Propofol para la sedación durante la colonoscopia
Referencias
Referencias de los estudios incluidos en esta revisión
Referencias de los estudios excluidos de esta revisión
Referencias adicionales
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | Single center, randomised, controlled trial | |
| Participants | Number of eligible patients: Not stated | |
| Interventions | Group A (n=50): Propofol (Mean (SD) dose: 161.06 (52.2) mg) and Midazolam 2 mg (pre‐medication) | |
| Outcomes | Patients' level of sedation, pain, recovery time, discharge time, patient satisfaction, vital signs, gastroenterologist satisfaction | |
| Notes | Definitions: | |
| Methods | Randomized study 1) Allocation concealment: Unclear/Not stated | |
| Participants | Number of eligible patients: Not stated | |
| Interventions | Group A (n=518): Propofol (mean total dose: 5.98 (2.67) mg/kg/hr) and Meperidine (mean total dose: 1.74 (1.05) mg/kg/hr) | |
| Outcomes | Primary outcome: colonoscopy completion rate | |
| Notes | For the secondary outcomes, no scores or values are provided; only p values provided for some of the secondary outcomes. Hence results for the secondary outcomes from this study were not included in the meta‐analysis. | |
| Methods | Multi center, randomised, controlled trial | |
| Participants | Number of eligible patients: 82 | |
| Interventions | Group A (n=34): PCS Propofol (Median (range) dose: 5.5 (1.9‐12.8) mg/kg/hr) and Alfentanil (Median (range) dose: 13.8 (4.8‐32) micrograms/kg/hr) | |
| Outcomes | Procedure time, patients' level of sedation, pain, complications, recovery time, discharge time, patient satisfaction, impact on normal activities | |
| Notes | Definitions: | |
| Methods | Single center, randomised, controlled trial | |
| Participants | Number of eligible patients: Not stated | |
| Interventions | All patients were premedicated with 800mg of Cimetidine prior to the procedures | |
| Outcomes | Recovery at different time points, after end of sedation | |
| Notes | French language article | |
| Methods | Single center, randomised, controlled trial | |
| Participants | Number of eligible patients: Not stated | |
| Interventions | Group A (n=33): Propofol (Mean (SD) dose: 117 (65) mg) | |
| Outcomes | Drops in oxygen saturation, increase in transcutaneous PCO2, patient satisfaction, endoscopist satisfaction | |
| Notes | No major adverse events occurred during sedation. | |
| Methods | Single center, randomised, controlled trial | |
| Participants | Number of eligible patients: Not stated | |
| Interventions | Group A (n=41): Propofol (dose: not stated) | |
| Outcomes | Hypoventilation as measured by changes in the transcutaneous carbon dioxide tension; hypoxemia (SpO2<85%); apnea | |
| Notes | Definitions | |
| Methods | Single center, randomised, controlled trial | |
| Participants | Number of eligible patients: Not stated | |
| Interventions | Group A (n=4,005): Propofol | |
| Outcomes | Incidence of colonic perforations | |
| Notes | It is unclear whether only adult patients were included in the study | |
| Methods | Single center, randomised, controlled trial | |
| Participants | Number of eligible patients: Not stated | |
| Interventions | Group A (n=19: Propofol (Bolus: mean (SD) dose: 1.3 (0.29) mg/kg; Infusion mean (SD) dose:76.5 (30) micro gm/kg/min) and Fentanyl (mean (SD) dose: 2.2 (0.48) micro gm/kg) | |
| Outcomes | Reliability of sedation | |
| Notes | Definitions: | |
| Methods | Single center, randomised, controlled trial | |
| Participants | Number of eligible patients: 166 | |
| Interventions | Group A (n=50): PCS with Propofol (median(IQR) dose: 78 (57‐119) mg) and Alfentanil ( 198 (144‐300) micrograms) | |
| Outcomes | Primary outcome: patient satisfaction with the degree of sedation during colonoscopy | |
| Notes | Definitions: | |
| Methods | Single center, randomised, controlled trial | |
| Participants | Number of eligible patients: 148 | |
| Interventions | Group A (n=Not stated): Propofol dose: 94 (30‐210) mg | |
| Outcomes | Primary outcome: Patient satisfaction scores, measured on a VAS, 4 hours after colonoscopy | |
| Notes | Numbers enrolled in each group not provided. Measure of variation and exact p value not provided for average satisfaction scores or procedure times | |
| Methods | Single center, randomised, controlled trial | |
| Participants | Number of eligible patients: Not stated | |
| Interventions | Group A (n=55): Mixture of Propofol 400 mg and Alfentanil 1mg delivered by a PCA pump in bolus of 2mg/ml (lockout period of 3 minutes) | |
| Outcomes | Procedure time; Recovery time | |
| Notes | Recovery time not defined | |
| Methods | Single center, randomised, controlled trial | |
| Participants | Number of eligible patients: 186 Incl. Criteria: 1) Patients scheduled for out patient colonoscopy only 2) Age 18‐65 years 3) ASA class I or II Country: Hong Kong | |
| Interventions | Group A (n=88): Nurse administered propofol sedation. A loading dose of 40‐60 mg or 0.8 mg/kg propofol, whichever was the higher, was given to patients 1 minute before the commencement of the procedure.. Then a PCA pump was used by the designated nurse to deliver a bolus dose of 1,5 ml mixture containing 14.3 mg propofol admixed with 35 microgram of alfentanil, with zero lock‐out for a goal OSSA score of 3 (assessed every 30 seconds). Median (range) dose; propofol 165 mg (52‐292); alfentanil 0.175 mg (0.035‐0.595) | |
