Hemodiafiltración, hemofiltración y hemodiálisis para la enfermedad renal terminal
Referencias
References to studies included in this review
References to studies excluded from this review
References to studies awaiting assessment
References to ongoing studies
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group
Control group
Total substitution volume: 60 L/patient/session Treatment duration: 12 months | |
| Outcomes | Intradialytic problems
Interdialytic problems
Other
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) | High risk | Not stated; probably not done |
| Blinding of outcome assessment (detection bias) | High risk | Not stated; probably not done |
| Incomplete outcome data (attrition bias) | High risk | Not using intention‐to‐treat analysis; loss to follow‐up 6/39 (23%) |
| Selective reporting (reporting bias) | Unclear risk | No protocol of the study available |
| Other bias | High risk | Carry over effect might be present because of the cross‐over design; data at the end of first phase of treatment not available; commercial sponsor listed as author |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group 1
Treatment group 2
Both treatment groups
Control group
Co‐interventions: NS | |
| Outcomes |
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) | High risk | Not stated; probably not done |
| Blinding of outcome assessment (detection bias) | High risk | Not stated; probably not done |
| Incomplete outcome data (attrition bias) | Unclear risk | No data about drop‐outs provided after different cross‐over phases; lost to follow‐up: 0/14 |
| Selective reporting (reporting bias) | High risk | Data at the end of first phase of treatment not available |
| Other bias | High risk | Carryover effect present because of the cross‐over design; data not extractable for meta‐analysis |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group
Control group
Co‐interventions: NS | |
| Outcomes |
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not stated; probably not done |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated; probably not done |
| Incomplete outcome data (attrition bias) | High risk | 33% loss to follow‐up (5/15 patients whose Hb dropped at monthly checks were withdrawn from the study) |
| Selective reporting (reporting bias) | High risk | Data at the end of first phase of treatment not available |
| Other bias | High risk | Carry over effect present because of the cross‐over design; data not available for meta‐analysis |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group
Control group
| |
| Outcomes |
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Low risk | Centrally and envelopes were used |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | High risk | Missing outcome data balanced across groups with similar reasons for missing data across groups but with 13/40 patients (32%) not included in the final analysis |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
| Other bias | High risk | Imbalanced ratio men/women between groups; interventions not matched; funded by industry |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group 1
Treatment group 2
Control group
| |
| Outcomes |
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Central computer generated: " randomisation list that was stratified by centre and prepared in advance by one author" |
| Allocation concealment (selection bias) | Unclear risk | Patients were centrally randomised using an email assignment from one of the authors |
| Blinding of participants and personnel (performance bias) | High risk | No blinding |
| Blinding of outcome assessment (detection bias) | High risk | No blinding |
| Incomplete outcome data (attrition bias) | High risk | 10/146 (14%) withdrew from study due to transfer to another technique, thrombosis or vascular access infection, withdrawal of consent, transfer to another centre, transfer to another study, infection) |
| Selective reporting (reporting bias) | High risk | Key patient relevant outcomes not reported |
| Other bias | High risk | Interventions and patient characteristics not matched; industry support provided |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group
Control group
| |
| Outcomes |
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information, the method of concealment is not stated. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | High risk | "no difference for dialysis tolerance between the groups"; no intention‐to‐treat analysis; lost to follow‐up (9; no clear description of drop‐outs, reasons or belonging) |
| Selective reporting (reporting bias) | High risk | Not all of the study's pre‐specified primary outcomes have been reported; data at the end of first phase of treatment not available |
| Other bias | High risk | Important difference between selected and analysed patients (e.g. dialysis vintage 249 months in the selected initial group versus 164 months in the analysed group); carry over effect present because of the cross‐over design |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group
Control group
Both groups
| |
| Outcomes |
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Centrally by blocks |
| Allocation concealment (selection bias) | Unclear risk | Centrally |
| Blinding of participants and personnel (performance bias) | High risk | Not done: "Because of the nature of the intervention, it was not possible to blind the patients, the local study nurses, or the investigators to the treatment assignment." |
| Blinding of outcome assessment (detection bias) | Low risk | Adjudication committee unaware of treatment assignment |
| Incomplete outcome data (attrition bias) | Low risk | All the results are available |
| Selective reporting (reporting bias) | Low risk | All important outcomes are reported |
| Other bias | High risk | Interventions not matched between treatment groups; early termination due to futility; funded by industry |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group
Control group
| |
| Outcomes |
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated |
| Allocation concealment (selection bias) | Unclear risk | The allocation was made by means of a computer generated sequence |
| Blinding of participants and personnel (performance bias) | High risk | Not stated. probably not done |
| Blinding of outcome assessment (detection bias) | High risk | Not stated, probably not done |
| Incomplete outcome data (attrition bias) | Unclear risk | Not sufficiently detailed |
| Selective reporting (reporting bias) | High risk | Insufficient information |
| Other bias | High risk | Abstract‐only publication |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group
Control group
Co‐interventions: not stated | |
| Outcomes |
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) | High risk | "A second patient refused to finish post‐HDF and insisted that AFB be tried because of his unbearable shoulder" |
| Incomplete outcome data (attrition bias) | High risk | No intention‐to‐treat analysis; 3 patients violated the study protocol (one patient's fistula failed during the second month of pre‐HDF; one refused to finish post‐HDF; one patient had severe headache |
| Selective reporting (reporting bias) | High risk | Data for end of first phase of treatment not available |
| Other bias | High risk | Carry over effect present because of the cross‐over design |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group
Control group
Co‐interventions
| |
| Outcomes |
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Patients were randomised according to the cause of their underlying CKD |
| Allocation concealment (selection bias) | High risk | Inadequate: names were drawn from individual subgroups at the following ratios 1:1 |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | Unclear risk | Insufficient data; no loss to follow‐up |
| Selective reporting (reporting bias) | High risk | Not all patient important outcomes reported |
| Other bias | High risk | Interventions and baseline patient characteristics not matched |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group
Control group
Both groups
| |
| Outcomes |
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A central computerised random‐generator |
| Allocation concealment (selection bias) | Unclear risk | Centrally |
| Blinding of participants and personnel (performance bias) | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) | High risk | Open‐label study |
| Incomplete outcome data (attrition bias) | High risk | 355/906 discontinued the study, 39% from the total number of included patients, 41% in the HDF arm |
| Selective reporting (reporting bias) | Low risk | All the prespecified outcomes were reported |
| Other bias | High risk | Commercial sponsor on authorship or involved in data management; interventions and baseline patient characteristics not matched |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group
Control group
Co‐interventions: not stated | |
| Outcomes |
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | "by coin toss", an insecure method |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | Unclear risk | Insufficient data; no loss to follow‐up |
| Selective reporting (reporting bias) | High risk | Data for end of first phase of treatment not available, no protocol of the study available |
| Other bias | High risk | Carry over effect present because of the cross‐over design; data not extractable for meta‐analysis |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group 1
Treatment group 2
Control group
| |
| Outcomes |
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | Unclear risk | Lost to follow‐up 2/24; no clear description of drop‐outs or reasons |
| Selective reporting (reporting bias) | High risk | Data at the end of first phase of treatment not available |
| Other bias | High risk | Carry over effect due to cross‐over design, interventions not matched |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group
Control group
| |
| Outcomes |
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) | Unclear risk | "The treatment modality was blinded to the patient by use of filter types unknown to the patients and not ordinarily used in the department. The tubing was mounted as to haemodiafiltration in all sessions, and the indicators showing the treatment modality on the console were covered." Investigators not blinded |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated, probably not done |
| Incomplete outcome data (attrition bias) | Unclear risk | Not clearly stated that all patients that performed one dialysis have done the second dialysis session as well |
| Selective reporting (reporting bias) | High risk | Data for end of first phase of treatment not available |
| Other bias | High risk | Study conducted in two consecutive dialysis sessions, "wash out effect" insufficient, carry over effect might be present because of the cross‐over design; data not extractable for meta‐analysis; interventions not matched |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group
Control group
Co‐interventions: not stated | |
| Outcomes |
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | High risk | Inadequate: "We used partially randomised patient preference (PRPP) design by incorporating patient preferences into this randomised trials" |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | Unclear risk | Not stated, insufficient information |
| Selective reporting (reporting bias) | High risk | Outcome/s of interest reported incompletely and cannot be used in meta‐analysis; protocol of the study unavailable; failure to report a key/expected outcome (mortality, major CV events) |
| Other bias | Low risk | Not additional risks apparent |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group 1
Treatment group 2
Treatment group 3
Treatment group 4
Co‐interventions: not stated | |
| Outcomes |
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Adequate: "Randomization was centralized at the Department of Nephrology at Lecco Hospital, using separate lists for each Center that were randomly divided into blocks of four for the assignment of two or four treatments (depending on the treatments available in the different Centers)." |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | High risk | 108/205 analysed (46%) (34% due to technical reasons, acute clinical reason, fistula‐related reason, treatment inadequacy) |
| Selective reporting (reporting bias) | High risk | Key outcomes not reported |
| Other bias | High risk | Interventions not matched and patient characteristics not matched at baseline; interventions and patient characteristics not matched; commercial sponsor involved in the conduct of this study |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group 1
Treatment group 2
Control group
Co‐interventions: not stated | |
| Outcomes |
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | Low risk | 0/8 lost to follow‐up |
| Selective reporting (reporting bias) | High risk | Insufficient information; protocol of the study not available; only data about B2 microglobulin were available |
| Other bias | High risk | Carry over effect present because of the cross‐over design; data not extractable for meta‐analysis |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group
Control group
| |
| Outcomes |
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Centrally randomised |
| Allocation concealment (selection bias) | Unclear risk | Centrally randomised |
| Blinding of participants and personnel (performance bias) | High risk | Probably not done |
| Blinding of outcome assessment (detection bias) | High risk | Probably not done |
| Incomplete outcome data (attrition bias) | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | High risk | Key patient outcomes not provided |
| Other bias | High risk | Carry over effect present because of the cross‐over design; commercial sponsor involved in authorship or data management |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group
Control group 1
Control group 2
| |
| Outcomes |
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) | High risk | Insufficient information. probably not done |
| Blinding of outcome assessment (detection bias) | High risk | Insufficient information. probably not done |
| Incomplete outcome data (attrition bias) | High risk | Missing outcome data; loss to follow‐up 3/17 (18%) (transplantation (2); lack of compliance (1)) |
| Selective reporting (reporting bias) | High risk | Data for end of first phase of treatment not available |
