புகைப்பிடிப்பதை விடுவதற்கான கைத் தொலைபேசி (மொபைல் போன்) சார்ந்த சிகிச்சை தலையீடுகள்
Información
- DOI:
- https://doi.org/10.1002/14651858.CD006611.pub4Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 10 abril 2016see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Tabaquismo
- Copyright:
-
- Copyright © 2016 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
RW is the lead author of this review.
For the most recent update:
-
RW and YG selected studies for inclusion.
-
HM reviewed the selected studies for inclusion.
-
RW and YG independently extracted data from the papers and undertook the analysis.
-
All authors contributed to the writing and editing of the review.
Sources of support
Internal sources
-
National Institute for Health Innovation (Auckland Uniservices), New Zealand.
-
Cancer Council Victoria, Australia.
External sources
-
No sources of support supplied
Declarations of interest
RW was co‐author of one paper on one of the included studies (Bramley 2005). She was a co‐investigator on two included studies (Free 2009; Free 2011), and principle investigator of a further included study (Whittaker 2011).
CB and HM were co‐authors of Whittaker 2011.
RB was co‐author of one of the trials (Borland 2013), and he led the development of the intervention.
AR was a lead author (Rodgers 2005), and a co‐author (Free 2009; Free 2011; Whittaker 2011), on included studies.
HM received honoraria from Johnson & Johnson and Pfizer for speaking at educational events and attending advisory group meetings. He has also received investigator initiated research funding from Pfizer.
RW's institution has received grant money to cover the costs of providing the text messaging intervention for the study described in Free 2011, and there is a grant pending to develop this intervention further for a different audience. The institution has also licensed the STOMP intervention described in Rodgers 2005 to HSAGlobal.
All other authors had no other known conflicts of interest.
Acknowledgements
We acknowledge the assistance of the Cochrane Tobacco Addiction Review Group Editorial base in the preparation of this review.
Version history
| Published | Title | Stage | Authors | Version |
| 2019 Oct 22 | Mobile phone text messaging and app‐based interventions for smoking cessation | Review | Robyn Whittaker, Hayden McRobbie, Chris Bullen, Anthony Rodgers, Yulong Gu, Rosie Dobson | |
| 2016 Apr 10 | Mobile phone‐based interventions for smoking cessation | Review | Robyn Whittaker, Hayden McRobbie, Chris Bullen, Anthony Rodgers, Yulong Gu | |
| 2012 Nov 14 | Mobile phone‐based interventions for smoking cessation | Review | Robyn Whittaker, Hayden McRobbie, Chris Bullen, Ron Borland, Anthony Rodgers, Yulong Gu | |
| 2009 Oct 07 | Mobile phone‐based interventions for smoking cessation | Review | Robyn Whittaker, Ron Borland, Chris Bullen, Ruey B Lin, Hayden McRobbie, Anthony Rodgers | |
| 2009 Jul 08 | Mobile phone‐based interventions for smoking cessation | Protocol | Robyn Whittaker, Ron Borland, Chris Bullen, Ray Lin, Hayden McRobbie, Anthony Rodgers | |
Differences between protocol and review
We have followed the change of policy of the Cochrane Tobacco Addiction Group, and now report our findings using Mantel‐Haenszel fixed‐effect risk ratios rather than as odds ratios. Previously, we had not pooled studies in the presence of substantial statistical heterogeneity as assessed by the I2 statistic, but in this update of the review, we report pooled results due to the homogenous nature of the included studies in terms of design, intervention and outcome.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
Medical Subject Headings Check Words
Adult; Humans;
PICO

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Forest plot of comparison: 1 Mobile phone intervention v ersus control, outcome: 1.1 26‐week cessation outcomes all studies.

Forest plot of comparison: 1 Mobile phone intervention versus control; outcome: 1.4 26‐week biochemically verified cessation outcomes (six studies).
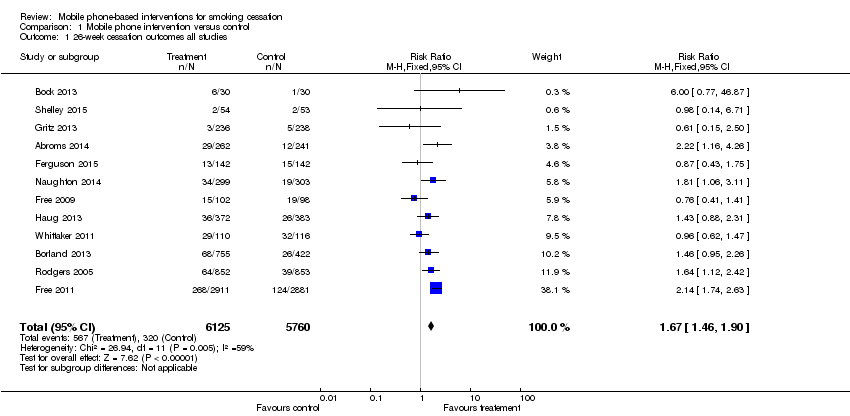
Comparison 1 Mobile phone intervention versus control, Outcome 1 26‐week cessation outcomes all studies.

Comparison 1 Mobile phone intervention versus control, Outcome 2 26‐week continuous abstinence.
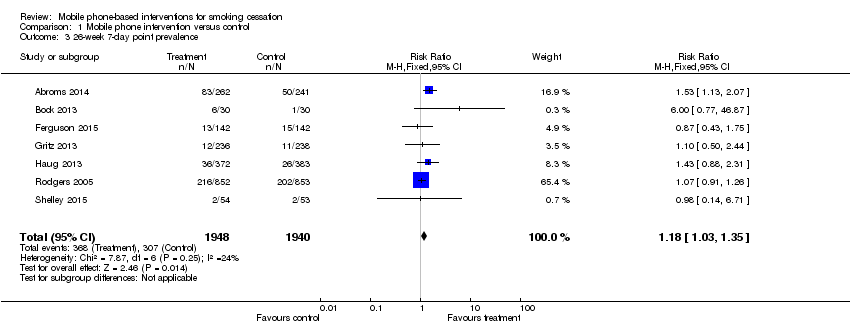
Comparison 1 Mobile phone intervention versus control, Outcome 3 26‐week 7‐day point prevalence.
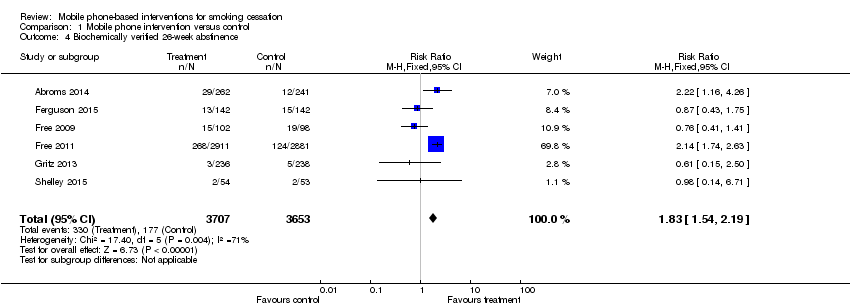
Comparison 1 Mobile phone intervention versus control, Outcome 4 Biochemically verified 26‐week abstinence.
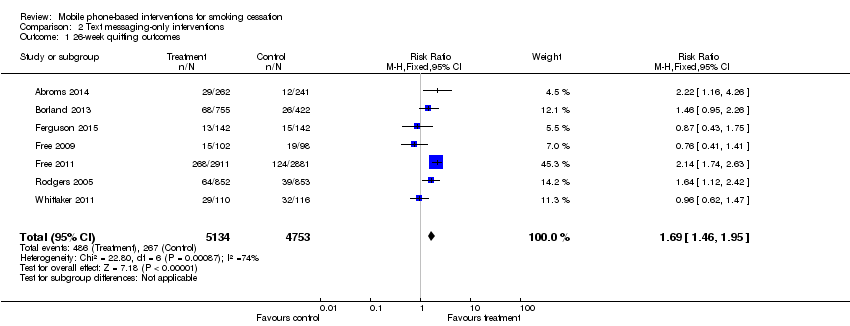
Comparison 2 Text messaging‐only interventions, Outcome 1 26‐week quitting outcomes.

Comparison 3 Text messaging plus face‐to‐face interventions, Outcome 1 Text message plus face‐to‐face interventions.
| Mobile phone‐based interventions for smoking cessation | ||||||
| Patient or population: people who smoke | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed quitters without intervention | Estimated quitters with mobile phone interventions | |||||
| 26‐week smoking cessation | Study population | RR 1.67 | 11,885 | ⊕⊕⊕⊝ | There was evidence of moderate heterogeneity across the included studies | |
| 56 per 1000 | 93 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 There was evidence of moderate heterogeneity. Sensitivity analyses around potential explanations for heterogeneity did not make substantial differences to the findings. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 26‐week cessation outcomes all studies Show forest plot | 12 | 11885 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.67 [1.46, 1.90] |
| 2 26‐week continuous abstinence Show forest plot | 8 | 10679 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.72 [1.50, 1.98] |
| 3 26‐week 7‐day point prevalence Show forest plot | 7 | 3888 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [1.03, 1.35] |
| 4 Biochemically verified 26‐week abstinence Show forest plot | 6 | 7360 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.83 [1.54, 2.19] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 26‐week quitting outcomes Show forest plot | 7 | 9887 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.69 [1.46, 1.95] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Text message plus face‐to‐face interventions Show forest plot | 5 | 1995 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.54 [1.12, 2.11] |

