Антипсихотические средства при кокаиновой зависимости
Referencias
References to studies included in this review
References to studies excluded from this review
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | Randomised double‐blind parallel trial | |
| Participants | Participants: 28, mean age 36 years, predominantly men (89%), 54% African‐American, 32% Hispanic,14% white, 26 participants current cannabis abuse/dependence and 20 cocaine dependence. Inclusion criteria: DSM‐IV criteria for cocaine dependence and schizophrenia or schizoaffective disorder. | |
| Interventions | (1) Risperidone 9 mg/day (14 participants) versus (2) olanzapine 20 mg/day (14 participants). Previously, in a 2‐week cross‐taper phase, participants were tapered off their previously prescribed medication onto the study medication with gradual increases in doses of risperidone (3 mg/day for days 1 – 3, 6 mg/day for days 4 – 7, 9 mg/day from day 8 until end of the study) and olanzapine (5 mg/day for days 1 – 3, 10 mg/day for days 4 – 7, 15 mg/day for days 8 – 12, 20 mg/day from day 13 until end of the study). Country of origin: USA | |
| Outcomes | Substance use (Quantitative Substance Use Inventory, urinalysis and self report), craving (Cocaine Craving Report), psychiatric decompensation (PANSS, HAM‐D, CGI), side effects (AIMS, Simpson, psychiatrist assessments), compliance, retention, percentage of study completers | |
| Notes | Dates when the study was conducted: not reported Funding from the National Institute of Drug Abuse and from MARSAD and Ely Lilly and Co, Indianapolis Confict of interest: Levin received support from Ortho. Mc Neil Pharmaceuticals, Ely Lilly & Company, UCB Pharma; he served as a consultant to Shire Phamaceuticals Inc, Astra Zeneca Pharmaceuticals and Ely Lilly & Company | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not enough information reported to make a judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement: method of concealment is not described |
| Blinding of participants and personnel (performance bias) | Unclear risk | Insufficient information to permit judgement; it only reports that the study was double‐blind |
| Blinding of participants and personnel (performance bias) | Low risk | Insufficient information, but objective outcomes unlikely to be biased by lack of blinding |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information to permit judgement; it only reports that the study was double‐blind |
| Blinding of outcome assessment (detection bias) | Low risk | Insufficient information, but objective outcomes unlikely to be biased by lack of blinding |
| Incomplete outcome data (attrition bias) | High risk | 43% of participants dropped out, with imbalance between groups; reasons for missing data provided separately for each group |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement; it seems that the published reports include all expected outcomes, but the study protocol is not available and we therefore do not know the prespecified outcomes. |
| Methods | Randomised double‐blind placebo‐controlled trial. | |
| Participants | Participants: 12, mean age 47.4 years, 50% men | |
| Interventions | (1) Quetiapine 400 mg to 800 mg/day (mean exit doses 428.57 mg/day) (7 participants) versus (2) placebo (5 participants) setting: Outpatient Country of origin: USA | |
| Outcomes | Attrition, side effects, compliance, cocaine use , craving (CCQ), amount of cocaine used (self‐reported g/week), psychiatric decompensation (YMRS, HAM‐D, CGI, PRD‐III) | |
| Notes | Dates when the study was conducted: not reported Funding from an investigator‐initiated grant from Astra Zeneca No conflict of interest declared | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about the sequence generation process to permit judgement; randomisation process is not described |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement; method of concealment is not described |
| Blinding of participants and personnel (performance bias) | Unclear risk | Insufficient information to permit judgement; it only reports that the study was double‐blind |
| Blinding of participants and personnel (performance bias) | Low risk | Insufficient information , but objective outcomes unlikely to be biased by lack of blinding |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information to permit judgement; it only reports that the study was double‐blind |
| Blinding of outcome assessment (detection bias) | Low risk | Insufficient information, but objective outcomes unlikely to be biased by lack of blinding |
| Incomplete outcome data (attrition bias) | Low risk | ITT analysis |
| Selective reporting (reporting bias) | Low risk | All the study’s prespecified primary outcomes have been reported |
| Methods | Randomised double‐blind placebo‐controlled trial | |
| Participants | Participants: 112, mean age 45.1 years in the lamotrigine group and 43.5 in the placebo group, 59.8% men Inclusion criteria: current diagnosis of cocaine dependence and reported cocaine use within 14 days before randomisation. Bipolar I, II or NOS disorder as determined by SCID‐CV. Baseline Hamilton rating scale for depression (HRSD17) score ≥ 10 | |
| Interventions | (1) Lamotrigine initiated at 25 mg/day and increased to 200 mg/day over 5 weeks. After that increased to a maximum of 400 mg/day (55 participants) versus (2) placebo (57 participants) Country of origin: USA | |
| Outcomes | Attrition, side effects, compliance, craving (CCQ), amount of cocaine used (self‐reported % days of use and money spent on cocaine) | |
| Notes | Dates when the study was conducted: not reported Funding from the Stanley Medical Research Institute, grant number 05T‐704 Confict of interest: Dr Brown received support from Stanley Medical Research Institute, Sunovion Pharmaceuticals, Forest Research Institute, GlaxoSmithKline and Astra Zeneca; Dr Sunderajan from Bristol‐MyersSquibb, Lilly USA, LLC, and Takeda Pharmaceuticals North America; and Dr Carmody from Cyberonics and the Institute for Chronic Illness | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was conducted by the study statistician through a computerised randomisation process |
| Allocation concealment (selection bias) | High risk | Randomisation was downloaded to a spreadsheet used by unblinded clinic staff to allocate medication |
| Blinding of participants and personnel (performance bias) | Low risk | All direct care staff (i.e. study physicians and raters) were blinded |
| Blinding of participants and personnel (performance bias) | Low risk | All direct care staff (i.e. study physicians and raters) were blinded |
| Blinding of outcome assessment (detection bias) | Low risk | All direct care staff (i.e. study physicians and raters) were blinded |
| Blinding of outcome assessment (detection bias) | Low risk | All direct care staff (i.e. study physicians and raters) were blinded |
| Incomplete outcome data (attrition bias) | Unclear risk | 120 participants randomised but "The number of subjects available for analysis was 112 (those with at least one post baseline assessment)". Not specified from which groups the 8 participants (6.6%) dropped out |
| Selective reporting (reporting bias) | Low risk | All the study’s prespecified primary outcomes have been reported |
| Methods | Randomised double‐blind placebo‐controlled trial | |
| Participants | Participants 96, mean age 36.9 years, 59.4% men, 79.2% white, 10.4% Hispanic, 10.4% black; mean educational level 12.3 years; 68% unemployed, 25% employed, 7% student or retired; use of cocaine: 20.9% less than once/week, 6.3% once a week, 38.5% several times/week, 28.1% once a day, 6.3% greater than 3 times/day | |
| Interventions | (1) Risperidone 2 mg/day (32 participants) (2) risperidone 4 mg/day (31 participants) versus (3) placebo (33 participants) | |
| Outcomes | Retention, side effects, cocaine use, changes in blood pressure, psychiatric decompensation (BDI) | |
| Notes | Dates when the study was conducted: not reported Funding from NIDA Grants DA P50‐9262, DA RO1‐6143 AND DA RO1 16302 No conflicts of interest existed for any author | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement; randomisation process not described |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement; method of allocation is not described |
| Blinding of participants and personnel (performance bias) | Unclear risk | Insufficient information to permit judgement; only reported that it is double‐blind |
| Blinding of participants and personnel (performance bias) | Low risk | Insufficient information, but objective outcomes unlikely to be biased by lack of blinding |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information to permit judgement; only reported that it is double‐blind |
| Blinding of outcome assessment (detection bias) | Low risk | Insufficient information, but objective outcomes unlikely to be biased by lack of blinding |
| Incomplete outcome data (attrition bias) | Low risk | ITT analysis |
| Selective reporting (reporting bias) | Low risk | All the study’s prespecified primary outcomes have been reported |
| Methods | Randomised double‐blind placebo‐controlled trial | |
| Participants | Participants 48, mean age 45.8 years, all male veterans, with 41 (85.4%) Africa‐American and 7 (14.6%) white. Means of cocaine use and money spent on drugs 30 days before study entry: 11.28 days and USD 357; reported routes of cocaine administration: 73.9% smoking, 10.9% nasal, 6.5% IV, 6.5% non‐IV injection, and 2.2% oral; other substances used 30 days before entry: alcohol (85.1%), heroin (6.4%), methadone (10.6%), other opioids (8.5%), sedative‐hypnotics (14.9%), and cannabis (25.5%), 87.2% reported using more than 1 substance per day; Inclusion criteria: ≥ 18 years, diagnosis of cocaine dependence as determined by a clinician interview using a checklist of DSM‐IV criteria and active use of cocaine within the past 30 days, determined by urine testing or self report | |
| Interventions | (1) starting 2.5 mg olanzapine per day, could be titrated up to a maximum daily dose of 20 mg (23 participants), versus (2) placebo (25 participants) Setting: Outpatient Country of origin: USA | |
| Outcomes | Cocaine use (urinalysis), craving (Craving Questionnaire), side effects (Barnes Akathisia Scale, Simpson‐Angus Scale, the AIMS, 7 ASI subscales), compliance (pill count), severity of dependence (ASI), attrition (number of sessions attended) | |
| Notes | Dates when the study was conducted: not reported Funding from an investigator‐initiated grant from Eli Lilly & Company, Indianapolis, Indiana No conflict of interest declared | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about the sequence generation process to permit judgement; only described that participants were randomised to receive in double‐blind fashion either olanzapine or placebo (1:1 ratio) |
| Allocation concealment (selection bias) | Low risk | A support staff member not involved with the treatment of participants obtained the randomisation assignment by opening a sealed opaque envelope and conveyed the assignment to a research pharmacist. |
| Blinding of participants and personnel (performance bias) | Low risk | A support staff member not involved with the treatment of participants obtained the randomisation assignment by opening a sealed opaque envelope and conveyed the assignment to a research pharmacist, who dispensed the study medication in coded containers and identical‐appearing placebo. |
| Blinding of participants and personnel (performance bias) | Low risk | A support staff member not involved with the treatment of participants obtained the randomisation assignment by opening a sealed opaque envelope and conveyed the assignment to a research pharmacist, who dispensed the study medication in coded containers and identical‐appearing placebo. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) | Low risk | Insufficient information, but objective outcomes unlikely to be biased by lack of blinding |
| Incomplete outcome data (attrition bias) | Low risk | 3 participants ( 6%) not included in the analysis for side effects |
| Selective reporting (reporting bias) | Low risk | All the study’s prespecified primary outcomes have been reported |
| Methods | Randomised double‐blind placebo‐controlled trial | |
| Participants | Participants 30, mean age 41 years, 73.3% men, 93.3% African‐American, 3.3% white, 3.3% Native American; mean educational level 12.33 years; cocaine use: past 30 days mean 12.5 days, use lifetime mean 12 years, numbers of prior treatments mean 2.5; route of administration: 10% intranasal, 86.6% smoked, 10% intravenous | |
