Use of plastic adhesive drapes during surgery for preventing surgical site infection
Abstract
Background
Surgical site infection has been estimated to occur in about 15% of clean surgery and 30% of contaminated surgery cases. Using plastic adhesive drapes to protect the wound from organisms that may be present on the surrounding skin during surgery is one strategy used to prevent surgical site infection. Results from non‐randomised studies have produced conflicting results about the efficacy of this approach. A systematic review was required to guide clinical practice.
Objectives
To assess the effect of adhesive drapes used during surgery on surgical site infection, cost, mortality and morbidity.
Search methods
For this fourth update we searched the Cochrane Wounds Group Specialised Register (searched 4th March 2015); the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2015, Issue 2); Ovid MEDLINE (2012 to 3rd March 2015); Ovid MEDLINE (In‐Process & Other Non‐Indexed Citations, 2012 to 3rd March 2015); Ovid EMBASE (2012 to 3rd March 2015); and EBSCO CINAHL (2012 to 4th March 2015).
Selection criteria
Randomised controlled trials comparing any plastic adhesive drape with no plastic adhesive drape, used alone or in combination with woven (material) drapes or disposable (paper) drapes, in patients undergoing any type of surgery. Ring drapes were excluded.
Data collection and analysis
Two review authors independently selected and assessed studies for trial quality and both independently extracted data. We contacted study authors for additional information.
Main results
We identified no new studies for this fourth update. The review includes five studies involving 3082 participants comparing plastic adhesive drapes with no drapes and two studies involving 1113 participants comparing iodine‐impregnated adhesive drapes with no drapes. A significantly higher proportion of patients in the adhesive drape group developed a surgical site infection when compared with no drapes (risk ratio (RR) 1.23, 95% confidence interval (CI) 1.02 to 1.48, P = 0.03). Iodine‐impregnated adhesive drapes had no effect on the surgical site infection rate (RR 1.03, 95% CI 0.06 to 1.66, P = 0.89). Length of hospital stay was similar in the adhesive drape and non‐adhesive drape groups.
Authors' conclusions
There was no evidence from the seven trials that plastic adhesive drapes reduce surgical site infection rates, and some evidence that they increase infection rates. Further trials may be justified, using blinded outcome assessment to examine the effect of adhesive drapes on surgical site infection, based on different wound classifications.
PICO
Plain language summary
Use of plastic adhesive drapes during surgery for preventing surgical site infection
Following surgery, up to 30% of wounds may become infected. This complication of surgery may cause distress for the patient and lead to higher treatment costs. Many interventions have been designed to reduce postoperative infections. One of these is the use of a drape which adheres to the skin, and through which the surgeon cuts. It is thought that adhesive drapes prevent germs (which may be on the skin) from entering the open wound. This updated review of over 4000 patients, in seven separate trials could find no evidence that adhesive drapes reduce surgical site infection rates, and some evidence that they may increase infection rates.
Authors' conclusions
Summary of findings
| Adhesive drapes compared with no adhesive drapes for preventing surgical site infection | ||||||
| Patient or population: Patients undergoing surgery Comparison: No adhesive drapes | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No. of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Adhesive drapes versus no adhesive drapes | |||||
| Surgical site infection (all wound classifications) | Medium risk population | RR 1.23 | 3082 | ⊕⊕⊕⊕ | ||
| 109 per 1000 | 134 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Various definitions of infection were used; we accepted the authors definition in each case. | ||||||
| Iodophore‐impregnated adhesive drapes compared with no adhesive drapes for preventing surgical site infection | ||||||
| Patient or population: Patients undergoing surgery | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No. of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| No adhesive drapes | Iodophore‐impregnated adhesive drapes | |||||
| Surgical site infection | Medium risk population | RR 1.03 | 1113 | ⊕⊕⊕⊝ | ||
| 45 per 1000 | 46 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 A number of definitions of wound infection were used across the trials. We accepted the authors definition in all cases. | ||||||
Background
Description of the condition
Surgical site infection (SSI) is one of the most common postoperative complications, and has been estimated to occur in about 15% of cases of clean surgery and 30% of contaminated surgery cases (Bruce 2001). SSI is associated with longer recovery and further risks of additional complications, therefore increasing the risk of morbidity and mortality (Mangram 1999). However the incidence rate depends on a number of factors, including the definition of infection used, the intensity of surveillance, whether patients are followed up after discharge, and the prevalence of risk factors in the population studied (Smyth 2000). Risk factors associated with SSI have been grouped into two main categories: patient‐ or host‐related and operation‐ or procedure‐related (Mangram 1999; Smyth 2000). Patient characteristics include age, obesity, co morbidities such as diabetes, remote infection, American Society of Anestheologists score (ASA) status, immunosuppressive therapy and length of preoperative hospital stay. Operative risk factors include length of surgery, skin preparation (including shaving and antiseptic skin preparation), type of procedure, antimicrobial prophylaxis and surgical technique (Mangram 1999; Smyth 2000).