| Other bias | High risk | Carry over effect present because of the cross‐over design; in the reported results is included a comparison between random and non‐randomly allocated groups; commercial sponsor involved in authorship or data management |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group
Control group
Co‐interventions: not stated | |
| Outcomes |
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | High risk | 3/12 patients died |
| Selective reporting (reporting bias) | High risk | Data for end of first phase of treatment not available |
| Other bias | High risk | Carry over effect present because of the cross‐over design |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group
Control group
Co‐interventions: not stated | |
| Outcomes |
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | Unclear risk | Missing outcome data balanced across groups; 0/5 lost to follow‐up |
| Selective reporting (reporting bias) | High risk | Data for end of first phase of treatment not available; no protocol of the study available |
| Other bias | High risk | Carry over effect present because of the cross‐over design; data not extractable for meta‐analysis and interventions not matched |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group
Control group
| |
| Outcomes |
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | Unclear risk | Unclear; insufficient information provided about losses to follow‐up |
| Selective reporting (reporting bias) | High risk | Data about mortality events were missing |
| Other bias | Low risk | No additional risks identified |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group
Control group
| |
| Outcomes |
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "randomised by central telephone into a 1:1 ratio" |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) | High risk | Not performed |
| Blinding of outcome assessment (detection bias) | High risk | Not performed |
| Incomplete outcome data (attrition bias) | High risk | 10% lost for follow‐up, 4% due to access failures (unclear whether imbalanced between groups) |
| Selective reporting (reporting bias) | High risk | Data for end of first phase of treatment not available |
| Other bias | High risk | Carry over effect present because of the cross‐over design; commercial sponsorship on authorship or data management; interventions not matched |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group
Control group
| |
| Outcomes |
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "patients were randomised to treatment with either HF or HD using an online computer‐based program stratified by age and diabetes " |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) | High risk | Probably not done |
| Blinding of outcome assessment (detection bias) | Low risk | Echocardiograms read by an observer blinded to treatment |
| Incomplete outcome data (attrition bias) | High risk | Missing outcome data balanced across groups but attrition is 29%; randomised (48), analysed (34), finished the 24 months follow‐up (17, 35%) |
| Selective reporting (reporting bias) | High risk | Study protocol available (ISRCTN83264534) and all pre‐specified outcomes have been reported. All key patient outcomes not provided |
| Other bias | High risk | Commercial sponsor on authorship and/or involved in data management, interventions not matched |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group
Control group
| |
| Outcomes |
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Centrally: "An independent person performed randomisation for the sequence of treatment. " |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) | High risk | Probably not done |
| Blinding of outcome assessment (detection bias) | High risk | Probably not done |
| Incomplete outcome data (attrition bias) | Low risk | Missing outcome data balanced across groups but no intention‐to‐treat analysis |
| Selective reporting (reporting bias) | High risk | Data for end of first phase of treatment not available; insufficient information, no study protocol available |
| Other bias | High risk | Carry over effect present because of the cross‐over design; interventions not matched |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group
Control group
| |
| Outcomes |
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomisation was done centrally (with a computerized random‐number generator) using the balanced block randomisation technique with a 1:1 ratio, stratification according to the clinical centre concerned and a block size of eight" |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not stated, probably not done |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated, probably not done |
| Incomplete outcome data (attrition bias) | High risk | 21% loss to follow‐up and dropouts censored at time of termination |
| Selective reporting (reporting bias) | High risk | Outcomes of interest are not reported |
| Other bias | High risk | Interventions not matched and patient baseline characteristics not matched |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group
Control group
| |
| Outcomes |
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients were centrally randomised, 1:1 ratio |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) | High risk | "There was no blinding of participants, investigators, or outcome assessors." |
| Blinding of outcome assessment (detection bias) | High risk | "There was no blinding of participants, investigators, or outcome assessors." |
| Incomplete outcome data (attrition bias) | High risk | 20% withdrew from study due to personal reasons, transferred to another centre to centre not able to offer treatment (HF); imbalance between arms |
| Selective reporting (reporting bias) | High risk | Study protocol unavailable; outcomes of interest reported incompletely |
| Other bias | High risk | Baseline patient characteristics not matched |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group
Control group
Co‐interventions: not stated | |
| Outcomes |
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | Unclear risk | Insufficient information |
| Selective reporting (reporting bias) | High risk | Limited amount of data reported for a 2 year study |
| Other bias | High risk | Data not extractable for data analysis, intervention not matched |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group
Control group
| |
| Outcomes |
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | "by coin flip", an insecure method |
| Allocation concealment (selection bias) | High risk | Not stated |
| Blinding of participants and personnel (performance bias) | High risk | "Unblinded' |
| Blinding of outcome assessment (detection bias) | High risk | "Unblinded' |
| Incomplete outcome data (attrition bias) | Low risk | 3% loss to follow‐up due to move away from dialysis centre; similar in both groups |
| Selective reporting (reporting bias) | High risk | Outcomes of interest not reported |
| Other bias | Low risk | Carry over effect present because of the cross‐over design but we included in our analysis only the data available about the first phase of the study |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group
Control group
Co‐interventions: not stated | |
| Outcomes |
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Each pair of patients was randomised to either AFB or HD |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | High risk | 13% lost to follow‐up due to allergy, refusal or side‐effects. Unclear whether imbalance between groups |
| Selective reporting (reporting bias) | High risk | Not all the expected outcomes were reported |
| Other bias | Low risk | No additional risks identified |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group
Control group
| |
| Outcomes |
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) | High risk | Probably not done |
| Blinding of outcome assessment (detection bias) | High risk | Probably not done |
| Incomplete outcome data (attrition bias) | High risk | Insufficient reporting |
| Selective reporting (reporting bias) | High risk | Study protocol unavailable and data for end of first phase of treatment not available |
| Other bias | High risk | Patients included were selected using two different inclusion criteria (prone to hypotension or stable patients) with no clear description of the initial number of analysed number. Carry over effect present because of the cross‐over design; data not extractable for meta‐analysis and interventions not matched |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group
Control group
| |
| Outcomes |
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) | Low risk | "The study was patient‐blinded and partially investigator‐blinded" |
| Blinding of outcome assessment (detection bias) | Low risk | The interviewers did not know which treatment was performed |
| Incomplete outcome data (attrition bias) | High risk | 20% loss to follow‐up (pain, intracerebral bleeding, patient request, and dialysis access problems) |
| Selective reporting (reporting bias) | High risk | Study protocol unavailable; outcomes of interest not all reported |
| Other bias | High risk | Carry over effect present because of the cross‐over design; interventions not matched |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group
Control group
Co‐interventions: not stated | |
| Outcomes |
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | High risk | 15% loss to follow‐up (declined by patient and personal reasons) |
| Selective reporting (reporting bias) | High risk | Study protocol unavailable but no data available to be included |
| Other bias | High risk | Carry over effect present because of the cross‐over design; data not extractable for meta‐analysis, interventions not matched |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group
Control group
Co‐interventions: not stated | |
| Outcomes |
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | Unclear risk | Unclear; no loss to follow‐up |
| Selective reporting (reporting bias) | High risk | Data for end of first phase of treatment not available |
| Other bias | High risk | Carry over effect present because of the cross‐over design; data not extractable for meta‐analysis |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group
Control group
Co‐interventions: not stated | |
| Outcomes |
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) | Low risk | No blinding but review authors judge that outcome measurement not likely influenced |
| Incomplete outcome data (attrition bias) | Unclear risk | Short duration of study, < 10% attrition |
| Selective reporting (reporting bias) | High risk | Data for end of first phase of treatment not available |
| Other bias | High risk | Carry over effect present because of the cross‐over design; data not extractable for meta‐analysis; abstract only publication |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group
Control group
| |
| Outcomes |
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) | High risk | Open‐label |
| Blinding of outcome assessment (detection bias) | High risk | Open label |
| Incomplete outcome data (attrition bias) | High risk | 20% loss to follow‐up plus imbalance in loss to follow‐up due to vascular access problems |
| Selective reporting (reporting bias) | Low risk | Study protocol available and all patient important outcomes were reported |
| Other bias | High risk | Commercial sponsorship of study |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group
Control group
| |
| Outcomes |
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | A random code was used, with a separate list for each study centre |
| Allocation concealment (selection bias) | Unclear risk | Centrally performed by an independent institute |
| Blinding of participants and personnel (performance bias) | High risk | "Open" |
| Blinding of outcome assessment (detection bias) | High risk | "Open" |
| Incomplete outcome data (attrition bias) | High risk | 46% lost and dropped out patients "not replaced"; lost to follow‐up: 20 drop‐outs (4 died, 11 were transplanted and other reasons for 5) and 49 withdrawn patients |
| Selective reporting (reporting bias) | High risk | Insufficient information about patient important outcomes |
| Other bias | High risk | Commercial sponsor involved in authorship and data management; interventions not matched |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group
Control group
Co‐interventions: not stated | |
| Outcomes |
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | High risk | 20% loss to follow‐up |
| Selective reporting (reporting bias) | High risk | Outcome/s of interest reported incompletely, cannot be used in meta‐analysis; "Intradialysis status P = 0.003"; study protocol unavailable |
| Other bias | High risk | Carry over effect present because of the cross‐over design; data not extractable for meta‐analysis and interventions not matched |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group
Control group
Co‐interventions: not stated | |
| Outcomes |
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Each pair of patients was randomised to either AFB or HD |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | High risk | 22% patients lost‐to‐follow‐up |
| Selective reporting (reporting bias) | High risk | All outcomes of interest not reported |
| Other bias | High risk | Patient baseline characteristics not matched |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group
Control group
Co‐interventions: not stated | |
| Outcomes |
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | High risk | 18% loss to follow‐up |
| Selective reporting (reporting bias) | High risk | Study protocol unavailable and outcomes of interest reported incompletely, cannot be used in meta‐analysis (e.g. BP) |
| Other bias | High risk | Sponsor involved in authorship and/or data management; interventions not matched |
ACEi ‐ angiotensin‐converting enzyme inhibitor; AFB ‐ acetate‐free biofiltration; BD ‐ conventional bicarbonate dialysis; BP ‐ blood pressure; CKD ‐ chronic kidney disease; CrCl ‐ creatinine clearance; CV ‐ cardiovascular; EPO ‐ erythropoietin; ESKD ‐ end‐stage kidney disease; Hb ‐ haemoglobin; HCT ‐ haematocrit; HD ‐ haemodialysis; HF ‐ haemofiltration; HDF ‐ haemodiafiltration; HTN ‐ hypertension; LVMi ‐ left ventricular mass index; MAP ‐ mean arterial pressure; MI ‐ myocardial infarction; QB ‐ blood flow rate; QD ‐ dialysate flow rate; QoL ‐ quality of life; rHuEPO: recombinant human EPO; RRT ‐ renal replacement therapy; TIA ‐ transient Ischaemic attack; URR ‐ urea reduction ratio