| Outcomes | The primary end‐point of the study was the patient's level of sedation (measured by OSSA score) during colonoscopy intubation and when the cecum was reached. Secondary outcomes: 1) Time to cecal intubation and total procedure time 2) Patient, endoscopist and nurse satisfaction scores with regard to sedation 3) Overall pain score 4) Patients' willingness to repeat colonoscopy with the same sedation 5) Complications related to sedation 6) Cost comparison | |
| Notes | Nurses and endoscopists involved in the study were first trained by an anaesthesiologist regarding propofol delivery, patient monitoring using OSSA score and airway management. Concealment of allocation is judged to be adequate as first written informed consent was obtained and then randomizations carried out by a designated nurse, using computer generated numbers inside concealed envelopes. Definitions: 2) Complications related to sedation: Hypotension (systolic BP < 90 mm Hg); Oxygen desaturation<90%; Bradycardia (pulse< 50/min). 3) Full recovery= Fully alert (able to calculate serial subtraction in 7s from 100), hemodynamically stable (BP and HR within 20% of baseline and oxygen saturation >90% at room air) and ambulant. 4) Patient, nurse and endoscopist satisfaction: After full recovery of the patient, satisfaction scores regarding sedation from the patient, nurse and endoscopist were documented, using a 10 cm VAS, ranging from 0 (very unsatisfied) to 10 (very satisfied) 5) Pain: as reported by the patients on a similar scale. The continuous outcome variables (procedure time, recovery time, patient satisfaction, pain score, level of sedation) were reported as median and range. As per the Cochrane collaboration handbook, ranges should not be used to calculate SDs and hence these were not included in the meta‐analyses. For the review, we extracted the level of sedation as dichotomous outcome, with "failure to sedate" considered as those with OSSA score of 5 during colonoscopy intubation. | |
| Methods | Single center, randomised, controlled trial | |
| Participants | Number of eligible patients: Not stated ASA score : Not provided for all patients (ASA score III: Groups A:2 and group B:1) | |
| Interventions | Group A (n=25): Propofol (10 mg/ml) and Remifentanil (10 micrograms/ml): Initial bolus of 2.5 ml with demand of 0.75 ml at zero lockout Group B (n=24): Midazolam (0.5 mg/ml) and Fentanyl (12.5 micrograms/ml): Initial bolus of 4 ml with demand of 1ml at 1' lockout | |
| Outcomes | Procedure time, time to sedation, recovery time, SaO2 < 85% for 60", patient satisfaction | |
| Notes | Block size of 10 for first ten cases and then 40 for the rest of the cases was used. No mention, whether the study was performed on adult patients, but the mean age and use of PCS suggests that patients were adults. Anesthesiologist intervention for oxygen desaturation recorded 2) Recovery time= time to ambulation‐time at removal of colonoscope; however the time reported in the study and abstracted by us is the "recovery room time", which was not explicitly defined in the study. 3) Procedure time=time between colonoscope removal and insertion. 4) Safety endpoints included: arterial desaturation (85% for more than 60 s); hypotension (90 mmHg systolic or 20% decrease from baseline persisting on repeat determination 1 min later )or inability to tolerate the procedure. 5) Time to sedation: time between insertion of the colonoscope and initiation of the sedation. | |
| Methods | Randomized, controlled trial | |
| Participants | Number of eligible patients: Not stated | |
| Interventions | Group A (n=103): Remiphentanyl (0.1 micrograms/ Kg/min) followed by bolus of Propofol (0.5 mg/ Kg) | |
| Outcomes | Procedure time, recovery time, colonoscopy completion rate, endoscopist assessment of procedure difficulty and adequacy of sedation, and patient assessment of sedation. | |
| Notes | Age of patients not given in the abstract. | |
| Methods | Single center, randomised, controlled trial | |
| Participants | Number of eligible patients: Not stated | |
| Interventions | Group A (n=20): Propofol (Mean (range) dose: 273 (153‐490) mg) | |
| Outcomes | Primary outcome: Recovery time | |
| Notes | Definitions: | |
| Methods | Single center, randomised, controlled trial | |
| Participants | Number of eligible patients: Not stated | |
| Interventions | Group A (n=14): Propofol (Mean dose: 191.79 mg) | |
| Outcomes | Recovery time, discharge time, post procedure amnesia, patient satisfaction, endoscopist satisfaction | |
| Notes | Abstract DDW 1994 | |
| Methods | Single center, randomised, controlled trial | |
| Participants | Number of eligible patients: Not stated | |
| Interventions | Group A (n=44): Propofol (Mean (SD) dose: 98.20(36.74) mg) | |
| Outcomes | Primary outcomes: Endoscopist satisfaction, patient cooperation, deepest sedation score, sedation score 30 minutes after surgery and time to discharge | |
| Notes | 1) Discharge time: Patients deemed fit according to existing protocol in endoscopy suite. | |
| Methods | Single center, randomised, controlled trial | |
| Participants | Number of eligible patients: Not stated | |
| Interventions | Group A (n=64): Propofol (Median (range) dose: 80 (40‐150) mg) and Midazolam ( 2‐3 mg) | |
| Outcomes | Complications (a decline in oxygen saturation <90% for more than 10 s, change in MABP > 10 mm Hg, change in HR < or > 20%) | |
| Notes | Number of withdrawals is unclear, as only complete colonoscopies were included. There might have been some patients with incomplete colonoscopy, who were withdrawn after enrolment. | |
| Methods | Single center, randomised, controlled trial | |
| Participants | Number of eligible patients: Not stated | |
| Interventions | Group A (n=49): Propofol: Initial bolus 2.5 mg/kg body weight (for participants 18‐50 years age), 1.7 mg/kg (51‐74 years) or 1.1 mg/kg (75‐93 years). Subsequent infusion 6 mg/kg/hr (18‐50 years ), 4.5 mg/kg/hr (51‐74 years) or 3.8 mg/kg/hr (75‐93 years) | |