Surgical wounds are frequently classified as either 'clean', 'clean contaminated', 'contaminated' or 'dirty‐infected' with the latter categories associated with a higher infection rate (Lilani 2005). Many countries now benchmark their SSI rate using the National Nosocomial Infections Surveillance (NNIS) system risk index, in which wound classification is combined with the ASA status, length of surgery and whether surgery was undertaken laparoscopically to assess risk of SSI (Gaynes 2001). The additional per patient cost of SSI has been estimated to be between GBP 959 for abdominal hysterectomy to GBP 6103 for limb amputation (Coello 2005), and over USD 14,000 for an organ space SSI (Kashimura 2012). In the Unites States the estimated annual cost of SSIs is USD 3.5 billion to USD 10 billion (Thompson 2011).
Description of the intervention
The high additional costs associated with SSI have led to the adoption of strategies that could reduce the incidence of SSI. These strategies include administration of prophylactic antibiotics, use of antiseptic solutions for skin preparation, and the use of sterile disposable materials. One of the commonly used operative strategies to reduce SSI is the plastic adhesive drape (referred to hereafter as adhesive drape). This was first tested 50 years ago on a cohort of patients undergoing a range of abdominal surgeries (Payne 1956). The study had three main aims: 1) to test adherence of a polyvinyl drape to the skin; 2) to assess the level of wound contamination; and 3) to assess skin and wound reaction to the drape. Problems were found with adherence of the drape to the skin, despite trialing a number of skin preparation solutions. Positive cultures were recovered from two of the 51 wounds but no skin or wound reactions to the polyvinyl sheet were recorded. Since that time, use of adhesive drapes has become widespread and the product has undergone modifications to improve effectiveness (Ritter 1988; Yoshimura 2003). This review will focus on plastic (defined as polyethylene, polyurethane or polyvinyl) adhesive drapes (e.g. OpSite (Smith and Nephew), Ioban (3M Company, USA), Steridrape (3M United Kingdom) through which an incision is made. Drapes may be either plain or impregnated with an antibacterial agent such as iodine.
How the intervention might work
For most SSIs, the source of the invading pathogen (or disease causing biological agent) is the patient's skin (Nichols 1996). Consequently, preoperative skin preparation is intended to render the skin as free as possible from bacteria that may enter the surgical wound. Although skin disinfection prior to surgery drastically reduces the number of bacteria on the skin's surface, recolonisation with bacteria from deeper skin layers and hair follicles may occur during the operation (Fleischmann 1996). Sterile surgical drapes, made of either linen or impervious paper, are used to prevent any contact with unprepared surfaces. Adhesive drapes are also used for this purpose and, are generally used in combination with other draping techniques, but they have an additional function; theoretically, they act as a microbial barrier, to prevent migration of contaminating bacteria from the skin to the operative site, for which there is some evidence (French 1976; Ha'eri 1983).
Why it is important to do this review
Although there is theoretical plausibility for the use of adhesive drapes, conflicting reports have been published regarding their usefulness in limiting bacteria around the surgical site (Katthagen 1992; Lilly 1970) and for preventing SSI (Ritter 1988; Swenson 2008). Recolonisation of the skin following antiseptic preparation is also more rapid under adhesive drapes compared with using no adhesive drapes in healthy volunteers (Falk‐Brynhildsen 2013) and in cardiac surgery (Falk‐Brynhildsen 2013a). Moreover, allergic reactions to povidone iodine are not unknown, and there is at least one case report of allergic contact dermatitis associated with the use of iodophor‐impregnated incise drapes (Zokaie 2011). In a related systematic review, Edwards 2009 found no benefit in using iodophor‐impregnated adhesive drapes to prevent postoperative surgical wound infection, when they were used as part of preoperative skin antisepsis. In light of these controversies, and because their use is widespread, a systematic review of the possible benefits and harms of adhesive drapes is justified to guide clinical practice.
Objectives
The primary objective of this systematic review was to assess the effect of plastic adhesive drapes used during surgery on surgical site infection (SSI) rates.
The secondary objectives were:
-
to determine the cost effectiveness of using plastic adhesive drapes;
-
to assess if there were any adverse effects associated with the use of plastic adhesive drapes; and
-
to determine whether different types of plastic adhesive drapes (polyethylene/polyurethane/polyvinyl) have differential effects on SSI rates.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) that evaluated the effectiveness of adhesive drapes (used alone or in combination with other drapes), in preventing SSI.
