Sistemas de recordatorio e intervenciones para rescatar pacientes inasistentes para el diagnóstico y tratamiento de la tuberculosis
Referencias
Referencias de los estudios incluidos en esta revisión
Referencias de los estudios excluidos de esta revisión
Referencias adicionales
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | Quasi‐randomized controlled trial Generation of allocation sequence: randomized by day of the week Allocation concealment: unclear Blinding of outcome assessors: unclear Blinding of providers and participants: not possible Inclusion of randomized participants in the analysis: 627/627 (100%) Protection against contamination: unclear | |
| Participants | Number: 627 randomized Inclusion criteria: consecutive children ages 1 to 12 years due for a tuberculosis test in an urban children's hospital outpatient department; 1 child per family enrolled Exclusion criteria: not stated | |
| Interventions | Intervention of interest Other interventions Control All families received education regarding the importance of skin testing for tuberculosis and the need for follow up to read the results. Instructions were given to return to the clinic in 48 to 72 hours | |
| Outcomes | Non‐adherence to return visit for Mantoux test reading | |
| Notes | Location: USA Baseline data: comparable | |
| Methods | Randomized controlled trial Generation of allocation sequence: unclear Allocation concealment: unclear Blinding of outcome assessors: unclear Blinding of providers and participants: not possible Inclusion of randomized participants in the analysis: 150/170 (89%); 20 participants excluded from main analysis because of death (8), lost to follow up (6), chemotherapy change (3), or transfer to more accessible clinics (3) Protection against contamination: unclear | |
| Participants | Number: 170 randomized; 150 analysed Inclusion criteria: patients with symptoms reporting at the Institute of Tuberculosis and Chest Diseases in Madras; with radiographic evidence of tuberculosis but negative smears; aged ≥12 years; prescribed national tuberculosis programme recommended regimen; living within a radius of about 5 km from the clinic; bona fide residents of Madras city and regarded as stable (expected to remain in the city for at least 1 year) Exclusion criteria: not stated | |
| Interventions | Intervention Control | |
| Outcomes | 1. Failure to retrieve the defaulters with the first action for the first episode of default | |
| Notes | Location: South India Baseline data: comparable Default: defined by the trial authors as failure of the patient to collect his/her supply of drugs on the due date or within the next 3 days | |
| Methods | Randomized controlled trial Generation of allocation sequence: random‐numbers table Allocation concealment: sequentially numbered and sealed opaque envelopes Blinding of outcome assessors: yes Blinding of providers and participants: not possible Inclusion of randomized participants in the analysis: 480/480 (100%) Protection against contamination: done | |
| Participants | Number: 480 randomized Inclusion criteria: new smear‐positive pulmonary tuberculosis (PTB); never been treated previously; delayed coming to collect drugs at the health centre for at least 3 days after scheduled appointment; identified from official patient record cards Exclusion criteria: re‐treatment patients | |
| Interventions | Intervention Control | |
| Outcomes | 1. Patient who did not complete treatment | |
| Notes | Location: Iraq Baseline data: not reported Default: defined by the author as treatment interrupted for ≥ 2 consecutive months | |
| Methods | Randomized controlled trial Generation of allocation sequence: random‐numbers table Allocation concealment: centralized randomization by a third party Blinding of outcome assessors: no Blinding of providers and participants: no Inclusion of randomized participants in the analysis: 200/200 (100%) Protection against contamination: done | |
| Participants | Number: 200 randomized Inclusion criteria: newly diagnosed adult pulmonary tuberculosis patients; sputum positive for acid‐fast bacilli (AFB); no treatment or < 15 days previous treatment; not in moribund condition or suffering from disorders like diabetes, cardiac failure, or renal failure; willing to stay in the hospital for the initial 1‐month intensive phase of treatment Exclusion criteria: not stated | |
| Interventions | Intervention Control | |
| Outcomes | 1. Number of patients who did not complete treatment | |
| Notes | Location: South India Baseline data: not reported Defaulter defined by author as a patient who failed to collect the drugs within 3 days after the due date of drug collection | |
| Methods | Randomized controlled trial Generation of allocation sequence: unclear Allocation concealment: unclear Blinding of outcome assessors: unclear Blinding of providers and participants: not possible Inclusion of all randomized participants in the analysis: 200/200 (100%) Protection against contamination: unclear | |
| Participants | Number: 200 randomized Inclusion criteria: volunteers who participated in a university‐sponsored tuberculosis detection drive; mostly college students Exclusion criteria: not stated | |
| Interventions | Intervention Control | |
| Outcomes | Number of participants who fail to return for skin‐test reading | |
| Notes | Location: USA Baseline data: comparable | |
| Methods | Randomized controlled trial Generation of allocation sequence: unclear Allocation concealment: unclear Blinding of outcome assessors: unclear Blinding of providers and participants: not possible Inclusion of all randomized participants in the analysis: 553/553 (100%) Contamination: unclear | |
| Participants | Number: 553 randomized Inclusion criteria: volunteers who participated in a university‐sponsored tuberculosis detection drive Exclusion criteria: not stated | |
| Interventions | Intervention Control | |
| Outcomes | Number of participants who fail to return for skin‐test reading | |
| Notes | Location: USA Baseline data: comparable | |
| Methods | Randomized controlled trial Generation of allocation sequence: unclear Allocation concealment: unclear Blinding of outcome assessors: unclear Blinding of providers and participants: not possible Inclusion of randomized participants in the analysis: 275/318 (85%); 43/318 (13.5%) withdrew from treatment Protection against contamination: unclear | |
| Participants | Number: 318 randomized Inclusion criteria: school children of both sexes in the first year of primary school in state‐run and private schools in the provinces of Barcelona, on anti‐tuberculosis chemoprophylaxis Exclusion criteria: children with active tuberculosis confirmed by medical examination and chest x‐ray | |
| Interventions | Intervention of interest Other interventions Control | |
| Outcomes | 1. Non‐adherence to final appointment | |
| Notes | Location: Spain Baseline data: not reported | |
| Methods | Quasi‐randomized controlled trial Generation of allocation sequence: within each 5‐week period each message variation was used once on each weekday, different variations were used each day of a given week by a computer‐generated system Allocation concealment: unclear Blinding of outcome assessors: unclear Blinding of providers and participants: not possible Inclusion of randomized participants in the analysis: 2008/2008 (100%) Protection against contamination: unclear | |
| Participants | Number: 2008 randomized Inclusion criteria: patients with scheduled appointments in the Tuberculosis Control Program of Santa Clara County Health Department over a period of 6 months Exclusion criteria: not stated | |
| Interventions | Interventions Control Appropriate recorded message was sent to patients between 1800 and 2100 the evening before the scheduled appointment. The system allows a message to be left on answering machines and to call back up to 5 times at half‐hour intervals if patients' lines were busy or there was no answer after 8 rings. For households whose primary language was English, Spanish, Vietnamese, or Tagalog, the message was sent in that language | |