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Population included not relevant to this review (patients with acute kidney injury | |
| Not RCT | |
| Not RCT | |
| Not RCT; interventions not relevant to this review | |
| Not RCT | |
| Not RCT | |
| Not RCT | |
| Not RCT | |
| Not RCT | |
| Not RCT | |
| Not RCT | |
| Not RCT | |
| Not RCT | |
| Not RCT | |
| Interventions not relevant to this review | |
| Outcomes not relevant to this review | |
| Outcomes not relevant to this review | |
| Not RCT | |
| Not RCT | |
| Interventions not relevant to this review | |
| Outcomes not relevant to this review | |
| Not RCT | |
| Interventions not relevant to this review | |
| Interventions not relevant to this review | |
| Not RCT | |
| Not RCT | |
| Outcomes not relevant to this review | |
| Not RCT | |
| Not RCT | |
| Not RCT | |
| Not RCT | |
| Not RCT | |
| Not an RCT | |
| Outcomes not relevant to this review | |
| Outcomes not relevant to this review | |
| Not RCT | |
| Not RCT | |
| Outcomes not relevant to this review | |
| Not RCT | |
| Interventions not relevant to this review | |
| Interventions not relevant to this review | |
| Not RCT | |
| Not RCT | |
| Not RCT | |
| Interventions not relevant to this review | |
| Not RCT | |
| Interventions not relevant to this review | |
| Interventions not relevant to this review | |
| Interventions not relevant to this review | |
| Outcomes not relevant to review | |
| Not RCT | |
| Outcomes not relevant to review | |
| Interventions not relevant to this review | |
| Not RCT | |
| Interventions not relevant to this review | |
| Not RCT | |
| Outcomes not relevant to this review | |
| Outcomes not relevant to this review | |
| Interventions not relevant to this review | |
| Not RCT | |
| Interventions not relevant to this review | |
| Not RCT | |
| Not RCT | |
| Outcomes not relevant to review | |
| Interventions not relevant to this review | |
| Interventions not relevant to this review | |
| Interventions not relevant to this review | |
| Not RCT | |
| Not RCT | |
| Interventions not relevant to this review | |
| Outcomes not relevant to this review (serum AGE level reduction rates) | |
| Not RCT | |
| Not RCT | |
| Not RCT | |
| Not RCT | |
| Not RCT (review article) | |
| Not RCT | |
| Interventions not relevant to this review | |
| Not RCT | |
| Not RCT (review article) | |
| Interventions not relevant to this review | |
| Not RCT | |
| Not RCT | |
| Interventions not relevant to this review | |
| Interventions not relevant to this review | |
| Not RCT | |
| Not RCT | |
| Interventions not relevant to this review | |
| Interventions not relevant to this review | |
| Not RCT | |
| Interventions not relevant to this review | |
| Not RCT | |
| Not RCT | |
| Not RCT | |
| Not RCT | |
| Not RCT | |
| Interventions not relevant to this review | |
| Interventions not relevant to this review | |
| Interventions not relevant to this review | |
| Interventions not relevant to this review | |
| Interventions not relevant to this review | |
| Interventions not relevant to this review | |
| Outcomes not relevant to this review | |
| Not RCT | |
| Not RCT | |
| Interventions not relevant to this review | |
| Interventions not relevant to this review | |
| Interventions not relevant to this review | |
| Interventions not relevant to this review | |
| Interventions not relevant to this review | |
| Interventions not relevant to this review | |
| Interventions and outcomes not relevant to this review | |
| Outcomes not relevant to this review | |
| Not RCT | |
| Interventions not relevant to this review | |
| Outcomes not relevant to review | |
| Not RCT | |
| Not RCT | |
| Interventions not relevant to this review | |
| Outcomes not relevant to this review | |
| Outcomes not relevant to review | |
| Not RCT | |
| Not RCT | |
| Outcomes not relevant to review | |
| Not RCT | |
| Outcomes not relevant to review | |
| Outcomes not relevant to review | |
| Outcomes not relevant to review | |
| Outcomes not relevant to review | |
| Not RCT (review article) | |
| Not RCT | |
| Not RCT | |
| Not RCT |
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
| Methods | Country: Netherlands Enrolment: not reported |
| Participants | Ages eligible for study: 18 to 80 years Inclusion criteria prevalent conventional HD patients; AV‐fistula enabling double‐needle vascular access with blood flow rate of at least 350 mL/min; informed consent; age more than 18 years Exclusion criteria withdrawal of consent; acute intercurrent illness (infection, malignancy, cardiovascular event, uncontrolled diabetes) |
| Interventions | Assigned interventions 4‐hour HD, 4‐hour HDF, 8‐hour HD and 8‐hour HDF Prevalent conventional HD (CHD) patients (dialysing 3 days a week during 4 hours per dialysis session) will undergo, in random order, a mid‐week 4‐hour HD session, a mid‐week 4‐hour HDF session, a mid‐week 8‐hour HD session, and a mid‐week 8‐hour HDF session with a 2‐week interval between every session to assess the influence of treatment duration and of convection on the removal of uraemic toxins and on the haemodynamic responses and autonomic nervous regulation In between the study dialysis sessions these patients will receive routine CHD treatments |
| Outcomes | Primary outcome measures Removal of uraemic toxins Haemodynamic response: BP, heart rate, heart rate variability, cardiac output and systemic vascular resistance will be measured. Skin microcirculation will be measured with laser Doppler flowmetry. |
| Notes | source: http://clinicaltrials.gov/ct2/show/study/NCT01328119?term=NCT01328119&rank=1 |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Tolerance to hemodialysis in insulin‐requiring diabetic patients: BD vs AFB with blood volume biofeedback (THIRD) |
| Methods | Country: Italy Enrolment: 55 patients |
| Participants | Ages eligible for study: 18 to 85 years Inclusion criteria End stage renal disease patients; patients affected by diabetic nephropathy with insulin therapy, for, at least, 6 months; patients with renal replacement therapy with haemodialysis three time a week, for, at least, 6 months; age between 18 and 85 years Exclusion criteria Patients affected by neoplasm and/or mental illness; patients with residual diuresis > 500 mL/d; patients in single needle bicarbonate dialysis |
| Interventions | The study, 9 months long, is aimed to verify the treatment tolerance of insulin requiring diabetic patients, by using standard bicarbonate dialysis (BD), or acetate free biofiltration (AFB) and/or a blood volume control (BVC). The study is divided in three phases: the first one, three months long, is the baseline in standard bicarbonate dialysis, then all the patients are shifted to AFB with BVC, for other three months, while the last three months long phase, after a randomisation, has the aim to identify the relative contribution of each factor (absence of acetate in the bath or BVC) in the treatment tolerance improvement (if any). The treatment tolerance will be evaluated considering the frequency of intradialytic hypotensive events. |
| Outcomes | Primary outcome measures tolerance to dialysis. (time frame: 3 months) The treatment tolerance is measured by the number of intra dialytic hypotensive events Secondary outcome measures The secondary outcome measure is to evaluate the relative efficiency of each factor (AFB in the bath and blood volume control) to reach this result. (time frame: 3 months) The evaluation will be done on: frequency of hypotensive events; number of nurse interventions (defined as ultrafiltration rate stop, or saline infusion); antihypertensive drugs. |
| Starting date | March 2006 |
| Contact information | Dott. Ezio Movilli, Dept of Nephrology ‐Brescia, Italy |
| Notes | Study details as provided by Università deg li Studi di Brescia. source: http://clinicaltrials.gov/ct2/show/NCT01098149 |
| Trial name or title | Tolerance of "on Line" hemodiafiltration in chronic renal failure patients (on‐line‐HDF) |
| Methods | Country: France Enrolment: 600 patients |
| Participants | Ages eligible for study: 65 to 90 years Inclusion criteria: patient who has signed the written consent form; aged > 65 and < 90 years; creatinine clearance < 10 mL/min; on dialysis for a minimum of 3 months; with 3 times/week haemodialysis sessions; erythropoietin dosage needed to maintain haemoglobin at a constant level (range of haemoglobin: 9 to 13 g/dL without any variation of more than 2g/dL for less than 3 months); without any problem of vascular access Exclusion criteria: patient aged < 65 and > 90 years; presence of severe malnutrition (albumin < 20 g/L);, unstable clinical condition; unipuncture or failed vascular access flow; known problems of coagulation |
| Interventions | Active arm: on‐line haemodiafiltration Haemodialysis patients treated with on line HDF technic Procedure: on line haemodiafiltration 3 sessions/week; 3‐4 hours per session Comparator: haemodialysis Haemodialysis patients treated with conventional haemodialysis technic using high‐flux dialyzers Procedure: haemodialysis 3 sessions/week; 3‐4 hours per session; high‐flux dialyzers |
| Outcomes | Primary outcome measures Tolerance of "on line" HDF treatment versus conventional high‐flux haemodialysis in terms of adverse events occurring during dialysis sessions (time frame: between day 30 and day 120 of treatment) Quality of life evaluated with the KDQOL questionnaire (time frame: day 0, 180, 365, 730) measure of pro‐inflammatory cytokines and acute phase reactant proteins measure of oxidative stress markers (AOPP, AGE) and antioxidant systems (vitamin E) |
| Starting date | May 2005 |
| Contact information | Prof Bernard CANAUD, Centre Hospitalier Universitaire Montpellier France Sponsors and Collaborators: University Hospital, Montpellier Ministry of Health, France |
| Notes | Estimated study completion date: December 2012 source: http://clinicaltrials.gov/ct2/results?term=NCT01327391&Search=Search |
| Trial name or title | Acute brain volume changes in haemodialysis: comparison of low flux haemodialysis with pre‐dilution haemodiafiltration |
| Methods | Country: Denmark Enrolment: 12 patients |
| Participants | Ages eligible for study: 18 years and older Inclusion criteria Age ≥ 18 years Informed consent; patient with end‐stage renal disease (ESRD); stabile haemodialysis treatment (Kt/V ≥ 1.3); no contraindications against MRI (pacemaker or other metal implants, claustrophobia, severe adiposity); weight < 140 kg Exclusion criteria Clinical signs of new structural, thromboembolic or vascular brain disease the last 3 month before entering the study; changes in corticosteroid treatment during the last two weeks; change in diuretics during the last two weeks; non‐compliant with regard to salt and fluid intake; acute disease |
| Interventions | Assigned intervention Procedure: HDF during the first examination The patient will receive treatment with pre‐dilution HDF during the first examination. During the second examination the patient will receive treatment with low‐flux hemodialysis. MRI of the brain will be performed before and after the treatment. The MRI‐data will later be processed to determine the degree of brain volume change due to the treatment. Assigned comparison Procedure: HD during the first examination. The patient will receive treatment with low‐flux haemodialysis during the first examination. During the second examination the patient will receive treatment with pre‐dilution hemodiafiltration. MRI of the brain will be performed before and after the treatment. The MRI‐data will later be processed to determine the degree of brain volume change due to the treatment. |
| Outcomes | Primary outcome measures Percent brain volume change (PBVC), brain volume before and after one haemodialysis session (4,5 hours) and one session of HDF (4,5 hours) |
| Starting date | July 2011 |
| Contact information | Study director: Jens D. Jensen, MD, PhD, Department of Renal Medicine C, Aarhus University Hospital, Skejby, Denmark Principal Investigator: Niels Johansen, Department of Renal Medicine C, Aarhus University Hospital, Skejby, Denmark |
| Notes | Study completion date: February 2012 source: http://clinicaltrials.gov/ct2/show/NCT01396863?term=NCT01396863&rank=1 |
| Trial name or title | Solute removal with high volume hemodiafiltration versus long high flux hemodialysis |
| Methods | Country: Belgium Enrolment: 10 patients |
| Participants | Ages eligible for study: 18 years and older Inclusion criteria Chronic kidney disease (CKD) stage 5 with haemodialysis or HDF treatment for more than three months; no vascular access related problems (Arteriovenous (A/V) fistula, graft or bi‐flow catheter); double needle/lumen vascular access; no ongoing infection; signed informed consent form Exclusion criteria Inclusion criteria not met; known HIV or active hepatitis B or C infection (Positive Polymerisation Chain Reaction (PCR)); pregnancy; unstable clinical condition (e.g. cardiac or vascular instability); known coagulation problems; patients participating in another study interfering with the planned study |
| Interventions | Intervention: high volume post dilution HDF This is a prospective cross‐over study including 10 stable haemodialysis patients with chronic kidney disease stage 5. The cross‐over study lasts 2 weeks with the study dialysis sessions at midweek. During one session, the patient will be dialyzed during 4 hours with high volume post dilution haemodiafiltration (HDF) with an FX800 haemodialyser (Fresenius Medical Care) and a blood flow of 300mL/min, dialysate flow of 500mL/min, and substitution flow of 75 mL/min. During the other midweek session, the patient will be dialyzed during 8 hours with high‐flux haemodialysis (HD) with an FX80 haemodialyser (Fresenius Medical Care) and a blood flow of 200mL/min and a dialysate flow of 500mL/min. |
| Outcomes | Primary outcome measures Uraemic retention solute concentrations from pre and post dialysis blood samples, dialyzer inlet and outlet blood samples, and spent dialysate samples. (time frame: during 4 hours) |
| Starting date | April 2012 |
| Contact information | Raymond Vanholder, PhD, MD University Hospital Ghent, Ghent, Belgium, 9000 |
| Notes | Estimated study completion date: December 2012 |
| Trial name or title | Prospective randomized study comparing the hemodiafiltration on‐line and conventional hemodialysis in terms of cost‐benefit |
| Methods | Country: Canada Allocation: randomised Endpoint classification: efficacy study Intervention model: parallel assignment Masking: open label Primary purpose: treatment |