Types of participants
We considered for inclusion, trials recruiting people of any age or gender, undergoing any type of inpatient or outpatient surgery.
Types of interventions
The primary intervention was adhesive drapes (polyethylene, polyurethane or polyvinyl), through which an incision is made. Adhesive drapes may have been used alone or in combination with other drapes: woven (material) drapes or disposable (paper) drapes and with any antiseptic skin preparation. The comparison intervention was no adhesive drapes; other drapes such as woven (material) drapes or disposable (paper) drapes may have been used. We excluded trials evaluating plastic 'ring drapes' or 'V' drapes as the incision is not made through the drape.
Comparisons included:
-
adhesive drapes (without added antimicrobial properties) compared with no adhesive drapes; and
-
adhesive drapes (with added antimicrobial properties) compared with no adhesive drapes.
Types of outcome measures
Primary outcomes
Rates of surgical site infection (SSI). For the purposes of this review we accepted the definition of SSI used in the trial.
Secondary outcomes
-
Mortality (any cause)
-
Length of hospital stay
-
Costs
-
Hospital readmissions
-
Adverse reactions (e.g. contact dermatitis, anaphylaxis)
-
Other serious infection or infectious complication such as septicaemia or septic shock
Search methods for identification of studies
Electronic searches
For an outline of the search methods used in the third update of this review see Appendix 1.
For this fourth update we searched the following electronic databases.
-
The Cochrane Wounds Group Specialised Register (searched 4th March 2015).
-
The Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2015, Issue 2).
-
Ovid MEDLINE (2012 to 3rd March 2015).
-
Ovid MEDLINE (In‐Process & Other Non‐Indexed Citations, 2012 to 3rd March 2015).
-
Ovid EMBASE (2012 to 3rd March 2015).
-
EBSCO CINAHL (2012 to 4th March 2015).
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) using the following strategy.
#1 MeSH descriptor Surgical Wound Infection explode all trees
#2 MeSH descriptor Surgical Wound Dehiscence explode all trees
#3 MeSH descriptor Infection Control explode all trees
#4 (surg* NEAR/5 infect*):ti,ab,kw
#5 (surg* NEAR/5 wound*):ti,ab,kw
#6 (surg* NEAR/5 site*):ti,ab,kw
#7 (surg* NEAR/5 incision*):ti,ab,kw
#8 (surg* NEAR/5 dehisc*):ti,ab,kw
#9 (wound* NEAR/5 dehisc*):ti,ab,kw
#10 (wound NEAR/5 complication*):ti,ab,kw
#11 (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10)
#12 (plastic NEAR/3 drape*):ti,ab,kw
#13 (adhes* NEAR/3 drape*):ti,ab,kw
#14 (skin NEAR/3 drape*):ti,ab,kw
#15 (incis* NEAR/3 drape*):ti,ab,kw
#16 (iodophor NEAR/3 drape*):ti,ab,kw
#17 (iodine NEAR/3 drape*):ti,ab,kw
#18 (opsite or steridrape or ioban):ti,ab,kw
#19 (#12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18)
#20 (#11 AND #19)
The search strategies for Ovid MEDLINE, Ovid EMBASE and EBSCO CINAHL can be found in Appendix 2, Appendix 3 and Appendix 4 respectively. We combined the Ovid MEDLINE search with the Cochrane Highly Sensitive Search Strategy for identifying randomised trials in MEDLINE: sensitivity‐ and precision‐maximising version (2008 revision); Ovid format (Lefebvre 2011). We combined the EMBASE search with the Ovid EMBASE filter developed by the UK Cochrane Centre, which is also cited in the Cochrane Handbook (Lefebvre 2011). We combined the CINAHL searches with the trial filters developed by the Scottish Intercollegiate Guidelines Network (SIGN) (SIGN 2012). We did not apply any date or language restrictions.
Searching other resources
We contacted researchers and manufactures in order to obtain any unpublished data. We also searched reference lists of potentially useful articles.
Data collection and analysis
Selection of studies
For the initial review, two authors (JW, AA) independently assessed the title and abstracts of references identified by the search strategy. We then retrieved full reports of all potentially relevant trials for further assessment of eligibility, based on the inclusion criteria. We settled differences of opinion by consensus or referral to the editorial base of the Wounds Group. There was no blinding of authorship. For this updated review, JW excluded trials and the Managing Editor of the Wounds Group verified their exclusion.