| Outcomes | Non‐attendance for a scheduled appointment: if a patient had > 1 appointment during the course of the study, only data from the first appointment were included | |
| Notes | Location: USA Baseline data: not reported | |
| Methods | Tanke 1994 results for patients in the Reactor Clinic for diagnosis | |
| Participants | — | |
| Interventions | — | |
| Outcomes | — | |
| Notes | — | |
| Methods | Tanke 1994 results for patients in the INH [isoniazid] Clinic for prophylaxis | |
| Participants | — | |
| Interventions | — | |
| Outcomes | — | |
| Notes | — | |
| Methods | Tanke 1994 results for patients in the Case Clinic for treatment | |
| Participants | — | |
| Interventions | — | |
| Outcomes | — | |
| Notes | — | |
| Methods | Randomized controlled trial Generation of allocation sequence: unclear Allocation concealment: unclear Blinding of outcome assessors: unclear Blinding of providers and participants: not possible Inclusion of randomized participants in the analysis: 701/701 (100%) Protection against contamination: unclear | |
| Participants | Number: 701 randomized Inclusion criteria: persons undergoing tuberculin skin test at the 2 largest clinics of Santa Clara (California) County Immunization Program Exclusion criteria: not stated | |
| Interventions | Intervention Control | |
| Outcomes | 1. Total return failures | |
| Notes | Location: USA Baseline data: Not reported | |
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| Case‐control study design | |
| Intervention did not include reminders or late patient tracers | |
| Most participants did not have need for screening, prophylaxis, or treatment for tuberculosis, and results for the individuals in these categories were not presented separately | |
| Intervention did not include reminders or late patient tracers | |
| Intervention did not include reminders or late patient tracers | |
| Intervention did not include reminders or late patient tracers | |
| Review article | |
| Cohort study design | |
| Intervention did not include reminders or late patient tracers, except for those routinely provided and also applied to the control group | |
| Intervention did not include reminders or late patient tracers | |
| Process of late patient tracers not described, and the main objective was to assess predictors of latent tuberculosis infection completion by using structural equation modelling among homeless adults | |
| Reminders or late patient tracers not adequately described or systematically applied |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Patients who did not complete treatment Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.1  Comparison 1 Late patient tracers vs no late patient tracer, Outcome 1 Patients who did not complete treatment. | ||||
| 1.1 Home visit plus health education vs usual care (directly observed therapy, short‐course) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Reminder letter vs usual care | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Failure of patients to return to treatment after first missed appointment Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.2  Comparison 1 Late patient tracers vs no late patient tracer, Outcome 2 Failure of patients to return to treatment after first missed appointment. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Patients who did not complete treatment Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.1  Comparison 2 Late patient tracers (home visit plus health education) vs usual care, Outcome 1 Patients who did not complete treatment. | ||||
| 2 Treatment interrupted for 2 consecutive months or more Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.2  Comparison 2 Late patient tracers (home visit plus health education) vs usual care, Outcome 2 Treatment interrupted for 2 consecutive months or more. | ||||
| 3 Treatment failure Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.3  Comparison 2 Late patient tracers (home visit plus health education) vs usual care, Outcome 3 Treatment failure. | ||||
| 4 Death Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.4  Comparison 2 Late patient tracers (home visit plus health education) vs usual care, Outcome 4 Death. | ||||
| 5 Sputum‐smear positive follow up Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.5  Comparison 2 Late patient tracers (home visit plus health education) vs usual care, Outcome 5 Sputum‐smear positive follow up. | ||||
| 5.1 2 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 5 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.3 End of treatment | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Patients who did not complete treatment Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 3.1  Comparison 3 Late patient tracers (home visit) vs letter, Outcome 1 Patients who did not complete treatment. | ||||
| 2 Failure of patients to return for treatment after missed appointment Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 3.2  Comparison 3 Late patient tracers (home visit) vs letter, Outcome 2 Failure of patients to return for treatment after missed appointment. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Non‐attendance at clinic appointment Show forest plot | 3 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 4.1  Comparison 4 Reminders (automated telephone message) vs no message, Outcome 1 Non‐attendance at clinic appointment. | ||||
| 1.1 Basic message vs no message | 3 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Message + authority vs no message | 3 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Message + importance statement vs no message | 3 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 Message + authority + importance vs no message | 3 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.5 Any type of message vs no message | 3 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Non‐adherence to Mantoux test reading Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 5.1  Comparison 5 Reminders (non‐automated reminder phone call) vs no reminder, Outcome 1 Non‐adherence to Mantoux test reading. | ||||
| 1.1 Reminder phone call vs no call | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Non‐adherence to final clinic appointment Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 6.1  Comparison 6 Reminder plus health education vs usual care, Outcome 1 Non‐adherence to final clinic appointment. | ||||
| 1.1 Phone call plus health education vs usual care | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Home visit plus health education vs usual care | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Failed to return for skin test reading Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 7.1  Comparison 7 Reminder vs other types of reminders and no reminder, Outcome 1 Failed to return for skin test reading. | ||||
| 1.1 Expert vs non‐expert | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Take‐home card vs postcard | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Take‐home card vs telephone call | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 Take‐home card vs person‐to‐person | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.5 Postcard vs telephone call | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.6 Postcard vs person‐to‐person | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.7 Telephone call vs person‐to‐person | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Failed to return for skin test reading Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 7.2  Comparison 7 Reminder vs other types of reminders and no reminder, Outcome 2 Failed to return for skin test reading. | ||||