| Participants | Chronic renal failure; haemodialysis |
| Interventions | Active comparator: conventional haemodialysis Active comparator: haemodiafiltration on‐line haemodiafiltration |
| Outcomes | Compare the medication cost between the 2 groups (HD and HDF) (time frame: 3 years) Demonstrate lower cost of erythropoietin in HDF, with same control of anaemia to HD group (time frame: 3 years) Demonstrate lower cost of phosphate binder in HDF, with same control of phospho‐calcium balance to HD group (time frame: 3 years) Demonstrate lower need of erythropoietin and best control of anaemia in HDF (time frame: 3 years) Demonstrate lower need of phosphate binder and best control of phospho‐calcium balance in HDF (time frame: 3 years) Demonstrate less hospitalisation stay and cost related in HDF group (time frame: 3 years) Stabilisation or regression of left ventricular hypertrophy (time frame: 3 years) |
| Starting date | January 2011 |
| Contact information | Renée Lévesque, MD, [email protected] Marie‐Line Caron, B.Sc, marie‐[email protected] |
| Notes | Estimated completion date: June 2016 Source: clinicaltrials.gov/ct2/show/study/NCT02374372 |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 1 All‐cause mortality Show forest plot | 11 | 3396 | Risk Ratio (IV, Random, 95% CI) | 0.87 [0.72, 1.05] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 1.1  Comparison 1 Convection (haemofiltration/haemodiafiltration/acetate‐free biofiltration) versus haemodialysis, Outcome 1 All‐cause mortality. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 2 Cardiovascular mortality Show forest plot | 6 | 2889 | Risk Ratio (IV, Random, 95% CI) | 0.75 [0.61, 0.92] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 1.2  Comparison 1 Convection (haemofiltration/haemodiafiltration/acetate‐free biofiltration) versus haemodialysis, Outcome 2 Cardiovascular mortality. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3 Nonfatal cardiovascular event (rate/person‐years follow‐up) Show forest plot | 1 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 1.3  Comparison 1 Convection (haemofiltration/haemodiafiltration/acetate‐free biofiltration) versus haemodialysis, Outcome 3 Nonfatal cardiovascular event (rate/person‐years follow‐up). | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 4 Hospitalisation Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 1.4  Comparison 1 Convection (haemofiltration/haemodiafiltration/acetate‐free biofiltration) versus haemodialysis, Outcome 4 Hospitalisation. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 4.1 Hospital admissions/year | 1 | 45 | Mean Difference (IV, Random, 95% CI) | 0.20 [‐0.07, 0.47] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 4.2 Days spent in hospital | 2 | 67 | Mean Difference (IV, Random, 95% CI) | ‐1.22 [‐7.47, 5.03] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 5 Hospitalisation (rate/person‐years follow‐up) Show forest plot | 2 | 400 | Risk Ratio (IV, Random, 95% CI) | 1.23 [0.93, 1.63] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 1.5  Comparison 1 Convection (haemofiltration/haemodiafiltration/acetate‐free biofiltration) versus haemodialysis, Outcome 5 Hospitalisation (rate/person‐years follow‐up). | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 6 Change of dialysis modality Show forest plot | 5 | 2919 | Risk Ratio (IV, Random, 95% CI) | 0.87 [0.41, 1.84] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 1.6  Comparison 1 Convection (haemofiltration/haemodiafiltration/acetate‐free biofiltration) versus haemodialysis, Outcome 6 Change of dialysis modality. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 7 Hypotension during dialysis (rate/person‐years follow‐up) Show forest plot | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 1.7
Comparison 1 Convection (haemofiltration/haemodiafiltration/acetate‐free biofiltration) versus haemodialysis, Outcome 7 Hypotension during dialysis (rate/person‐years follow‐up). | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 8 Dialysis sessions with hypotension Show forest plot | 2 | 42 | Mean Difference (IV, Random, 95% CI) | ‐4.05 [‐15.39, 7.30] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 1.8  Comparison 1 Convection (haemofiltration/haemodiafiltration/acetate‐free biofiltration) versus haemodialysis, Outcome 8 Dialysis sessions with hypotension. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 9 Predialysis blood pressure Show forest plot | 7 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 1.9 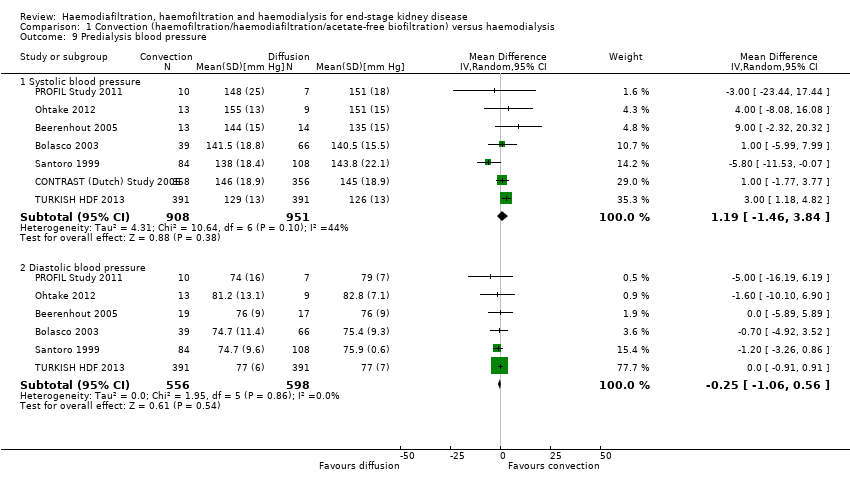 Comparison 1 Convection (haemofiltration/haemodiafiltration/acetate‐free biofiltration) versus haemodialysis, Outcome 9 Predialysis blood pressure. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 9.1 Systolic blood pressure | 7 | 1859 | Mean Difference (IV, Random, 95% CI) | 1.19 [‐1.46, 3.84] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 9.2 Diastolic blood pressure | 6 | 1154 | Mean Difference (IV, Random, 95% CI) | ‐0.25 [‐1.06, 0.56] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 10 Maximal drop in blood pressure during dialysis Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 1.10  Comparison 1 Convection (haemofiltration/haemodiafiltration/acetate‐free biofiltration) versus haemodialysis, Outcome 10 Maximal drop in blood pressure during dialysis. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 10.1 Systolic blood pressure | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 11 Kidney diseases questionnaire and well‐being scores Show forest plot | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 1.11  Comparison 1 Convection (haemofiltration/haemodiafiltration/acetate‐free biofiltration) versus haemodialysis, Outcome 11 Kidney diseases questionnaire and well‐being scores. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 11.1 Inter‐dialysis patient well‐being score | 1 | 67 | Mean Difference (IV, Random, 95% CI) | 0.60 [0.30, 0.90] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 11.2 Physical symptoms | 2 | 121 | Mean Difference (IV, Random, 95% CI) | ‐0.54 [‐1.52, 0.44] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 11.3 Fatigue | 1 | 45 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐0.98, 0.98] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 11.4 Depression | 1 | 45 | Mean Difference (IV, Random, 95% CI) | 0.20 [‐0.50, 0.90] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 11.5 Relationships | 1 | 45 | Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.73, 0.93] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 11.6 Frustration | 1 | 45 | Mean Difference (IV, Random, 95% CI) | ‐0.20 [‐1.61, 1.21] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 12 Kt/V Show forest plot | 14 | 2022 | Mean Difference (IV, Random, 95% CI) | 0.07 [‐0.00, 0.14] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 1.12  Comparison 1 Convection (haemofiltration/haemodiafiltration/acetate‐free biofiltration) versus haemodialysis, Outcome 12 Kt/V. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 13 Urea reduction ratio Show forest plot | 3 | 879 | Std. Mean Difference (IV, Random, 95% CI) | 0.39 [0.06, 0.72] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 1.13  Comparison 1 Convection (haemofiltration/haemodiafiltration/acetate‐free biofiltration) versus haemodialysis, Outcome 13 Urea reduction ratio. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 14 Predialysis serum B2 microglobulin Show forest plot | 12 | 1813 | Mean Difference (IV, Random, 95% CI) | ‐5.55 [‐9.11, ‐1.98] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 1.14  Comparison 1 Convection (haemofiltration/haemodiafiltration/acetate‐free biofiltration) versus haemodialysis, Outcome 14 Predialysis serum B2 microglobulin. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 15 B2 microglobulin clearance Show forest plot | 3 | 65 | Mean Difference (IV, Random, 95% CI) | 13.05 [‐5.94, 32.04] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 1.15  Comparison 1 Convection (haemofiltration/haemodiafiltration/acetate‐free biofiltration) versus haemodialysis, Outcome 15 B2 microglobulin clearance. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 16 Dialysate B2 microglobulin level Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 1.16  Comparison 1 Convection (haemofiltration/haemodiafiltration/acetate‐free biofiltration) versus haemodialysis, Outcome 16 Dialysate B2 microglobulin level. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 17 Data from cross‐over studies Show forest plot | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 1.17
Comparison 1 Convection (haemofiltration/haemodiafiltration/acetate‐free biofiltration) versus haemodialysis, Outcome 17 Data from cross‐over studies. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 17.1 Hospitalisation | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 17.2 Patients experiencing hypotension | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 17.3 Intradialytic hypotensive events | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 17.4 Symptomatic intradialytic hypotensive events | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 17.5 Predialysis systolic blood pressure (mm Hg) | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 17.6 Predialysis diastolic blood pressure (mm Hg) | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 17.7 Predialysis mean arterial pressure (mm Hg) | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 17.8 Postdialysis systolic blood pressure (mm Hg) | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 17.9 Postdialysis diastolic blood pressure (mm Hg) | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 17.10 Postdialysis fall in systolic blood pressure (mm Hg) | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 17.11 Postdialysis mean arterial pressure (mm Hg) | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 17.12 Difference between pre‐ and postdialysis systolic blood pressure (mm Hg) | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 17.13 Difference between pre‐ and postdialysis diastolic blood pressure (mm Hg) | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 17.14 Intradialysis mean systolic blood pressure (mm Hg) | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 17.15 Intradialysis mean diastolic blood pressure (mm Hg) | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 17.16 Intradialysis mean arterial pressure (mm Hg) | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 17.17 Kt/V | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 17.18 Urea reduction ratio | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 17.19 Predialysis serum B2 microglobulin level (mg/L) | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 1 All‐cause mortality Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 2.1  Comparison 2 Convection versus convection (haemofiltration versus haemodiafiltration), Outcome 1 All‐cause mortality. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 2 Predialysis blood pressure Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 2.2  Comparison 2 Convection versus convection (haemofiltration versus haemodiafiltration), Outcome 2 Predialysis blood pressure. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 2.1 Systolic blood pressure | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 2.2 Diastolic blood pressure | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3 Kt/V Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 2.3  Comparison 2 Convection versus convection (haemofiltration versus haemodiafiltration), Outcome 3 Kt/V. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 4 Data from cross‐over studies Show forest plot | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 2.4
Comparison 2 Convection versus convection (haemofiltration versus haemodiafiltration), Outcome 4 Data from cross‐over studies. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 4.1 Days spent in hospital | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 4.2 Average number of episodes of hypotension/patient/month | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 4.3 Number of patients experiencing hypotension | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 4.4 Predialysis systolic blood pressure (mm Hg) | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 4.5 Predialysis diastolic blood pressure (mm Hg) | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 4.6 Predialysis mean arterial pressure (mm Hg) | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 4.7 Postdialysis systolic blood pressure (mm Hg) | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 4.8 Postdialysis diastolic blood pressure (mm Hg) | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 4.9 Postdialysis mean arterial blood pressure (mm Hg) | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 4.10 Number of patients experiencing hypertension | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 4.11 Kt/V | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 4.12 Predialysis serum B2 microglobulin (mg/L) | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 4.13 B2 microglobulin clearance (mL/min) | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 1 Data from cross‐over studies Show forest plot | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 3.1
Comparison 3 Convection versus convection (haemodiafiltration versus acetate‐free biofiltration), Outcome 1 Data from cross‐over studies. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 1.1 Number of hospitalisations/patient during observation period | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 1.2 Length of hospitalisation stay/patient (days/patient) | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 1.3 Number of dialysis sessions with hypotension | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 1.4 Predialysis systolic blood pressure (mm Hg) | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 1.5 Predialysis mean arterial pressure (mm Hg) | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 1.6 Postdialysis systolic blood pressure (mm Hg) | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 1.7 Interdialysis symptom score | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 1.8 Kt/V | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 1.9 Urea reduction ratio | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 1.10 Predialysis B2 microglobulin (mg/L) | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 1.11 Number of dialysis sessions with side effects (nausea, vomiting, headaches) | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||

