para asegurar que solo se agruparan las intervenciones similares y los controles similares en los metanálisis.
Los análisis encontraron evidencia de certeza moderada (Resumen de resultados, tabla 1) de que las intervenciones de mensajería de texto son más efectivas que el apoyo mínimo para dejar de fumar (Abroms 2014; Abroms 2017; Borland 2013; Chan 2015; Cobos‐Campos 2017; Ferguson 2015; Free 2009; Free 2011; Haug 2013; Liao 2018; Rodgers 2005; Whittaker 2011; Yu 2017). Los mensajes de texto añadidos a otras intervenciones para dejar de fumar también parecieron ser más efectivos que las otras intervenciones para dejar de fumar solas (Bock 2013; Naughton 2014; Tseng 2017; Yu 2017; Resumen de resultados, tabla 2).
Sin embargo, cuando la mensajería de texto se comparó con otras intervenciones para el abandono del hábito de fumar, el análisis no encontró evidencia de que la intervención con mensajería de texto o las otras intervenciones para el abandono del hábito de fumar produjeran tasas superiores de abandono del hábito. Es importante destacar que solo hubo dos estudios en este análisis y que cada uno tuvo contextos ligeramente diferentes: Borland 2013 incluyó a personas que no buscaban apoyo para dejar de fumar y a los participantes se les dieron "sugerencias acerca de los recursos a utilizar"; Chan 2015 se realizó en el contexto de un concurso Quit & Win.
También fue posible evaluar el efecto de los mensajes de texto de intensidad mayor versus menor sobre las tasas de abstinencia a largo plazo, mediante el uso de los datos agrupados de tres estudios que proporcionaron comparaciones directas (Augustson 2017; Liao 2018; Squiers 2017). La frecuencia de la mensajería difirió de algún modo entre los estudios (p.ej., Augustson 2017 utilizó un promedio de 15 versus un mensaje de texto por semana; Liao 2018 utilizó de 21 a 35 versus tres a cinco mensajes por semana; y Squiers 2017 utilizó, en promedio, 16 versus cinco versus un mensaje de texto por semana), pero en general, este análisis no proporcionó evidencia de que la intensidad de la intervención de la mensajería de texto impactara en las tasas de abstinencia. En promedio, las intervenciones de alta intensidad dieron lugar a tasas de abstinencia del 26,6% versus 27,1% en las intervenciones de baja intensidad.
Los estudios de aplicaciones para teléfonos inteligentes también incluyeron varios programas control. No se encontró evidencia de un efecto beneficioso de las aplicaciones de teléfonos inteligentes de alta intensidad en comparación con las aplicaciones para dejar de fumar de baja intensidad (BinDhim 2018; Garrison 2018; Herbec 2019), o un apoyo mínimo para dejar de fumar sin aplicaciones (Baskerville 2018; Peiris 2019), pero se considera que la evidencia es de certeza muy baja, lo que significa que hay muy poca confianza en la estimación del efecto (Resumen de resultados, tabla 3).
Danaher 2019 fue la única intervención que utilizó la mensajería de texto y una aplicación para teléfonos inteligentes y encontró que esta combinación dio lugar a tasas de abandono más altas que una intervención para dejar de fumar basada en la web.
Compleción y aplicabilidad general de las pruebas
La revisión incluye 26 estudios con 33 849 participantes. En comparación con las revisiones anteriores, ahora hay un número mucho mayor de estudios elegibles, con un mayor tamaño de las muestras y que incluyen una mayor diversidad de contextos y países. También se encontró un gran número de estudios en curso (n = 34), cuyos resultados probablemente aumentarán aún más la diversidad de contextos.
Esta es la primera actualización de esta revisión donde se pudieron incluir ensayos controlados aleatorizados de aplicaciones para teléfonos inteligentes. En 2011, una revisión de las aplicaciones disponibles para dejar de fumar encontró que no cumplían las guías ni la teoría para el abandono del hábito de fumar (Abroms 2011). En esta revisión las aplicaciones para teléfonos inteligentes incluidas, aunque escasas en número, tendieron a basarse en la evidencia o en la teoría y se probaron en ensayos controlados aleatorizados de alta calidad.
Se ha criticado el hecho de que las aplicaciones para teléfonos inteligentes no sean ampliamente accesibles para todos, ya que pueden depender de un cierto grado de alfabetización digital y de un acceso a la tecnología que puede no ser muy amplio entre la población. Es importante señalar que en los estudios incluidos de aplicaciones para teléfonos inteligentes había niveles de educación razonablemente altos: el 84% de los participantes en Garrison 2018 tenía una educación superior a la secundaria; en BinDhim 2018, el 53,7% tenía un nivel de educación media o superior; en Baskerville 2018, el 55,5% tenía una educación posterior a la secundaria o superior; Danaher 2019 incluyó el 70% con una educación media y superior; y en Herbec 2019, el 68,7% tenía una calificación de más de 16 años.