Late patient tracers vs no late patient tracer: Patients who did not complete treatment

Late patient tracers (home visit) vs letter: Patients who did not complete treatment
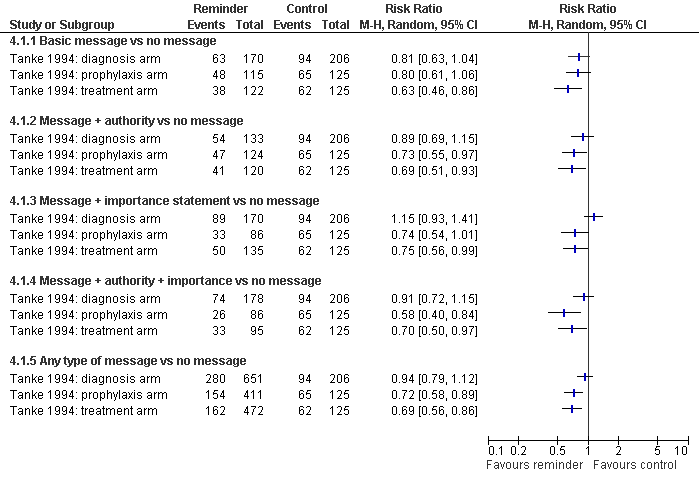
Reminders (automated telephone message) vs no message: Non‐attendance at clinic appointment