| Outcomes | Patients' level of sedation, pain, ability to cooperate, acceptability of procedure, antegrade amnesia, complications (a decline in oxygen saturation, drop in systolic and diastolic pressure, apnea) | |
| Notes | German language article | |
| Methods | Single center, randomised, controlled trial | |
| Participants | Number of eligible patients: Not stated | |
| Interventions | Group A (n=47): Propofol (Median (IQR) dose: 100 (53‐145) mg) and initially 2 mg of midazolam | |
| Outcomes | Recovery time, Patients' cognitive function (before and after the colonoscopy), procedure time, patient satisfaction, endoscopist assessment of quality of sedation, severe oxygen desaturation (SaO2 <85%), change in BP (MAP), HR or RR | |
| Notes | Although " the study was planned to include 150 patients", no information is provided on the sample size calculation | |
| Methods | Single center, randomised, controlled trial | |
| Participants | Number of eligible patients: 86 | |
| Interventions | Group A (n=33): PCS Propofol (Median dose: 105 mg) and Alfentanil (Median dose: 0.13 mg) | |
| Outcomes | Procedure time, patients' level of sedation, pain, complications, recovery time, patient satisfaction | |
| Notes | Definitions: | |
| Methods | Single center, randomised, controlled trial | |
| Participants | Number of eligible patients: 82 | |
| Interventions | Group A (n=40): Propofol (Mean (SD) dose: 214 (94) mg) Bolus administration | |
| Outcomes | Primary outcomes: sedation time, recovery time, discharge time, patient satisfaction scores (10 cm VAS) | |
| Notes | Before starting the study, the sequence of sedation treatments was created with a coin toss and blocks of 4. The block size of 4 may have been too small to prevent deciphering of sequence. | |
| Methods | Single center, randomised, controlled trial | |
| Participants | Number of eligible patients: Not stated | |
| Interventions | Group A (n=50): Propofol (Mean (SD) dose: 277 (105) mg) Bolus administration | |
| Outcomes | Primary outcomes: sedation time, recovery time, discharge time, patient satisfaction scores (10 cm VAS) | |
| Notes | Definitions: | |
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| Non‐randomised study. " The first group consisted of 102 consecutive patients". | |
| Duplicate report. Patients in RCT study, reported in Ulmer 2003 | |
| Duplicate reference. Conference abstract. Full article published in Canadian Journal of Gastroenterology 1994 | |
| An Anesthesiologist was present in the endoscopy room for all patients. All patients received propofol. | |
| Comparison of use of propofol to inhalational anaesthesia with nitrous oxide (Entonox). | |
| Study randomised patients undergoing upper or lower use gastrointestinal endoscopy. Results for those undergoing colonoscopy not reported separately. | |
| Comparative group was not traditional sedation. Comparison of combination of propofol/fentanyl/midazolam to inhalational anaesthesia with sevoflurane/nitrous oxide | |
| Retrospective Study | |
| Study randomised patients undergoing upper or lower gastrointestinal endoscopy. Results for those undergoing colonoscopy not reported separately. |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size | ||||||||||||||||||||||||||||||
| 1 Recovery time Show forest plot | 11 | 776 | Mean Difference (IV, Random, 95% CI) | ‐16.59 [‐24.99, ‐8.18] | ||||||||||||||||||||||||||||||
| Analysis 1.1  Comparison 1 Propofol Versus Traditional Agents, Outcome 1 Recovery time. | ||||||||||||||||||||||||||||||||||
| 1.1 Propofol alone | 4 | 249 | Mean Difference (IV, Random, 95% CI) | ‐14.68 [‐19.79, ‐9.58] | ||||||||||||||||||||||||||||||
| 1.2 Propofol combined with another agent | 7 | 527 | Mean Difference (IV, Random, 95% CI) | ‐17.36 [‐29.39, ‐5.34] | ||||||||||||||||||||||||||||||
| 2 Recovery time (minutes) in studies, which reported recovery time in formats which could not be meta‐analyzyed Show forest plot | Other data | No numeric data | ||||||||||||||||||||||||||||||||
| Analysis 1.2
Comparison 1 Propofol Versus Traditional Agents, Outcome 2 Recovery time (minutes) in studies, which reported recovery time in formats which could not be meta‐analyzyed. | ||||||||||||||||||||||||||||||||||
| 3 Discharge time Show forest plot | 7 | 542 | Mean Difference (IV, Random, 95% CI) | ‐20.86 [‐30.94, ‐10.78] | ||||||||||||||||||||||||||||||
| Analysis 1.3  Comparison 1 Propofol Versus Traditional Agents, Outcome 3 Discharge time. | ||||||||||||||||||||||||||||||||||
| 3.1 Propofol alone | 4 | 297 | Mean Difference (IV, Random, 95% CI) | ‐19.06 [‐28.08, ‐10.04] | ||||||||||||||||||||||||||||||
| 3.2 Propofol combined with another agent | 3 | 245 | Mean Difference (IV, Random, 95% CI) | ‐32.17 [‐64.84, 0.50] | ||||||||||||||||||||||||||||||
| 4 Procedure duration Show forest plot | 9 | 736 | Mean Difference (IV, Random, 95% CI) | 0.84 [‐1.02, 2.71] | ||||||||||||||||||||||||||||||
| Analysis 1.4  Comparison 1 Propofol Versus Traditional Agents, Outcome 4 Procedure duration. | ||||||||||||||||||||||||||||||||||
| 4.1 Propofol alone | 2 | 168 | Mean Difference (IV, Random, 95% CI) | ‐1.98 [‐6.12, 2.17] | ||||||||||||||||||||||||||||||
| 4.2 Propofol combined with another agent | 7 | 568 | Mean Difference (IV, Random, 95% CI) | 1.85 [‐0.26, 3.97] | ||||||||||||||||||||||||||||||
| 5 Cecal intubation Show forest plot | 9 | 1840 | Odds Ratio (M‐H, Random, 95% CI) | 0.41 [0.22, 0.76] | ||||||||||||||||||||||||||||||