Data extraction and management
Two review authors (JW,AA) independently extracted the following data, using a piloted data extraction sheet: type of study, country, study setting, number of participants, sex, mean age, type of surgery, preoperative wound classification, predisposing risk factors by treatment groups, type of drape, draping procedure, type of preoperative skin preparation, prophylactic or therapeutic antibiotic use, all primary and secondary outcome measures reported and authors' conclusions. Clarification about aspects of the trial were required from all of the authors; five were untraceable (Chiu 1993; Cordtz 1989; Jackson 1971; Psaila 1977; Ward 2001). Additional trial details were received from Dewan 1987 and from the second author of the Segal 2002 trial. We also contacted manufacturers of plastic adhesive drapes (Johnson & Johnson, 3M Company and Smith & Nephew) to request details of any unpublished trials. A representative of each of these manufacturers responded; no current trials are underway and they were unaware of any unpublished trials.
Assessment of risk of bias in included studies
Two review authors independently assessed the quality of eligible trials, using a predefined quality assessment form, based on the assessment criteria outlined below. Disagreements between review authors were again resolved by consensus or referral to the editorial base of the Wounds Group. We contacted investigators of included trials to resolve any ambiguities. Each included study was assessed using the Cochrane Collaboration's tool for assessing risk of bias (Higgins 2011). This tool addresses six specific domains, namely sequence generation, allocation concealment, blinding, incomplete outcome data, selective outcome reporting, and other issues (e.g. extreme baseline imbalance) (see Appendix 5 for details of criteria on which the judgement was based). We assessed blinding and completeness of outcome data for each outcome separately. We completed a risk of bias table for each eligible study. We discussed any disagreement amongst authors to achieve a consensus.
We presented an assessment of risk of bias using a 'Risk of bias' summary figure, which presents all of the judgments in a cross‐tabulation of study by entry. This display of internal validity indicates the weight the reader may give the results of each study.
We defined high quality trials as those receiving a 'low risk of bias' rating for the criterion of allocation concealment (central computerised randomisation service or sealed opaque envelopes), and for blinding of outcome assessment.
Measures of treatment effect
For dichotomous outcomes, we calculated risk ratio (RR) plus 95% confidence intervals (CI). For continuous outcomes we calculated mean difference (MD) plus 95% confidence intervals.
Unit of analysis issues
Individual patients were the analytic units in all trials, so there were no unit of analysis issues.
Dealing with missing data
If there was evidence of missing data, we contacted the study authors to request the information. Where trial authors could not provide missing data, we assessed the risk of bias of the missing data and decided if the missing data were of 'low' or 'high' risk of bias according to our risk of bias criteria (Higgins 2011). Or, if data were considered to be missing at random, we analysed the available information.
Assessment of heterogeneity
We assessed heterogeneity using the Chi2 statistic with significance being set at P < 0.10. In addition, we investigated the degree of heterogeneity by calculating the I2 statistic (Higgins 2002). If we identified evidence of significant heterogeneity (> 50%), we explored potential sources of heterogeneity and a random‐effects approach to the analysis was undertaken. We conducted a narrative review of eligible studies where statistical synthesis of data from more than one study was not possible or considered not appropriate.
Assessment of reporting biases
We completed a 'Risk of bias' table for each eligible study and present an assessment of risk of bias using a 'Risk of bias' summary figure (Figure 1) which presents the judgements in a cross‐tabulation. This display of internal validity indicates the weight the reader may give to the results of each study.

Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
Data synthesis
We analysed data using Review manager software (RevMan 2011). One review author (JW) entered the data and the other author (AA) cross‐checked the printout against their own data extraction forms. We calculated risk ratios (RRs) and 95% confidence intervals (CIs) for dichotomous outcomes (risk ratio is the risk of infection in the intervention group divided by the risk of infection in the control group; a risk ratio of less than one indicates fewer infections in the intervention or adhesive drape group). We calculated mean differences (MDs) and 95% CIs for continuous outcomes. Where appropriate, we pooled the results of comparable trials using a fixed‐effect model, and we reported the pooled estimate together with its 95% CI.
We included all eligible trials in the initial analysis and carried out preplanned sensitivity analyses to evaluate the effect of trial quality. This was done by excluding trials most susceptible to bias (based on the quality assessment): those with inadequate allocation concealment and uncertain or unblinded outcome assessment.
Subgroup analysis and investigation of heterogeneity
We had planned the following four subgroup analyses.
-
Clean surgery compared with contaminated surgery.
-
Individual compared with cluster allocation.
-
Prophylactic antibiotic compared with no prophylaxis.
-
Hair clipping compared with shaving.
The only subgroup analysis that was possible, based on available data, was of clean compared with contaminated surgery. Nor was it possible to undertake a planned sensitivity analysis based on the type of material the drape was made from due to insufficient detail about the products.