Reminder plus health education vs usual care: Non‐adherence to final clinic appointment

Comparison 1 Late patient tracers vs no late patient tracer, Outcome 1 Patients who did not complete treatment.
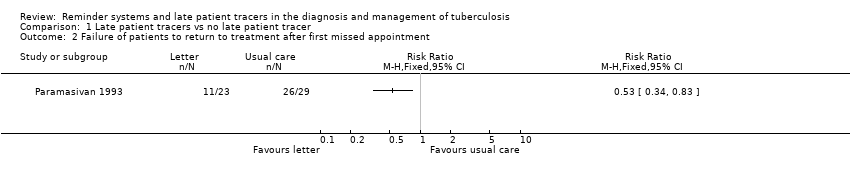
Comparison 1 Late patient tracers vs no late patient tracer, Outcome 2 Failure of patients to return to treatment after first missed appointment.

Comparison 2 Late patient tracers (home visit plus health education) vs usual care, Outcome 1 Patients who did not complete treatment.
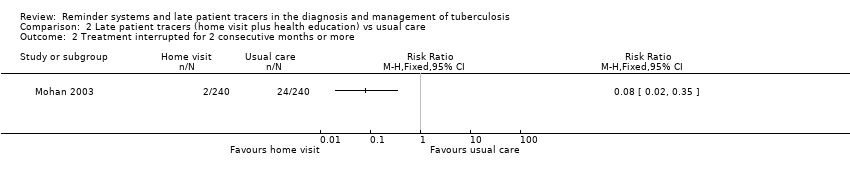
Comparison 2 Late patient tracers (home visit plus health education) vs usual care, Outcome 2 Treatment interrupted for 2 consecutive months or more.

