Tratamiento de la invaginación intestinal en niños
Información
- DOI:
- https://doi.org/10.1002/14651858.CD006476.pub3Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 01 junio 2017see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Colorrectal
- Copyright:
-
- Copyright © 2017 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
SG: running searches, selecting studies, extracting data, analysing results, and writing the main review.
JK: drafting the protocol, identifying studies, and providing content area advice.
ACW: drafting the protocol and providing methodological advice.
RGM: drafting the protocol, running searches, selecting studies, extracting data, analysing results, and writing the main review.
Declarations of interest
Review authors have no conflicts of interest to declare.
Acknowledgements
Jooly Joseph and Madan Mohan Palliyil wrote an initial protocol for this review in 2007. We would like to thank Tanvir Kapoor for helping to draft the updated protocol. We would also like to thank the Cochrane Colorectal Cancer Review Group (Managing editor, Information specialist, editors and referees) for support and guidance provided.
Version history
| Published | Title | Stage | Authors | Version |
| 2017 Jun 01 | Management for intussusception in children | Review | Steven Gluckman, Jonathan Karpelowsky, Angela C Webster, Richard G McGee | |
| 2013 Oct 30 | Surgical and non‐surgical management for intussusception in children | Protocol | Tanvir Kapoor, Richard G McGee, Jonathan Karpelowsky, Michael Su, Angela C Webster | |
| 2007 Apr 18 | Surgical and non‐surgical management for intussusception in children | Protocol | Tanvir Kapoor, Richard G McGee, Jonathan Karpelowsky, Michael Su, Angela C Webster | |
Differences between protocol and review
In contrast to our published protocol, we decided to conduct the analysis using fixed‐effect meta‐analysis because it is more conservative in the presence of heterogeneity and small‐study effects. Although not specifically stipulated in the protocol, we saw both quasi‐RCTs and cluster RCTs as fit for inclusion in this review.
When data were missing, and intention‐to‐treat analysis was not possible, we planned to use available‐case or per‐protocol analysis.
Although we did not discuss these matters in the protocol, we used the GRADE approach and 'Summary of findings' tables to summarise our findings.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
- Air;
- Dexamethasone [*therapeutic use];
- Enema [*methods];
- Gastrointestinal Agents [*therapeutic use];
- Glucagon [*therapeutic use];
- Glucocorticoids [*therapeutic use];
- Intestinal Perforation [etiology];
- Intussusception [surgery, *therapy];
- Postoperative Complications;
- Randomized Controlled Trials as Topic;
- Recurrence;
- Secondary Prevention [methods];
Medical Subject Headings Check Words
Child; Humans;
PICO

Study flow diagram for identification of randomised trials exploring management of intussusception in children.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
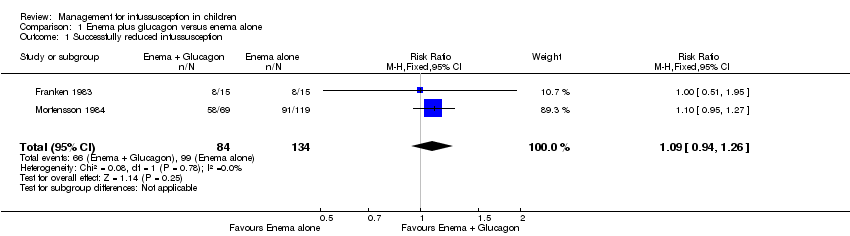
Comparison 1 Enema plus glucagon versus enema alone, Outcome 1 Successfully reduced intussusception.
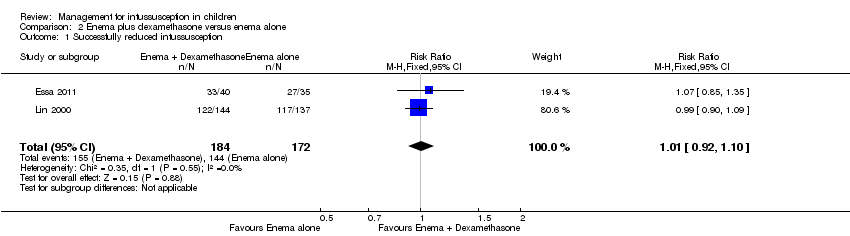
Comparison 2 Enema plus dexamethasone versus enema alone, Outcome 1 Successfully reduced intussusception.

Comparison 2 Enema plus dexamethasone versus enema alone, Outcome 2 Bowel perforation(s).

Comparison 2 Enema plus dexamethasone versus enema alone, Outcome 3 Recurrent intussusception.