Results
Description of studies
Results of the search
The initial search identified 84 possibly relevant titles, and after screening the titles we considered 18 as potentially useful. Both review authors independently retrieved abstracts or full‐texts and reviewed them against the inclusion criteria. Eleven studies did not meet the inclusion criteria and we excluded them from the review. We excluded two further studies in subsequent updates (Breitner 1986; Swenson 2008). For this fourth update, we identified 49 new trials using the search strategy and follow‐up of reference lists. Only one of these trials was potentially relevant but was later excluded (Falk‐Brynhildsen 2013a), see Characteristics of excluded studies.
Included studies
From the initial search, seven RCTs (Chiu 1993; Cordtz 1989; Dewan 1987; Jackson 1971; Psaila 1977; Segal 2002; Ward 2001) met the inclusion criteria (see Characteristics of included studies). We included these seven trials of 4195 participants in the review, with individual trial sizes ranging between 141 to 1340 participants. Five of the trials compared an adhesive drape with no adhesive drape (Chiu 1993; Cordtz 1989; Jackson 1971; Psaila 1977; Ward 2001) and two compared an iodine‐impregnated adhesive drape with no adhesive drape (Dewan 1987; Segal 2002). One study was a multi‐centre trial (Cordtz 1989); the remaining trials were single centre. An a priori sample size calculation, based on a 50% reduction in the infection rate, was reported in one study (Ward 2001). Segal 2002 reported a sample size calculation based on an analysis of results of a pilot study of 120 patients, the trial was then continued, recruiting a further 64 patients.
Surgical procedures included caesarean section (Cordtz 1989; Ward 2001), general or abdominal surgery (Dewan 1987; Jackson 1971; Psaila 1977), hip surgery (Chiu 1993) and cardiac surgery (Segal 2002). Surgical site infection (SSI) was not defined in one study (Chiu 1993); the Characteristics of included studies table contains details of other definitions used.
Four trials used iodine and alcohol to prepare the operative site (Chiu 1993; Cordtz 1989; Dewan 1987; Jackson 1971); one used Savlon and alcoholic chlorhexidine (Psaila 1977); an iodophor/alcohol water insoluble film was used in the Segal 2002 trial; and in the Ward 2001 trial, skin was swabbed with alcoholic chlorhexidine. In the Cordtz 1989 trial, participants were also randomised to have their wound re‐disinfected prior to wound closure. Jackson 1971 ran a concurrent test of antibiotic spray in random cases.
Prophylactic cephalosporin was given to each patient at anaesthetic induction in the Chiu 1993 trial and all patients in the Ward 2001 trial received 1g of cephazolin when the baby's cord was clamped, unless antibiotics were already being administered for therapy or prophylaxis. Antibiotic use was recorded by Cordtz 1989 and Segal 2002 but not reported by group. No information about antibiotic use was provided by other authors (Dewan 1987; Jackson 1971; Psaila 1977).
Excluded studies
The Characteristics of excluded studies table contains reasons for excluding 14 of these studies. In summary, six were not RCTs (Breitner 1986; Duvvi 2005; Fairclough 1986; Maxwell 1969; Swenson 2008; Yoshimura 2003), three did not report SSI rates (French 1976; Ha'eri 1983; Manncke 1984; Falk‐Brynhildsen 2013a), one did not report the number of participants in each group (Lewis 1984) and an adhesive drape was not used in the remaining three trials (Nystrom 1980; Nystrom 1984; Williams 1972). We excluded one trial from the first review update which was waiting assessment, as it reported colonisation rates but not SSI rates (Breitner 1986).
Risk of bias in included studies
(See risk of bias Figure 1; Figure 2 and Appendix 5)
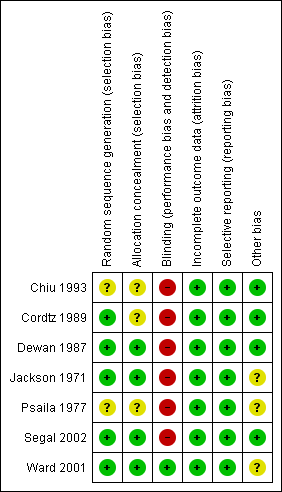
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
Random sequence generation
In all trials, the trial authors stated that participants were randomly allocated to the intervention. It was unclear how the allocation sequence was generated in three trials (Chiu 1993; Psaila 1977; Segal 2002). In the Cordtz 1989 trial, the National Centre for Hospital Hygiene was responsible for the randomisation process; Dewan 1987 and Ward 2001 used a random number table and in the Jackson 1971 trial, a 'spin of the coin' was used.