Comparison 2 Late patient tracers (home visit plus health education) vs usual care, Outcome 3 Treatment failure.
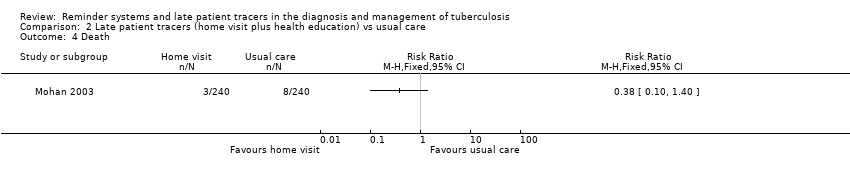
Comparison 2 Late patient tracers (home visit plus health education) vs usual care, Outcome 4 Death.

Comparison 2 Late patient tracers (home visit plus health education) vs usual care, Outcome 5 Sputum‐smear positive follow up.

Comparison 3 Late patient tracers (home visit) vs letter, Outcome 1 Patients who did not complete treatment.

Comparison 3 Late patient tracers (home visit) vs letter, Outcome 2 Failure of patients to return for treatment after missed appointment.

Comparison 4 Reminders (automated telephone message) vs no message, Outcome 1 Non‐attendance at clinic appointment.
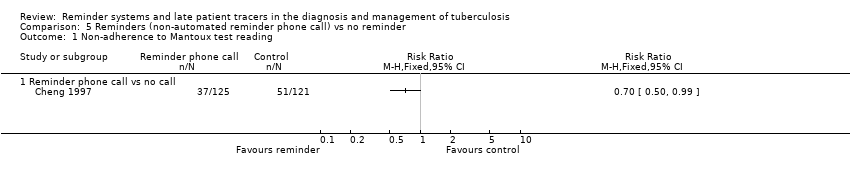
Comparison 5 Reminders (non‐automated reminder phone call) vs no reminder, Outcome 1 Non‐adherence to Mantoux test reading.
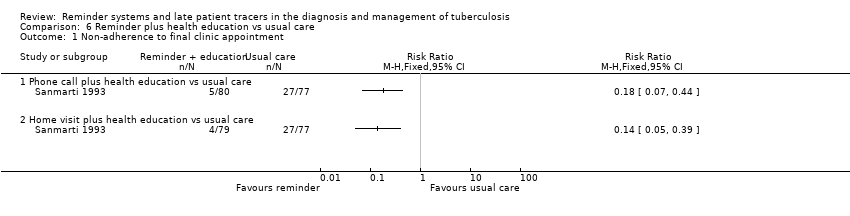
Comparison 6 Reminder plus health education vs usual care, Outcome 1 Non‐adherence to final clinic appointment.