Comparison 2 Enema plus dexamethasone versus enema alone, Outcome 4 Bowel resection.
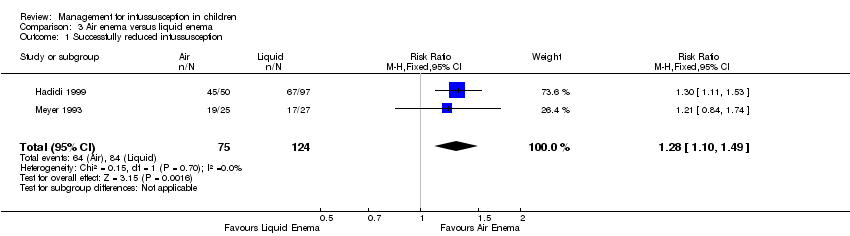
Comparison 3 Air enema versus liquid enema, Outcome 1 Successfully reduced intussusception.
| Enema plus glucagon versus enema alone summary of findings table | |||||
| Patient or population: children with intussusception | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Quality of the evidence | |
| Risk with liquid enema alone | Risk with liquid enema plus glucagon | ||||
| Successfully reduced intussusception | Study population | RR 1.09 | 218 | Lowa | |
| 739 per 1000 | 805 per 1000 | ||||
| Moderate | |||||
| 649 per 1000 | 707 per 1000 | ||||
| Bowel perforation(s) | Outcome not reported in any studies | ||||
| Recurrent intussusception (follow‐up: 6 months) | Outcome not reported in any studies | ||||
| Bowel resection | Outcome not reported in any studies | ||||
| Postoperative complication(s) | Outcome not reported in any studies | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on assumed risk in the comparison group and relative effect of the intervention (and its 95% CI) | |||||
| GRADE Working Group grades of evidence | |||||
| aDowngraded two levels for serious concerns for high risk of selection, attrition, and performance bias | |||||
| Enema plus dexamethasone versus enema alone summary of findings table | |||||
| Patient or population: children with intussusception | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Quality of the evidence | |
| Risk with enema alone | Risk with enema plus dexamethasone | ||||
| Successfully reduced intussusception | Study population | RR 1.01 | 356 | Lowa | |
| 157 per 1000 | 159 per 1000 | ||||
| Moderate | |||||
| 771 per 1000 | 779 per 1000 | ||||
| Bowel perforation(s) | Study population | RR 2.63 | 75 | Lowb,c | |
| 125 per 1000 | 329 per 1000 | ||||
| Moderate | |||||
| 125 per 1000 | 48 per 1000 | ||||
| Recurrent intussusception (follow‐up: 6 months) | Study population | RR 0.14 | 299 | Lowa | |
| 69 per 1000 | 10 per 1000 | ||||
| Moderate | |||||
| 370 per 1000 | 52 per 1000 | ||||
| Bowel resection | Study population | RR 0.88 | 75 | Lowb,c | |
| 86 per 1000 | 75 per 1000 | ||||
| Moderate | |||||
| 375 per 1000 | 330 per 1000 | ||||
| Postoperative complication(s) | Outcome not reported in any studies | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on assumed risk in the comparison group and relative effect of the intervention (and its 95% CI) | |||||
| GRADE Working Group grades of evidence | |||||
| aDowngraded two levels for serious concerns for high risk of attrition and performance bias bDowngraded one level for serious imprecision (95% CI is wide and includes null effect) cDowngraded one level for concerns for high risk of performance bias | |||||
| Air enema versus liquid enema summary of findings table | |||||
| Patient or population: children with intussusception | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Quality of the evidence | |
| Risk with liquid contrast enema | Risk with air enema | ||||
| Successfully reduced intussusception | Study population | RR 1.28 | 199 | Lowa | |
| 677 per 1000 | 867 per 1000 | ||||
| Moderate | |||||
| 712 per 1000 | 911 per 1000 | ||||
| Bowel perforation(s) | Outcome not reported in any studies | ||||
| Recurrence of intussusception (follow‐up: 6 months) | Outcome not reported in any studies | ||||
| Bowel resection | Outcome not reported in any studies | ||||
| Postoperative complication(s) | Outcome not reported in any studies | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on assumed risk in the comparison group and relative effect of the intervention (and its 95% CI) | |||||
| GRADE Working Group grades of evidence | |||||
| aDowngraded two levels for serious concerns for high risk of selection, performance, and detection bias | |||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Successfully reduced intussusception Show forest plot | 2 | 218 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.94, 1.26] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Successfully reduced intussusception Show forest plot | 2 | 356 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.92, 1.10] |
| 2 Bowel perforation(s) Show forest plot | 1 | 75 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.63 [0.11, 62.66] |
| 3 Recurrent intussusception Show forest plot | 2 | 299 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.03, 0.60] |
| 4 Bowel resection Show forest plot | 1 | 75 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.19, 4.06] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Successfully reduced intussusception Show forest plot | 2 | 199 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.28 [1.10, 1.49] |