Allocation concealment
Allocation concealment was adequate in three studies. Segal 2002 asked surgeons participating in the trial to draw the treatment allocation from a 'closed sack' at the beginning of surgery and Ward 2001 and Dewan 1987 used sealed envelopes for group allocation. In other studies the information was not available to judge (unclear), although we contacted trial authors where possible (Chiu 1993; Cordtz 1989; Jackson 1971; Psaila 1977).
Blinding
It was impossible for surgeons to be blinded to the intervention. In the Ward 2001 and Dewan 1987 trials, outcomes were assessed by staff who were unaware of group assignment. The study investigators inspected wounds for signs of infection in the Jackson 1971 and Segal 2002 trials. In all other trials it was unclear who was responsible for assessing outcomes, and whether those who did inspect wounds for signs of infection were aware of group assignment (Chiu 1993; Cordtz 1989; Psaila 1977).
Incomplete outcome data
One trial did not indicate the period of follow‐up (Psaila 1977). In the remaining trials, follow‐up ranged between five days and six months (Characteristics of included studies table). In the Dewan 1987 trial, 46 patients (4.2%) were unable to be tracked and were excluded from the analysis. Based on reported data, follow‐up appeared to be complete in all of the other included trials. However, the absence of detailed participant flow charts, or any reference to the number who started the trial and were unable to be followed up, makes assessment of rates difficult, particularly as the follow‐up periods were lengthy in some studies, increasing the likelihood of incomplete follow‐up.
Selective reporting
Results for all expected outcomes were reported in all of the trials.
Other bias
Intention‐to‐treat analysis
None of the trials reported group assignment violations, and so it is difficult to assess whether patient outcomes were analysed in the group to which they were assigned. None of the trials specifically reported that they used an intention‐to‐treat analysis.
Baseline comparability
No information was available about baseline comparability for five trials (Chiu 1993; Cordtz 1989; Jackson 1971; Psaila 1977; Segal 2002). In the Dewan 1987 trial, the author stated that groups were similar for all risk factors but no data was presented. Ward 2001 stated that, apart from age and parity, groups were comparable at baseline but again, no data were available for comparison.
Conflict of interest
No conflict of interests issues were reported by any of the trial authors
Effects of interventions
See: Summary of findings for the main comparison ; Summary of findings 2
This review includes seven studies involving 4195 participants of whom 2133 were in the treatment group and 2062 formed the control group. All seven trials recorded incidence of surgical site infection (SSI) as an outcome. Surgical procedures included general or abdominal surgery (Dewan 1987; Jackson 1971; Psaila 1977), caesarean section (Cordtz 1989; Ward 2001), cardiac surgery (Segal 2002) and hip surgery (Chiu 1993). Based on our quality criteria, we considered the trials of Dewan 1987 and Ward 2001 to have a low risk of bias. The remaining five trials (Chiu 1993; Cordtz 1989; Jackson 1971; Psaila 1977; Segal 2002) contained a moderate risk of bias. However, as results from all trials were not dissimilar, we combined all of the eligible trials in the meta‐analyses.
We undertook two comparisons: adhesive drapes compared with no adhesive drapes (Data and analyses Table 1) (Chiu 1993; Cordtz 1989; Jackson 1971; Psaila 1977; Ward 2001) and iodine‐impregnated adhesive drapes compared with no adhesive drapes (Analysis 2.1) (Dewan 1987; Segal 2002).
Adhesive drapes compared with no adhesive drapes (Analysis: 1)
Primary outcome
Surgical site infection (SSI)
Five studies were included in this comparison (Cordtz 1989; Chiu 1993; Jackson 1971; Psaila 1977; Ward 2001). These studies included 3082 participants, of whom 1556 were in the adhesive drape group and 1526 were in the no adhesive drape group. Although the studies covered a 30‐year time span and included a range of different types of surgery, we did not detect any heterogeneity (I2 = 0%). Pooling these studies (fixed‐effect model) indicated significantly more SSIs in the adhesive drape group, (RR 1.23, 95% CI 1.02 to 1.48, P = 0.03, Analysis 1.1). The overall event rate was 13.7% and 11.2% in the adhesive drape group and no drape group, respectively.