| Analysis 1.5  Comparison 1 Propofol Versus Traditional Agents, Outcome 5 Cecal intubation. | ||||||||||||||||||||||||||||||||||
| 5.1 Propofol alone | 3 | 268 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | ||||||||||||||||||||||||||||||
| 5.2 Propofol combined with another agent | 6 | 1572 | Odds Ratio (M‐H, Random, 95% CI) | 0.41 [0.22, 0.76] | ||||||||||||||||||||||||||||||
| 6 Patient Dissatisfication (dichotomous data) Show forest plot | 6 | 449 | Odds Ratio (M‐H, Random, 95% CI) | 0.22 [0.11, 0.44] | ||||||||||||||||||||||||||||||
| Analysis 1.6  Comparison 1 Propofol Versus Traditional Agents, Outcome 6 Patient Dissatisfication (dichotomous data). | ||||||||||||||||||||||||||||||||||
| 6.1 Propofol alone | 2 | 117 | Odds Ratio (M‐H, Random, 95% CI) | 0.25 [0.09, 0.72] | ||||||||||||||||||||||||||||||
| 6.2 Propofol combined with another agent | 4 | 332 | Odds Ratio (M‐H, Random, 95% CI) | 0.19 [0.07, 0.50] | ||||||||||||||||||||||||||||||
| 7 Patient Satisfication (continuous data) Show forest plot | 4 | 370 | Std. Mean Difference (IV, Random, 95% CI) | 0.43 [‐0.00, 0.85] | ||||||||||||||||||||||||||||||
| Analysis 1.7  Comparison 1 Propofol Versus Traditional Agents, Outcome 7 Patient Satisfication (continuous data). | ||||||||||||||||||||||||||||||||||
| 7.1 Propofol alone | 3 | 220 | Std. Mean Difference (IV, Random, 95% CI) | 0.50 [‐0.17, 1.17] | ||||||||||||||||||||||||||||||
| 7.2 Propofol combined with another agent | 1 | 150 | Std. Mean Difference (IV, Random, 95% CI) | 0.32 [‐0.02, 0.66] | ||||||||||||||||||||||||||||||
| 8 Patient Dissatisfication (combined) Show forest plot | 7 | Odds Ratio (Random, 95% CI) | 0.35 [0.23, 0.53] | |||||||||||||||||||||||||||||||
| Analysis 1.8  Comparison 1 Propofol Versus Traditional Agents, Outcome 8 Patient Dissatisfication (combined). | ||||||||||||||||||||||||||||||||||
| 8.1 Propofol Alone | 4 | Odds Ratio (Random, 95% CI) | 0.33 [0.18, 0.60] | |||||||||||||||||||||||||||||||
| 8.2 Propofol combined with another agent | 3 | Odds Ratio (Random, 95% CI) | 0.33 [0.14, 0.80] | |||||||||||||||||||||||||||||||
| 9 Pain Control (continuous outcome) Show forest plot | 6 | 633 | Std. Mean Difference (IV, Random, 95% CI) | 0.38 [0.03, 0.74] | ||||||||||||||||||||||||||||||
| Analysis 1.9  Comparison 1 Propofol Versus Traditional Agents, Outcome 9 Pain Control (continuous outcome). | ||||||||||||||||||||||||||||||||||
| 9.1 Propofol alone | 2 | 187 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.14 [‐0.53, 0.26] | ||||||||||||||||||||||||||||||
| 9.2 Propofol combined with another agent | 4 | 446 | Std. Mean Difference (IV, Random, 95% CI) | 0.51 [0.17, 0.84] | ||||||||||||||||||||||||||||||
| 10 Pain Control (dichotomous outcome) Show forest plot | 5 | 344 | Odds Ratio (M‐H, Random, 95% CI) | 1.12 [0.21, 5.97] | ||||||||||||||||||||||||||||||
| Analysis 1.10  Comparison 1 Propofol Versus Traditional Agents, Outcome 10 Pain Control (dichotomous outcome). | ||||||||||||||||||||||||||||||||||
| 10.1 Propofol alone | 3 | 220 | Odds Ratio (M‐H, Random, 95% CI) | 0.20 [0.02, 1.79] | ||||||||||||||||||||||||||||||
| 10.2 Propofol combined with another agent | 2 | 124 | Odds Ratio (M‐H, Random, 95% CI) | 2.64 [0.39, 18.04] | ||||||||||||||||||||||||||||||
| 11 Pain Control (combined) Show forest plot | 9 | Odds Ratios (Random, 95% CI) | 1.71 [1.02, 2.88] | |||||||||||||||||||||||||||||||
| Analysis 1.11  Comparison 1 Propofol Versus Traditional Agents, Outcome 11 Pain Control (combined). | ||||||||||||||||||||||||||||||||||
| 11.1 Propofol Alone | 3 | Odds Ratios (Random, 95% CI) | 0.67 [0.34, 1.33] | |||||||||||||||||||||||||||||||
| 11.2 Propofol combined with another agent | 6 | Odds Ratios (Random, 95% CI) | 2.27 [1.42, 3.63] | |||||||||||||||||||||||||||||||
| 12 Hypoxia Show forest plot | 15 | 1408 | Odds Ratio (M‐H, Random, 95% CI) | 0.80 [0.48, 1.31] | ||||||||||||||||||||||||||||||
| Analysis 1.12  Comparison 1 Propofol Versus Traditional Agents, Outcome 12 Hypoxia. | ||||||||||||||||||||||||||||||||||
| 12.1 Propofol alone | 5 | 407 | Odds Ratio (M‐H, Random, 95% CI) | 0.69 [0.25, 1.89] | ||||||||||||||||||||||||||||||
| 12.2 Propofol combined with another agent | 10 | 1001 | Odds Ratio (M‐H, Random, 95% CI) | 0.83 [0.47, 1.48] | ||||||||||||||||||||||||||||||
| 13 Apnea Show forest plot | 11 | 918 | Odds Ratio (M‐H, Random, 95% CI) | 1.35 [0.56, 3.24] | ||||||||||||||||||||||||||||||
| Analysis 1.13  Comparison 1 Propofol Versus Traditional Agents, Outcome 13 Apnea. | ||||||||||||||||||||||||||||||||||
| 13.1 Propofol alone | 5 | 407 | Odds Ratio (M‐H, Random, 95% CI) | 1.35 [0.56, 3.24] | ||||||||||||||||||||||||||||||
| 13.2 Propofol combined with another agent | 6 | 511 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | ||||||||||||||||||||||||||||||
| 14 Respiratory depression requiring intervention Show forest plot | 10 | 898 | Odds Ratio (M‐H, Random, 95% CI) | 0.67 [0.15, 2.89] | ||||||||||||||||||||||||||||||
| Analysis 1.14  Comparison 1 Propofol Versus Traditional Agents, Outcome 14 Respiratory depression requiring intervention. | ||||||||||||||||||||||||||||||||||
| 14.1 Propofol alone | 3 | 268 | Odds Ratio (M‐H, Random, 95% CI) | 0.33 [0.01, 8.21] | ||||||||||||||||||||||||||||||
| 14.2 Propofol combined with another agent | 7 | 630 | Odds Ratio (M‐H, Random, 95% CI) | 0.81 [0.16, 4.18] | ||||||||||||||||||||||||||||||
| 15 Arrhythmias Show forest plot | 7 | 684 | Odds Ratio (M‐H, Random, 95% CI) | 0.70 [0.31, 1.55] | ||||||||||||||||||||||||||||||
| Analysis 1.15  Comparison 1 Propofol Versus Traditional Agents, Outcome 15 Arrhythmias. | ||||||||||||||||||||||||||||||||||
| 15.1 Propofol alone | 3 | 220 | Odds Ratio (M‐H, Random, 95% CI) | 0.55 [0.09, 3.46] | ||||||||||||||||||||||||||||||