Una crítica habitual a los ensayos controlados aleatorizados es que, aunque pueden proporcionar evidencia de efectividad en el contexto de un ensayo clínico, estos datos no son aplicables a los contextos del "mundo real". Se está consciente de que muchos países implementan intervenciones mCessation y estimulan el seguimiento y la evaluación habitual de estos programas, lo que proporcionará evidencia importante del "mundo real" para su consideración junto con la evidencia de los estudios de investigación.
Certeza de la evidencia
Hubo evidencia de certeza moderada de que los mensajes de texto aumentan las tasas de abandono del hábito de fumar en aproximadamente el 50% en comparación con el apoyo mínimo para el abandono del hábito de fumar (Resumen de resultados, tabla 1). La evidencia se redujo un nivel debido a la inconsistencia, ya que hubo heterogeneidad estadística significativa e inexplicable. Lo cual significa que es probable que el efecto verdadero esté próximo al efecto estimado, aunque existe la posibilidad de que difiera de manera significativa.
También hubo evidencia de certeza moderada de que los mensajes de texto aumentan las tasas de abandono del hábito de fumar en aproximadamente el 60% cuando se prueban como complemento de otro apoyo para el abandono del hábito de fumar (Resumen de resultados, tabla 2). Los resultados se redujeron un nivel debido a la imprecisión: hubo menos de 300 eventos en general, y los intervalos de confianza incluyeron beneficios mínimos y beneficios significativos.
Hubo evidencia de certeza muy baja con respecto al efecto de las aplicaciones de los teléfonos inteligentes en comparación con el apoyo de baja intensidad (Resumen de resultados, tabla 3). Esto se debe a la inconsistencia (considerable heterogeneidad estadística inexplicada) y a una imprecisión muy importante, con intervalos de confianza que abarcan efectos perjudiciales clínicamente significativos y efectos beneficiosos clínicamente significativos.
Sesgos potenciales en el proceso de revisión
Una amplia variabilidad de los programas de los grupos control es potencialmente importante para asegurar que los estudios puedan proporcionar la mejor información para los responsables de la toma de decisiones que deseen comparar mCessation con lo que ya existe en su contexto. Sin embargo, también podría dar lugar a dificultades en la interpretación de los resultados. En algunos casos, los grupos control recibieron un apoyo significativo para dejar de fumar y los detalles de este apoyo no siempre estuvieron claros. Lo anterior se apoya en el hecho de que en algunos casos se observaron tasas de abandono altas, por encima de lo que cabía esperar, en los grupos control (Rodgers 2005; Squiers 2017), con tasas altas en los grupos de intervención y control en otro estudio (Augustson 2017). Esto podría indicar algún grado de efecto del ensayo (a todos les va mejor con solo participar en un estudio de investigación), sesgo de conveniencia social o que las intervenciones mínimas con teléfonos móviles (solo para recordatorios, avisos u obtención de datos) también pueden ser efectivas para producir cambios de comportamiento. Los grupos control de alta intensidad que dan lugar a altas tasas de abandono del hábito de fumar podrían haber subestimado el efecto relativo de las intervenciones con teléfonos móviles.
Aunque se realizaron búsquedas en los registros de ensayos, existe el riesgo de que haya estudios elegibles, pero no publicados, que no se pudieron identificar. De manera tranquilizadora, un gráfico en embudo (Figura 3), no mostró evidencia de asimetría.
Acuerdos y desacuerdos con otros estudios o revisiones
Esta revisión concuerda con otras revisiones de los efectos beneficiosos de los mensajes de texto para apoyar un cambio de comportamiento saludable (Armanasco 2017; Thakkar 2016; Scott‐Sheldon 2016). Varios estudios han mostrado resultados mixtos con respecto a la efectividad de las aplicaciones de los teléfonos inteligentes en el cambio de comportamiento, con problemas significativos relacionados con el tamaño y la calidad de los estudios (Byambasuren 2018; Dirieto 2016; Lunde 2018; Schoeppe 2016; Zhao 2016).

