Profilaxis con betamiméticos orales para la prevención del trabajo de parto prematuro en embarazos de un feto único
Referencias
Referencias de los estudios incluidos en esta revisión
Referencias de los estudios excluidos de esta revisión
Referencias adicionales
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | Performance bias: blinding of participants ‐ yes; blinding of caregiver ‐ yes; blinding of outcome assessment ‐ yes. | |
| Participants | Country: UK. | |
| Interventions | Isoxsuprine 30 mg 4 times a day versus placebo. | |
| Outcomes | Incidence of: | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| Comparison of preterm delivery rates of asthmatic women routinely taking betamimetics and those on no betamimetics. Observational study not a RCT. | |
| Trial of isoxsuprine to inhibit acute preterm labour. | |
| Trial tested the effects of oral isoxsuprine in preventing preterm labour in low‐risk women. | |
| Trial of the effect of betamimetics on fetal umbilical artery velocimetry in low‐risk pregnancy. | |
| Trial of the effect of isoxsuprine on uterine contractions in women in normal and preterm labour. Observational study not a RCT. | |
| Trial of maintenance tocolytic therapy. | |
| Trial comparing the effects of oral ritodrine and placebo on preterm delivery rates of primigravid women in whom the internal os of the cervix was one or more fingerbreadths dilated at 28 to 32 weeks' gestation. Whilst cervical change on digital examination was thought at the time this study was conducted to be an accurate predictor of preterm delivery subsequent work has shown that routine digital examination, which is a subjective and non‐specific examination, has a poor positive predictive value and may influence the number of unnecessary hospital admissions or tocolytic use. |
RCT: randomised controlled trial
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Perinatal mortality Show forest plot | 1 | 64 | Odds Ratio (M‐H, Fixed, 95% CI) | 4.74 [0.50, 45.00] |
| Analysis 1.1  Comparison 1 Betamimetic versus placebo, Outcome 1 Perinatal mortality. | ||||
| 2 Preterm labour Show forest plot | 1 | 64 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.14, 8.09] |
| Analysis 1.2  Comparison 1 Betamimetic versus placebo, Outcome 2 Preterm labour. | ||||
| 3 Side effects sufficient to stop therapy Show forest plot | 1 | 64 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.51 [0.59, 10.76] |
| Analysis 1.3  Comparison 1 Betamimetic versus placebo, Outcome 3 Side effects sufficient to stop therapy. | ||||
| 4 Birth before 37 completed weeks Show forest plot | 1 | 64 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.14, 8.09] |
| Analysis 1.4  Comparison 1 Betamimetic versus placebo, Outcome 4 Birth before 37 completed weeks. | ||||
| 5 Birthweight less than 2500 grams Show forest plot | 1 | 64 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.74 [0.44, 6.87] |
| Analysis 1.5  Comparison 1 Betamimetic versus placebo, Outcome 5 Birthweight less than 2500 grams. | ||||
| 6 Neonatal death Show forest plot | 1 | 64 | Odds Ratio (M‐H, Fixed, 95% CI) | 4.74 [0.50, 45.00] |
| Analysis 1.6  Comparison 1 Betamimetic versus placebo, Outcome 6 Neonatal death. | ||||
| 7 Side effects and adverse effects of betamimetics not sufficient to stop medication Show forest plot | 1 | 64 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.5 [0.88, 7.08] |
| Analysis 1.7  Comparison 1 Betamimetic versus placebo, Outcome 7 Side effects and adverse effects of betamimetics not sufficient to stop medication. | ||||

Comparison 1 Betamimetic versus placebo, Outcome 1 Perinatal mortality.

Comparison 1 Betamimetic versus placebo, Outcome 2 Preterm labour.
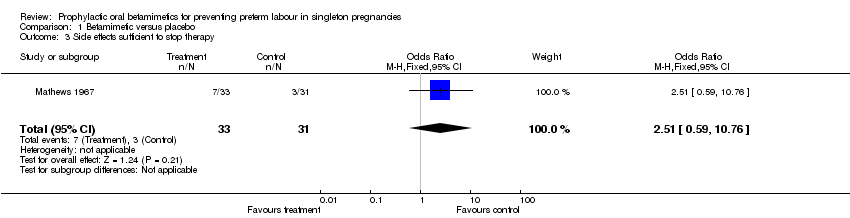
Comparison 1 Betamimetic versus placebo, Outcome 3 Side effects sufficient to stop therapy.

Comparison 1 Betamimetic versus placebo, Outcome 4 Birth before 37 completed weeks.

Comparison 1 Betamimetic versus placebo, Outcome 5 Birthweight less than 2500 grams.
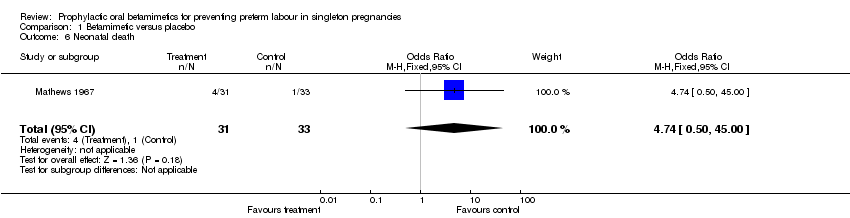
Comparison 1 Betamimetic versus placebo, Outcome 6 Neonatal death.

Comparison 1 Betamimetic versus placebo, Outcome 7 Side effects and adverse effects of betamimetics not sufficient to stop medication.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Perinatal mortality Show forest plot | 1 | 64 | Odds Ratio (M‐H, Fixed, 95% CI) | 4.74 [0.50, 45.00] |
| 2 Preterm labour Show forest plot | 1 | 64 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.14, 8.09] |
| 3 Side effects sufficient to stop therapy Show forest plot | 1 | 64 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.51 [0.59, 10.76] |
| 4 Birth before 37 completed weeks Show forest plot | 1 | 64 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.14, 8.09] |
| 5 Birthweight less than 2500 grams Show forest plot | 1 | 64 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.74 [0.44, 6.87] |
| 6 Neonatal death Show forest plot | 1 | 64 | Odds Ratio (M‐H, Fixed, 95% CI) | 4.74 [0.50, 45.00] |
| 7 Side effects and adverse effects of betamimetics not sufficient to stop medication Show forest plot | 1 | 64 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.5 [0.88, 7.08] |