Study flow diagram.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
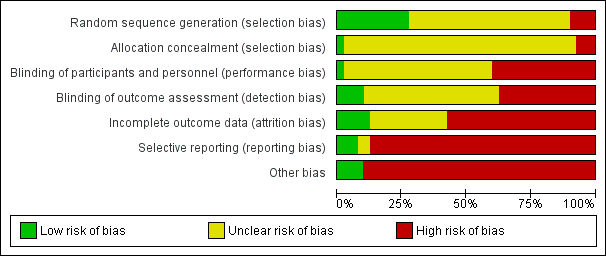
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
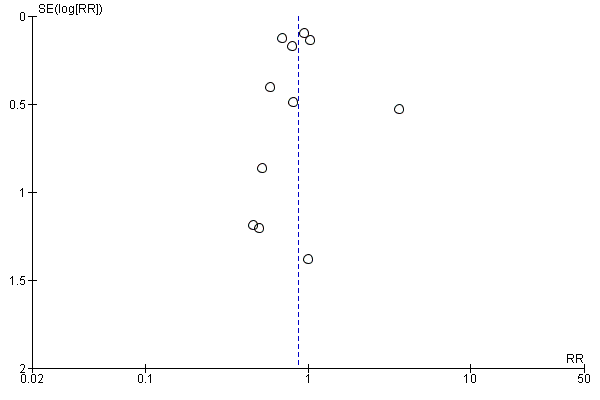
Funnel plot of comparison: 1 Convection (haemofiltration/HDF/acetate‐free biofiltration) versus haemodialysis, outcome: 1.1 All‐cause mortality.