| 15.2 Propofol combined with another agent | 4 | 464 | Odds Ratio (M‐H, Random, 95% CI) | 0.74 [0.30, 1.80] | ||||||||||||||||||||||||||||||
| 16 Hypotension Show forest plot | 6 | 548 | Odds Ratio (M‐H, Random, 95% CI) | 0.97 [0.55, 1.71] | ||||||||||||||||||||||||||||||
| Analysis 1.16  Comparison 1 Propofol Versus Traditional Agents, Outcome 16 Hypotension. | ||||||||||||||||||||||||||||||||||
| 16.1 Propofol alone | 2 | 144 | Odds Ratio (M‐H, Random, 95% CI) | 1.03 [0.28, 3.83] | ||||||||||||||||||||||||||||||
| 16.2 Propofol combined with another agent | 4 | 404 | Odds Ratio (M‐H, Random, 95% CI) | 0.96 [0.51, 1.79] | ||||||||||||||||||||||||||||||
| 17 Blood pressure drop or lowest blood pressure during the procedure Show forest plot | 5 | 494 | Mean Difference (IV, Random, 95% CI) | 2.19 [‐2.55, 6.94] | ||||||||||||||||||||||||||||||
| Analysis 1.17  Comparison 1 Propofol Versus Traditional Agents, Outcome 17 Blood pressure drop or lowest blood pressure during the procedure. | ||||||||||||||||||||||||||||||||||
| 17.1 Propofol alone | 2 | 199 | Mean Difference (IV, Random, 95% CI) | 0.45 [‐9.40, 10.30] | ||||||||||||||||||||||||||||||
| 17.2 Propofol combined with another agent | 3 | 295 | Mean Difference (IV, Random, 95% CI) | 3.63 [‐4.47, 11.72] | ||||||||||||||||||||||||||||||
| 18 Colonic perforations Show forest plot | 1 | 7286 | Odds Ratio (M‐H, Random, 95% CI) | 2.87 [0.60, 13.83] | ||||||||||||||||||||||||||||||
| Analysis 1.18  Comparison 1 Propofol Versus Traditional Agents, Outcome 18 Colonic perforations. | ||||||||||||||||||||||||||||||||||
| 18.1 Propofol alone | 1 | 7286 | Odds Ratio (M‐H, Random, 95% CI) | 2.87 [0.60, 13.83] | ||||||||||||||||||||||||||||||
| 18.2 Propofol combined with another agent | 0 | 0 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | ||||||||||||||||||||||||||||||
| 19 Sedation (failure to sedate) Show forest plot | 2 | 277 | Odds Ratio (M‐H, Random, 95% CI) | 0.03 [0.00, 1.43] | ||||||||||||||||||||||||||||||
| Analysis 1.19  Comparison 1 Propofol Versus Traditional Agents, Outcome 19 Sedation (failure to sedate). | ||||||||||||||||||||||||||||||||||
| 19.1 Propofol alone | 1 | 99 | Odds Ratio (M‐H, Random, 95% CI) | 0.20 [0.07, 0.60] | ||||||||||||||||||||||||||||||
| 19.2 Propofol combined with another agent | 1 | 178 | Odds Ratio (M‐H, Random, 95% CI) | 0.00 [0.00, 0.01] | ||||||||||||||||||||||||||||||
| 20 Sedation Show forest plot | 6 | 521 | Std. Mean Difference (IV, Random, 95% CI) | 0.41 [‐0.45, 1.27] | ||||||||||||||||||||||||||||||
| Analysis 1.20  Comparison 1 Propofol Versus Traditional Agents, Outcome 20 Sedation. | ||||||||||||||||||||||||||||||||||
| 20.1 Propofol alone | 3 | 268 | Std. Mean Difference (IV, Random, 95% CI) | 1.38 [0.93, 1.82] | ||||||||||||||||||||||||||||||
| 20.2 Propofol combined with another agent | 3 | 253 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.53 [‐0.98, ‐0.09] | ||||||||||||||||||||||||||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size | ||||||||||||
| 1 Patient Satisfication Show forest plot | Other data | No numeric data | ||||||||||||||
| Analysis 2.1
Comparison 2 Non‐anesthesiologist Versus Anesthesiologist, Outcome 1 Patient Satisfication. | ||||||||||||||||
| 2 Procedure duration (minutes) Show forest plot | Other data | No numeric data | ||||||||||||||
| Analysis 2.2
Comparison 2 Non‐anesthesiologist Versus Anesthesiologist, Outcome 2 Procedure duration (minutes). | ||||||||||||||||
| 3 Hypoxia Show forest plot | Other data | No numeric data | ||||||||||||||
| Analysis 2.3
Comparison 2 Non‐anesthesiologist Versus Anesthesiologist, Outcome 3 Hypoxia. | ||||||||||||||||
| 4 Hypotension Show forest plot | Other data | No numeric data | ||||||||||||||
| Analysis 2.4
Comparison 2 Non‐anesthesiologist Versus Anesthesiologist, Outcome 4 Hypotension. | ||||||||||||||||

Comparison 1 Propofol Versus Traditional Agents, Outcome 1 Recovery time.
| Study | Group A | Group B | Group C | Comments |
| Kulling 2001 | 45 min: 0 (IQR ‐0.5 ‐0.5) | In between A and C | 45 min: 1.0 (IQR 0.0 ‐9.0) | Difference from baseline of the score on Triegger dot‐joining test. Less difference, better recovery. Recovery‐‐therefore better recovery in Group A (PCS propofol), as compared with C at 45 and 90 mins |
| Liu 2009 | 2.5 (0‐15.0) | 0 (0‐7.5) | No group C in this study | Recovery time was reported as median (minutes) and range. Recovery time was s ignificantly longer in group A (p<0.0001) |
| Martinez‐Palli 2005 | 24 | 38 | 32 | No measures of variance provided |
| Paspatis 2002 | 5 min: 9.5±0.6 | 5 min: 8.3±1.3 | No group C in this study | Significantly higher Aldrete scores at 5, 10 and 30 minutes in Group A (Propofol) |
Comparison 1 Propofol Versus Traditional Agents, Outcome 2 Recovery time (minutes) in studies, which reported recovery time in formats which could not be meta‐analyzyed.

Comparison 1 Propofol Versus Traditional Agents, Outcome 3 Discharge time.

Comparison 1 Propofol Versus Traditional Agents, Outcome 4 Procedure duration.

Comparison 1 Propofol Versus Traditional Agents, Outcome 5 Cecal intubation.

Comparison 1 Propofol Versus Traditional Agents, Outcome 6 Patient Dissatisfication (dichotomous data).

Comparison 1 Propofol Versus Traditional Agents, Outcome 7 Patient Satisfication (continuous data).

Comparison 1 Propofol Versus Traditional Agents, Outcome 8 Patient Dissatisfication (combined).

Comparison 1 Propofol Versus Traditional Agents, Outcome 9 Pain Control (continuous outcome).

Comparison 1 Propofol Versus Traditional Agents, Outcome 10 Pain Control (dichotomous outcome).