| Interventions | (1) Olanzapine 10 mg/day (15 participants) versus (2) placebo (15 participants) | |
| Outcomes | Retention, craving (BSCS), use of cocaine (self‐reported as days used in past 30 days ), amount of cocaine used (money spent in past 30 days), side effects, severity of dependence (ASI, CGI), anxiety (HAM‐A), depression (HAM‐D), withdrawal symptom (CSSA) | |
| Notes | Dates when the study was conducted: not reported Funding by a grant from the Eli Lilly Company No conflict of interest declared | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Urn randomisation method has been used |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement; method of allocation is not described |
| Blinding of participants and personnel (performance bias) | Unclear risk | Insufficient information to permit judgement; only reported that it is double‐blind |
| Blinding of participants and personnel (performance bias) | Low risk | Insufficient information, but objective outcomes unlikely to be biased by lack of blinding |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information to permit judgement; only reported that it is double‐blind |
| Blinding of outcome assessment (detection bias) | Low risk | Insufficient information, but objective outcomes unlikely to be biased by lack of blinding |
| Incomplete outcome data (attrition bias) | Low risk | 3/30 participants dropped out, balanced between groups |
| Selective reporting (reporting bias) | Low risk | All the study’s prespecified primary outcomes have been reported |
| Methods | Randomised double‐blind placebo‐controlled trial | |
| Participants | Participants 14, mean age 39.9 years, 71% men, 43% African‐American, 43% Hispanic, 14% white; mean educational level 13.6 years; cocaine use: past 30 days mean 16.1 days, mean amount spent last 30 days USD 70.3; route of administration: 50% intranasal, 50% intravenous/freebase | |
| Interventions | (1) Risperidone mean 2.1 mg/day (9 participants) versus (2) placebo (5 participants) | |
| Outcomes | Dropouts, cocaine use (urinalysis and self report), craving (VAS), side effects | |
| Notes | Dates when the study was conducted: not reported Funding by grants K20 DA‐00214 (Dr. Levin), K02 DA‐00288 (Dr. Nunes), and P50 DA‐09236 from the No conflict of interest declared | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement; randomisation process not described |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement; method of allocation is not described |
| Blinding of participants and personnel (performance bias) | Unclear risk | Insufficient information to permit judgement; only reported that it is double‐blind |
| Blinding of participants and personnel (performance bias) | Low risk | insufficient information, but objective outcomes unlikely to be biased by lack of blinding |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information to permit judgement; only reported that it is double‐blind |
| Blinding of outcome assessment (detection bias) | Low risk | Insufficient information, but objective outcomes unlikely to be biased by lack of blinding |
| Incomplete outcome data (attrition bias) | Low risk | Missing outcome data imputed in analysis and reasons balanced in numbers across intervention groups |
| Selective reporting (reporting bias) | Low risk | All the study’s prespecified primary outcomes have been reported. |
| Methods | Randomised double‐blind placebo‐controlled trial | |
| Participants | Participants 31, mean age 44.1 in the risperidone group and 42.4 in the placebo group, all men. Mean baseline frequency of cocaine use 12.6 days in the past 30 days Inclusion criteria: men, age 18 to 60 years, with DSM‐IV criteria for cocaine dependence, use of cocaine by self report of at least once every week and 2 urine samples positive for cocaine Exclusion criteria: diagnostic criteria of schizophrenia, bipolar disorder, current severe major depressive disorder or HIV infection, head trauma with loss of consciousness, corrected QT interval > 450 msec or another unstable medical condition | |
| Interventions | (1) Risperidone (long‐acting) 25 mg IM every other week (16 participants) and behavioural intervention, versus (2) placebo and behavioural intervention (15 participants) | |
| Outcomes | Dropouts, side effects (SATEEGI), cocaine use (urinary concentration of cocaine metabolites and self report), compliance, craving (University of Minnesota Cocaine Craving Scale), withdrawal symptoms (CSSA), severity of dependence (ASI), psychiatric symptoms (SHPS, HAM‐D) | |
| Notes | Dates when the study was conducted: October 2005‐ September 2006 Funding by a grant from Janssen Pharmaceutica to Dr. Evins and by an invest fellowship from the NIDA International programme to Dr. Loebl No conflict of interest declared | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about the sequence generation process to permit judgement; only described that participants were randomised |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement; method of concealment is not described |
| Blinding of participants and personnel (performance bias) | Unclear risk | Insufficient information to permit judgement; it only reports that the study was double‐blind |
| Blinding of participants and personnel (performance bias) | Low risk | Insufficient information, but objective outcomes unlikely to be biased by lack of blinding |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information to permit judgement; it only reports that the study was double‐blind |
| Blinding of outcome assessment (detection bias) | Low risk | Insufficient information, but objective outcomes unlikely to be biased by lack of blinding |
| Incomplete outcome data (attrition bias) | Low risk | ITT analysis |
| Selective reporting (reporting bias) | Low risk | All the study’s prespecified primary outcomes have been reported |
| Methods | Randomised clinical trial (open label) | |
| Participants | Participants 28, 22 men, mean age 33.4 years. 75% were employed and 93% were living with their family or with their partner, 43% had more than 8 years of education Inclusion criteria: Out‐patients, age 18 to 65 years, diagnosis of cocaine dependence ICD‐9‐CM criteria (304.20 code), at least 3 preadmission urine samples positive for cocaine metabolites throughout the latest month preceding enrolment, carried out every 72 ‐ 96 hours. Exclusion criteria: diagnosis of cocaine abuse (305.60 ICD‐9‐CM code), or remission of cocaine dependence (304.23 ICD‐9‐CM code), or any other current Axis I Disorder (DSM‐IV‐TR), organic mental disorders or patients at serious suicidal risk or with significant auto‐aggressive behaviour. Also chronic medical illnesses, pregnancy or nursing, hyperglycaemia, lactose intolerance or malabsorption, malignant neuroleptic syndrome, epileptic seizures, any kind of pharmacological treatment, psychotherapy treatment, therapeutic communities treatment or detention. But not opioid dependence comorbidity if treated with a constant dosage of methadone, or remission of life‐time psychiatric disorders. | |
| Interventions | (1) Aripriprazol 10 mg/day (16 participants) versus (2) ropinirole 1.5 mg x 3/day (12 participants) Setting: Outpatient Country of origin: Italy | |
| Outcomes | Dropouts, side effects, craving (VAS), cocaine use (urinalysis), amount of cocaine used (self‐reported g/week), severity of dependence (CGI severity score) | |
| Notes | Dates when the study was conducted: between May 2008 and June 2009 Funding not reported The authors report no conflicts of interest | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The randomisation list was generated by a statistician using an ad hoc procedure in SPSS and kept by an administrative staffer of the co‐ordinating centre not involved in participant recruitment. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement; only specified that randomisation was concealed until inclusion criteria were determined and then communicated to the participating centres |
| Blinding of participants and personnel (performance bias) | High risk | Open‐label design |
| Blinding of participants and personnel (performance bias) | Low risk | Insufficient information, but objective outcomes unlikely to be biased by lack of blinding |
| Blinding of outcome assessment (detection bias) | High risk | Open‐label design and not otherwise specified |
| Blinding of outcome assessment (detection bias) | Low risk | Insufficient information, but objective outcomes unlikely to be biased by lack of blinding |
| Incomplete outcome data (attrition bias) | High risk | 46.6% dropout. ITT analysis performed only for percentage of urine positive |
| Selective reporting (reporting bias) | Low risk | All the study’s prespecified primary outcomes have been reported |
| Methods | Randomised double‐blind placebo‐controlled trial. | |
| Participants | Participants 63, mean age 38.7 years, 50 men, 11 white, 51 black, 1 other; mean educational level 13 years, use of cocaine, lifetime men 14 years, last 30 days mean 16.8 days; route of administration: 20.8% intranasal, 76% smoked, 3% injected | |
| Interventions | Olanzapine 10 mg/day (16 participants), versus placebo (15 participants) | |
| Outcomes | Dropouts, side effects, use of cocaine self‐reported (TLFB), craving (BSCS, CCQ), severity of dependence (ASI, CGIS), anxiety (HAM‐A), depression (HAM‐D) | |
| Notes | Dates when the study was conducted: not reported Funding by a contract from the National Institute on Drug Abuse: YO1 DA 50038 No conflict of interest declared | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement; randomisation process not described |
| Allocation concealment (selection bias) | Low risk | Study medications were dispensed by, and returned to, a non‐blinded pharmacist. All other study staff and investigators were blinded to treatment assignment |
| Blinding of participants and personnel (performance bias) | Low risk | Study medications were dispensed by, and returned to, a non‐blinded pharmacist. All other study staff and investigators were blinded to treatment assignment |
| Blinding of participants and personnel (performance bias) | Low risk | Insufficient information, but objective outcomes unlikely to be biased by lack of blinding |
| Blinding of outcome assessment (detection bias) | Low risk | Study medications were dispensed by, and returned to, a non‐blinded pharmacist. All other study staff and investigators were blinded to treatment assignment |
| Blinding of outcome assessment (detection bias) | Low risk | Insufficient information, but objective outcomes unlikely to be biased by lack of blinding |
| Incomplete outcome data (attrition bias) | Low risk | ITT analysis performed |
| Selective reporting (reporting bias) | Low risk | All the study’s prespecified primary outcomes have been reported |
| Methods | Randomised double blind controlled trial | |
| Participants | Participants 35, mean age 41.2, cocaine use in the last 30 days: mean 5.4; years of cocaine use: 6.3 | |
| Interventions | Risperidone 1 mg/day (19 participants), placebo (16 participants). | |
| Outcomes | Dropouts, craving (VCCQ) | |
| Notes | Dates when the study was conducted: not reported The project was supported by the VISN 3 Mental Illness, Research and Clinical Center, the VA New Jersey Health Care System and Janssen Research Foundation No conflict of interest declared | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement; randomisation process not described |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement; only specified that randomisation was concealed until inclusion criteria were determined and then communicated to the participating centres |
| Blinding of participants and personnel (performance bias) | Unclear risk | Insufficient information to permit judgement; it only reports that the study was double‐blind |
| Blinding of participants and personnel (performance bias) | Low risk | Insufficient information, but objective outcomes unlikely to be biased by lack of blinding |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information to permit judgement; it only reports that the study was double‐blind |
| Blinding of outcome assessment (detection bias) | Low risk | Insufficient information, but objective outcomes unlikely to be biased by lack of blinding |