Study flow diagram for this update
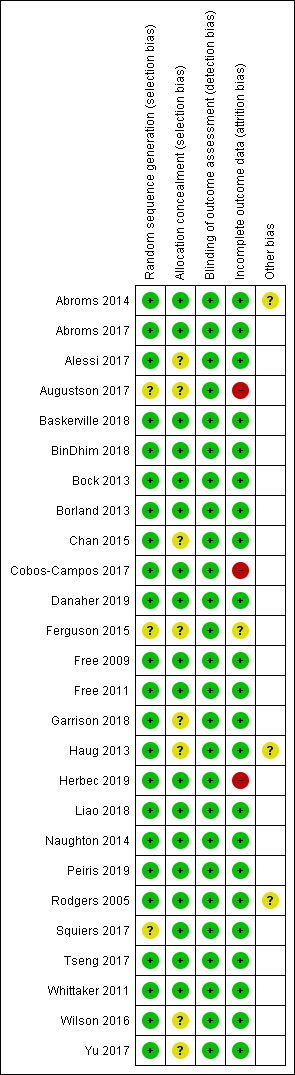
'Risk of bias' summary: review authors' judgements about each 'Risk of bias' item for each included study

Funnel plot of comparison 1. Text messaging versus minimal smoking cessation support, outcome: 1.1 long‐term abstinence (all randomised))

Comparison 1 Text messaging versus minimal smoking cessation support, Outcome 1 Long‐term abstinence (all randomised)).

Comparison 1 Text messaging versus minimal smoking cessation support, Outcome 2 Long‐term abstinence (complete case).
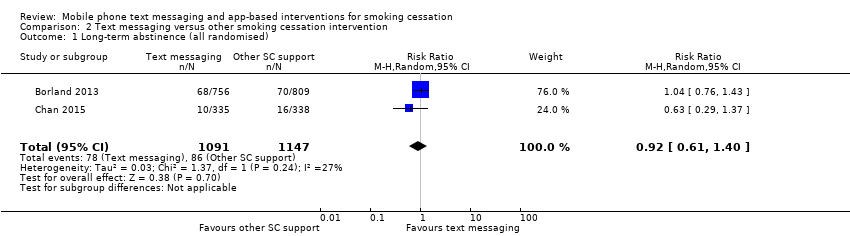
Comparison 2 Text messaging versus other smoking cessation intervention, Outcome 1 Long‐term abstinence (all randomised).
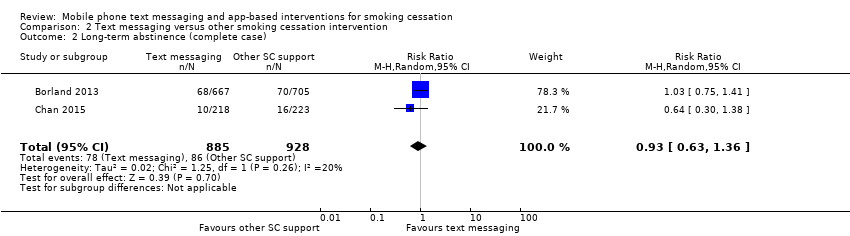
Comparison 2 Text messaging versus other smoking cessation intervention, Outcome 2 Long‐term abstinence (complete case).
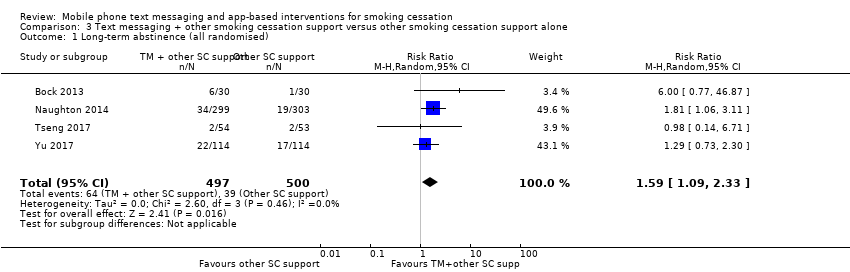
Comparison 3 Text messaging + other smoking cessation support versus other smoking cessation support alone, Outcome 1 Long‐term abstinence (all randomised).