Funnel plot of comparison: 1 Convection (haemofiltration/haemodiafiltration/acetate‐free biofiltration) versus haemodialysis, outcome: 1.2 Cardiovascular mortality.

Funnel plot of comparison: 1 Convection (haemofiltration/HDF/acetate‐free biofiltration) versus haemodialysis, outcome: 1.6 Change of dialysis modality.

Comparison 1 Convection (haemofiltration/haemodiafiltration/acetate‐free biofiltration) versus haemodialysis, Outcome 1 All‐cause mortality.

Comparison 1 Convection (haemofiltration/haemodiafiltration/acetate‐free biofiltration) versus haemodialysis, Outcome 2 Cardiovascular mortality.
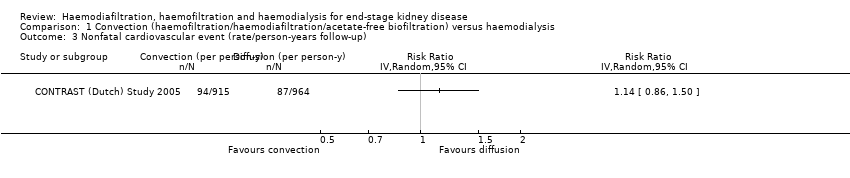
Comparison 1 Convection (haemofiltration/haemodiafiltration/acetate‐free biofiltration) versus haemodialysis, Outcome 3 Nonfatal cardiovascular event (rate/person‐years follow‐up).

Comparison 1 Convection (haemofiltration/haemodiafiltration/acetate‐free biofiltration) versus haemodialysis, Outcome 4 Hospitalisation.

Comparison 1 Convection (haemofiltration/haemodiafiltration/acetate‐free biofiltration) versus haemodialysis, Outcome 5 Hospitalisation (rate/person‐years follow‐up).
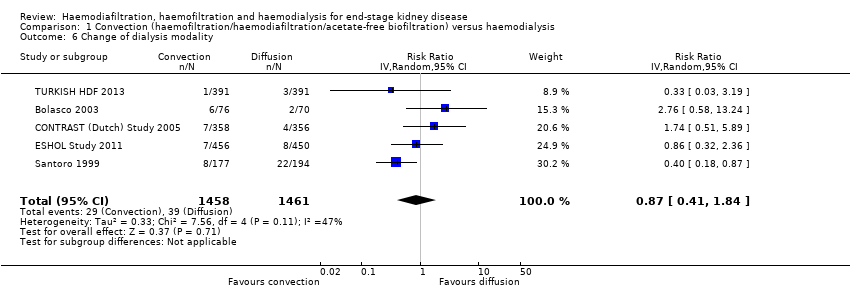
Comparison 1 Convection (haemofiltration/haemodiafiltration/acetate‐free biofiltration) versus haemodialysis, Outcome 6 Change of dialysis modality.
| Study | Treatment effect | No. of participants |
| ESHOL Study 2011 | In this study which reporting the number of hypotensive events/person‐years follow‐up, convective dialysis reduced the rate of hypotension during dialysis (906 participants: RR 0.72, 95% CI 0.66 to 0.80) | 906 |
Comparison 1 Convection (haemofiltration/haemodiafiltration/acetate‐free biofiltration) versus haemodialysis, Outcome 7 Hypotension during dialysis (rate/person‐years follow‐up).

Comparison 1 Convection (haemofiltration/haemodiafiltration/acetate‐free biofiltration) versus haemodialysis, Outcome 8 Dialysis sessions with hypotension.

Comparison 1 Convection (haemofiltration/haemodiafiltration/acetate‐free biofiltration) versus haemodialysis, Outcome 9 Predialysis blood pressure.
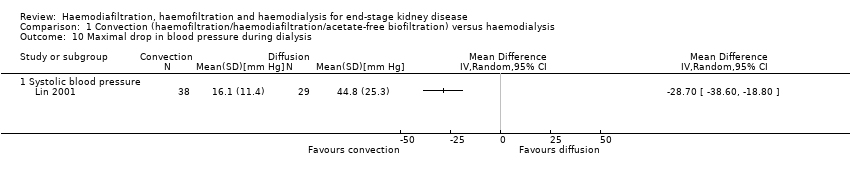
Comparison 1 Convection (haemofiltration/haemodiafiltration/acetate‐free biofiltration) versus haemodialysis, Outcome 10 Maximal drop in blood pressure during dialysis.

Comparison 1 Convection (haemofiltration/haemodiafiltration/acetate‐free biofiltration) versus haemodialysis, Outcome 11 Kidney diseases questionnaire and well‐being scores.

Comparison 1 Convection (haemofiltration/haemodiafiltration/acetate‐free biofiltration) versus haemodialysis, Outcome 12 Kt/V.

Comparison 1 Convection (haemofiltration/haemodiafiltration/acetate‐free biofiltration) versus haemodialysis, Outcome 13 Urea reduction ratio.

Comparison 1 Convection (haemofiltration/haemodiafiltration/acetate‐free biofiltration) versus haemodialysis, Outcome 14 Predialysis serum B2 microglobulin.

Comparison 1 Convection (haemofiltration/haemodiafiltration/acetate‐free biofiltration) versus haemodialysis, Outcome 15 B2 microglobulin clearance.
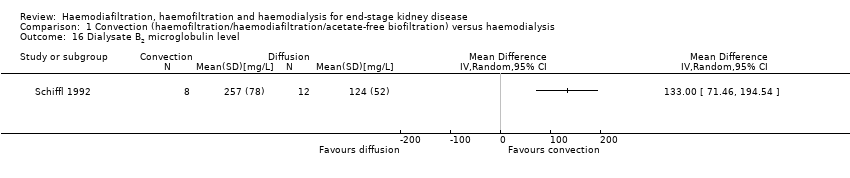
Comparison 1 Convection (haemofiltration/haemodiafiltration/acetate‐free biofiltration) versus haemodialysis, Outcome 16 Dialysate B2 microglobulin level.
| Study | Convective therapy | Diffusive therapy | P value from paper |
| Hospitalisation | |||
| Verzetti 1998 | 8 | 17 | Not reported |
| Patients experiencing hypotension | |||
| Fox 1993 | 1/9 | 0/9 | Not reported |
| Karamperis 2005 | 0/12 | 0/12 | Not significant |
| Pedrini 2011a | 2/62 | 5/62 | Not reported |
| Teo 1987 | 0/10 | 0/10 | Not reported |
| Intradialytic hypotensive events | |||
| Selby 2006a | 23 | 37 | Not significant |
| Stefansson 2012 | 32 dialysis sessions with hypotension from a total of 520 sessions | 28 dialysis sessions with hypotension from a total of 520 sessions | Not significant |
| Symptomatic intradialytic hypotensive events | |||
| Selby 2006a | 2 | 2 | Not significant |
| Predialysis systolic blood pressure (mm Hg) | |||
| Karamperis 2005 | 12 patients | 12 patients | Not significant |
| Noris 1998 | 5 patients | 5 patients | P > 0.05 |
| Pedrini 2011a | 62 patients | 62 patients | P = 0.014 |
| Stefansson 2012 | 20 patients | 20 patients | Not reported |
| Todeschini 2002 | 9 patients | 9 patients | P > 0.05 |
| Predialysis diastolic blood pressure (mm Hg) | |||
| Karamperis 2005 | 12 patients | 12 patients | Not significant |
| Noris 1998 | 5 patients | 5 patients | P > 0.05 |
| Pedrini 2011a | 62 patients | 62 patients | P = 0.05 |
| Stefansson 2012 | 20 patients | 20 patients | Not reported |
| Todeschini 2002 | 9 patients | 9 | P > 0.05 |
| Predialysis mean arterial pressure (mm Hg) | |||
| Karamperis 2005 | 12 patients | 12 patients | Not significant |
| Teo 1987 | 10 patients | 10 patients | "Statistically insignificant" |
| Postdialysis systolic blood pressure (mm Hg) | |||
| Karamperis 2005 | 12 patients | 12 patients | Not significant |
| Noris 1998 | 5 patients | 5 patients | P > 0.05 |
| Pedrini 2011a | 62 patients | 62 patients | "not differ significantly" |
| Stefansson 2012 | 20 patients | 20 patients | Not reported |
| Todeschini 2002 | 9 patients | 9 patients | P > 0.05 |
| Postdialysis diastolic blood pressure (mm Hg) | |||
| Karamperis 2005 | 12 patients | 12 patients | Not significant |
| Pedrini 2011a | 62 patients | 62 patients | "not differ significantly" |
| Stefansson 2012 | 20 patients | 20 patients | Not reported |
| Postdialysis fall in systolic blood pressure (mm Hg) | |||
| Todeschini 2002 | 9 patients | 9 patients | P > 0.05 |
| Postdialysis mean arterial pressure (mm Hg) | |||
| Karamperis 2005 | 12 patients | 12 patients | Not significant |
| Teo 1987 | 10 patients | 10 patients | "Statistically insignificant" |
| Difference between pre‐ and postdialysis systolic blood pressure (mm Hg) | |||
| Noris 1998 | 5 patients | 5 patients | P > 0.05 |
| Difference between pre‐ and postdialysis diastolic blood pressure (mm Hg) | |||
| Noris 1998 | 5 patients | 5 patients | P > 0.05 |
| Todeschini 2002 | 9 patients | 9 patients | P > 0.05 |
| Intradialysis mean systolic blood pressure (mm Hg) | |||
| Selby 2006a | 12 patients | 12 patients | P < 0.0001 |
| Intradialysis mean diastolic blood pressure (mm Hg) | |||
| Selby 2006a | 12 patients | 12 patients | P = 0.005 |
| Intradialysis mean arterial pressure (mm Hg) | |||
| Selby 2006a | 12 patients | 12 patients | P < 0.0001 |
| Teo 1987 | 10 patients | 10 patients | "statistically insignificant decrease" |
| Kt/V | |||
| Basile 2001 | 10 patients | 10 patients | No significant difference |
| Kantartzi 2013 | 48 patients | 48 patients | P = 0.33 |
| Karamperis 2005 | 12 patients | 12 patients | No significant difference |
| Noris 1998 | 5 patients | 5 patients | P > 0.05 |
| Pedrini 2011a | 62 patients Mean (± SE): 1.60 (0.31) | 62 patients Mean (± SE): 1.44 (0.26) | P < 0.0001 |
| Righetti 2010 | 24 patients | 24 patients | P < 0.01 |
| Selby 2006a | 12 patients Mean (± SE): 1.37 (0.28) | 12 patients Mean (± SE): 1.38 (0.32) | P = 0.91 |
| Stefansson 2012 | 20 patients | 20 patients | Not reported |
| Todeschini 2002 | 9 patients | 9 patients | P > 0.05 |
| Tuccillo 2002 | 12 patients | 12 patients | P > 0.05 |
| Urea reduction ratio | |||
| Righetti 2010 | 24 patients | 24 patients | P < 0.01 |
| Predialysis serum B2 microglobulin level (mg/L) | |||
| Kantartzi 2013 | 48 patients | 48 patients | P < 0.01 |
| Pedrini 2011a | 62 patients | 62 patients | P < 0.0001 |
| Righetti 2010 | 24 patients | 24 patients | P < 0.01 |
| Stefansson 2012 | 20 patients | 20 patients | Not reported |
Comparison 1 Convection (haemofiltration/haemodiafiltration/acetate‐free biofiltration) versus haemodialysis, Outcome 17 Data from cross‐over studies.

Comparison 2 Convection versus convection (haemofiltration versus haemodiafiltration), Outcome 1 All‐cause mortality.

Comparison 2 Convection versus convection (haemofiltration versus haemodiafiltration), Outcome 2 Predialysis blood pressure.

Comparison 2 Convection versus convection (haemofiltration versus haemodiafiltration), Outcome 3 Kt/V.
| Study | Haemodiafiltraton | Haemofiltration | P value from paper |
| Days spent in hospital | |||
| Altieri 2004 | 30 patients | 30 patients | Not significant |
| Average number of episodes of hypotension/patient/month | |||
| Altieri 2004 | 30 patients | 30 patients | P = 0.0169 |
| Number of patients experiencing hypotension | |||
| Altieri 2004 | 2/30 | 0/30 | P > 0.05 |
| Predialysis systolic blood pressure (mm Hg) | |||
| Altieri 2004 | 30 patients | 30 patients | P = 0.044 |
| Predialysis diastolic blood pressure (mm Hg) | |||
| Altieri 2004 | 30 patients | 30 patients | P > 0.05 |
| Predialysis mean arterial pressure (mm Hg) | |||
| Altieri 2004 | 30 patients | 30 patients | P > 0.05 |
| Postdialysis systolic blood pressure (mm Hg) | |||
| Altieri 2004 | 30 patients | 30 patients | P > 0.05 |
| Postdialysis diastolic blood pressure (mm Hg) | |||
| Altieri 2004 | 30 patients | 30 patients Mean (± SD): 74.5 (7.9) | P > 0.05 |
| Postdialysis mean arterial blood pressure (mm Hg) | |||
| Altieri 2004 | 30 patients | 30 patients | P > 0.05 |
| Number of patients experiencing hypertension | |||
| Altieri 2004 | 6/30 | 7/30 | P > 0.05 |
| Kt/V | |||
| Altieri 2004 | 30 patients | 30 patients | P < 0.001 |
| Predialysis serum B2 microglobulin (mg/L) | |||
| Altieri 2004 | 30 patients | 30 patients | Not significant |
| B2 microglobulin clearance (mL/min) | |||
| Meert 2009 | 14 patients | 14 patients | P < 0.017 |
Comparison 2 Convection versus convection (haemofiltration versus haemodiafiltration), Outcome 4 Data from cross‐over studies.
| Study | Haemodiafiltration | Acid‐free biofiltration | P value from paper |
| Number of hospitalisations/patient during observation period | |||
| Movilli 1996 | 12 patients | 12 patients | Not significant |
| Length of hospitalisation stay/patient (days/patient) | |||
| Movilli 1996 | 12 patients | 12 patients | Not significant |
| Number of dialysis sessions with hypotension | |||
| Coll 2009 | 21 patients 7/545 sessions | 21 patients 46/545 sessions | "On‐line HDF was associated with fewer hypotensive episodes than treatment with on‐line HDF without acetate (P=0.019)" |
| Movilli 1996 | 12 patients | 12 patients | Not significant |
| Predialysis systolic blood pressure (mm Hg) | |||
| Ding 2002 | 9 patients | 9 patients | Not significant |
| Predialysis mean arterial pressure (mm Hg) | |||
| Ding 2002 | 9 patients | 9 patients | Not reported |
| Postdialysis systolic blood pressure (mm Hg) | |||
| Ding 2002 | 9 patients | 9 patients | Not significant |
| Interdialysis symptom score | |||
| Ding 2002 | 9 patients | 9 patients | Not significant |
| Kt/V | |||
| Movilli 1996 | 12 patients | 12 patients | Not significant |
| Urea reduction ratio | |||
| Ding 2002 | 9 patients | 9 patients | Not significant |
| Predialysis B2 microglobulin (mg/L) | |||
| Coll 2009 | 21 patients Mean (± SD): 27.7 (7.2) | 21 patients Mean (± SD): 27.4 (6.7) | Not significant |
| Ding 2002 | 9 patients | 9 patients | Not significant |
| Number of dialysis sessions with side effects (nausea, vomiting, headaches) | |||
| Movilli 1996 | 12 patients | 12 patients | Not significant |
Comparison 3 Convection versus convection (haemodiafiltration versus acetate‐free biofiltration), Outcome 1 Data from cross‐over studies.
| Convective compared with diffusive dialysis modalities for men and women with end‐stage kidney disease | ||||||
| Patient or population: men and women with end‐stage kidney disease Intervention: convective dialysis Comparison: diffusive dialysis | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Diffusion | Convection | |||||
| All‐cause mortality | 200 per 1000 | Not significant | RR 0.87 (0.72 to 1.05) | 11 (3396) | ⊕⊕⊝⊝ | Convective therapy has little or no effect on all‐cause mortality |
| Cardiovascular mortality | 100 per 1000 | 75 per 1000 | RR 0.75 (0.81 to 0.92) | 6 (2889) | ⊕⊕⊝⊝ | Convective therapy may reduce cardiovascular mortality |
| Nonfatal cardiovascular events | 130 per 1000 | Not significant | RR 1.23 (0.93‐1.63) | 2 (1688) | ⊕⊝⊝⊝ | Convective therapy has uncertain effects on non‐fatal cardiovascular events |
| Health‐related quality of life | Not estimable | Not estimable | Not estimable | 8 (988) | ⊕⊕⊝⊝ | Convective therapy has uncertain effects on health‐related quality of life |
| *The assumed risk (e.g. the median control group risk across studies) is derived from data within dialysis registries for all‐cause mortality and cardiovascular mortality and the reported event rate in the available study for nonfatal cardiovascular events (CONTRAST (Dutch) Study 2005). The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk Ratio | ||||||
| GRADE (Grading of Recommendations Assessment, Development, and Evaluation) Working Group grades of evidence (Guyatt 2011). | ||||||
| Study ID | Intervention | Duration | Number of patients |
| HDF versus HF | 12 months | 39 | |
| HDF versus HD | 2 weeks | 14 | |
| AFB versus HD | 12 months | 11 | |
| HF versus HD | 12 months | 40 | |
| HF versus HDF versus HD | 18 months | 146 | |
| AFB versus HDF | 15 months | 30 | |
| HDF versus HD | 36 months | 714 | |
| HDF versus HD | 1 session | 12 | |
| HDF versus AFB | 36 weeks | 12 | |
| AFB versus HD | 12 months | 20 | |
| HDF versus HD | 36 months | 906 | |
| HF versus HD | 1 session | 9 | |
| HDF versus HD | 3 months | 24 | |
| HDF versus HD | 2 sessions | 12 | |
| HDF versus HD | 15 months | 67 | |
| HDF versus HD | 24 months | 205 | |
| HDF versus HD | 1 session | 8 | |
| HDF versus HD | 6 weeks | 8 | |
| HDF versus HF | 9 weeks | 14 | |
| HDF versus AFB | 6 months | 12 | |
| AFB versus HD | 1 week | 5 | |
| HDF versus HD | 12 months | 22 | |
| HDF versus HD | 12 months | 69 | |
| HF versus HD | 24 months | 48 | |
| HDF versus HD | 18 months | 24 | |
| AFB versus HD | 48 months | 371 | |
| HF versus HD | 36 months | 64 | |
| HF versus HD | 48 months | 32 | |
| HDF versus HD | 48 months | 76 | |
| AFB versus HD | 12 months | 24 | |
| AFB versus HD | 4 weeks | 12 | |
| HDF versus HD | 4 months | 20 | |
| HDF versus HD | 8 months | 13 | |
| AFB versus HD | 3 sessions | 9 | |
| HDF versus HD | 3 months | 12 | |
| HDF versus HD | 24 months | 782 | |
| HDF versus HD | 48 weeks | 129 | |
| AFB versus HD | 12 months | 41 | |
| HDF versus HD | 12 months | 50 | |
| HDF versus HD | 24 months | 44 | |
| AFB ‐ acetate‐free biofiltration; HDF ‐ haemodiafiltration; HD ‐ haemodialysis; HF ‐ haemofiltration | |||
| Categories of intervention | Study | Total number of studies | Total number of patients |
| HDF versus HD | Bammens 2004; Lin 2001; Locatelli 1994; Lornoy 1998; Teo 1987; Tuccillo 2002; Ward 2000; Wizemann 2000; Bolasco 2003; CONTRAST (Dutch) Study 2005; Cristofano 2004; Karamperis 2005; Mandolfo 2008; Pedrini 2011a; Righetti 2010; Schiffl 2007; Stefansson 2012; TURKISH HDF 2013; Vaslaki 2006ESHOL Study 2011; Kantartzi 2013; Ohtake 2012 | 22 | 3299 |
| HF versus HD | Beerenhout 2005; Fox 1993; Schiffl 1992; Bolasco 2003; Santoro 2005a; PROFIL Study 2011 | 6 | 325 |
| AFB versus HD | Basile 2001; Santoro 1999; Eiselt 2000; Noris 1998; Schrander vd Meer 1998; Selby 2006a; Todeschini 2002; Verzetti 1998 | 8 | 487 |
| HDF versus AFB | 3 | 59 | |
| HDF versus HF | 3 | 199 | |
| More than two treatment arms | 3 | 383 | |
| AFB ‐ acetate‐free biofiltration; HDF ‐ haemodiafiltration; HD ‐ haemodialysis; HF ‐ haemofiltration | |||
| Study ID | Comparison | Quality of Life scale used | Time of assessment | End of study result | Selective reporting of quality of life dimensions |
| HF versus HD | Kidney Disease Questionnaire | Before randomisation, at 6 months and at 1 year | No significant difference in scores in all five components of the scoring system between interventions | Yes | |
| HDF versus HD | Kidney Disease Quality of Life‐Short Form | Median follow‐up | There were no significant differences in changes in health‐related quality of life over time between groups (generic or kidney‐disease specific domains) | No | |
| HDF versus HD | SF‐36 | At 3 months | There were statistical significant differences in QoL for the total SF‐36 (36.1 (26.7 to 45.7) and 40.7 (30.2 to 62.8)), for classic low‐flux HD and high‐flux HDF, for bodily pain (45 (26.9 to 66.9) and 55 (35.6 to 87.5)), and No data were available for the end of the first phase of treatment | No | |
| HDF versus HD | Patient well‐being score | Once weekly for 15 months | Patients on HDF had significantly better scores ((physical well‐being score) MD 0.60, 95% CI 0.30 to 0.90). | No | |
| HDF versus HD | Kidney Disease | after 52 weeks | None of the other dimensions of the KDQ showed a change during the course of the study No data were available for the end of the first phase of treatment | Yes | |
| HDF versus HD | Physical functioning domain of IQOLA SF‐36 questionnaire | At day 60 | With the exception of a lower score for social functioning with HDF (P < 0.05), there was no significant difference in quality of life between HD and HDF No data were available for the end of the first phase of treatment | No | |
| AFB | Subjective well‐being | Monthly | Reported well‐being significantly higher in patients receiving AFB in multivariate analysis although unclear whether between‐groups comparison was reported No data were available for the end of the first phase of treatment | No | |
| HDF versus HD | Kidney Disease Questionnaire | At 6 months and 1 year | No significant difference in scores in all five components of the scoring system between interventions | No | |
| AFB ‐ acetate‐free biofiltration; HDF ‐ haemodiafiltration; HD ‐ haemodialysis; HF ‐ haemofiltration; SF‐36 ‐ Short‐Form Health Survey with 36 questions | |||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All‐cause mortality Show forest plot | 11 | 3396 | Risk Ratio (IV, Random, 95% CI) | 0.87 [0.72, 1.05] |
| 2 Cardiovascular mortality Show forest plot | 6 | 2889 | Risk Ratio (IV, Random, 95% CI) | 0.75 [0.61, 0.92] |
| 3 Nonfatal cardiovascular event (rate/person‐years follow‐up) Show forest plot | 1 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 4 Hospitalisation Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 Hospital admissions/year | 1 | 45 | Mean Difference (IV, Random, 95% CI) | 0.20 [‐0.07, 0.47] |
| 4.2 Days spent in hospital | 2 | 67 | Mean Difference (IV, Random, 95% CI) | ‐1.22 [‐7.47, 5.03] |
| 5 Hospitalisation (rate/person‐years follow‐up) Show forest plot | 2 | 400 | Risk Ratio (IV, Random, 95% CI) | 1.23 [0.93, 1.63] |
| 6 Change of dialysis modality Show forest plot | 5 | 2919 | Risk Ratio (IV, Random, 95% CI) | 0.87 [0.41, 1.84] |
| 7 Hypotension during dialysis (rate/person‐years follow‐up) Show forest plot | Other data | No numeric data | ||
| 8 Dialysis sessions with hypotension Show forest plot | 2 | 42 | Mean Difference (IV, Random, 95% CI) | ‐4.05 [‐15.39, 7.30] |
| 9 Predialysis blood pressure Show forest plot | 7 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 9.1 Systolic blood pressure | 7 | 1859 | Mean Difference (IV, Random, 95% CI) | 1.19 [‐1.46, 3.84] |
| 9.2 Diastolic blood pressure | 6 | 1154 | Mean Difference (IV, Random, 95% CI) | ‐0.25 [‐1.06, 0.56] |
| 10 Maximal drop in blood pressure during dialysis Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 10.1 Systolic blood pressure | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 11 Kidney diseases questionnaire and well‐being scores Show forest plot | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 11.1 Inter‐dialysis patient well‐being score | 1 | 67 | Mean Difference (IV, Random, 95% CI) | 0.60 [0.30, 0.90] |
| 11.2 Physical symptoms | 2 | 121 | Mean Difference (IV, Random, 95% CI) | ‐0.54 [‐1.52, 0.44] |
| 11.3 Fatigue | 1 | 45 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐0.98, 0.98] |
| 11.4 Depression | 1 | 45 | Mean Difference (IV, Random, 95% CI) | 0.20 [‐0.50, 0.90] |
| 11.5 Relationships | 1 | 45 | Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.73, 0.93] |
| 11.6 Frustration | 1 | 45 | Mean Difference (IV, Random, 95% CI) | ‐0.20 [‐1.61, 1.21] |
| 12 Kt/V Show forest plot | 14 | 2022 | Mean Difference (IV, Random, 95% CI) | 0.07 [‐0.00, 0.14] |
| 13 Urea reduction ratio Show forest plot | 3 | 879 | Std. Mean Difference (IV, Random, 95% CI) | 0.39 [0.06, 0.72] |
| 14 Predialysis serum B2 microglobulin Show forest plot | 12 | 1813 | Mean Difference (IV, Random, 95% CI) | ‐5.55 [‐9.11, ‐1.98] |
| 15 B2 microglobulin clearance Show forest plot | 3 | 65 | Mean Difference (IV, Random, 95% CI) | 13.05 [‐5.94, 32.04] |
| 16 Dialysate B2 microglobulin level Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 17 Data from cross‐over studies Show forest plot | Other data | No numeric data | ||
| 17.1 Hospitalisation | Other data | No numeric data | ||
| 17.2 Patients experiencing hypotension | Other data | No numeric data | ||
| 17.3 Intradialytic hypotensive events | Other data | No numeric data | ||
| 17.4 Symptomatic intradialytic hypotensive events | Other data | No numeric data | ||
| 17.5 Predialysis systolic blood pressure (mm Hg) | Other data | No numeric data | ||
| 17.6 Predialysis diastolic blood pressure (mm Hg) | Other data | No numeric data | ||
| 17.7 Predialysis mean arterial pressure (mm Hg) | Other data | No numeric data | ||
| 17.8 Postdialysis systolic blood pressure (mm Hg) | Other data | No numeric data | ||
| 17.9 Postdialysis diastolic blood pressure (mm Hg) | Other data | No numeric data | ||
| 17.10 Postdialysis fall in systolic blood pressure (mm Hg) | Other data | No numeric data | ||
| 17.11 Postdialysis mean arterial pressure (mm Hg) | Other data | No numeric data | ||
| 17.12 Difference between pre‐ and postdialysis systolic blood pressure (mm Hg) | Other data | No numeric data | ||
| 17.13 Difference between pre‐ and postdialysis diastolic blood pressure (mm Hg) | Other data | No numeric data | ||
| 17.14 Intradialysis mean systolic blood pressure (mm Hg) | Other data | No numeric data | ||
| 17.15 Intradialysis mean diastolic blood pressure (mm Hg) | Other data | No numeric data | ||
| 17.16 Intradialysis mean arterial pressure (mm Hg) | Other data | No numeric data | ||
| 17.17 Kt/V | Other data | No numeric data | ||
| 17.18 Urea reduction ratio | Other data | No numeric data | ||
| 17.19 Predialysis serum B2 microglobulin level (mg/L) | Other data | No numeric data | ||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All‐cause mortality Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2 Predialysis blood pressure Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.1 Systolic blood pressure | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Diastolic blood pressure | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Kt/V Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4 Data from cross‐over studies Show forest plot | Other data | No numeric data | ||
| 4.1 Days spent in hospital | Other data | No numeric data | ||
| 4.2 Average number of episodes of hypotension/patient/month | Other data | No numeric data | ||
| 4.3 Number of patients experiencing hypotension | Other data | No numeric data | ||
| 4.4 Predialysis systolic blood pressure (mm Hg) | Other data | No numeric data | ||
| 4.5 Predialysis diastolic blood pressure (mm Hg) | Other data | No numeric data | ||
| 4.6 Predialysis mean arterial pressure (mm Hg) | Other data | No numeric data | ||
| 4.7 Postdialysis systolic blood pressure (mm Hg) | Other data | No numeric data | ||
| 4.8 Postdialysis diastolic blood pressure (mm Hg) | Other data | No numeric data | ||
| 4.9 Postdialysis mean arterial blood pressure (mm Hg) | Other data | No numeric data | ||
| 4.10 Number of patients experiencing hypertension | Other data | No numeric data | ||
| 4.11 Kt/V | Other data | No numeric data | ||
| 4.12 Predialysis serum B2 microglobulin (mg/L) | Other data | No numeric data | ||
| 4.13 B2 microglobulin clearance (mL/min) | Other data | No numeric data | ||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Data from cross‐over studies Show forest plot | Other data | No numeric data | ||
| 1.1 Number of hospitalisations/patient during observation period | Other data | No numeric data | ||
| 1.2 Length of hospitalisation stay/patient (days/patient) | Other data | No numeric data | ||
| 1.3 Number of dialysis sessions with hypotension | Other data | No numeric data | ||
| 1.4 Predialysis systolic blood pressure (mm Hg) | Other data | No numeric data | ||
| 1.5 Predialysis mean arterial pressure (mm Hg) | Other data | No numeric data | ||
| 1.6 Postdialysis systolic blood pressure (mm Hg) | Other data | No numeric data | ||
| 1.7 Interdialysis symptom score | Other data | No numeric data | ||
| 1.8 Kt/V | Other data | No numeric data | ||
| 1.9 Urea reduction ratio | Other data | No numeric data | ||
| 1.10 Predialysis B2 microglobulin (mg/L) | Other data | No numeric data | ||
| 1.11 Number of dialysis sessions with side effects (nausea, vomiting, headaches) | Other data | No numeric data | ||