| Incomplete outcome data (attrition bias) | Low risk | 8.5% dropout, balanced between groups |
| Selective reporting (reporting bias) | Low risk | All the study’s prespecified primary outcomes have been reported |
| Methods | Randomised controlled trial | |
| Participants | Participants 31, mean age 42.9, cocaine use in the last 30 days: mean 8.4; age of first cocaine use mean 29.3 Exclusion criteria: DSM‐IV criteria for any additional AXIS I disorder and dependence (excluded nicotine), taking prescribed medication that could affect the central nervous system, history of seizures, pregnancy, chronic disease of the central nervous system other than schizophrenia | |
| Interventions | Olanzapine 10 mg/day (16 participants), haloperidol 10 mg/day (15 participants) | |
| Outcomes | Dropouts, craving (VCCQ), psychopathology (PANSS), withdrawal symptoms (PANSS) | |
| Notes | Dates when the study was conducted: not reported Funding by an investigator‐initiated grant from Eli Lilly and grants from Substance Abuse and Mental Health Service Administration (H79 TI16576), National Institute of Complimentary and Alternative Medicine Conflict of interest; the authors received support from Eli Lilly (David Smelson), Astra Zeneca (David Smelson), Bristol Meyers Squib (David Smelson, Douglas Ziedonis), and Janssen (David Smelson, Douglas Ziedonis, Maureen Kaune), Forest (Douglas Ziedonis), and New Jersey Comprehensive Tobacco Control Program (Jill Williams) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about the sequence generation process to permit judgement; only described that participants were randomised |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement; method of concealment is not described |
| Blinding of participants and personnel (performance bias) | Unclear risk | Insufficient information to permit judgement; it only reports that the study was double‐blind |
| Blinding of participants and personnel (performance bias) | Low risk | Insufficient information, but objective outcomes unlikely to be biased by lack of blinding |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information to permit judgement; it only reports that the study was double‐blind |
| Blinding of outcome assessment (detection bias) | Low risk | Insufficient information, but objective outcomes unlikely to be biased by lack of blinding |
| Incomplete outcome data (attrition bias) | High risk | 42% dropout, balanced between groups |
| Selective reporting (reporting bias) | Low risk | All the study’s prespecified primary outcomes have been reported |
| Methods | Randomised double‐blind placebo‐controlled trial | |
| Participants | Participants 60, mean age 47.9, 86.6% men Inclusion criteria: Has used cocaine within the 30 days prior to screening Exclusion criteria: DSM‐IV criteria for any psychotic disorder (bipolar, schizophrenia, etc.) or who were psychiatrically or medically unstable, pregnant or nursing | |
| Interventions | (1) Quetiapine 400 mg/day (29 participants) versus placebo (31 participants) Setting:outpatient | |
| Outcomes | Dropouts, side effects, compliance, craving (BSCS), cocaine use: end‐of‐trial abstinence measured by urinalysis and self‐reported use (TLFB), amount of cocaine used (self‐reported in dollars spent/week, days of cocaine use/week and g/week) | |
| Notes | Dates when the study was conducted: not reported Funded by the Seattle Institute for Biomedical and Clinical Research and in collaboration with the VA Puget Sound Health Care System and Astra Zeneca Confict of interest: Dr. Tapp received support from Astra Zeneca | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random allocation computer software was used for a randomised block design with randomly assigned block sizes of 2, 4, and 6 |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) | Unclear risk | Insufficient information to permit judgement; it only reports that the study was double‐blind |
| Blinding of participants and personnel (performance bias) | Low risk | Insufficient information, but objective outcomes unlikely to be biased by lack of blinding |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information to permit judgement; it only reports that the study was double‐blind |
| Blinding of outcome assessment (detection bias) | Low risk | Insufficient information, but objective outcomes unlikely to be biased by lack of blinding |
| Incomplete outcome data (attrition bias) | High risk | 68% dropped out, unbalanced in numbers or reasons reported between groups |
| Selective reporting (reporting bias) | Low risk | All the study’s prespecified primary outcomes have been reported |
| Methods | Randomised double‐blind placebo‐controlled trial | |
| Participants | Participants 119, mean age 41.2 in the reserpine group and 40.7 in the placebo group, 70% men | |
| Interventions | (1) Reserpine 0.50 mg (60 participants) versus placebo (59 participants) Setting:outpatient | |
| Outcomes | Retention, side effects, cocaine non‐use days (self‐reported and by urine drug test), compliance, craving (BSCS), severity of addiction (ASI), depression (HAM‐D) | |
| Notes | Dates when the study was conducted: not reported Funded by the National Institutes of Health, National Institute on Drug Abuse through contract N01‐DA‐9‐8095 (E. Somoza) No conflict of interest declared | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stratified randomisation, balancing for gender and self report of cocaine use was used to assign eligible participants to reserpine or placebo within each study site |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) | Unclear risk | Insufficient information to permit judgement; it only reports that the study was double‐blind |
| Blinding of participants and personnel (performance bias) | Low risk | Insufficient information, but objective outcomes unlikely to be biased by lack of blinding |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information to permit judgement; it only reports that the study was double‐blind |
| Blinding of outcome assessment (detection bias) | Low risk | Insufficient information, but objective outcomes unlikely to be biased by lack of blinding |
| Incomplete outcome data (attrition bias) | High risk | 34% dropout, balanced between groups |
| Selective reporting (reporting bias) | Low risk | All the study’s prespecified primary outcomes have been reported |
ASI: Addiction Severity Index
BDI: Beck Depression Inventory
BSCS: Brief Substance Craving Scale
CCQ: Cocaine Craving Questionnaire
CGIS: Clinical Global Impression Scale
CREST:Cocaine Rapid Efficacy Screening Trial
CSSA: Cocaine Selective Severity Assessment
DSM: Diagnostic and Statistical Manual of Mental Disorders
IDS‐C‐30: Clinical‐Rated Inventory of Depressive Symptomatology
HAM‐A: Hamilton Anxiety Rating Scale
HAM‐D: Hamilton Depression Rating Scale
ITT: intention‐to‐treat
PANSS: Positive and Negative Syndrome Scale
SATEEGI: Systematic Assessment for Treatment Emergent Events General Inquiry
SHPS: Snaith Hamilton Pleasure Scale
SCQ‐10: Stimulant Craving Questionaire
TLFB: Timeline Followback Interview
VAS Scale: Visual Analogue Scale
VCCQ: Voris Cocaine Craving Questionnaire
WSRS: Within Session Rating Scale
YMRS: Young Mania Rating Scale
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| Excluded, as the outcome was not in the inclusion criteria: cross‐over study with 12 participants assessing craving up to 1 hour after the administration of a cue exposure. | |
| Excluded as the objective of the study and the outcomes were not in the inclusion criteria: attentional bias for stimulant‐related words was measured | |
| Excluded as the objective of the study and the outcomes were not in the inclusion criteria: drug and cocaine given simultaneously by the researcher to assess their effects on the cardiovascular system and subjective response to cocaine | |
| Excluded as the objective of the study and the outcomes were not in the inclusion criteria: drug and cocaine given simultaneously by the researcher to assess their effects on the cardiovascular, nervous system and subjective response to cocaine | |
| Excluded as it was impossible to extract useful data from the study: number of participants allocated to each group not stated | |
| Excluded as the objective of the study was not in the inclusion criteria.This trial was included and classified as ongoing in the original review and has been excluded from this update, because of a modification of the study protocol before initiating the original trial in 2007, substituting the pharmacological intervention with an antipsychotic drug (aripiprazol) with one of an antidepressant (citalopram). | |
| Excluded as the objective of the study and the outcomes were not in the inclusion criteria: drug and cocaine given simultaneously by the researcher to assess their effects on the cardiovascular system and subjective response to cocaine. This study was identified as ongoing in the original review. | |
| Excluded as the objective of the study and the outcomes were not in the inclusion criteria: cocaine consumption assessed in opiate‐dependent patients in Methadone Maintenance Therapy (MMT) who use cocaine (not cocaine dependence) | |
| Excluded as it was impossible to extract useful data from the study: no raw data for outcome measures, only P‐values | |
| Excluded as the objective of the study and the outcomes were not in the inclusion criteria: drug and cocaine given simultaneously by the researcher to assess the safety and tolerability of intranasal cocaine during maintenance with the drug. | |
| Excluded as the objective of the study and the outcomes were not in the inclusion criteria: drug and cocaine given simultaneously by the researcher to assess their effects on the cardiovascular system and subjective response to cocaine. | |
| Excluded as the objective of the study and the outcomes were not in the inclusion criteria: drug and cocaine given simultaneously by the researcher to assess their effects on the reinforcing efficacy of cocaine. | |
| Excluded as the objective of the study and the outcomes were not in the inclusion criteria: drug given by the researcher to assess their effects on the reinforcing efficacy of cocaine. | |
| Excluded as the objective of the study and the outcomes were not in the inclusion criteria: looking to assess the value of amisulpride as antipsychotic treatment. | |
| Excluded as the objective of the study and the outcomes were not in the inclusion criteria: results provided for drug use defined as cocaine or metamphetamine use, without reporting separately results for cocaine use. This study was identified as ongoing in the original review. | |
| Excluded as the objective of the study and the outcomes were not in the inclusion criteria: aripiprazole effects on cigarette smoking among cocaine users were measured. | |
| Excluded as the objective of the study and the outcomes were not in the inclusion criteria: drug and cocaine given simultaneously by the researcher to assess their effects on the cardiovascular, nervous system and subjective response to cocaine. | |
| Excluded as it was impossible to extract useful data from the study: data not separately reported for cocaine‐dependent participants. | |
| Excluded as it was impossible to extract useful data from the study: data not separately reported for cocaine‐dependent participants. | |
| Excluded as the objective of the study and the outcomes were not in the inclusion criteria: drug and cocaine given simultaneously by the researcher to assess their effects on the cardiovascular, nervous system and subjective response to cocaine. | |
| Excluded as it was impossible to extract useful data from the study: number of participants allocated to each group not stated and no raw data for outcome measures reported, only P‐values. | |
| Excluded as the objective of the study and the outcomes were not in the inclusion criteria: drug and cocaine given simultaneously by the researcher to assess their effects on the cardiovascular system and subjective response to cocaine. | |
| Excluded as the objective of the study and the outcomes were not in the inclusion criteria: drug and cocaine given simultaneously by the researcher to assess their safety, tolerability, and subject‐rated effects. | |
| Excluded as only 4 participants have been included in the study and the results have been presented only for 3. |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Dropouts Show forest plot | 8 | 397 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.57, 0.97] |
| Analysis 1.1  Comparison 1 Any antipsychotic versus placebo, Outcome 1 Dropouts. | ||||
| 2 Side effects Show forest plot | 6 | 291 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.93, 1.10] |
| Analysis 1.2  Comparison 1 Any antipsychotic versus placebo, Outcome 2 Side effects. | ||||
| 3 Number of participants using cocaine during the treatment (as days/week by urine tests or self report) Show forest plot | 2 | 91 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.65, 1.62] |
| Analysis 1.3 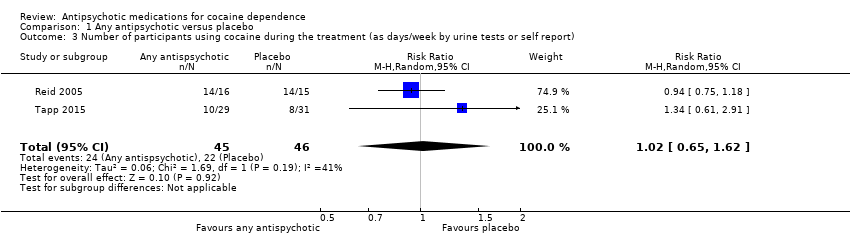 Comparison 1 Any antipsychotic versus placebo, Outcome 3 Number of participants using cocaine during the treatment (as days/week by urine tests or self report). | ||||
| 4 Continuous abstinence (number of participants who maintained negative drug screens for 2 ‐ 3 weeks) Show forest plot | 3 | 139 | Risk Ratio (M‐H, Random, 95% CI) | 1.30 [0.73, 2.32] |
| Analysis 1.4  Comparison 1 Any antipsychotic versus placebo, Outcome 4 Continuous abstinence (number of participants who maintained negative drug screens for 2 ‐ 3 weeks). | ||||
| 5 Craving (Brief Substance Craving Scale) Show forest plot | 4 | 240 | Mean Difference (IV, Random, 95% CI) | 0.13 [‐1.08, 1.35] |
| Analysis 1.5  Comparison 1 Any antipsychotic versus placebo, Outcome 5 Craving (Brief Substance Craving Scale). | ||||
| 6 Severity of dependence (Addiction Severity Index) Show forest plot | 4 | 211 | Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.01, 0.04] |
| Analysis 1.6  Comparison 1 Any antipsychotic versus placebo, Outcome 6 Severity of dependence (Addiction Severity Index). | ||||
| 7 Severity of dependence (Clinical Global Impression Scale) Show forest plot | 3 | 180 | Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.38, 0.39] |
| Analysis 1.7  Comparison 1 Any antipsychotic versus placebo, Outcome 7 Severity of dependence (Clinical Global Impression Scale). | ||||
| 8 Use of cocaine during the treatment (self‐reported as g/week) Show forest plot | 2 | 72 | Mean Difference (IV, Random, 95% CI) | ‐0.54 [‐0.92, ‐0.16] |
| Analysis 1.8 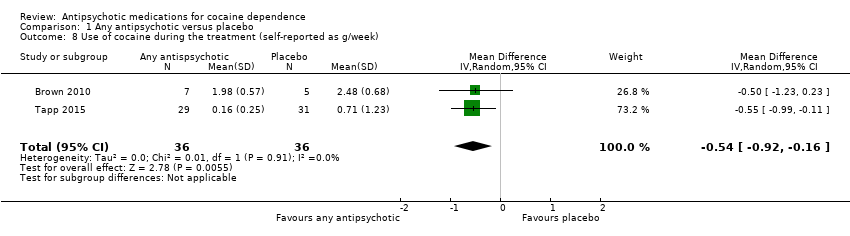 Comparison 1 Any antipsychotic versus placebo, Outcome 8 Use of cocaine during the treatment (self‐reported as g/week). | ||||
| 9 Depression (Hamilton Depression Rating Scale) Show forest plot | 4 | 192 | Mean Difference (IV, Random, 95% CI) | ‐0.82 [‐3.19, 1.55] |
| Analysis 1.9  Comparison 1 Any antipsychotic versus placebo, Outcome 9 Depression (Hamilton Depression Rating Scale). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Dropouts Show forest plot | 4 | 176 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.63, 1.04] |
| Analysis 2.1  Comparison 2 Risperidone versus placebo, Outcome 1 Dropouts. | ||||
| 2 Severity of dependence (Addiction Severity Index) Show forest plot | 1 | 31 | Mean Difference (IV, Random, 95% CI) | 0.03 [‐0.04, 0.10] |
| Analysis 2.2 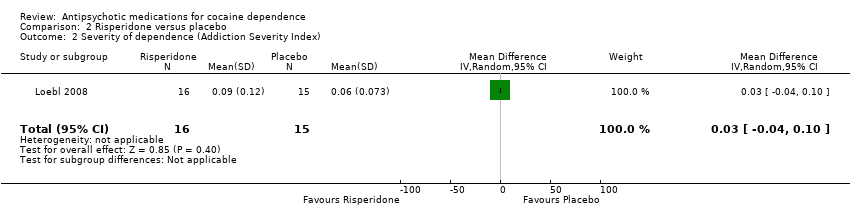 Comparison 2 Risperidone versus placebo, Outcome 2 Severity of dependence (Addiction Severity Index). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Dropouts Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 3.1 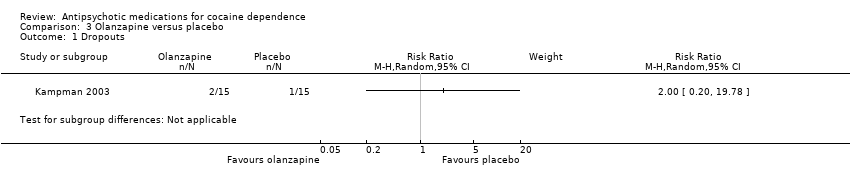 Comparison 3 Olanzapine versus placebo, Outcome 1 Dropouts. | ||||
| 2 Side effects Show forest plot | 2 | 79 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.92, 1.11] |
| Analysis 3.2 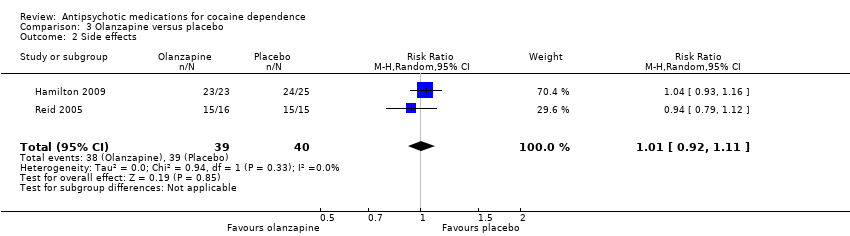 Comparison 3 Olanzapine versus placebo, Outcome 2 Side effects. | ||||
| 3 Use of cocaine during the treatment (self‐reported as days/week) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 3.3 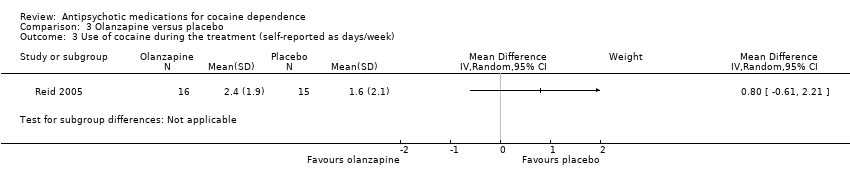 Comparison 3 Olanzapine versus placebo, Outcome 3 Use of cocaine during the treatment (self‐reported as days/week). | ||||
| 4 Use of cocaine during the treatment (self‐reported as days/past 30 days) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 3.4  Comparison 3 Olanzapine versus placebo, Outcome 4 Use of cocaine during the treatment (self‐reported as days/past 30 days). | ||||
| 5 Continuous abstinence (participants who maintained negative drug screens throughout the treatment period ) Show forest plot | 2 | 79 | Risk Ratio (M‐H, Random, 95% CI) | 1.37 [0.71, 2.61] |
| Analysis 3.5 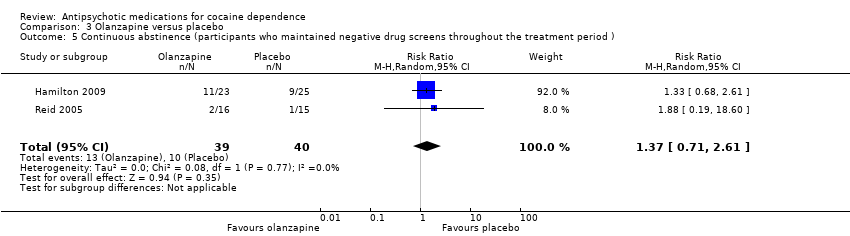 Comparison 3 Olanzapine versus placebo, Outcome 5 Continuous abstinence (participants who maintained negative drug screens throughout the treatment period ). | ||||
| 6 Craving (Brief Substance Craving Scale) Show forest plot | 2 | 61 | Mean Difference (IV, Random, 95% CI) | 1.33 [‐0.91, 3.58] |
| Analysis 3.6 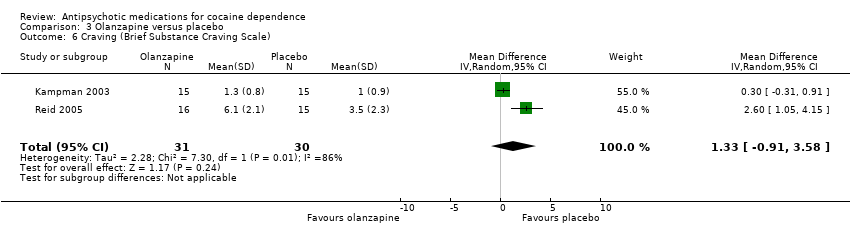 Comparison 3 Olanzapine versus placebo, Outcome 6 Craving (Brief Substance Craving Scale). | ||||
| 7 Severity of dependence (Addiction Severity Index) Show forest plot | 2 | 61 | Mean Difference (IV, Random, 95% CI) | 0.03 [‐0.01, 0.07] |
| Analysis 3.7  Comparison 3 Olanzapine versus placebo, Outcome 7 Severity of dependence (Addiction Severity Index). | ||||
| 8 Severity of dependence (Clinical Global Impression Scale) Show forest plot | 2 | 61 | Mean Difference (IV, Random, 95% CI) | 0.17 [‐0.51, 0.85] |
| Analysis 3.8  Comparison 3 Olanzapine versus placebo, Outcome 8 Severity of dependence (Clinical Global Impression Scale). | ||||
| 9 Amount of of cocaine use during the treatment (self‐reported as dollars spent/past 30 days) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 3.9 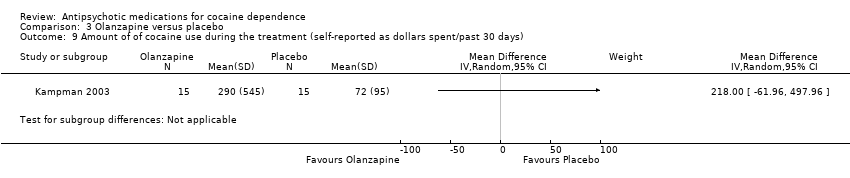 Comparison 3 Olanzapine versus placebo, Outcome 9 Amount of of cocaine use during the treatment (self‐reported as dollars spent/past 30 days). | ||||
| 10 Depression (Hamilton Depression Rating Scale) Show forest plot | 2 | 61 | Mean Difference (IV, Random, 95% CI) | 1.34 [‐3.84, 6.52] |
| Analysis 3.10 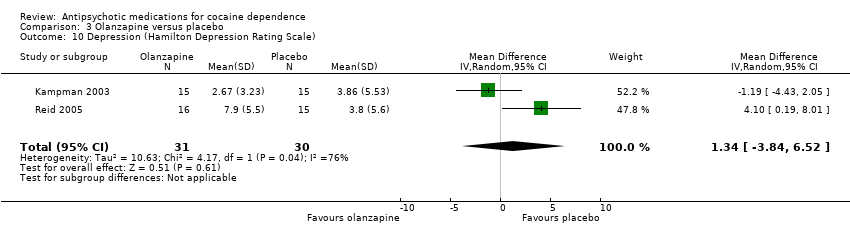 Comparison 3 Olanzapine versus placebo, Outcome 10 Depression (Hamilton Depression Rating Scale). | ||||
| 11 Anxiety (Hamilton Anxiety Rating Scale) Show forest plot | 2 | 61 | Mean Difference (IV, Random, 95% CI) | 1.37 [‐3.02, 5.75] |
| Analysis 3.11 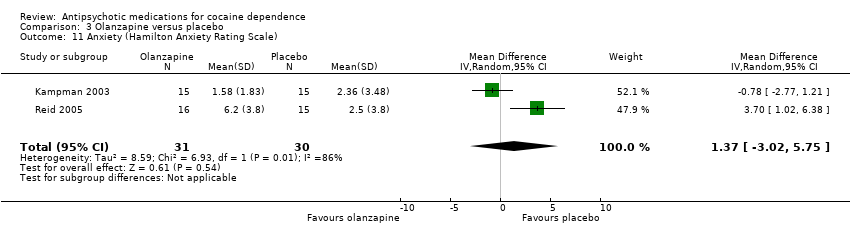 Comparison 3 Olanzapine versus placebo, Outcome 11 Anxiety (Hamilton Anxiety Rating Scale). | ||||
| 12 Withdrawal symptoms (Cocaine Selective Severity Assessment) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 3.12 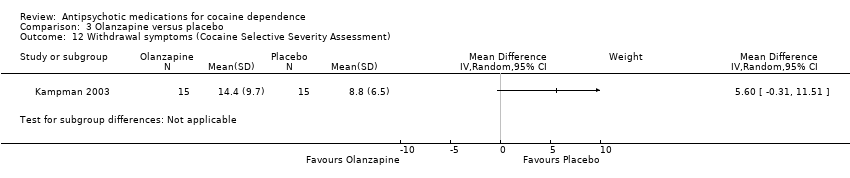 Comparison 3 Olanzapine versus placebo, Outcome 12 Withdrawal symptoms (Cocaine Selective Severity Assessment). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Dropouts Show forest plot | 2 | 72 | Risk Ratio (M‐H, Random, 95% CI) | 0.64 [0.20, 2.03] |
| Analysis 4.1 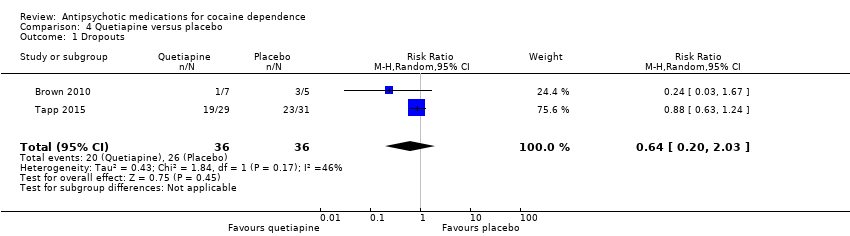 Comparison 4 Quetiapine versus placebo, Outcome 1 Dropouts. | ||||
| 2 Side effects Show forest plot | 2 | 72 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.77, 1.27] |
| Analysis 4.2  Comparison 4 Quetiapine versus placebo, Outcome 2 Side effects. | ||||
| 3 Use of cocaine during the treatment (self‐reported as days/week) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 4.3 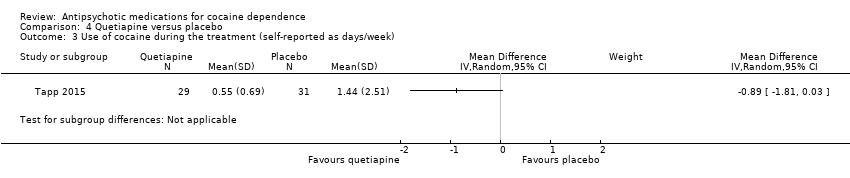 Comparison 4 Quetiapine versus placebo, Outcome 3 Use of cocaine during the treatment (self‐reported as days/week). | ||||
| 4 Craving (Brief Substance Craving Scale) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 4.4 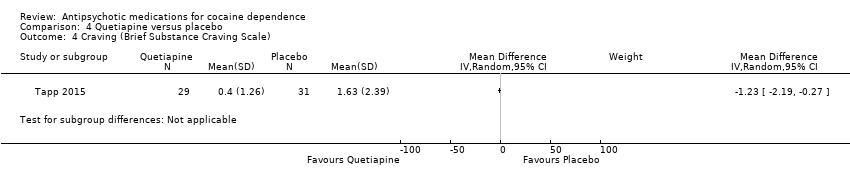 Comparison 4 Quetiapine versus placebo, Outcome 4 Craving (Brief Substance Craving Scale). | ||||
| 5 Amount of of cocaine use during the treatment (self‐reported as g/week) Show forest plot | 2 | 72 | Mean Difference (IV, Random, 95% CI) | ‐0.54 [‐0.92, ‐0.16] |
| Analysis 4.5 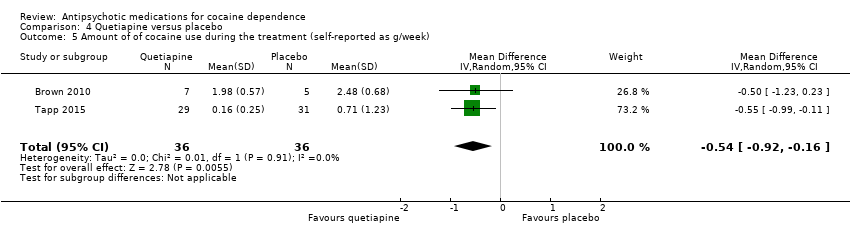 Comparison 4 Quetiapine versus placebo, Outcome 5 Amount of of cocaine use during the treatment (self‐reported as g/week). | ||||
| 6 Amount of of cocaine use during the treatment (self‐reported as dollars spent/week) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 4.6  Comparison 4 Quetiapine versus placebo, Outcome 6 Amount of of cocaine use during the treatment (self‐reported as dollars spent/week). | ||||
| 7 Depression (Hamilton Depression Rating Scale) Show forest plot | 1 | 12 | Mean Difference (IV, Random, 95% CI) | ‐3.67 [‐6.19, ‐1.15] |
| Analysis 4.7  Comparison 4 Quetiapine versus placebo, Outcome 7 Depression (Hamilton Depression Rating Scale). | ||||
| 8 Depression (Quick Inventory of Depressive Symptomatology) Show forest plot | 1 | 12 | Mean Difference (IV, Random, 95% CI) | ‐1.27 [‐9.61, 7.07] |
| Analysis 4.8  Comparison 4 Quetiapine versus placebo, Outcome 8 Depression (Quick Inventory of Depressive Symptomatology). | ||||
| 9 Manic and hypomanic symptoms (Young Mania Rating Scale) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 4.9 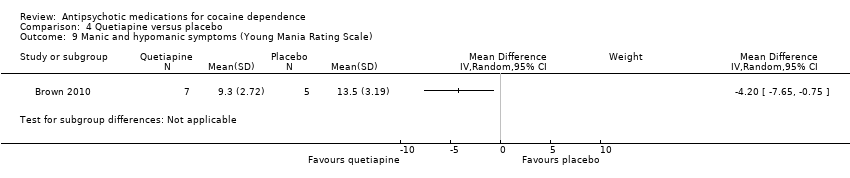 Comparison 4 Quetiapine versus placebo, Outcome 9 Manic and hypomanic symptoms (Young Mania Rating Scale). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Side effects Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 5.1  Comparison 5 Lamotrigine versus placebo, Outcome 1 Side effects. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Dropouts Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 6.1 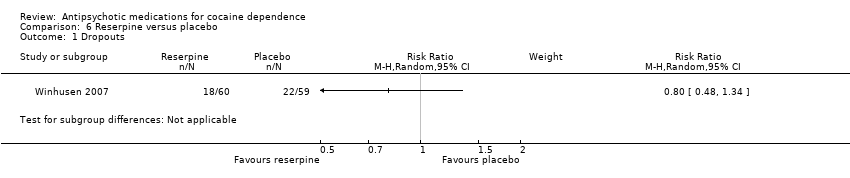 Comparison 6 Reserpine versus placebo, Outcome 1 Dropouts. | ||||
| 2 Craving (Brief Substance Craving Scale) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 6.2  Comparison 6 Reserpine versus placebo, Outcome 2 Craving (Brief Substance Craving Scale). | ||||
| 3 Severity of dependence (Addiction Severity Index) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 6.3  Comparison 6 Reserpine versus placebo, Outcome 3 Severity of dependence (Addiction Severity Index). | ||||
| 4 Severity of dependence (Clinical Global Impression Scale) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 6.4 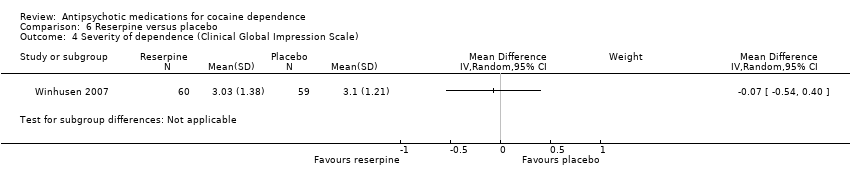 Comparison 6 Reserpine versus placebo, Outcome 4 Severity of dependence (Clinical Global Impression Scale). | ||||
| 5 Depression (Hamilton Depression Rating Scale) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 6.5 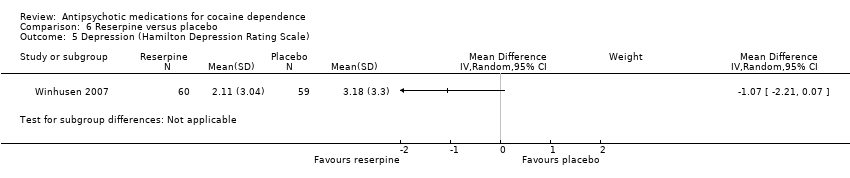 Comparison 6 Reserpine versus placebo, Outcome 5 Depression (Hamilton Depression Rating Scale). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Dropouts Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 7.1 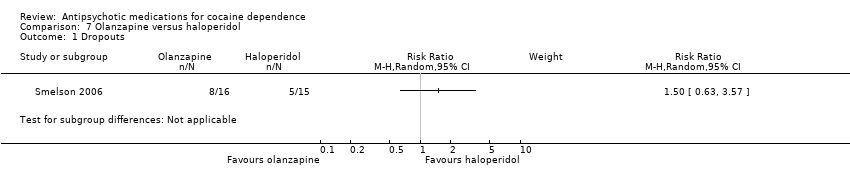 Comparison 7 Olanzapine versus haloperidol, Outcome 1 Dropouts. | ||||
| 2 Psychopathology (Positive and Negative Syndrome Scale) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 7.2  Comparison 7 Olanzapine versus haloperidol, Outcome 2 Psychopathology (Positive and Negative Syndrome Scale). | ||||
| 3 Craving (Voris Cocaine Craving Questionnaire‐Intensity subscale) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 7.3  Comparison 7 Olanzapine versus haloperidol, Outcome 3 Craving (Voris Cocaine Craving Questionnaire‐Intensity subscale). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Dropouts Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 8.1  Comparison 8 Olanzapine versus risperidone, Outcome 1 Dropouts. | ||||
| 2 Depression (Hamilton Depression Rating Scale) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 8.2 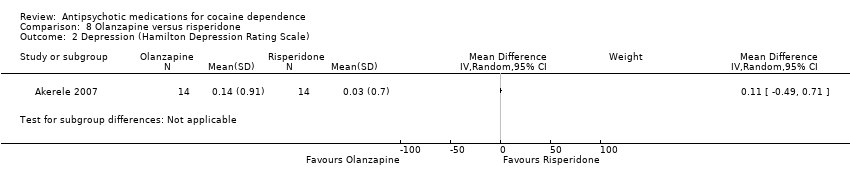 Comparison 8 Olanzapine versus risperidone, Outcome 2 Depression (Hamilton Depression Rating Scale). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Dropouts Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 9.1  Comparison 9 Aripiprazol versus ropinirol, Outcome 1 Dropouts. | ||||
| 2 Side effects Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 9.2 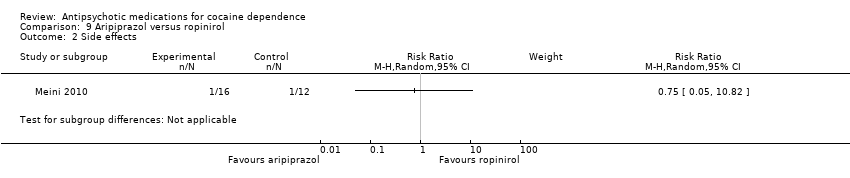 Comparison 9 Aripiprazol versus ropinirol, Outcome 2 Side effects. | ||||
| 3 Craving (VAS Scale) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 9.3  Comparison 9 Aripiprazol versus ropinirol, Outcome 3 Craving (VAS Scale). | ||||
| 4 Severity of dependence (Clinical Global Impression Scale) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 9.4 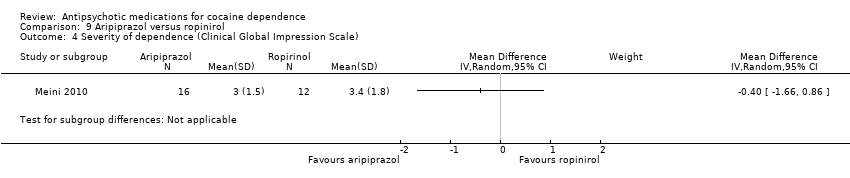 Comparison 9 Aripiprazol versus ropinirol, Outcome 4 Severity of dependence (Clinical Global Impression Scale). | ||||
| 5 Amount of of cocaine use during the treatment (self‐reported as g/week) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 9.5  Comparison 9 Aripiprazol versus ropinirol, Outcome 5 Amount of of cocaine use during the treatment (self‐reported as g/week). | ||||

Study flow diagram. Review update 2015.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Any antipsychotic versus placebo, Outcome 1 Dropouts.

Comparison 1 Any antipsychotic versus placebo, Outcome 2 Side effects.

Comparison 1 Any antipsychotic versus placebo, Outcome 3 Number of participants using cocaine during the treatment (as days/week by urine tests or self report).
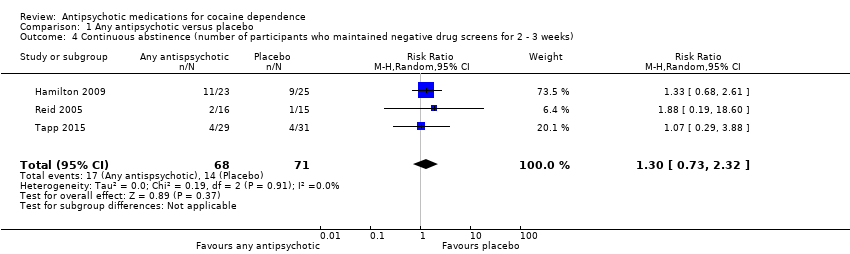
Comparison 1 Any antipsychotic versus placebo, Outcome 4 Continuous abstinence (number of participants who maintained negative drug screens for 2 ‐ 3 weeks).
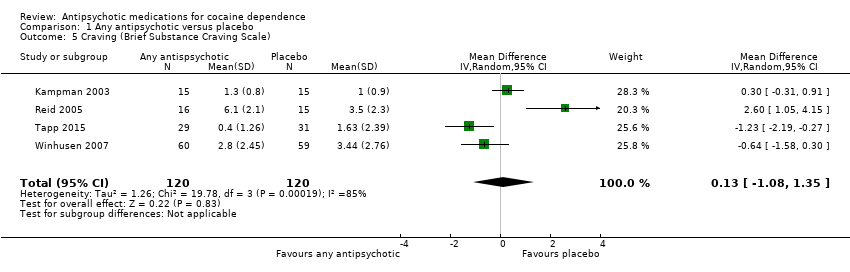
Comparison 1 Any antipsychotic versus placebo, Outcome 5 Craving (Brief Substance Craving Scale).
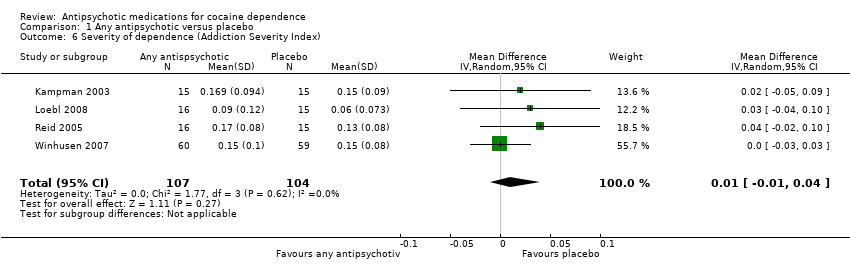
Comparison 1 Any antipsychotic versus placebo, Outcome 6 Severity of dependence (Addiction Severity Index).

Comparison 1 Any antipsychotic versus placebo, Outcome 7 Severity of dependence (Clinical Global Impression Scale).

Comparison 1 Any antipsychotic versus placebo, Outcome 8 Use of cocaine during the treatment (self‐reported as g/week).
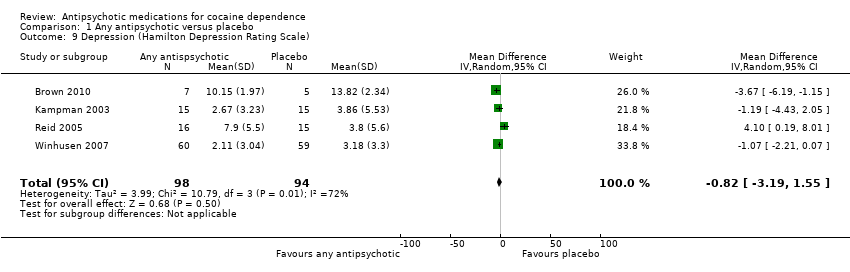
Comparison 1 Any antipsychotic versus placebo, Outcome 9 Depression (Hamilton Depression Rating Scale).

Comparison 2 Risperidone versus placebo, Outcome 1 Dropouts.

Comparison 2 Risperidone versus placebo, Outcome 2 Severity of dependence (Addiction Severity Index).
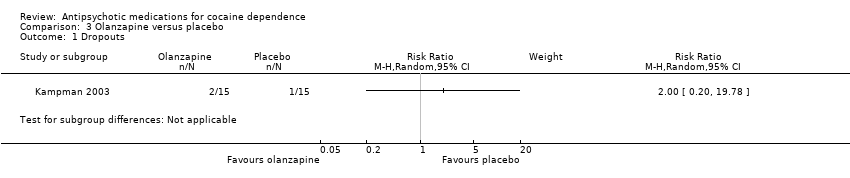
Comparison 3 Olanzapine versus placebo, Outcome 1 Dropouts.
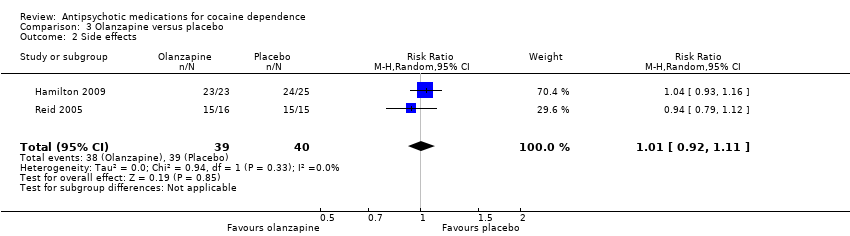
Comparison 3 Olanzapine versus placebo, Outcome 2 Side effects.

Comparison 3 Olanzapine versus placebo, Outcome 3 Use of cocaine during the treatment (self‐reported as days/week).
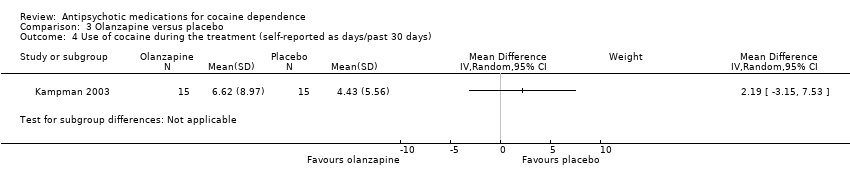
Comparison 3 Olanzapine versus placebo, Outcome 4 Use of cocaine during the treatment (self‐reported as days/past 30 days).

Comparison 3 Olanzapine versus placebo, Outcome 5 Continuous abstinence (participants who maintained negative drug screens throughout the treatment period ).

Comparison 3 Olanzapine versus placebo, Outcome 6 Craving (Brief Substance Craving Scale).
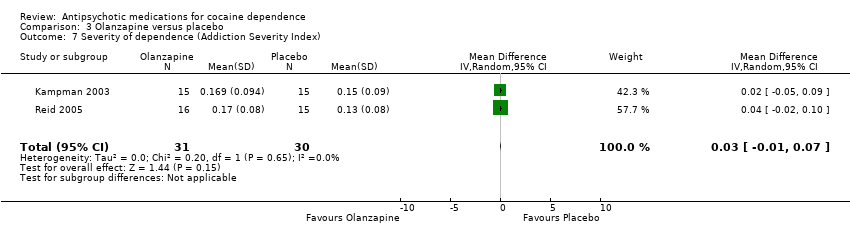
Comparison 3 Olanzapine versus placebo, Outcome 7 Severity of dependence (Addiction Severity Index).
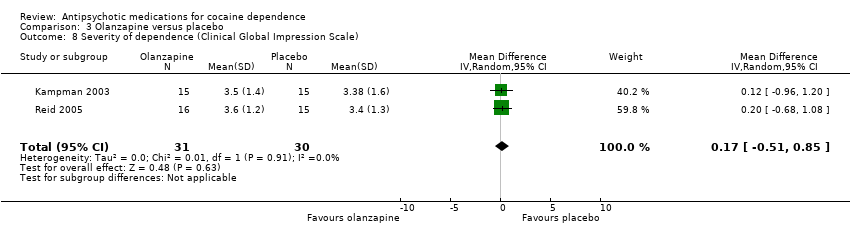
Comparison 3 Olanzapine versus placebo, Outcome 8 Severity of dependence (Clinical Global Impression Scale).

Comparison 3 Olanzapine versus placebo, Outcome 9 Amount of of cocaine use during the treatment (self‐reported as dollars spent/past 30 days).
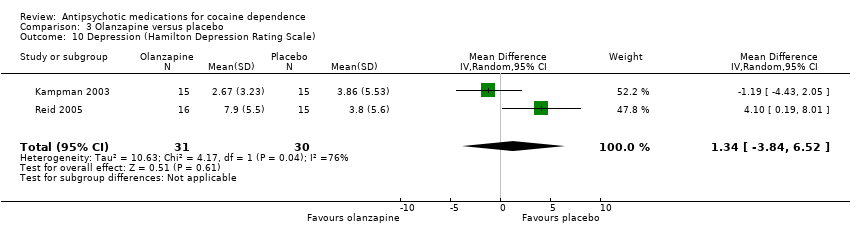
Comparison 3 Olanzapine versus placebo, Outcome 10 Depression (Hamilton Depression Rating Scale).
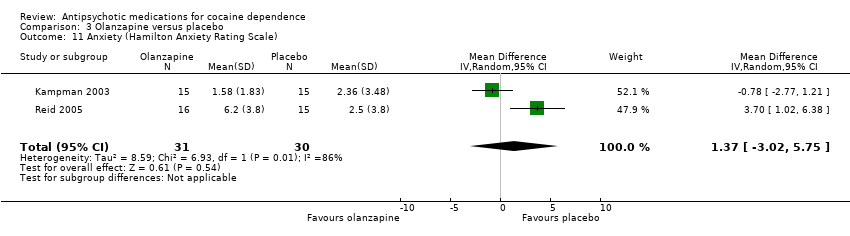
Comparison 3 Olanzapine versus placebo, Outcome 11 Anxiety (Hamilton Anxiety Rating Scale).

Comparison 3 Olanzapine versus placebo, Outcome 12 Withdrawal symptoms (Cocaine Selective Severity Assessment).
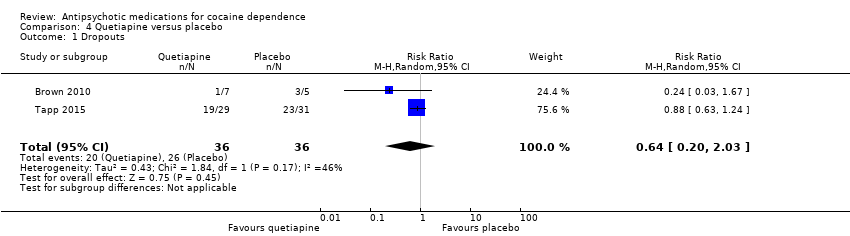
Comparison 4 Quetiapine versus placebo, Outcome 1 Dropouts.
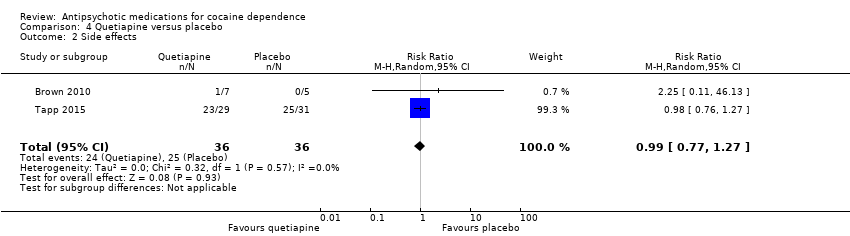
Comparison 4 Quetiapine versus placebo, Outcome 2 Side effects.

Comparison 4 Quetiapine versus placebo, Outcome 3 Use of cocaine during the treatment (self‐reported as days/week).
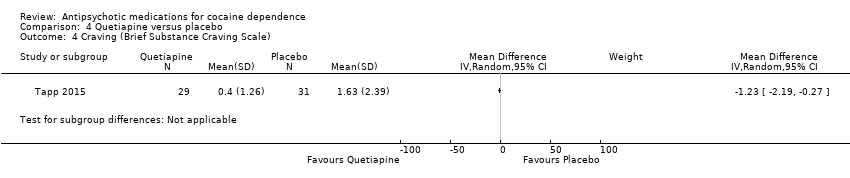
Comparison 4 Quetiapine versus placebo, Outcome 4 Craving (Brief Substance Craving Scale).

Comparison 4 Quetiapine versus placebo, Outcome 5 Amount of of cocaine use during the treatment (self‐reported as g/week).

Comparison 4 Quetiapine versus placebo, Outcome 6 Amount of of cocaine use during the treatment (self‐reported as dollars spent/week).
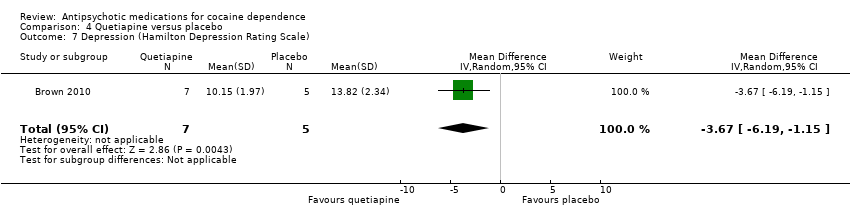
Comparison 4 Quetiapine versus placebo, Outcome 7 Depression (Hamilton Depression Rating Scale).
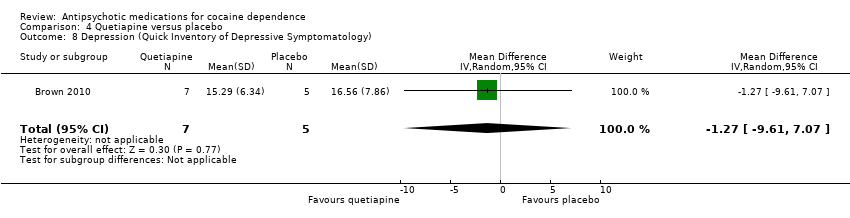
Comparison 4 Quetiapine versus placebo, Outcome 8 Depression (Quick Inventory of Depressive Symptomatology).
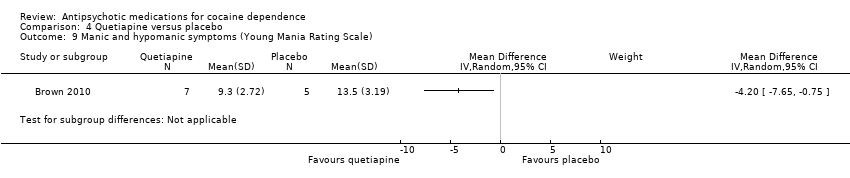
Comparison 4 Quetiapine versus placebo, Outcome 9 Manic and hypomanic symptoms (Young Mania Rating Scale).

Comparison 5 Lamotrigine versus placebo, Outcome 1 Side effects.

Comparison 6 Reserpine versus placebo, Outcome 1 Dropouts.

Comparison 6 Reserpine versus placebo, Outcome 2 Craving (Brief Substance Craving Scale).
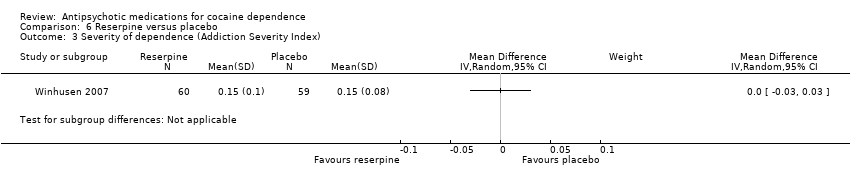
Comparison 6 Reserpine versus placebo, Outcome 3 Severity of dependence (Addiction Severity Index).

Comparison 6 Reserpine versus placebo, Outcome 4 Severity of dependence (Clinical Global Impression Scale).
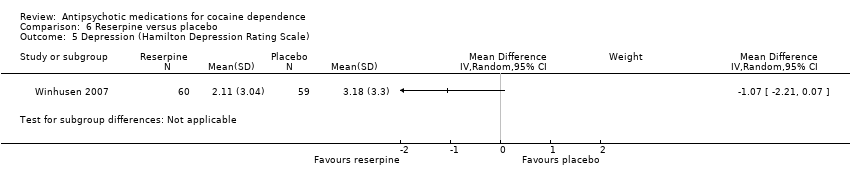
Comparison 6 Reserpine versus placebo, Outcome 5 Depression (Hamilton Depression Rating Scale).

Comparison 7 Olanzapine versus haloperidol, Outcome 1 Dropouts.

Comparison 7 Olanzapine versus haloperidol, Outcome 2 Psychopathology (Positive and Negative Syndrome Scale).
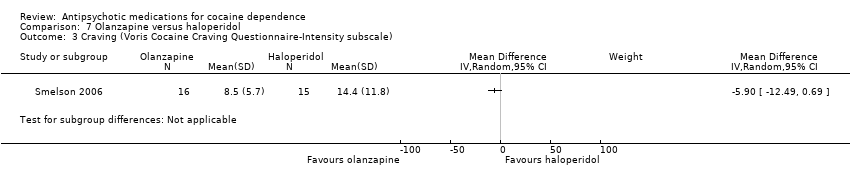
Comparison 7 Olanzapine versus haloperidol, Outcome 3 Craving (Voris Cocaine Craving Questionnaire‐Intensity subscale).
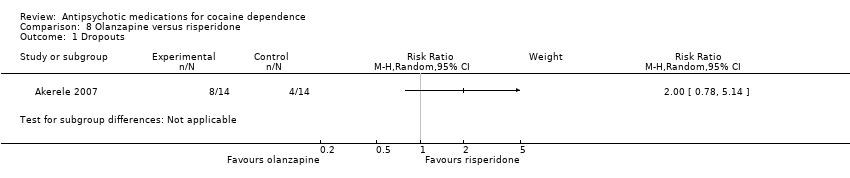
Comparison 8 Olanzapine versus risperidone, Outcome 1 Dropouts.
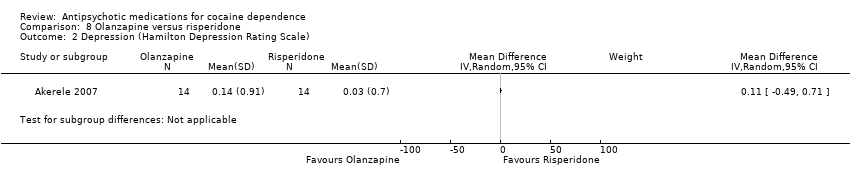
Comparison 8 Olanzapine versus risperidone, Outcome 2 Depression (Hamilton Depression Rating Scale).

Comparison 9 Aripiprazol versus ropinirol, Outcome 1 Dropouts.
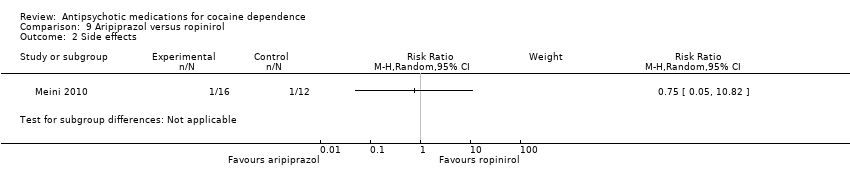
Comparison 9 Aripiprazol versus ropinirol, Outcome 2 Side effects.
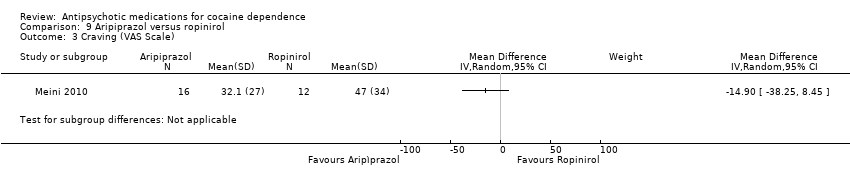
Comparison 9 Aripiprazol versus ropinirol, Outcome 3 Craving (VAS Scale).

Comparison 9 Aripiprazol versus ropinirol, Outcome 4 Severity of dependence (Clinical Global Impression Scale).
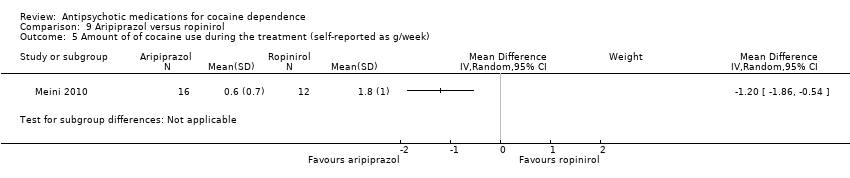
Comparison 9 Aripiprazol versus ropinirol, Outcome 5 Amount of of cocaine use during the treatment (self‐reported as g/week).
| Any antipsychotic versus placebo for cocaine dependence | ||||||
| Patient or population: people with cocaine dependence | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Any antipsychotic versus placebo | |||||
| Dropouts | Study population | RR 0.75 | 397 | ⊕⊕⊕⊝ | ||
| 547 per 1000 | 411 per 1000 | |||||
| Moderate | ||||||
| 500 per 1000 | 375 per 1000 | |||||
| Side effects | Study population | RR 1.01 | 291 | ⊕⊕⊝⊝ | ||
| 497 per 1000 | 502 per 1000 | |||||
| Moderate | ||||||
| 465 per 1000 | 470 per 1000 | |||||
| Number of participants using cocaine during the treatment (as days/week by urine tests or self report) | Study population | RR 1.02 | 91 | ⊕⊕⊝⊝ | ||
| 478 per 1000 | 488 per 1000 | |||||
| Moderate | ||||||
| 596 per 1000 | 608 per 1000 | |||||
| Continuous abstinence (number of participants who maintained negative drug screens for 2 ‐ 3 weeks) | Study population | RR 1.30 | 139 | ⊕⊕⊝⊝ | ||
| 197 per 1000 | 256 per 1000 | |||||
| Moderate | ||||||
| 129 per 1000 | 168 per 1000 | |||||
| Craving (Brief Substance Craving Scale) | The mean craving (brief substance craving scale) in the control groups was | The mean craving (Brief Substance Craving Scale) in the intervention groups was | 240 | ⊕⊕⊝⊝ | ||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1All the studies were at unclear risk of selection bias. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Dropouts Show forest plot | 8 | 397 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.57, 0.97] |
| 2 Side effects Show forest plot | 6 | 291 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.93, 1.10] |
| 3 Number of participants using cocaine during the treatment (as days/week by urine tests or self report) Show forest plot | 2 | 91 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.65, 1.62] |
| 4 Continuous abstinence (number of participants who maintained negative drug screens for 2 ‐ 3 weeks) Show forest plot | 3 | 139 | Risk Ratio (M‐H, Random, 95% CI) | 1.30 [0.73, 2.32] |
| 5 Craving (Brief Substance Craving Scale) Show forest plot | 4 | 240 | Mean Difference (IV, Random, 95% CI) | 0.13 [‐1.08, 1.35] |
| 6 Severity of dependence (Addiction Severity Index) Show forest plot | 4 | 211 | Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.01, 0.04] |
| 7 Severity of dependence (Clinical Global Impression Scale) Show forest plot | 3 | 180 | Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.38, 0.39] |
| 8 Use of cocaine during the treatment (self‐reported as g/week) Show forest plot | 2 | 72 | Mean Difference (IV, Random, 95% CI) | ‐0.54 [‐0.92, ‐0.16] |
| 9 Depression (Hamilton Depression Rating Scale) Show forest plot | 4 | 192 | Mean Difference (IV, Random, 95% CI) | ‐0.82 [‐3.19, 1.55] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Dropouts Show forest plot | 4 | 176 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.63, 1.04] |
| 2 Severity of dependence (Addiction Severity Index) Show forest plot | 1 | 31 | Mean Difference (IV, Random, 95% CI) | 0.03 [‐0.04, 0.10] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Dropouts Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2 Side effects Show forest plot | 2 | 79 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.92, 1.11] |
| 3 Use of cocaine during the treatment (self‐reported as days/week) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4 Use of cocaine during the treatment (self‐reported as days/past 30 days) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5 Continuous abstinence (participants who maintained negative drug screens throughout the treatment period ) Show forest plot | 2 | 79 | Risk Ratio (M‐H, Random, 95% CI) | 1.37 [0.71, 2.61] |
| 6 Craving (Brief Substance Craving Scale) Show forest plot | 2 | 61 | Mean Difference (IV, Random, 95% CI) | 1.33 [‐0.91, 3.58] |
| 7 Severity of dependence (Addiction Severity Index) Show forest plot | 2 | 61 | Mean Difference (IV, Random, 95% CI) | 0.03 [‐0.01, 0.07] |
| 8 Severity of dependence (Clinical Global Impression Scale) Show forest plot | 2 | 61 | Mean Difference (IV, Random, 95% CI) | 0.17 [‐0.51, 0.85] |
| 9 Amount of of cocaine use during the treatment (self‐reported as dollars spent/past 30 days) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 10 Depression (Hamilton Depression Rating Scale) Show forest plot | 2 | 61 | Mean Difference (IV, Random, 95% CI) | 1.34 [‐3.84, 6.52] |
| 11 Anxiety (Hamilton Anxiety Rating Scale) Show forest plot | 2 | 61 | Mean Difference (IV, Random, 95% CI) | 1.37 [‐3.02, 5.75] |
| 12 Withdrawal symptoms (Cocaine Selective Severity Assessment) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Dropouts Show forest plot | 2 | 72 | Risk Ratio (M‐H, Random, 95% CI) | 0.64 [0.20, 2.03] |
| 2 Side effects Show forest plot | 2 | 72 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.77, 1.27] |
| 3 Use of cocaine during the treatment (self‐reported as days/week) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4 Craving (Brief Substance Craving Scale) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5 Amount of of cocaine use during the treatment (self‐reported as g/week) Show forest plot | 2 | 72 | Mean Difference (IV, Random, 95% CI) | ‐0.54 [‐0.92, ‐0.16] |
| 6 Amount of of cocaine use during the treatment (self‐reported as dollars spent/week) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 7 Depression (Hamilton Depression Rating Scale) Show forest plot | 1 | 12 | Mean Difference (IV, Random, 95% CI) | ‐3.67 [‐6.19, ‐1.15] |
| 8 Depression (Quick Inventory of Depressive Symptomatology) Show forest plot | 1 | 12 | Mean Difference (IV, Random, 95% CI) | ‐1.27 [‐9.61, 7.07] |
| 9 Manic and hypomanic symptoms (Young Mania Rating Scale) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Side effects Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Dropouts Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2 Craving (Brief Substance Craving Scale) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3 Severity of dependence (Addiction Severity Index) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4 Severity of dependence (Clinical Global Impression Scale) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5 Depression (Hamilton Depression Rating Scale) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Dropouts Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2 Psychopathology (Positive and Negative Syndrome Scale) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3 Craving (Voris Cocaine Craving Questionnaire‐Intensity subscale) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Dropouts Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2 Depression (Hamilton Depression Rating Scale) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Dropouts Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2 Side effects Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3 Craving (VAS Scale) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4 Severity of dependence (Clinical Global Impression Scale) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5 Amount of of cocaine use during the treatment (self‐reported as g/week) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |