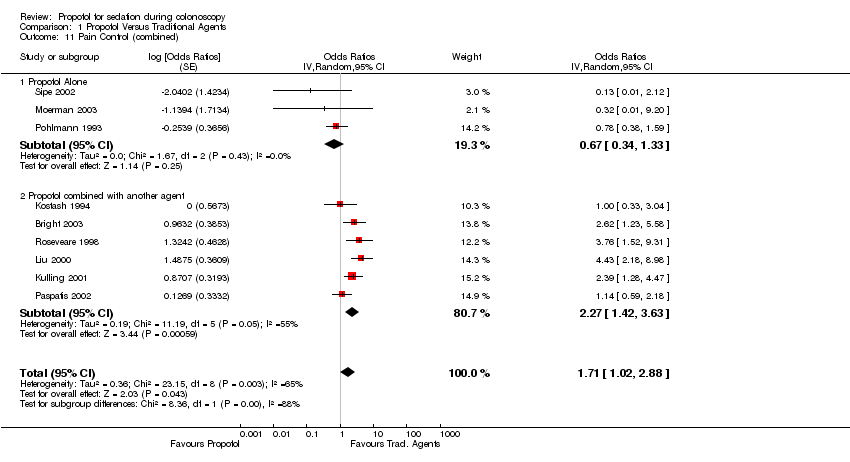
Comparison 1 Propofol Versus Traditional Agents, Outcome 11 Pain Control (combined).
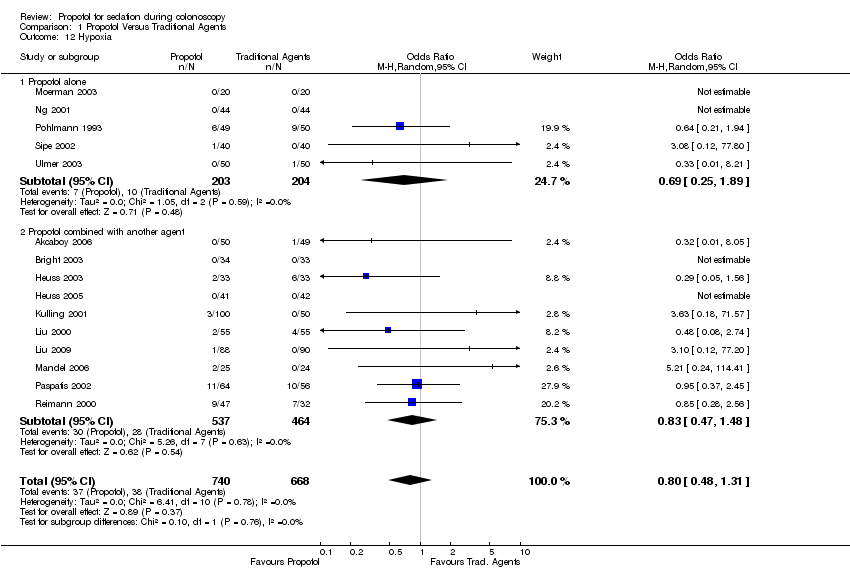
Comparison 1 Propofol Versus Traditional Agents, Outcome 12 Hypoxia.
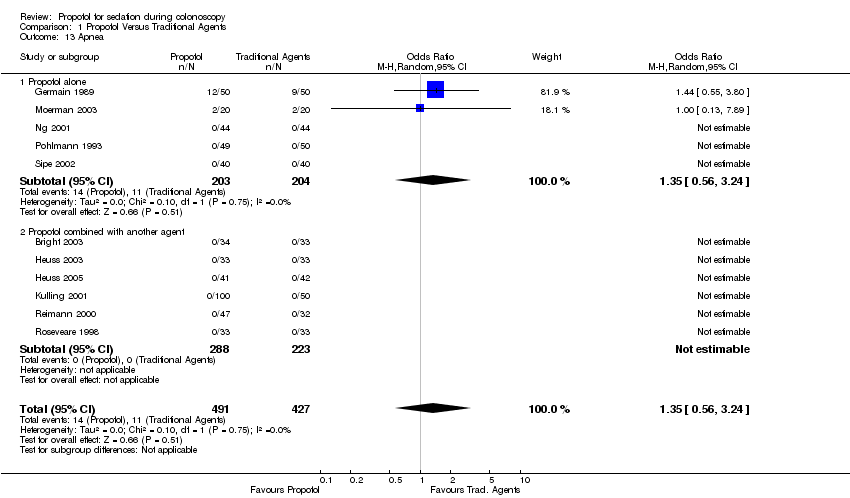
Comparison 1 Propofol Versus Traditional Agents, Outcome 13 Apnea.
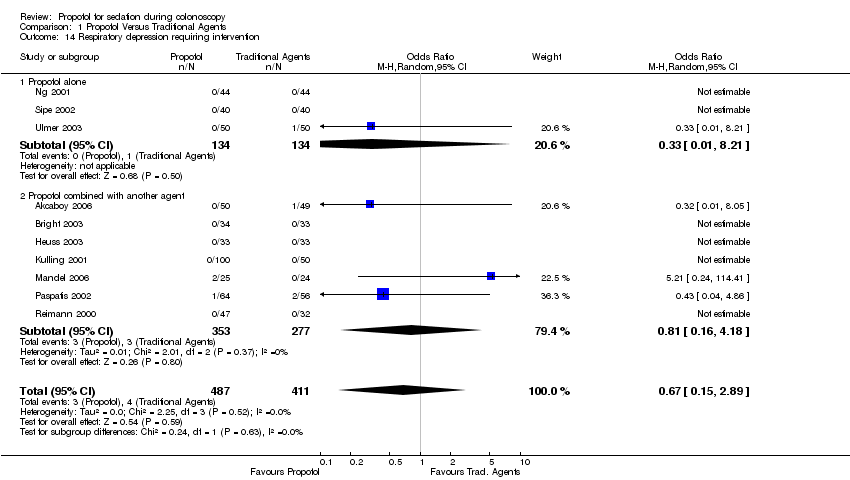
Comparison 1 Propofol Versus Traditional Agents, Outcome 14 Respiratory depression requiring intervention.

Comparison 1 Propofol Versus Traditional Agents, Outcome 15 Arrhythmias.
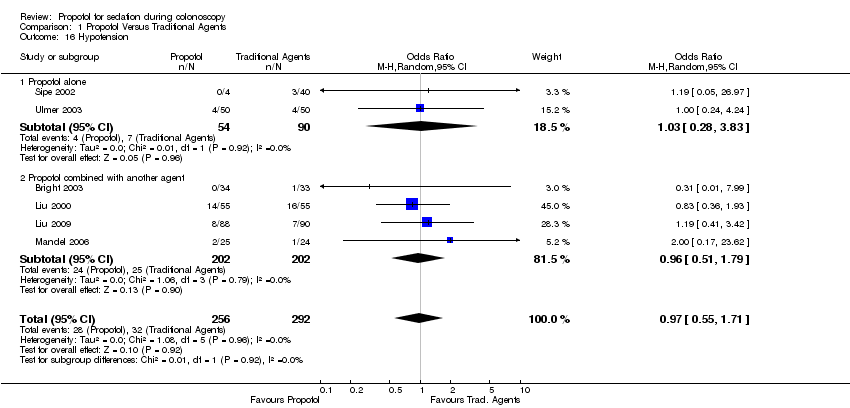
Comparison 1 Propofol Versus Traditional Agents, Outcome 16 Hypotension.

Comparison 1 Propofol Versus Traditional Agents, Outcome 17 Blood pressure drop or lowest blood pressure during the procedure.
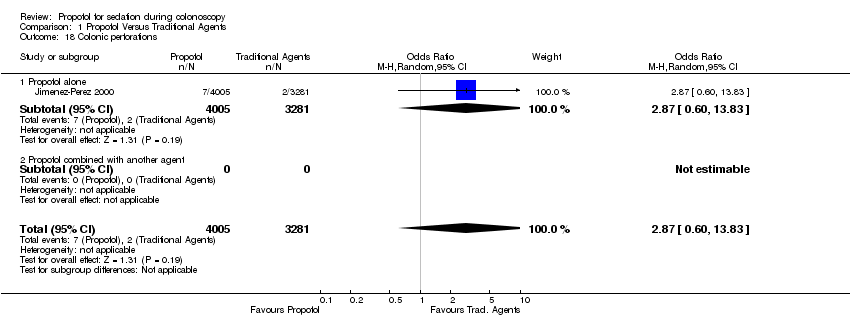
Comparison 1 Propofol Versus Traditional Agents, Outcome 18 Colonic perforations.

Comparison 1 Propofol Versus Traditional Agents, Outcome 19 Sedation (failure to sedate).
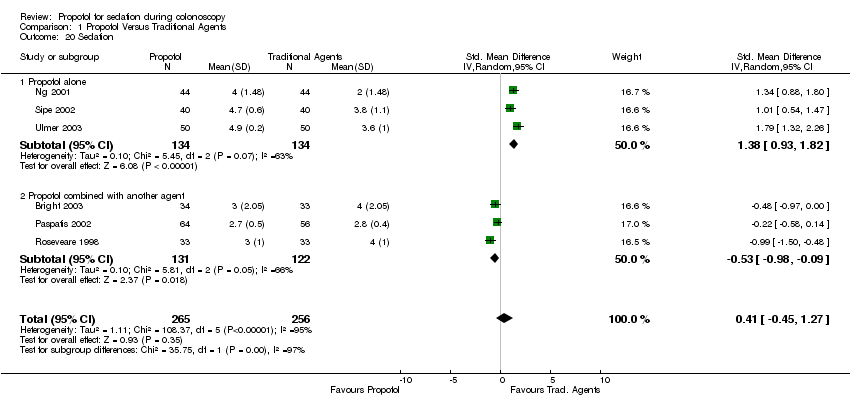
Comparison 1 Propofol Versus Traditional Agents, Outcome 20 Sedation.
| Study | Gastroenterologist | Anesthesiologist | Comments |
| Laquiere 2006 | Average score on VAS= 90.8 | Average score on VAS= 89 | Not significantly different |
Comparison 2 Non‐anesthesiologist Versus Anesthesiologist, Outcome 1 Patient Satisfication.
| Study | Gastroenterologist | Anesthesiologist | Comment |
| Laquiere 2006 | 16.7 | 17.7 | No significant difference |
Comparison 2 Non‐anesthesiologist Versus Anesthesiologist, Outcome 2 Procedure duration (minutes).
| Study | Gastroenterologist | Anesthesiologist | Comment |
| Laquiere 2006 | 6.6% | 35.5% | "Desaturation" not defined |
Comparison 2 Non‐anesthesiologist Versus Anesthesiologist, Outcome 3 Hypoxia.
| Study | Gastroenterologist | Anesthesiologist | Comment |
| Laquiere 2006 | 24.4% | 44% | Hypotension not defined |
Comparison 2 Non‐anesthesiologist Versus Anesthesiologist, Outcome 4 Hypotension.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Recovery time Show forest plot | 11 | 776 | Mean Difference (IV, Random, 95% CI) | ‐16.59 [‐24.99, ‐8.18] |
| 1.1 Propofol alone | 4 | 249 | Mean Difference (IV, Random, 95% CI) | ‐14.68 [‐19.79, ‐9.58] |
| 1.2 Propofol combined with another agent | 7 | 527 | Mean Difference (IV, Random, 95% CI) | ‐17.36 [‐29.39, ‐5.34] |
| 2 Recovery time (minutes) in studies, which reported recovery time in formats which could not be meta‐analyzyed Show forest plot | Other data | No numeric data | ||
| 3 Discharge time Show forest plot | 7 | 542 | Mean Difference (IV, Random, 95% CI) | ‐20.86 [‐30.94, ‐10.78] |
| 3.1 Propofol alone | 4 | 297 | Mean Difference (IV, Random, 95% CI) | ‐19.06 [‐28.08, ‐10.04] |
| 3.2 Propofol combined with another agent | 3 | 245 | Mean Difference (IV, Random, 95% CI) | ‐32.17 [‐64.84, 0.50] |
| 4 Procedure duration Show forest plot | 9 | 736 | Mean Difference (IV, Random, 95% CI) | 0.84 [‐1.02, 2.71] |
| 4.1 Propofol alone | 2 | 168 | Mean Difference (IV, Random, 95% CI) | ‐1.98 [‐6.12, 2.17] |
| 4.2 Propofol combined with another agent | 7 | 568 | Mean Difference (IV, Random, 95% CI) | 1.85 [‐0.26, 3.97] |
| 5 Cecal intubation Show forest plot | 9 | 1840 | Odds Ratio (M‐H, Random, 95% CI) | 0.41 [0.22, 0.76] |
| 5.1 Propofol alone | 3 | 268 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 Propofol combined with another agent | 6 | 1572 | Odds Ratio (M‐H, Random, 95% CI) | 0.41 [0.22, 0.76] |
| 6 Patient Dissatisfication (dichotomous data) Show forest plot | 6 | 449 | Odds Ratio (M‐H, Random, 95% CI) | 0.22 [0.11, 0.44] |
| 6.1 Propofol alone | 2 | 117 | Odds Ratio (M‐H, Random, 95% CI) | 0.25 [0.09, 0.72] |
| 6.2 Propofol combined with another agent | 4 | 332 | Odds Ratio (M‐H, Random, 95% CI) | 0.19 [0.07, 0.50] |
| 7 Patient Satisfication (continuous data) Show forest plot | 4 | 370 | Std. Mean Difference (IV, Random, 95% CI) | 0.43 [‐0.00, 0.85] |
| 7.1 Propofol alone | 3 | 220 | Std. Mean Difference (IV, Random, 95% CI) | 0.50 [‐0.17, 1.17] |
| 7.2 Propofol combined with another agent | 1 | 150 | Std. Mean Difference (IV, Random, 95% CI) | 0.32 [‐0.02, 0.66] |
| 8 Patient Dissatisfication (combined) Show forest plot | 7 | Odds Ratio (Random, 95% CI) | 0.35 [0.23, 0.53] | |
| 8.1 Propofol Alone | 4 | Odds Ratio (Random, 95% CI) | 0.33 [0.18, 0.60] | |
| 8.2 Propofol combined with another agent | 3 | Odds Ratio (Random, 95% CI) | 0.33 [0.14, 0.80] | |
| 9 Pain Control (continuous outcome) Show forest plot | 6 | 633 | Std. Mean Difference (IV, Random, 95% CI) | 0.38 [0.03, 0.74] |
| 9.1 Propofol alone | 2 | 187 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.14 [‐0.53, 0.26] |
| 9.2 Propofol combined with another agent | 4 | 446 | Std. Mean Difference (IV, Random, 95% CI) | 0.51 [0.17, 0.84] |
| 10 Pain Control (dichotomous outcome) Show forest plot | 5 | 344 | Odds Ratio (M‐H, Random, 95% CI) | 1.12 [0.21, 5.97] |
| 10.1 Propofol alone | 3 | 220 | Odds Ratio (M‐H, Random, 95% CI) | 0.20 [0.02, 1.79] |
| 10.2 Propofol combined with another agent | 2 | 124 | Odds Ratio (M‐H, Random, 95% CI) | 2.64 [0.39, 18.04] |
| 11 Pain Control (combined) Show forest plot | 9 | Odds Ratios (Random, 95% CI) | 1.71 [1.02, 2.88] | |
| 11.1 Propofol Alone | 3 | Odds Ratios (Random, 95% CI) | 0.67 [0.34, 1.33] | |
| 11.2 Propofol combined with another agent | 6 | Odds Ratios (Random, 95% CI) | 2.27 [1.42, 3.63] | |
| 12 Hypoxia Show forest plot | 15 | 1408 | Odds Ratio (M‐H, Random, 95% CI) | 0.80 [0.48, 1.31] |
| 12.1 Propofol alone | 5 | 407 | Odds Ratio (M‐H, Random, 95% CI) | 0.69 [0.25, 1.89] |
| 12.2 Propofol combined with another agent | 10 | 1001 | Odds Ratio (M‐H, Random, 95% CI) | 0.83 [0.47, 1.48] |
| 13 Apnea Show forest plot | 11 | 918 | Odds Ratio (M‐H, Random, 95% CI) | 1.35 [0.56, 3.24] |
| 13.1 Propofol alone | 5 | 407 | Odds Ratio (M‐H, Random, 95% CI) | 1.35 [0.56, 3.24] |
| 13.2 Propofol combined with another agent | 6 | 511 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Respiratory depression requiring intervention Show forest plot | 10 | 898 | Odds Ratio (M‐H, Random, 95% CI) | 0.67 [0.15, 2.89] |
| 14.1 Propofol alone | 3 | 268 | Odds Ratio (M‐H, Random, 95% CI) | 0.33 [0.01, 8.21] |
| 14.2 Propofol combined with another agent | 7 | 630 | Odds Ratio (M‐H, Random, 95% CI) | 0.81 [0.16, 4.18] |
| 15 Arrhythmias Show forest plot | 7 | 684 | Odds Ratio (M‐H, Random, 95% CI) | 0.70 [0.31, 1.55] |
| 15.1 Propofol alone | 3 | 220 | Odds Ratio (M‐H, Random, 95% CI) | 0.55 [0.09, 3.46] |
| 15.2 Propofol combined with another agent | 4 | 464 | Odds Ratio (M‐H, Random, 95% CI) | 0.74 [0.30, 1.80] |
| 16 Hypotension Show forest plot | 6 | 548 | Odds Ratio (M‐H, Random, 95% CI) | 0.97 [0.55, 1.71] |
| 16.1 Propofol alone | 2 | 144 | Odds Ratio (M‐H, Random, 95% CI) | 1.03 [0.28, 3.83] |
| 16.2 Propofol combined with another agent | 4 | 404 | Odds Ratio (M‐H, Random, 95% CI) | 0.96 [0.51, 1.79] |
| 17 Blood pressure drop or lowest blood pressure during the procedure Show forest plot | 5 | 494 | Mean Difference (IV, Random, 95% CI) | 2.19 [‐2.55, 6.94] |
| 17.1 Propofol alone | 2 | 199 | Mean Difference (IV, Random, 95% CI) | 0.45 [‐9.40, 10.30] |
| 17.2 Propofol combined with another agent | 3 | 295 | Mean Difference (IV, Random, 95% CI) | 3.63 [‐4.47, 11.72] |
| 18 Colonic perforations Show forest plot | 1 | 7286 | Odds Ratio (M‐H, Random, 95% CI) | 2.87 [0.60, 13.83] |
| 18.1 Propofol alone | 1 | 7286 | Odds Ratio (M‐H, Random, 95% CI) | 2.87 [0.60, 13.83] |
| 18.2 Propofol combined with another agent | 0 | 0 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 19 Sedation (failure to sedate) Show forest plot | 2 | 277 | Odds Ratio (M‐H, Random, 95% CI) | 0.03 [0.00, 1.43] |
| 19.1 Propofol alone | 1 | 99 | Odds Ratio (M‐H, Random, 95% CI) | 0.20 [0.07, 0.60] |
| 19.2 Propofol combined with another agent | 1 | 178 | Odds Ratio (M‐H, Random, 95% CI) | 0.00 [0.00, 0.01] |
| 20 Sedation Show forest plot | 6 | 521 | Std. Mean Difference (IV, Random, 95% CI) | 0.41 [‐0.45, 1.27] |
| 20.1 Propofol alone | 3 | 268 | Std. Mean Difference (IV, Random, 95% CI) | 1.38 [0.93, 1.82] |
| 20.2 Propofol combined with another agent | 3 | 253 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.53 [‐0.98, ‐0.09] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Patient Satisfication Show forest plot | Other data | No numeric data | ||
| 2 Procedure duration (minutes) Show forest plot | Other data | No numeric data | ||
| 3 Hypoxia Show forest plot | Other data | No numeric data | ||
| 4 Hypotension Show forest plot | Other data | No numeric data | ||