Comparison 3 Text messaging + other smoking cessation support versus other smoking cessation support alone, Outcome 2 Long‐term abstinence (complete case).

Comparison 4 High‐frequency versus low‐frequency text messaging, Outcome 1 Long‐term abstinence (all randomised).

Comparison 4 High‐frequency versus low‐frequency text messaging, Outcome 2 Long‐term abstinence (complete case).

Comparison 5 Smartphone app versus lower‐intensity smoking cessation support, Outcome 1 Long‐term abstinence (all randomised).
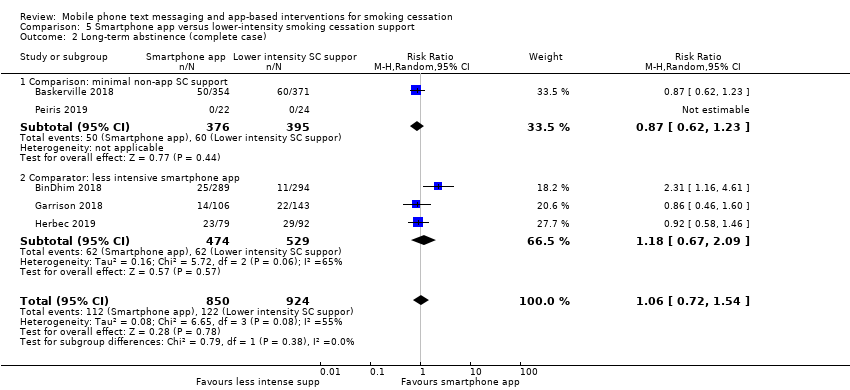
Comparison 5 Smartphone app versus lower‐intensity smoking cessation support, Outcome 2 Long‐term abstinence (complete case).
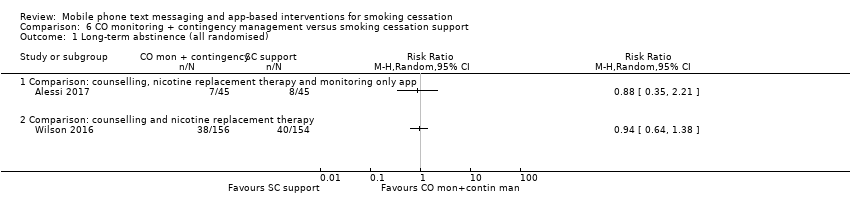
Comparison 6 CO monitoring + contingency management versus smoking cessation support, Outcome 1 Long‐term abstinence (all randomised).

Comparison 6 CO monitoring + contingency management versus smoking cessation support, Outcome 2 Long‐term abstinence (complete case).

Comparison 7 Smartphone app + text messaging versus web‐based intervention, Outcome 1 Long‐term abstinence (all randomised).
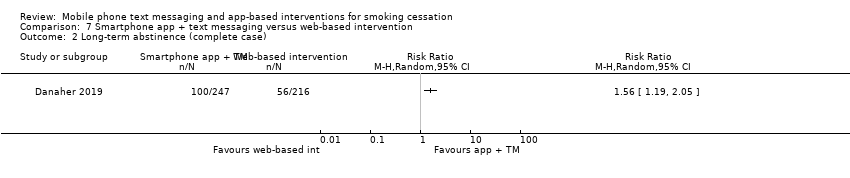
Comparison 7 Smartphone app + text messaging versus web‐based intervention, Outcome 2 Long‐term abstinence (complete case).
| Text messaging compared to minimal support for smoking cessation | ||||||
| Patient or population: people who smoke | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
| Risk with minimal SC support | Risk with text messaging | |||||
| Long‐term abstinence (all randomised) Measured with self‐report and biochemical validation at 6 to 12 months | Study population | RR 1.54 | 14,133 | ⊕⊕⊕⊝ | ||
| 6 per 100 | 9 per 100 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| aDowngraded one level due to inconsistency: substantial unexplained heterogeneity (I2 = 71%). | ||||||
| Text messaging in addition to other smoking cessation support compared to other smoking cessation support alone for smoking cessation | ||||||
| Patient or population: people who smoke | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
| Risk with other SC support alone | Risk with text messaging + other SC support | |||||
| Long‐term abstinence (all randomised) Measured as self‐reported and biochemical validation at 6 to 12 months | Study population | RR 1.59 | 997 | ⊕⊕⊕⊝ | ||
| 8 per 100 | 12 per 100 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| aDowngraded one level due to imprecision: fewer than 300 events overall. | ||||||
| Smartphone app compared to lower‐intensity support for smoking cessation | ||||||
| Patient or population: people who smoke Intervention: smartphone app | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
| Risk with lower intensity SC support | Risk with Smartphone app | |||||
| Long‐term abstinence (all randomised) Measured with self‐report and biochemical validation at 6 months | Study population | RR 1.00 | 3079 | ⊕⊝⊝⊝ | ||
| 8 per 100 | 8 per 100 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| aDowngraded one level due to inconsistency: considerable unexplained statistical heterogeneity (I2 = 59%). | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Long‐term abstinence (all randomised)) Show forest plot | 13 | 14133 | Risk Ratio (M‐H, Random, 95% CI) | 1.54 [1.19, 2.00] |
| 2 Long‐term abstinence (complete case) Show forest plot | 13 | 11969 | Risk Ratio (M‐H, Random, 95% CI) | 1.56 [1.21, 2.02] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Long‐term abstinence (all randomised) Show forest plot | 2 | 2238 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.61, 1.40] |
| 2 Long‐term abstinence (complete case) Show forest plot | 2 | 1813 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.63, 1.36] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Long‐term abstinence (all randomised) Show forest plot | 4 | 997 | Risk Ratio (M‐H, Random, 95% CI) | 1.59 [1.09, 2.33] |
| 2 Long‐term abstinence (complete case) Show forest plot | 4 | 796 | Risk Ratio (M‐H, Random, 95% CI) | 1.63 [1.12, 2.37] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Long‐term abstinence (all randomised) Show forest plot | 3 | 12985 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.95, 1.06] |
| 2 Long‐term abstinence (complete case) Show forest plot | 3 | 6798 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [1.00, 1.09] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Long‐term abstinence (all randomised) Show forest plot | 5 | 3079 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.66, 1.52] |
| 1.1 Comparison: minimal non‐app SC support | 2 | 1645 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.56, 1.18] |
| 1.2 Comparator: less intensive smartphone app | 3 | 1434 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.60, 2.09] |
| 2 Long‐term abstinence (complete case) Show forest plot | 5 | 1774 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.72, 1.54] |
| 2.1 Comparison: minimal non‐app SC support | 2 | 771 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.62, 1.23] |
| 2.2 Comparator: less intensive smartphone app | 3 | 1003 | Risk Ratio (M‐H, Random, 95% CI) | 1.18 [0.67, 2.09] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Long‐term abstinence (all randomised) Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.1 Comparison: counselling, nicotine replacement therapy and monitoring only app | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Comparison: counselling and nicotine replacement therapy | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Long‐term abstinence (complete case) Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.1 Comparison: counselling, nicotine replacement therapy and monitoring only app | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Comparison: counselling and nicotine replacement therapy | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Long‐term abstinence (all randomised) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2 Long‐term abstinence (complete case) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |

