| 1 Pregnancy per woman Show forest plot | 1 | 201 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
|
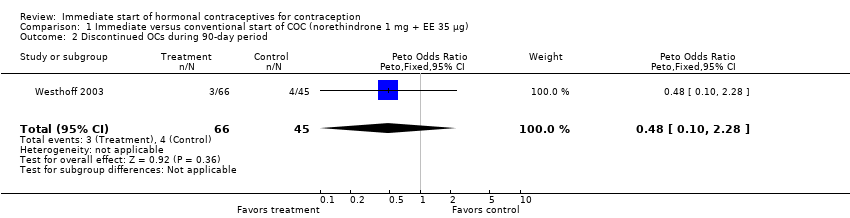
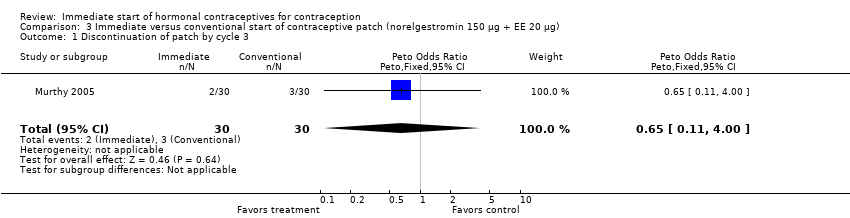
| 2 Discontinued method in 84‐day period Show forest plot | 1 | 174 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.84 [0.33, 2.18] |
|
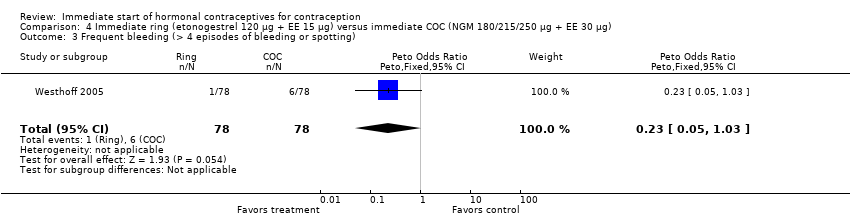
| 3 Frequent bleeding (> 4 episodes of bleeding or spotting) Show forest plot | 1 | 156 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.23 [0.05, 1.03] |
|
| 4 Irregular bleeding (bleeding‐free interval > 17 days) Show forest plot | 1 | 156 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.77 [0.33, 1.75] |
|
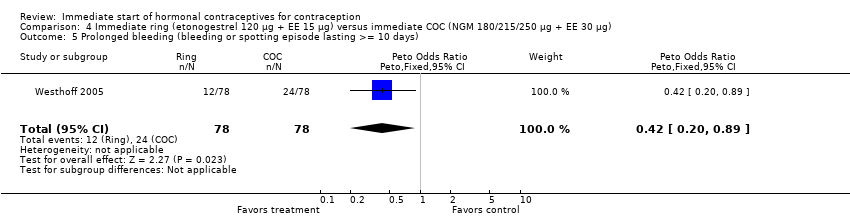
| 5 Prolonged bleeding (bleeding or spotting episode lasting >= 10 days) Show forest plot | 1 | 156 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.42 [0.20, 0.89] |
|
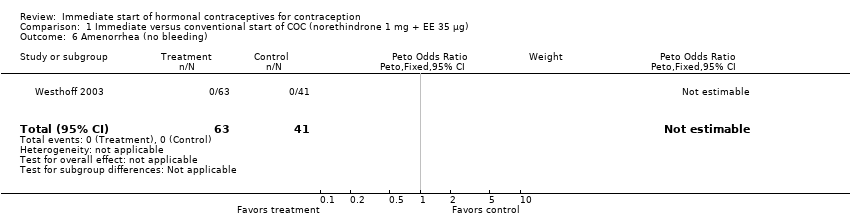
| 6 Amenorrhea Show forest plot | 1 | 156 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
|
| 7 Very satisfied with method Show forest plot | 1 | 174 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.88 [1.59, 5.22] |
|
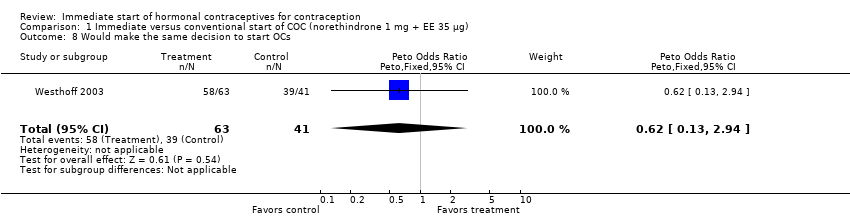
| 8 Planned to use method Show forest plot | 1 | 174 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.51 [1.32, 4.77] |
|
| 9 Reported bad change in weight Show forest plot | 1 | 174 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.42 [0.21, 0.87] |
|
| 10 Reported bad change in bleeding Show forest plot | 1 | 174 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.28 [0.14, 0.55] |
|
| 11 Reported bad change in headache Show forest plot | 1 | 174 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.53 [0.24, 1.18] |
|
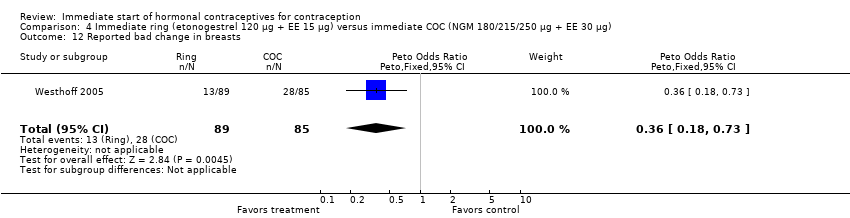
| 12 Reported bad change in breasts Show forest plot | 1 | 174 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.36 [0.18, 0.73] |
|
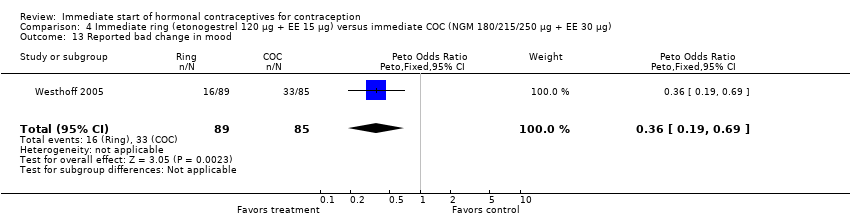
| 13 Reported bad change in mood Show forest plot | 1 | 174 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.36 [0.19, 0.69] |
|
| 14 Reported bad change in acne Show forest plot | 1 | 174 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.39 [0.59, 3.29] |
|
| 15 Reported bad change in appetite Show forest plot | 1 | 174 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.44 [0.21, 0.95] |
|
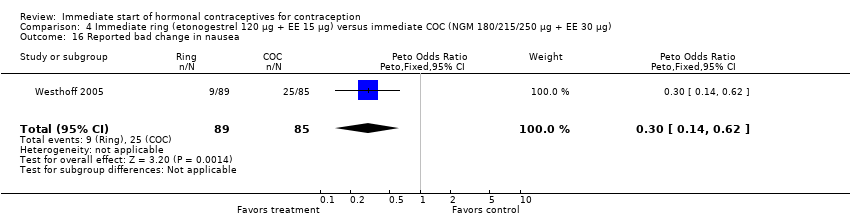
| 16 Reported bad change in nausea Show forest plot | 1 | 174 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.30 [0.14, 0.62] |
|
| 17 Reported bad change in cramps Show forest plot | 1 | 145 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.79 [0.37, 1.67] |
|
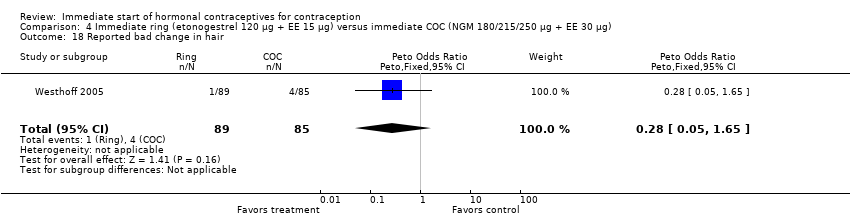
| 18 Reported bad change in hair Show forest plot | 1 | 174 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.28 [0.05, 1.65] |
|
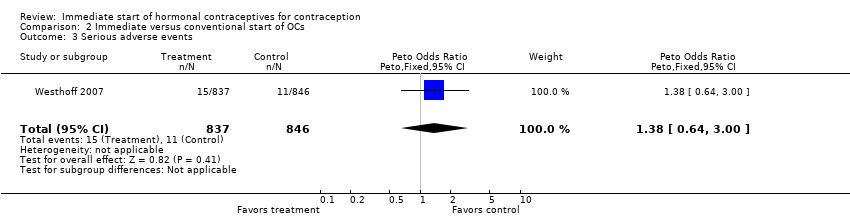
| 19 Serious adverse events (total) Show forest plot | 1 | 174 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
|