Surgical site infection ‐ by preoperative wound classification
A single trial of 921 participants analysed infection rates based on preoperative infection risk classifications (Jackson 1971). In this trial there was no significant effect of using an adhesive drape overall, although infection rates were lower for the no adhesive drape group. Results did not vary depending on baseline risk of infection: RR (overall) 1.20, 95% CI 0.86 to 1.66; RR (for clean wounds) 1.37, 95% CI 0.53 to 3.53; RR (for potentially infected wounds) 1.24, 95% CI 0.80 to 1.92; and RR (for infected wounds) 1.03, 95% CI 0.60 to 1.75 (Analysis 1.2). We have reported results from this trial as they were presented in the published paper, even though there was a minor discrepancy between results in the text and those in the tables. For example, in the text, 52 of the 448 cases in the no adhesive drape group became infected. In the table, when cases were classified as clean, potentially infected and infected, totals were 51 infections among 445 cases. Similarly in the adhesive drape group, 67 infections were reported in 473 patients in the text and 67 of 476 in the tables. Attempts to contact investigators were unsuccessful, however, using either set of results did not affect the overall level of significance for this outcome.
Secondary outcome
Length of stay
Ward 2001 was the only trial to report length of stay. The analysis was divided into two subgroups: length of stay for those with a SSI (n = 64) and those without a SSI (n = 539). In the infected subgroup, the mean length of stay in the adhesive drape group was 10.4 days (standard deviation (SD) 3.9 days), this was not statistically different from the mean length of stay in the no adhesive drape group (10.2 days, SD 3.9 days). Length of stay was much shorter among those without a SSI. In the adhesive drape group it was 5.2 days (SD 1.3 days) and also 5.2 days (SD 1.3 days) in the no adhesive drape group. We did not find any statistical difference in length of stay between the adhesive drape and no adhesive drape groups in either of these subgroups (Analysis 1.3).
None of the trials provided information about any of the other predefined secondary outcomes (mortality, cost, hospital readmissions, adverse reactions e.g. contact dermatitis, anaphylaxis), or other serious infection or infectious complication, such as septicaemia or septic shock.
Iodine‐impregnated adhesive drapes compared with no adhesive drapes (Analysis:2)
Primary outcome
Surgical site infection (SSI)
Two studies compared iodine‐impregnated adhesive drapes with no adhesive drapes (Dewan 1987; Segal 2002). These studies included 1113 participants, of whom 577 were in the iodine‐impregnated adhesive drape group and 536 were in the no adhesive drape group. In the absence of heterogeneity (I² = 0%) we pooled the studies. There was no significant difference in SSI rates between the two groups (RR 1.03, 95% CI 0.66 to 1.60, P = 0.89 Analysis 2.1).
Discussion
The conclusions from the original version of this review remain unchanged in this update. Although adhesive drapes are widely used in surgery to prevent surgical site infections (SSIs), the most recent recommendations for control of SSIs remains equivocal regarding the use of adhesive drapes for this purpose (Alexander 2011). Consequently, the primary focus of this review was to address the effectiveness of adhesive drapes in preventing SSI. We identified seven studies, including 4195 patients. The main finding of this review is that adhesive drapes are not associated with a reduced infection rate compared with no adhesive drapes, and appear to be associated with an increased risk of infection. The most obvious explanation for this result is that, if adequately disinfected prior to surgery, the patient's skin is unlikely to be a primary cause of SSI; so attempts to isolate the skin from the wound, using an adhesive drape, may be pointless and potentially harmful as excessive moisture under plastic drapes may encourage bacteria residing in hair follicles to migrate to the surface and multiply (Chiu 1993).
In the only trial to report on length of stay, the use of adhesive drapes did not appear to affect the duration of hospitalisation. There was no available evidence for our other preplanned outcomes of interest; mortality, cost, hospital readmissions or adverse reactions.
Three of the trials included in the review had concurrent interventions. Segal 2002 had four arms to the study, two of which did not involve a comparison between draping methods. In the analysis, we included the two arms of the study that included a draping comparison only. We believe it is unlikely that this design would have had an impact on the outcome as patients were mutually exclusive. Similarly, in the Psaila 1977 trial, ring drapes were used in a third group. Cordtz 1989 allocated patients to four groups, adhesive drape or no adhesive drape combined with re‐disinfection or no re‐disinfection. Although there was a lower rate of SSI in the re‐disinfection group, the reduction was similar irrespective of the type of drape used.
Studies were of variable quality with only two trials (Dewan 1987; Ward 2001) meeting our criteria for high quality (receiving an A rating for the criterion of allocation concealment and for blinding of outcome assessment). The reporting aspects of other trials were poor, making it difficult to assess study quality. However, results of all but one of the trials were in a similar direction, favouring no adhesive drapes, providing some confidence in results. Although verification remains a problem, with many older studies, where contact with authors is impossible. Only the Psaila 1977 trial had a non‐significant trend favouring adhesive drapes. This was a small study of 116 participants. The authors randomly allocated patients to two groups (adhesive drape and ring drape) and then stated, "in a control group, linen towels alone were used". We included outcomes from the control group in this study as the 'no adhesive drape' group in our analysis, but it was unclear how this group was selected. We are uncertain if any publication bias affected results; we did not find any unpublished studies.
Finally, it is unclear if all of the products used in the trials were similar. Trade names of adhesive drapes have changed over the 30‐year time span this review covers. Whether this has led to a qualitative improvement in the product is unclear. No specific details were provided about, for example, the density of the material or its adherability. Irrespective of this, results have remained consistent over time suggesting that any improvements or changes to the product have not affected SSI rates.
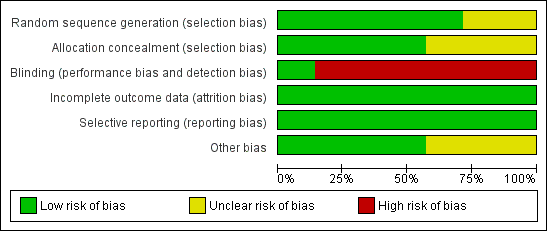
Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.

Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
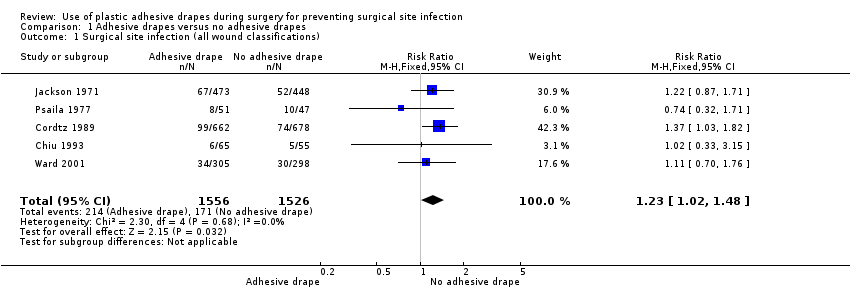
Comparison 1 Adhesive drapes versus no adhesive drapes, Outcome 1 Surgical site infection (all wound classifications).

Comparison 1 Adhesive drapes versus no adhesive drapes, Outcome 2 Surgical site infection (by wound classification).

Comparison 1 Adhesive drapes versus no adhesive drapes, Outcome 3 Length of hospital stay.
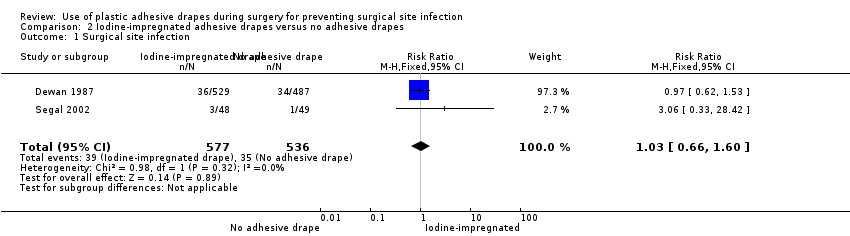
Comparison 2 Iodine‐impregnated adhesive drapes versus no adhesive drapes, Outcome 1 Surgical site infection.
| Adhesive drapes compared with no adhesive drapes for preventing surgical site infection | ||||||
| Patient or population: Patients undergoing surgery Comparison: No adhesive drapes | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No. of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Adhesive drapes versus no adhesive drapes | |||||
| Surgical site infection (all wound classifications) | Medium risk population | RR 1.23 | 3082 | ⊕⊕⊕⊕ | ||
| 109 per 1000 | 134 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Various definitions of infection were used; we accepted the authors definition in each case. | ||||||
| Iodophore‐impregnated adhesive drapes compared with no adhesive drapes for preventing surgical site infection | ||||||
| Patient or population: Patients undergoing surgery | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No. of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| No adhesive drapes | Iodophore‐impregnated adhesive drapes | |||||
| Surgical site infection | Medium risk population | RR 1.03 | 1113 | ⊕⊕⊕⊝ | ||
| 45 per 1000 | 46 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 A number of definitions of wound infection were used across the trials. We accepted the authors definition in all cases. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Surgical site infection (all wound classifications) Show forest plot | 5 | 3082 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.23 [1.02, 1.48] |
| 2 Surgical site infection (by wound classification) Show forest plot | 1 | 921 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.20 [0.86, 1.66] |
| 2.1 Clean | 1 | 363 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.37 [0.53, 3.53] |
| 2.2 Potentially infected | 1 | 486 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.24 [0.80, 1.92] |
| 2.3 Infected | 1 | 72 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.60, 1.75] |
| 3 Length of hospital stay Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 Infected wound | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 No infected wound | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Surgical site infection Show forest plot | 2 | 1113 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.66, 1.60] |