Comparison 7 Reminder vs other types of reminders and no reminder, Outcome 1 Failed to return for skin test reading.
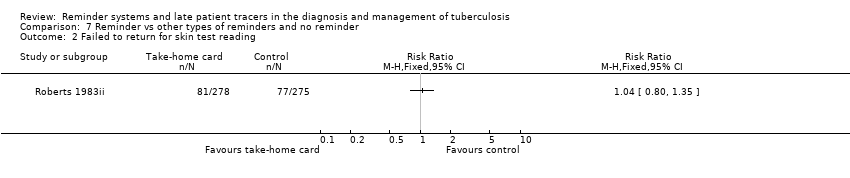
Comparison 7 Reminder vs other types of reminders and no reminder, Outcome 2 Failed to return for skin test reading.
| Search set | Cochrane SRa | CENTRAL | MEDLINEb | EMBASEb | LILACSb | SCI‐EXPANDED & SSCI | CINAHL |
| 1 | tuberculosis | tuberculosis | tuberculosis | tuberculosis | tuberculosis | tuberculosis | tuberculosis |
| 2 | adherence | PATIENT COMPLIANCE | TUBERCULOSIS/DRUG THERAPY/PREVENTION AND CONTROL | TUBERCULOSIS | adherence | adherence | adherence |
| 3 | compliance | PATIENT DROPOUTS | PATIENT COMPLIANCE | PATIENT‐COMPLIANCE | compliance | compliance | compliance |
| 4 | monitor* | REMINDER SYSTEMS | PATIENT DROPOUTS | medication adherence | monitor* | monitor* | monitor* |
| 5 | reminder* | TREATMENT REFUSAL | COOPERATIVE BEHAVIOUR | REMINDER‐SYSTEM | reminder* | reminder* | reminder* |
| 6 | 2 or 3 or 4 or 5 or 6 | DIRECTLY OBSERVED THERAPY | TREATMENT REFUSAL | TREATMENT‐REFUSAL | 2 or 3 or 4 or 5 | non‐adherence | non‐adherence |
| 7 | 1 and 6 | medication adherence | medication adherence | DIRECTLY‐OBSERVED‐THERAPY | 1 and 6 | late patient tracer | late patient tracer |
| 8 | — | electronic monitoring | REMINDER SYSTEMS | electronic monitoring | — | 2‐7/or | 2‐7/or |
| 9 | — | nonadherence | electronic monitoring | nonadherence | — | 1 and 8 | 1 and 8 |
| 10 | — | non‐adherence | nonadherence | non‐adherence | — | — | — |
| 11 | — | late patient tracer | non‐adherence | late patient tracer | — | — | — |
| 12 | — | 2‐11/or | DIRECTLY OBSERVED THERAPY | 1 or 2 | — | — | — |
| 13 | — | 1 and 12 | late patient tracer | 3‐11/or | — | — | — |
| 14 | — | — | 1 or 2 | 13 and 14 | — | — | — |
| 15 | — | — | 3‐13/or | — | — | — | — |
| 16 | — | — | 14 and 15 | — | — | — | — |
| aCochrane Infectious Diseases Group Specialized Register and the Cochrane Effective Practice and Organisation of Care Group Specialized Register. | |||||||
| Intervention | Trial | Design | Generation of allocation sequence | Allocation concealment | Blinded assessment | Inclusion of randomized participants in the analysis | Protection against contamination |
| Late patient tracers | RCT | Unclear | Unclear | Unclear | Adequate | Unclear | |
| RCT | Adequate | Adequate | No | Adequate | Done | ||
| RCT | Adequate | Adequate | Yes | Adequate | Done | ||
| Reminder systems | RCT | Unclear | Unclear | Unclear | Adequate | Unclear | |
| RCT | Unclear | Unclear | Unclear | Adequate | Unclear | ||
| RCT | Unclear | Unclear | Unclear | Adequate | Unclear | ||
| Quasi‐RCT | Inadequate | Unclear | Unclear | Adequate | Unclear | ||
| Quasi‐RCT | Inadequate | Unclear | Unclear | Adequate | Unclear | ||
| RCT | Unclear | Unclear | Unclear | Adequate | Unclear | ||
| RCT: randomized controlled trial. | |||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Patients who did not complete treatment Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Home visit plus health education vs usual care (directly observed therapy, short‐course) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Reminder letter vs usual care | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Failure of patients to return to treatment after first missed appointment Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Patients who did not complete treatment Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Treatment interrupted for 2 consecutive months or more Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Treatment failure Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4 Death Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5 Sputum‐smear positive follow up Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5.1 2 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 5 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.3 End of treatment | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Patients who did not complete treatment Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Failure of patients to return for treatment after missed appointment Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Non‐attendance at clinic appointment Show forest plot | 3 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.1 Basic message vs no message | 3 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Message + authority vs no message | 3 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Message + importance statement vs no message | 3 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 Message + authority + importance vs no message | 3 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.5 Any type of message vs no message | 3 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Non‐adherence to Mantoux test reading Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Reminder phone call vs no call | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Non‐adherence to final clinic appointment Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Phone call plus health education vs usual care | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Home visit plus health education vs usual care | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Failed to return for skin test reading Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Expert vs non‐expert | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Take‐home card vs postcard | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Take‐home card vs telephone call | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 Take‐home card vs person‐to‐person | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.5 Postcard vs telephone call | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.6 Postcard vs person‐to‐person | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.7 Telephone call vs person‐to‐person | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Failed to return for skin test reading Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |

