Bifosfonatos para el cáncer de próstata avanzado
Referencias
Referencias de los estudios incluidos en esta revisión
Referencias de los estudios excluidos de esta revisión
Referencias adicionales
Referencias de otras versiones publicadas de esta revisión
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | Recruitment period:
End points:
Pain assessment tool:
Randomization:
| |
| Participants | Eligibility criteria:
Exclusion criteria:
Participants randomized:
Mean age:
Country of participants:
| |
| Interventions | Previous interventions:
Interventions during study period:
| |
| Outcomes | Reported and analyzed in this review:
| |
| Funding sources | Funding sources:
| |
| Declarations of interest | Conflicts of interest: see meeting.ascopubs.org/cgi/content/abstract/24/18_suppl/4638?sid=2d509f53‐6021‐4c00‐8de9‐6bbec9c7cf92.
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information on sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information on allocation concealment. |
| Blinding of participants and personnel (performance bias) | Low risk | Placebo‐controlled trial. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information on outcome assessment. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information on outcome assessment. |
| Incomplete outcome data (attrition bias) | Unclear risk | Information regarding discontinuations and ITT. |
| Selective reporting (reporting bias) | Unclear risk | No study protocol available. |
| Other bias | Unclear risk | Insufficient report on methods. |
| Methods | Recruitment period:
End points:
Pain assessment tool:
Randomization:
| |
| Participants | Eligibility criteria:
Exclusion criteria:
Participants randomized:
Mean age:
Country of participants:
| |
| Interventions | Previous interventions:
Interventions during study period:
| |
| Outcomes | Reported and analyzed in this review:
| |
| Funding sources | Funding sources:
| |
| Declarations of interest | Conflicts of interest:
| |
| Notes | Prematurely completed after corporate supporter withdrew study drug supply. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomized block design was used." |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information on allocation concealment. |
| Blinding of participants and personnel (performance bias) | Low risk | Quote from protocol: "Masking: Double Blind (Subject, Caregiver, Investigator, Outcomes Assessor)." |
| Blinding of outcome assessment (detection bias) | Low risk | Quote from protocol: "Masking: Double Blind (Subject, Caregiver, Investigator, Outcomes Assessor)." |
| Blinding of outcome assessment (detection bias) | Low risk | Quote from protocol: "Masking: Double Blind (Subject, Caregiver, Investigator, Outcomes Assessor)." |
| Incomplete outcome data (attrition bias) | Low risk | All participants with bone metastasis from prostate cancer were included in the analysis of efficacy and all participants on treatment were used for analysis of safety. |
| Selective reporting (reporting bias) | Low risk | Report on every end point (primary and secondary) mentioned in the original protocol. |
| Other bias | Low risk | No further information provided. |
| Methods | Recruitment period:
End points:
Pain assessment tool:
Randomization:
| |
| Participants | Eligibility criteria:
Exclusion criteria:
Participants randomized:
Mean age:
Country of participants:
| |
| Interventions | Previous interventions:
Interventions during study period:
| |
| Outcomes | Reported and analyzed in this review:
| |
| Funding sources | Funding sources:
| |
| Declarations of interest | Conflicts of interest:
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information on sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information on allocation concealment. |
| Blinding of participants and personnel (performance bias) | Low risk | Placebo‐controlled trial. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information on outcome assessment. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information on outcome assessment. |
| Incomplete outcome data (attrition bias) | Unclear risk | No information regarding discontinuations and ITT. |
| Selective reporting (reporting bias) | Unclear risk | No study protocol available. |
| Other bias | Unclear risk | Quote from the article: "We are grateful to the Finnish Cancer Foundation and to Leiras Pharmaceutical Company for their support of this work." |
| Methods | Recruitment period:
End points:
Pain assessment tool:
Randomization:
| |
| Participants | Eligibility criteria:
Exclusion criteria:
Participants randomized:
Median age:
Country of participants:
| |
| Interventions | Previous interventions:
Interventions during study period:
| |
| Outcomes | Reported and analyzed in this review:
| |
| Funding sources | Funding sources:
| |
| Declarations of interest | Conflicts of interest:
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were randomly assigned using a block‐randomization procedure with equal probability of assignment to either arm." |
| Allocation concealment (selection bias) | Low risk | "Patients were randomly assigned using a block‐randomization procedure with equal probability of assignment to either arm." |
| Blinding of participants and personnel (performance bias) | Low risk | "The treating staff and patients were blinded to treatment allocation." |
| Blinding of outcome assessment (detection bias) | Low risk | "The treating staff and patients were blinded to treatment allocation." |
| Blinding of outcome assessment (detection bias) | Low risk | "The treating staff and patients were blinded to treatment allocation." |
| Incomplete outcome data (attrition bias) | Unclear risk | Insufficient information regarding discontinuations and ITT. |
| Selective reporting (reporting bias) | Unclear risk | Protocol available (NCT00003232), but outcomes not prespecified in the protocol. |
| Other bias | Unclear risk | Quote: "Supported by a grant from Immunex Corporation, Seattle, WA, and Aventis Pharma, Laval, Quebec, Canada." |
| Methods | Recruitment period:
End points:
Pain assessment tool:
Randomization:
| |
| Participants | Eligibility criteria:
Exclusion criteria:
Participants randomized:
Mean age:
Country of participants:
| |
| Interventions | Previous interventions:
Interventions during study period:
| |
| Outcomes | Reported and analyzed in this review:
| |
| Funding sources | Funding sources:
| |
| Declarations of interest | Conflicts of interest:
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information on sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information on allocation concealment. |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "This was an open label, randomized, phase II study [...]" |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information on blinding of outcome assessor. |
| Blinding of outcome assessment (detection bias) | High risk | Quote: "This was an open label, randomized, phase II study [...]" |
| Incomplete outcome data (attrition bias) | Low risk | Complete analysis of all randomized participants. |
| Selective reporting (reporting bias) | Unclear risk | Protocol available (NCT00019695), more outcomes reported than prespecified in the protocol (e.g. overall survival). |
| Other bias | Low risk | No further information provided. |
| Methods | Recruitment period:
End points:
Pain assessment tool:
Randomization:
| |
| Participants | Eligibility criteria:
Exclusion criteria:
Participants randomized:
Mean age:
Country of participants:
| |
| Interventions | Previous interventions
Interventions during study period:
| |
| Outcomes | Reported and analyzed in this review:
| |
| Funding sources | Funding sources:
| |
| Declarations of interest | Conflicts of interest:
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information on sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information on allocation concealment. |
| Blinding of participants and personnel (performance bias) | Low risk | Placebo‐controlled trial. |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information on blinding of outcome assessor. |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information on blinding of outcome assessor. |
| Incomplete outcome data (attrition bias) | Unclear risk | Complete analysis of all randomized participants. |
| Selective reporting (reporting bias) | Unclear risk | Protocol available (NCT00019695), more outcomes reported than prespecified in the protocol (e.g. overall survival). |
| Other bias | Unclear risk | No information provided. |
| Methods | Recruitment period:
End points:
Pain assessment tool:
Randomization:
| |
| Participants | Eligibility criteria:
Exclusion criteria:
Participants randomized:
Mean age:
Country of participants:
| |
| Interventions | Previous interventions:
Interventions during study period:
| |
| Outcomes | Reported and analyzed in this review:
| |
| Funding sources | Funding sources:
| |
| Declarations of interest | Conflicts of interest:
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information on sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information on allocation concealment. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Insufficient information provided. |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information on blinding of investigated outcome. |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information on blinding of investigated outcome. |
| Incomplete outcome data (attrition bias) | Unclear risk | No information regarding discontinuations and ITT. |
| Selective reporting (reporting bias) | Unclear risk | No study protocol available. |
| Other bias | Unclear risk | Quote from the article: "We are grateful to [...] the Finnish Cancer Foundation and to Leiras Pharmaceutical Company for their support of this study." |
| Methods | Recruitment period:
End points:
Pain assessment tool:
Randomization:
| |
| Participants | Eligibility criteria:
Exclusion criteria:
Participants randomized:
Mean age:
Country of participants:
| |
| Interventions | Previous interventions:
Interventions during study period:
| |
| Outcomes | Reported and analyzed in this review:
| |
| Funding sources | Funding sources:
| |
| Declarations of interest | Conflicts of interest:
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information on sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information on allocation concealment. |
| Blinding of participants and personnel (performance bias) | Low risk | Placebo‐controlled trial. |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information on blinding of investigated outcome. |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information on blinding of investigated outcome. |
| Incomplete outcome data (attrition bias) | Unclear risk | No information regarding discontinuations and ITT. |
| Selective reporting (reporting bias) | Unclear risk | No study protocol available. |
| Other bias | Unclear risk | Quote: "This study was supported by the Finnish Academy of Sciences, Finnish Cancer Foundation, Finnish Medical Society Duodecim, Reino Lathikari Foundation and by Leiras Clinical Research." |
| Methods | Recruitment period:
End points:
Pain assessment tool:
Randomization:
| |
| Participants | Eligibility criteria:
Exclusion criteria:
Participants randomized:
Mean age:
Country of participants:
| |
| Interventions | Previous interventions:
Interventions during study period:
| |
| Outcomes | Reported and analyzed in this review:
| |
| Funding sources | Funding sources:
| |
| Declarations of interest | Conflicts of interest:
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information on sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information on allocation concealment. |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "This randomised, open label, phase II/III trial [...]." |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: "This randomised, open label, phase II/III trial [...]." |
| Blinding of outcome assessment (detection bias) | High risk | Quote: "This randomised, open label, phase II/III trial [...]." |
| Incomplete outcome data (attrition bias) | Low risk | All participants with bone metastasis from prostate cancer were included in the analysis of efficacy and safety. |
| Selective reporting (reporting bias) | Low risk | Protocol available (ISRCTN22844568), prespecified outcomes reported. |
| Other bias | Unclear risk | Quote: "Funding was provided by Grants from Sanofi‐Aventis." |
| Methods | Recruitment period:
End points:
Pain assessment tool:
Randomization:
| |
| Participants | Eligibility criteria:
Exclusion criteria:
Participants randomized:
Mean age:
Country of participants:
| |
| Interventions | Previous interventions:
Interventions during study period:
| |
| Outcomes | Reported and analyzed in this review:
| |
| Funding sources | Funding sources:
| |
| Declarations of interest | Conflicts of interest:
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient report on sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information on allocation concealment. |
| Blinding of participants and personnel (performance bias) | Low risk | Placebo‐controlled trial. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient report on blinding of outcome. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient report on blinding of outcome. |
| Incomplete outcome data (attrition bias) | Low risk | No participants lost to follow‐up. All participants were included in the ITT analysis. |
| Selective reporting (reporting bias) | Unclear risk | No study protocol available. |
| Other bias | Low risk | No further information provided. |
| Methods | Recruitment period:
End points:
Pain assessment tool:
Randomization:
| |
| Participants | Eligibility criteria:
Exclusion criteria:
Participants randomized:
Median age:
Country of participants:
| |
| Interventions | Previous interventions:
Interventions during study period:
| |
| Outcomes | Reported and analyzed in this review:
| |
| Funding sources | Quote: "This trial was sponsored by the U.K. Medical Research Council (MRC)." "The trial was initiated with the support of Boehringer Mannheim. The company provided trial tablets (Loron 520 and matching placebo) free of charge, plus financial support (£250) on a per patient basis, which was sufficient to contribute toward the administrative costs of the trial. The financial support was distributed proportionately between the participating clinicians and the coordinating center [...] During the trial, Boehringer Mannheim was taken over by Roche Products Ltd., which honored all commitments regarding this trial." | |
| Declarations of interest | Insufficient report on potential conflicts of interest. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information on sequence generation. |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomization was performed centrally at the MRC CTU [...] No patient information, other than their drug number and hospital, was revealed to the pharmaceutical companies." |
| Blinding of participants and personnel (performance bias) | Low risk | Placebo‐controlled trial. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information on blinding of outcome assessment. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information on blinding of outcome assessment. |
| Incomplete outcome data (attrition bias) | Low risk | All participants were included in the ITT analysis. |
| Selective reporting (reporting bias) | Low risk | Study protocol available. All prespecified outcomes were reported. |
| Other bias | Unclear risk | Quote: "The trial was initiated with the support of Boehringer Mannheim. The company provided trial tablets (Loron 520 and matching placebo) free of charge, plus financial support (£250) on a per patient basis, which was sufficient to contribute toward the administrative costs of the trial. The financial support was distributed proportionately between the participating clinicians and the coordinating center.[...] During the trial, Boehringer Mannheim was taken over by Roche Products Ltd., which honored all commitments regarding this trial." |
| Methods | Recruitment period:
End points:
Pain assessment tool:
Randomization:
| |
| Participants | Eligibility criteria:
Exclusion criteria:
Participants randomized:
Mean age:
Country of participants:
| |
| Interventions | Previous interventions:
Interventions during study period:
| |
| Outcomes | Reported and analyzed in this review:
| |
| Funding sources | Quote: "Supported by a grant from Novartis Pharmaceuticals Corporation, East Hanover, NJ." | |
| Declarations of interest | Quote: "The following have conducted or are currently conducting research sponsored by Novartis Pharmaceuticals Corp.: F. Saad, D. M. Gleason, R. Murray, L. Lacombe, J. L. Chin, and J. J. Vinholes. F. Saad is a consultant on an advisory board to Novartis Pharmaceuticals Corp." | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The 643 patients who met the inclusion criteria after the screening visit were randomly assigned to treatment according to a computer‐generated list of randomization numbers provided to each center." |
| Allocation concealment (selection bias) | Unclear risk | Insufficient report on allocation concealment. |
| Blinding of participants and personnel (performance bias) | Low risk | Placebo‐controlled trial. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient report on blinding of outcome assessment. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient report on blinding of outcome assessment. |
| Incomplete outcome data (attrition bias) | Low risk | All participants were included in ITT analysis. |
| Selective reporting (reporting bias) | Unclear risk | No study protocol available. |
| Other bias | Low risk | No further information provided. |
| Methods | Recruitment period:
End points:
Pain assessment tool:
Randomization:
| |
| Participants | Eligibility criteria:
Exclusion criteria:
Only CGP 032:
Participants randomized:
Median age:
Country of participants:
| |
| Interventions | Previous interventions:
Interventions during study period:
| |
| Outcomes | Reported and analyzed in this review:
| |
| Funding sources | Funding sources:
| |
| Declarations of interest | Conflicts of interest:
| |
| Notes | 2 multicenter, randomized, double‐blind, placebo‐controlled trials (INT‐05 as international trial and CGP 032 as national trial in the US) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient report on sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information on allocation concealment. |
| Blinding of participants and personnel (performance bias) | Low risk | Placebo‐controlled trial. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient report on blinding of outcome assessor. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient report on blinding of outcome assessor. |
| Incomplete outcome data (attrition bias) | High risk | Quote: "Because of protocol violations, 350 patients were included in the intent‐to‐treat efficacy analysis (169 patients in the pamidronate group and 181 patients in the placebo group)." |
| Selective reporting (reporting bias) | Unclear risk | No study protocol available. |
| Other bias | Unclear risk | Quote: "The following authors or their immediate family members have indicated a financial interest. No conflict exists for drugs or devices used in a study if they are not being evaluated as part of the investigation. Owns stock (not including shares held through a public mutual fund): John Seaman, Novartis Pharmaceuticals; Mildred Kowalski, Novartis Pharmaceuticals; Stephanie Petrone, Novartis Pharmaceuticals. Acted as a consultant within the last 2 years: Matthew Smith, Novartis Pharmaceuticals; Eric Small, Novartis Pharmaceuticals. Received more than $2,000 a year from a company for either of the last 2 years: John Seaman, Novartis Pharmaceuticals; Mildred Kowalski, Novartis Pharmaceuticals; Matthew Smith, Novartis Pharmaceuticals." |
| Methods | Recruitment period:
End points:
Pain assessment tool:
Randomization:
| |
| Participants | Eligibility criteria:
Exclusion criteria:
Participants randomized:
Mean age:
Country of participants:
| |
| Interventions | Previous interventions:
Interventions during study period:
| |
| Outcomes | Reported and analyzed in this review:
| |
| Funding sources | Funding sources:
| |
| Declarations of interest | Conflicts of interest:
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information on sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information on allocation concealment. |
| Blinding of participants and personnel (performance bias) | Low risk | Placebo‐controlled trial. |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information on blinding of investigated outcome. |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information on blinding of investigated outcome. |
| Incomplete outcome data (attrition bias) | High risk | Quote: "Six patients [...] were considered unevaluable because they failed to complete 1 month of treatment." |
| Selective reporting (reporting bias) | Unclear risk | No study protocol available. |
| Other bias | High risk | No statistical analysis of observed results. |
| Methods | Recruitment period:
End points:
Pain assessment tool:
Randomization:
| |
| Participants | Eligibility criteria:
Exclusion criteria:
Participants randomized:
Mean age:
Country of participants:
| |
| Interventions | Previous interventions:
Interventions during study period:
| |
| Outcomes | Reported and analyzed in this review:
| |
| Funding sources | Funding sources:
| |
| Declarations of interest | Conflicts of interest:
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient report on sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information on allocation concealment. |
| Blinding of participants and personnel (performance bias) | Low risk | Placebo‐controlled trial. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient report on blinding of outcome assessor. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient report on blinding of outcome assessor. |
| Incomplete outcome data (attrition bias) | High risk | Different report on number of randomized participants. In the text, 55 participants were randomized and, according to Table 1, 52 participants were randomized. |
| Selective reporting (reporting bias) | Unclear risk | No study protocol available. |
| Other bias | High risk | Quote: "The study had to be prematurely terminated before the planned number of patients were included in secondary to difficulties finding enough patients according to inclusion and exclusion criteria." "The work was supported by Leiras OY Finland and ASTRA Lakemedel Sweden." |
| Methods | Recruitment period:
End points:
Pain assessment tool:
Randomization:
| |
| Participants | Eligibility criteria:
Exclusion criteria:
Participants randomized:
Median age:
Country of participants:
| |
| Interventions | Previous interventions:
Interventions during study period:
| |
| Outcomes | Reported and analyzed in this review:
| |
| Funding sources | Funding sources:
| |
| Declarations of interest | Conflicts of interest:
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were stratified by investigation center and ECOG performance status at trial entry in a 1:1:1:1 allocation ratio using a computerized minimization algorithm accessed by telephone to the trials unit." |
| Allocation concealment (selection bias) | Low risk | Quote: "Patients were stratified by investigation center and ECOG performance status at trial entry in a 1:1:1:1 allocation ratio using a computerized minimization algorithm accessed by telephone to the trials unit." |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "TRAPEZE was a randomized, open‐label, phase 3 trial using a 2 × 2 factorial design." |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient report on blinding of outcome assessment. |
| Blinding of outcome assessment (detection bias) | High risk | Quote: "TRAPEZE was a randomized, open‐label, phase 3 trial using a 2 × 2 factorial design." |
| Incomplete outcome data (attrition bias) | Unclear risk | Insufficient report on outcome data. |
| Selective reporting (reporting bias) | High risk | No report on all prespecified outcomes (e.g. QoL). |
| Other bias | Low risk | No further information provided. |
| Methods | Recruitment period:
End points:
Pain assessment tool:
Randomization:
| |
| Participants | Eligibility criteria:
Exclusion criteria:
Participants randomized:
Mean age:
Country of participants:
| |
| Interventions | Previous interventions:
Interventions during study period:
| |
| Outcomes | Reported and analyzed in this review:
| |
| Funding sources | Funding sources:
| |
| Declarations of interest | Conflicts of interest:
| |
| Notes | Inclusion of "bone pain" in SREs. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information on sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information on allocation concealment. |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "This study was under a still ongoing randomized multicenter collaborative open‐labeled project [...]." |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information on blinding of the outcome assessor. |
| Blinding of outcome assessment (detection bias) | High risk | Quote: "This study was under a still ongoing randomized multicenter collaborative open‐labeled project [...]." |
| Incomplete outcome data (attrition bias) | Low risk | All participants were included in statistical analysis. |
| Selective reporting (reporting bias) | High risk | No report on survival data (planned per protocol). |
| Other bias | Low risk | No further information provided. |
| Methods | Recruitment period:
End points:
Pain assessment tool:
Randomization:
| |
| Participants | Eligibility criteria:
Exclusion criteria:
Participants randomized:
Median age:
Country of participants:
| |
| Interventions | Previous interventions:
Interventions during study period:
| |
| Outcomes | Reported and analyzed in this review:
| |
| Funding sources | Funding sources:
| |
| Declarations of interest | Conflicts of interest:
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Computer‐based randomization was conducted at the Translational Research Informatics Center (TRI; Kobe, Japan) with stratification according to the treatment institution, baseline PSA concentration (<200 or ≥200 ng/mL), baseline extent of disease (EOD) grade [13] (≤2 or ≥3), and biopsy Gleason score (≤7 or ≥8). [...] The system automatically evaluated the eligibility of each patient and randomly assigned participants to each group." |
| Allocation concealment (selection bias) | Low risk | Quote: "Computer‐based randomization was conducted at the Translational Research Informatics Center (TRI; Kobe, Japan) with stratification according to the treatment institution, baseline PSA concentration (<200 or ≥200 ng/mL), baseline extent of disease (EOD) grade [13] (≤2 or ≥3), and biopsy Gleason score (≤7 or ≥8). [...] The system automatically evaluated the eligibility of each patient and randomly assigned participants to each group." |
| Blinding of participants and personnel (performance bias) | High risk | Open‐label trial. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information on blinding of outcome assessor. |
| Blinding of outcome assessment (detection bias) | High risk | Open‐label trial. |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "All 224 patients who received at least one dose of LH–RH agonist were included in the Safety Assessment Set (SAS)." |
| Selective reporting (reporting bias) | High risk | Study investigators initially planned to analyze QoL and pain as outcomes, but the authors did not provide any data on these end points in their publications. |
| Other bias | Unclear risk | Quote: "The ZAPCA trial was supported by Grant for Urologic Research No. 200040700148 from Kyoto University Hospital. [...] Tomomi Kamba accepted an honorarium from Astellas Pharma. Toshiyuki Kamoto accepted research funding and honoraria from Astellas Pharma. Fuminori Sato accepted research funding from Janssen Pharmaceutical and Astellas Pharma. Naoya Masumori accepted honoraria from Novartis Pharma and Daiichi Sankyo, and research funding from Daiichi Sankyo. Shin Egawa accepted research funding from Astellas Pharma and Takeda Pharmaceutical. Hideki Sakai accepted research funding from Astellas Pharma and Takeda Pharmaceutical, and honoraria from Astellas Pharma and AstraZeneca. Osamu Ogawa accepted an honorarium from Astellas Pharma." |
ALT: alanine aminotransferase; AST: aspartate transaminase; BPI: Brief Pain Inventory; BUN: blood urea nitrogen; CNS: central nervous system; CT: computed tomography; ECG: electrocardiogram; ECOG: Eastern Cooperative Oncology Group; Hb: hemoglobin; Inst: institution; ITT: intention to treat; IV: intravenous; LHRH: luteinizing hormone releasing hormone; MRI: magnetic resonance imaging; PFS: progression‐free survival; PO: orally; PPI: Present Pain Intensity; PSA: prostate‐specific antigen; QoL: quality of life; SRE: skeletal‐related event; ULN: upper limit of normal; VAS: visual analog scale; WHO: World Health Organization.
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| No control arm. | |
| Active control group with different dosages of clodronate. | |
| No subgroup analysis for men with prostate cancer. | |
| No subgroup analysis for men with prostate cancer. | |
| No control arm. | |
| No control arm. | |
| No control arm. | |
| Randomized controlled study with histomorphometric outcomes. Pain not an outcome. | |
| Bisphosphonates compared to denosumab. | |
| Bisphosphonates compared to denosumab. | |
| Non‐randomized study. | |
| Non‐randomized study. | |
| Randomized study comparing intravenous pamidronate with oral clodronate in a mixed tumor population. Not specific for prostate cancer. | |
| No control arm. | |
| Randomized controlled study with biochemical outcomes, clinical outcomes including pain were reported in another article by Strang 1997, 1 of the included studies. | |
| Active control groups on different administration routes of zoledronic acid. | |
| Participants in both arms received zoledronic acid, early or delayed, no results for the comparison before receiving delayed treatment. | |
| No control arm. | |
| Participants with and without bone metastases included, no subgroup results for people with metastases. | |
| Randomized controlled study with histomorphometric outcomes. Pain not an outcome. | |
| No control arm. | |
| No control arm. | |
| Active control group with other bisphosphonate (zoledronic acid). |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
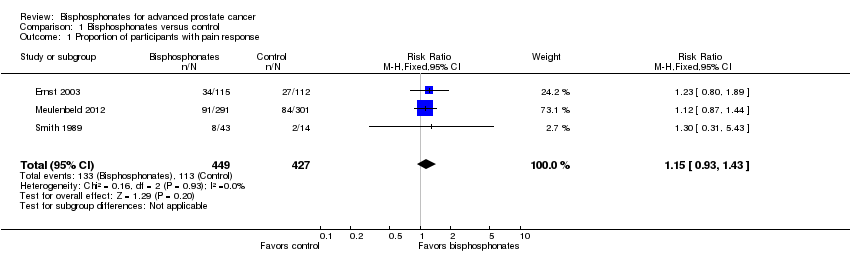
| 1 Proportion of participants with pain response Show forest plot | 3 | 876 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.93, 1.43] |
| Analysis 1.1  Comparison 1 Bisphosphonates versus control, Outcome 1 Proportion of participants with pain response. | ||||
| 2 Skeletal‐related events: any Show forest plot | 9 | 3153 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.81, 0.94] |
| Analysis 1.2  Comparison 1 Bisphosphonates versus control, Outcome 2 Skeletal‐related events: any. | ||||
| 3 Skeletal‐related events: pathologic fracture Show forest plot | 6 | 2226 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.53, 0.87] |
| Analysis 1.3 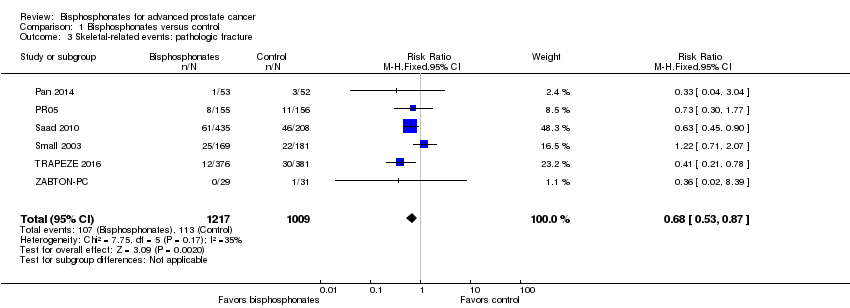 Comparison 1 Bisphosphonates versus control, Outcome 3 Skeletal‐related events: pathologic fracture. | ||||
| 4 Skeletal‐related events: pathologic fractures: vertebral fracture Show forest plot | 2 | 993 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.52, 1.36] |
| Analysis 1.4  Comparison 1 Bisphosphonates versus control, Outcome 4 Skeletal‐related events: pathologic fractures: vertebral fracture. | ||||
| 5 Skeletal‐related events: pathologic fractures: non‐vertebral fracture Show forest plot | 2 | 993 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.53, 1.10] |
| Analysis 1.5  Comparison 1 Bisphosphonates versus control, Outcome 5 Skeletal‐related events: pathologic fractures: non‐vertebral fracture. | ||||
| 6 Skeletal‐related events: spinal cord compression Show forest plot | 6 | 2226 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.50, 0.89] |
| Analysis 1.6  Comparison 1 Bisphosphonates versus control, Outcome 6 Skeletal‐related events: spinal cord compression. | ||||
| 7 Skeletal‐related events: bone radiation therapy Show forest plot | 6 | 1696 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.77, 1.06] |
| Analysis 1.7  Comparison 1 Bisphosphonates versus control, Outcome 7 Skeletal‐related events: bone radiation therapy. | ||||
| 8 Skeletal‐related events: bone surgery Show forest plot | 5 | 1915 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.29, 0.86] |
| Analysis 1.8 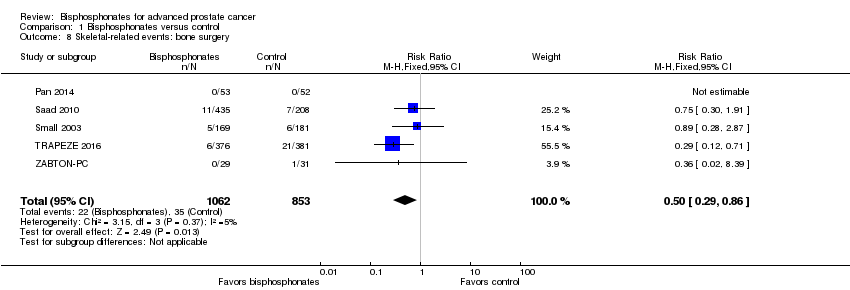 Comparison 1 Bisphosphonates versus control, Outcome 8 Skeletal‐related events: bone surgery. | ||||
| 9 Mortality Show forest plot | 9 | 2450 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.91, 1.04] |
| Analysis 1.9 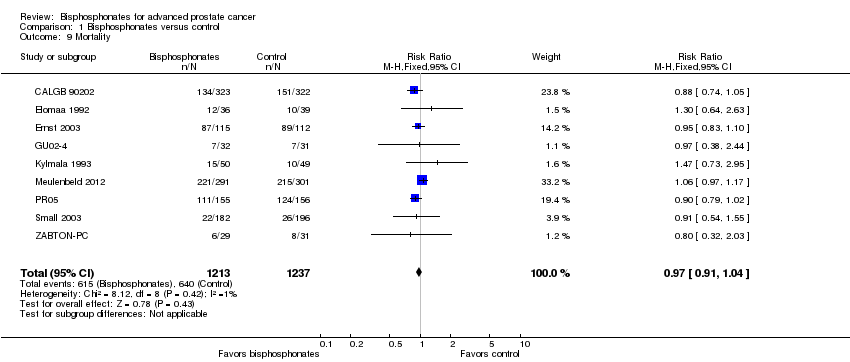 Comparison 1 Bisphosphonates versus control, Outcome 9 Mortality. | ||||
| 10 Adverse events: nausea Show forest plot | 9 | 3008 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [1.00, 1.41] |
| Analysis 1.10  Comparison 1 Bisphosphonates versus control, Outcome 10 Adverse events: nausea. | ||||
| 11 Adverse events: renal Show forest plot | 7 | 1794 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.65 [1.11, 2.46] |
| Analysis 1.11  Comparison 1 Bisphosphonates versus control, Outcome 11 Adverse events: renal. | ||||
| 12 Adverse events: bone pain Show forest plot | 5 | 1445 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.81, 1.06] |
| Analysis 1.12  Comparison 1 Bisphosphonates versus control, Outcome 12 Adverse events: bone pain. | ||||
| 13 Adverse events: osteonecrosis of the jaw Show forest plot | 5 | 1626 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.92 [0.75, 4.90] |
| Analysis 1.13 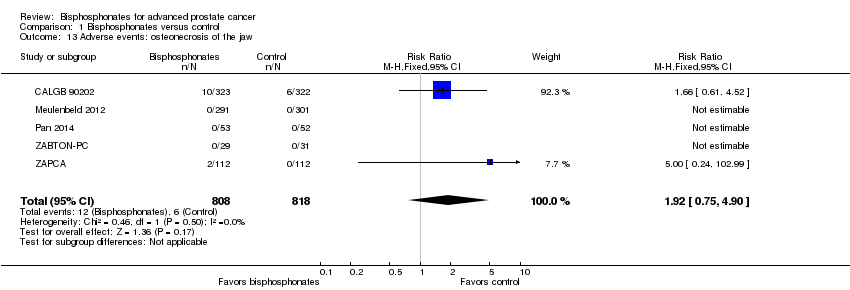 Comparison 1 Bisphosphonates versus control, Outcome 13 Adverse events: osteonecrosis of the jaw. | ||||
| 14 Proportion of participants with decreased analgesic consumption Show forest plot | 4 | 416 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.87, 1.63] |
| Analysis 1.14  Comparison 1 Bisphosphonates versus control, Outcome 14 Proportion of participants with decreased analgesic consumption. | ||||
| 15 Proportion of participants with disease progression Show forest plot | 7 | 2115 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.90, 0.98] |
| Analysis 1.15  Comparison 1 Bisphosphonates versus control, Outcome 15 Proportion of participants with disease progression. | ||||
| 16 Sensitivity analysis: pain response (low risk of bias vs high risk of bias) Show forest plot | 3 | 876 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.93, 1.43] |
| Analysis 1.16  Comparison 1 Bisphosphonates versus control, Outcome 16 Sensitivity analysis: pain response (low risk of bias vs high risk of bias). | ||||
| 16.1 Low risk of bias | 1 | 227 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.80, 1.89] |
| 16.2 High risk of bias | 2 | 649 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.88, 1.44] |
| 17 Subgroup analysis: pain response (amino‐bisphosphonate vs non‐amino‐bisphosphonate) Show forest plot | 3 | 876 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.93, 1.43] |
| Analysis 1.17  Comparison 1 Bisphosphonates versus control, Outcome 17 Subgroup analysis: pain response (amino‐bisphosphonate vs non‐amino‐bisphosphonate). | ||||
| 17.1 Amino‐bisphosphonate | 1 | 592 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.87, 1.44] |
| 17.2 Non‐amino‐bisphosphonate | 2 | 284 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.81, 1.87] |
| 18 Subgroup analysis: pain response (route of administration) Show forest plot | 3 | 876 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.93, 1.43] |
| Analysis 1.18 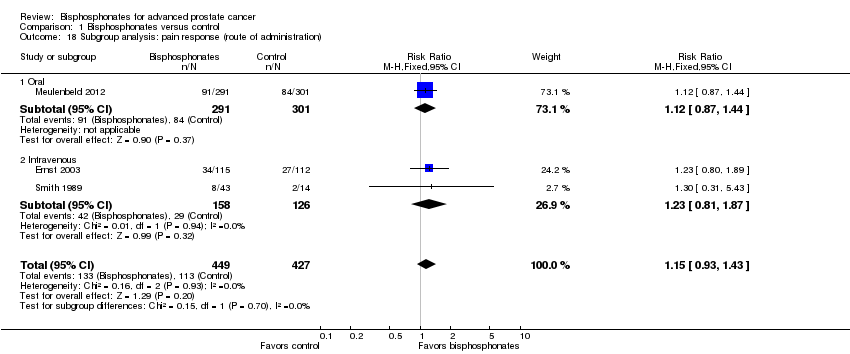 Comparison 1 Bisphosphonates versus control, Outcome 18 Subgroup analysis: pain response (route of administration). | ||||
| 18.1 Oral | 1 | 592 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.87, 1.44] |
| 18.2 Intravenous | 2 | 284 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.81, 1.87] |
| 19 Sensitivity analysis: skeletal‐related events (low risk of bias vs high risk of bias) Show forest plot | 9 | 3153 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.81, 0.94] |
| Analysis 1.19  Comparison 1 Bisphosphonates versus control, Outcome 19 Sensitivity analysis: skeletal‐related events (low risk of bias vs high risk of bias). | ||||
| 19.1 Low risk of bias | 5 | 1767 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.81, 0.99] |
| 19.2 High risk of bias | 4 | 1386 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.75, 0.94] |
| 20 Sensitivity analysis: skeletal‐related events (full‐text vs abstract publication) Show forest plot | 9 | 3153 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.81, 0.94] |
| Analysis 1.20  Comparison 1 Bisphosphonates versus control, Outcome 20 Sensitivity analysis: skeletal‐related events (full‐text vs abstract publication). | ||||
| 20.1 Full‐text publication | 8 | 3093 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.81, 0.95] |
| 20.2 Abstract publication | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.40 [0.12, 1.37] |
| 21 Subgroup analysis: skeletal‐related events (amino‐bisphosphonate versus non‐amino‐bisphosphonate) Show forest plot | 9 | 3153 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.81, 0.94] |
| Analysis 1.21 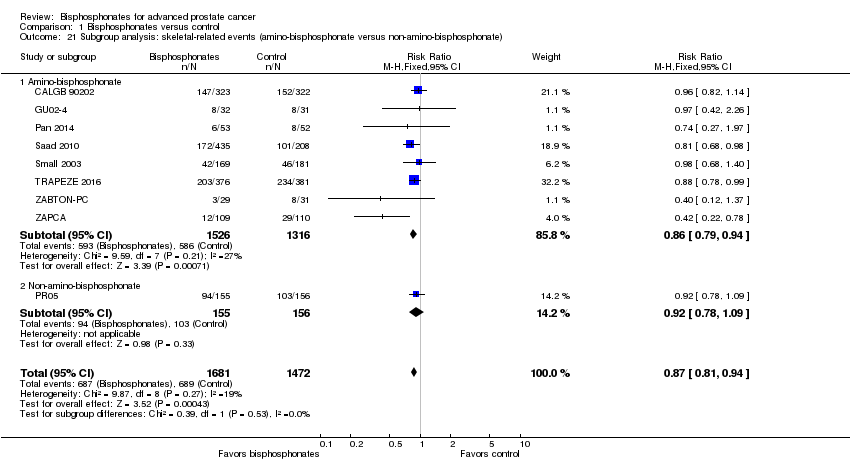 Comparison 1 Bisphosphonates versus control, Outcome 21 Subgroup analysis: skeletal‐related events (amino‐bisphosphonate versus non‐amino‐bisphosphonate). | ||||
| 21.1 Amino‐bisphosphonate | 8 | 2842 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.79, 0.94] |
| 21.2 Non‐amino‐bisphosphonate | 1 | 311 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.78, 1.09] |
| 22 Subgroup analysis: skeletal‐related events (route of administration) Show forest plot | 9 | 3153 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.81, 0.94] |
| Analysis 1.22  Comparison 1 Bisphosphonates versus control, Outcome 22 Subgroup analysis: skeletal‐related events (route of administration). | ||||
| 22.1 Oral | 2 | 374 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.78, 1.09] |
| 22.2 Intravenous | 7 | 2779 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.79, 0.94] |
| 23 Sensitivity analysis: mortality (low risk of bias vs high risk of bias)) Show forest plot | 9 | 2450 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.91, 1.04] |
| Analysis 1.23 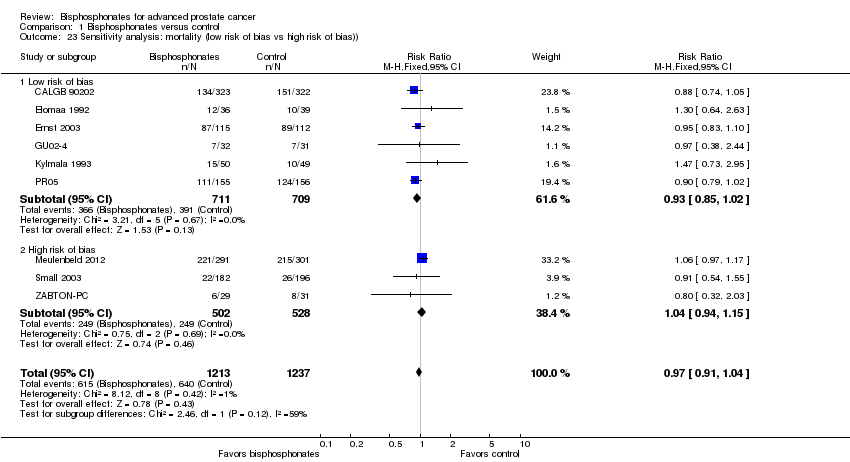 Comparison 1 Bisphosphonates versus control, Outcome 23 Sensitivity analysis: mortality (low risk of bias vs high risk of bias)). | ||||
| 23.1 Low risk of bias | 6 | 1420 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.85, 1.02] |
| 23.2 High risk of bias | 3 | 1030 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.94, 1.15] |
| 24 Sensitivity analysis: mortality (full‐text vs abstract publication) Show forest plot | 9 | 2450 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.91, 1.04] |
| Analysis 1.24  Comparison 1 Bisphosphonates versus control, Outcome 24 Sensitivity analysis: mortality (full‐text vs abstract publication). | ||||
| 24.1 Full‐text publication | 8 | 2390 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.91, 1.04] |
| 24.2 Abstract publication | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.32, 2.03] |
| 25 Subgroup analysis: mortality (amino‐bisphosphonate vs non‐amino‐bisphosphonate) Show forest plot | 9 | 2450 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.91, 1.04] |
| Analysis 1.25 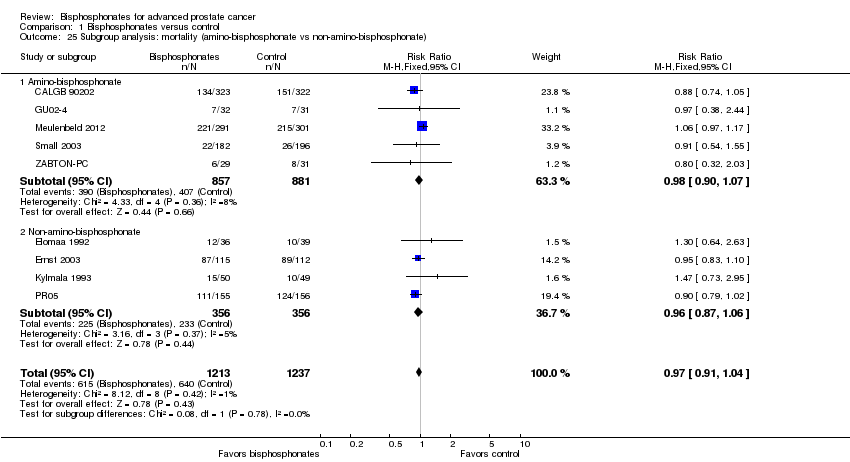 Comparison 1 Bisphosphonates versus control, Outcome 25 Subgroup analysis: mortality (amino‐bisphosphonate vs non‐amino‐bisphosphonate). | ||||
| 25.1 Amino‐bisphosphonate | 5 | 1738 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.90, 1.07] |
| 25.2 Non‐amino‐bisphosphonate | 4 | 712 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.87, 1.06] |
| 26 Subgroup analysis: mortality (route of administration) Show forest plot | 9 | 2450 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.91, 1.04] |
| Analysis 1.26  Comparison 1 Bisphosphonates versus control, Outcome 26 Subgroup analysis: mortality (route of administration). | ||||
| 26.1 Oral | 5 | 1140 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.95, 1.11] |
| 26.2 Intravenous | 4 | 1310 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.81, 1.02] |
| 27 Sensitivity analysis: adverse event: nausea (low risk of bias vs high risk of bias) Show forest plot | 9 | 3008 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [1.00, 1.41] |
| Analysis 1.27  Comparison 1 Bisphosphonates versus control, Outcome 27 Sensitivity analysis: adverse event: nausea (low risk of bias vs high risk of bias). | ||||
| 27.1 Low risk of bias | 7 | 2042 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.99, 1.40] |
| 27.2 High risk of bias | 2 | 966 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.41 [0.49, 4.03] |
| 28 Subgroup analysis: adverse event: nausea (amino‐bisphosphonate vs non‐amino‐bisphosphonate) Show forest plot | 9 | 3008 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [1.00, 1.41] |
| Analysis 1.28  Comparison 1 Bisphosphonates versus control, Outcome 28 Subgroup analysis: adverse event: nausea (amino‐bisphosphonate vs non‐amino‐bisphosphonate). | ||||
| 28.1 Amino‐bisphosphonate | 5 | 2332 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.98, 1.45] |
| 28.2 Non‐amino‐bisphosphonate | 4 | 676 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.83, 1.68] |
| 29 Subgroup analysis: adverse event: nausea (route of administration) Show forest plot | 9 | 3008 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [1.00, 1.41] |
| Analysis 1.29 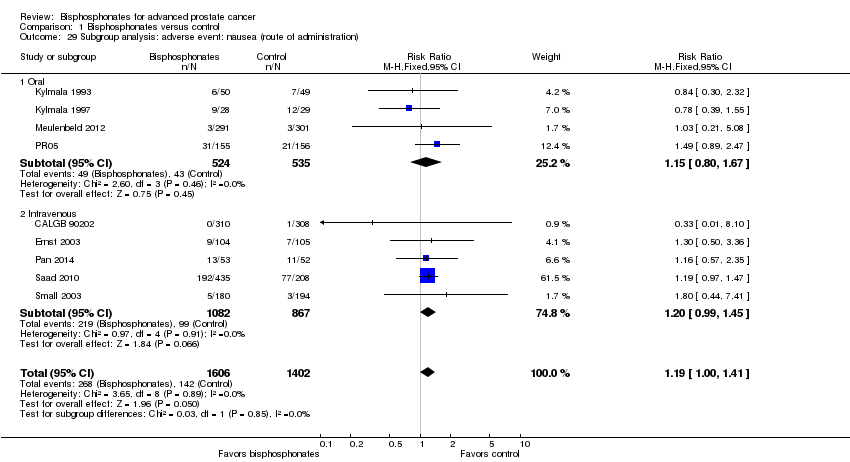 Comparison 1 Bisphosphonates versus control, Outcome 29 Subgroup analysis: adverse event: nausea (route of administration). | ||||
| 29.1 Oral | 4 | 1059 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.80, 1.67] |
| 29.2 Intravenous | 5 | 1949 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.20 [0.99, 1.45] |
| 30 Sensitivity analysis: renal (low risk of bias vs high risk of bias) Show forest plot | 7 | 1794 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.65 [1.11, 2.46] |
| Analysis 1.30 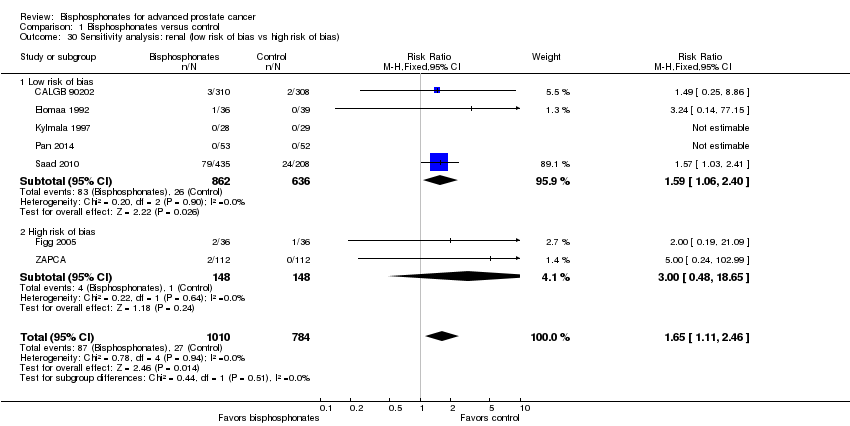 Comparison 1 Bisphosphonates versus control, Outcome 30 Sensitivity analysis: renal (low risk of bias vs high risk of bias). | ||||
| 30.1 Low risk of bias | 5 | 1498 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.59 [1.06, 2.40] |
| 30.2 High risk of bias | 2 | 296 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.48, 18.65] |
| 31 Subgroup analysis: renal (amino‐bisphosphonate vs non‐amino‐bisphosphonate) Show forest plot | 7 | 1794 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.65 [1.11, 2.46] |
| Analysis 1.31 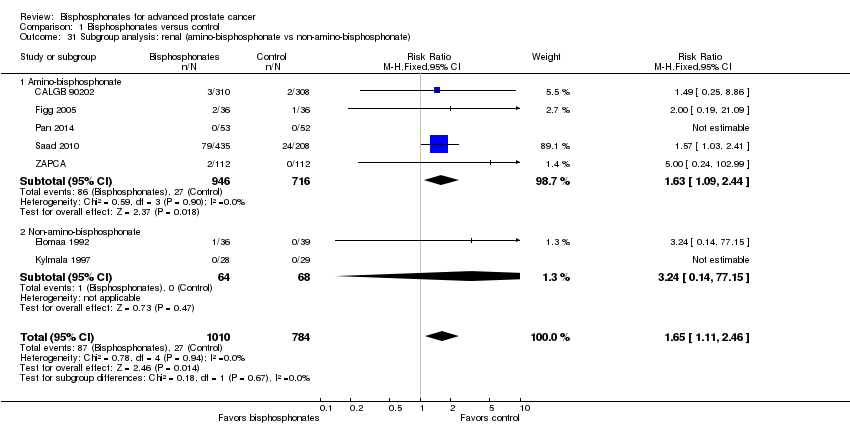 Comparison 1 Bisphosphonates versus control, Outcome 31 Subgroup analysis: renal (amino‐bisphosphonate vs non‐amino‐bisphosphonate). | ||||
| 31.1 Amino‐bisphosphonate | 5 | 1662 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.63 [1.09, 2.44] |
| 31.2 Non‐amino‐bisphosphonate | 2 | 132 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.24 [0.14, 77.15] |
| 32 Subgroup analysis: renal (route of administration) Show forest plot | 7 | 1794 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.65 [1.11, 2.46] |
| Analysis 1.32 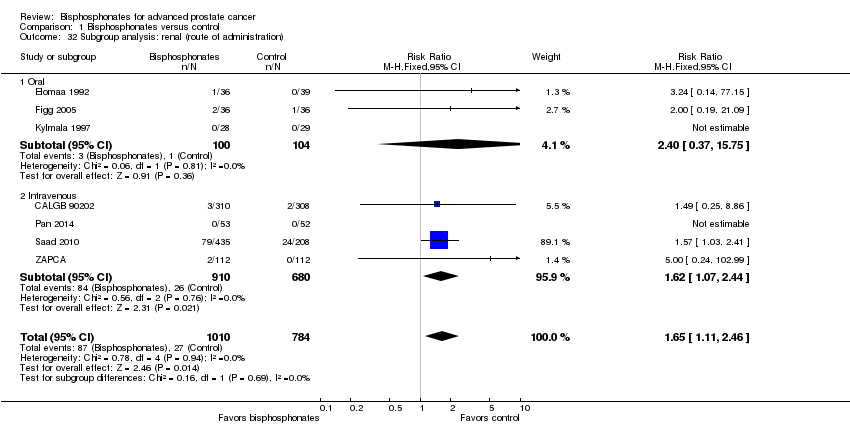 Comparison 1 Bisphosphonates versus control, Outcome 32 Subgroup analysis: renal (route of administration). | ||||
| 32.1 Oral | 3 | 204 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.40 [0.37, 15.75] |
| 32.2 Intravenous | 4 | 1590 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.62 [1.07, 2.44] |

Study flow diagram.
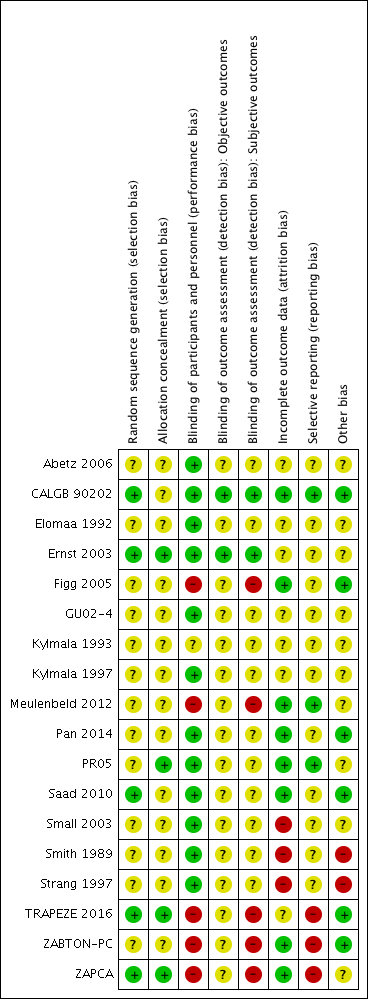
Risk of bias summary: review authors' judgments about each risk of bias item for each included study.

Forest plot of comparison: 1 Bisphosphonates versus control, outcome: 1.1 Proportion of participants with pain response.
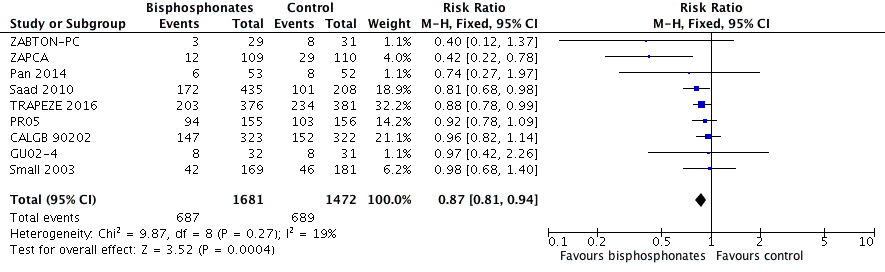
Forest plot of comparison: 1 Bisphosphonates versus control, outcome: 1.2 Skeletal‐related events: any.

Forest plot of comparison: 1 Bisphosphonates versus control, outcome: 1.9 Mortality.
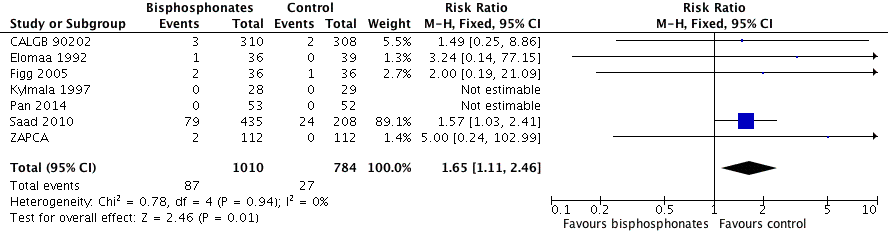
Forest plot of comparison: 1 Bisphosphonates versus control, outcome: 1.11 Adverse events: renal.
Comparison 1 Bisphosphonates versus control, Outcome 1 Proportion of participants with pain response.
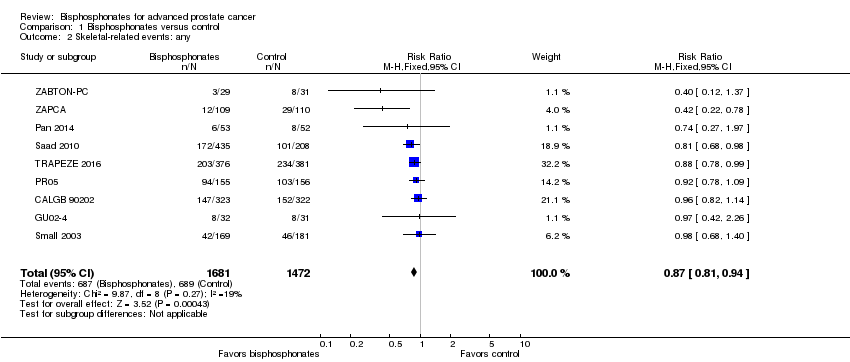
Comparison 1 Bisphosphonates versus control, Outcome 2 Skeletal‐related events: any.
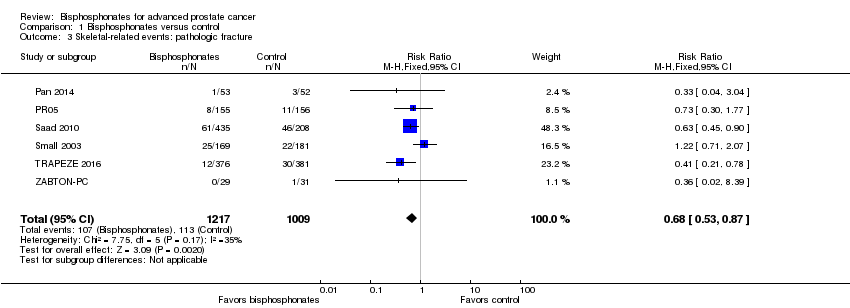
Comparison 1 Bisphosphonates versus control, Outcome 3 Skeletal‐related events: pathologic fracture.
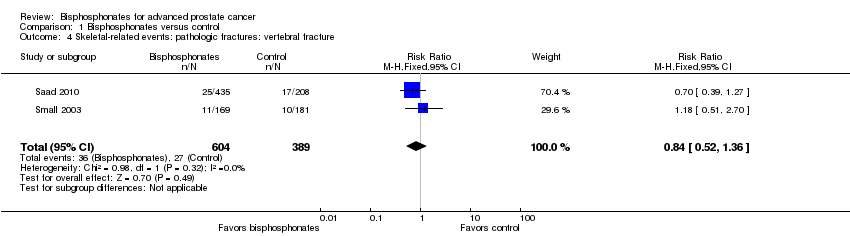
Comparison 1 Bisphosphonates versus control, Outcome 4 Skeletal‐related events: pathologic fractures: vertebral fracture.
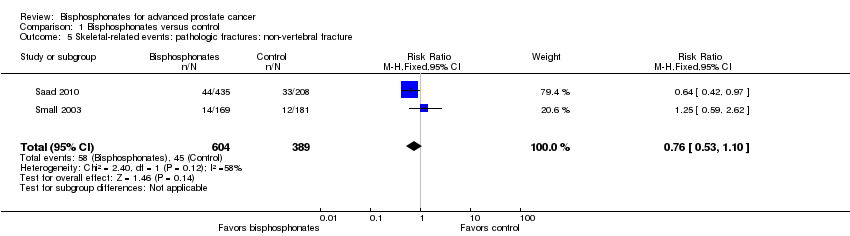
Comparison 1 Bisphosphonates versus control, Outcome 5 Skeletal‐related events: pathologic fractures: non‐vertebral fracture.
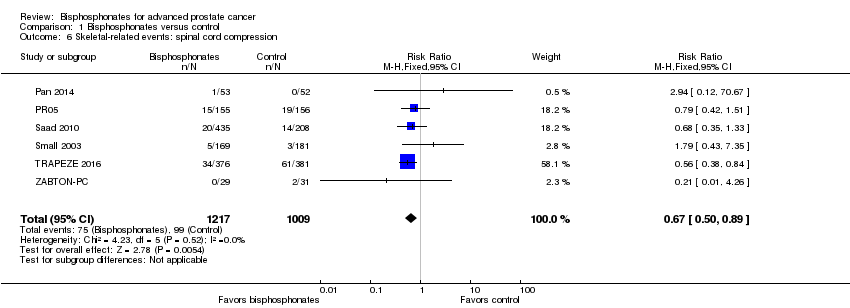
Comparison 1 Bisphosphonates versus control, Outcome 6 Skeletal‐related events: spinal cord compression.
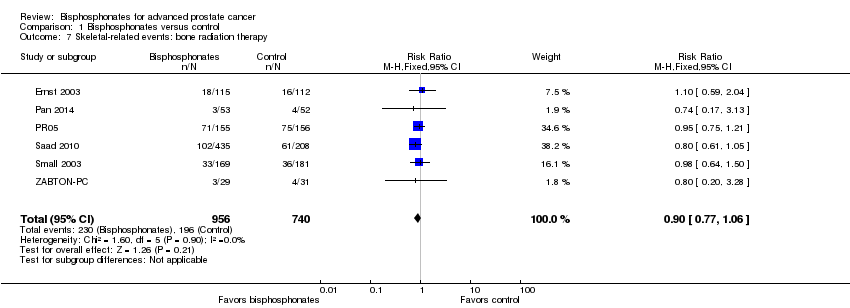
Comparison 1 Bisphosphonates versus control, Outcome 7 Skeletal‐related events: bone radiation therapy.

Comparison 1 Bisphosphonates versus control, Outcome 8 Skeletal‐related events: bone surgery.

Comparison 1 Bisphosphonates versus control, Outcome 9 Mortality.

Comparison 1 Bisphosphonates versus control, Outcome 10 Adverse events: nausea.

Comparison 1 Bisphosphonates versus control, Outcome 11 Adverse events: renal.
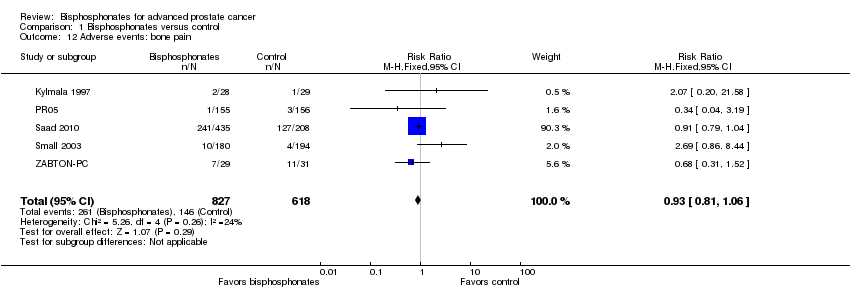
Comparison 1 Bisphosphonates versus control, Outcome 12 Adverse events: bone pain.

Comparison 1 Bisphosphonates versus control, Outcome 13 Adverse events: osteonecrosis of the jaw.

Comparison 1 Bisphosphonates versus control, Outcome 14 Proportion of participants with decreased analgesic consumption.

Comparison 1 Bisphosphonates versus control, Outcome 15 Proportion of participants with disease progression.

Comparison 1 Bisphosphonates versus control, Outcome 16 Sensitivity analysis: pain response (low risk of bias vs high risk of bias).

Comparison 1 Bisphosphonates versus control, Outcome 17 Subgroup analysis: pain response (amino‐bisphosphonate vs non‐amino‐bisphosphonate).
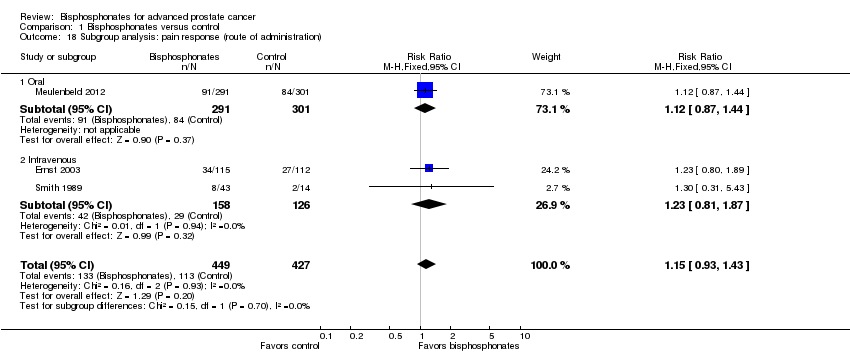
Comparison 1 Bisphosphonates versus control, Outcome 18 Subgroup analysis: pain response (route of administration).

Comparison 1 Bisphosphonates versus control, Outcome 19 Sensitivity analysis: skeletal‐related events (low risk of bias vs high risk of bias).
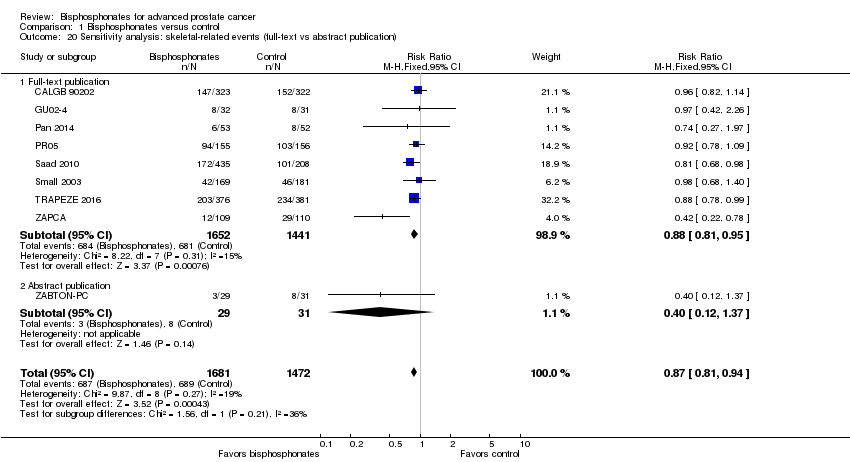
Comparison 1 Bisphosphonates versus control, Outcome 20 Sensitivity analysis: skeletal‐related events (full‐text vs abstract publication).

Comparison 1 Bisphosphonates versus control, Outcome 21 Subgroup analysis: skeletal‐related events (amino‐bisphosphonate versus non‐amino‐bisphosphonate).

Comparison 1 Bisphosphonates versus control, Outcome 22 Subgroup analysis: skeletal‐related events (route of administration).
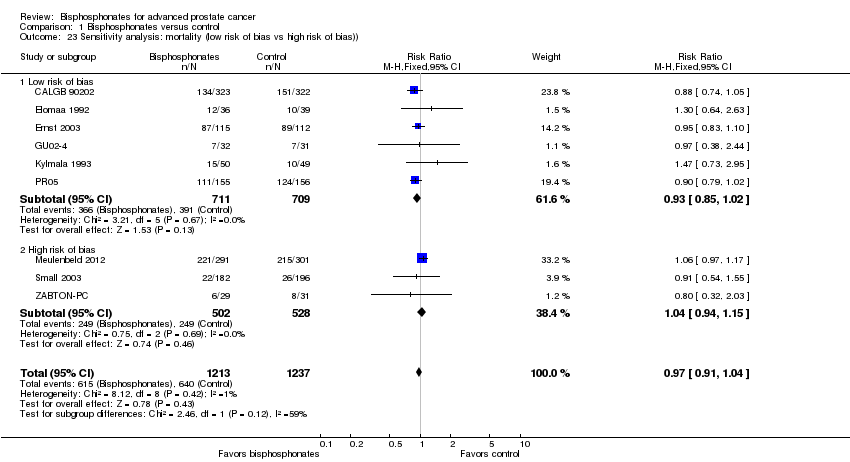
Comparison 1 Bisphosphonates versus control, Outcome 23 Sensitivity analysis: mortality (low risk of bias vs high risk of bias)).
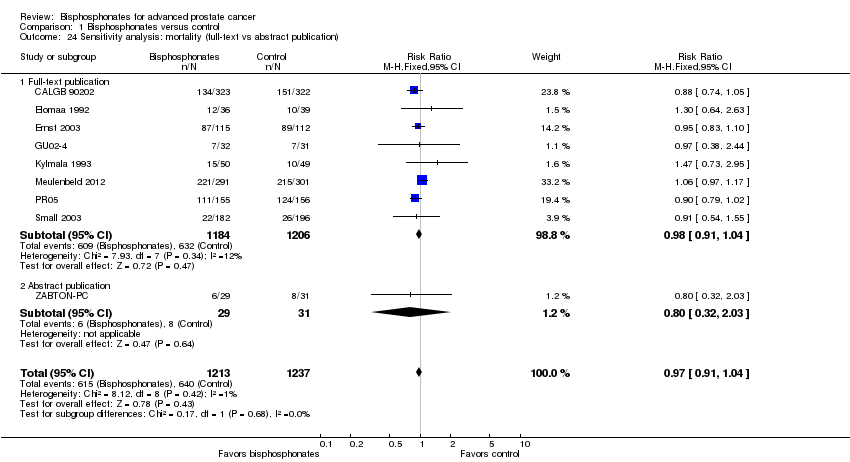
Comparison 1 Bisphosphonates versus control, Outcome 24 Sensitivity analysis: mortality (full‐text vs abstract publication).
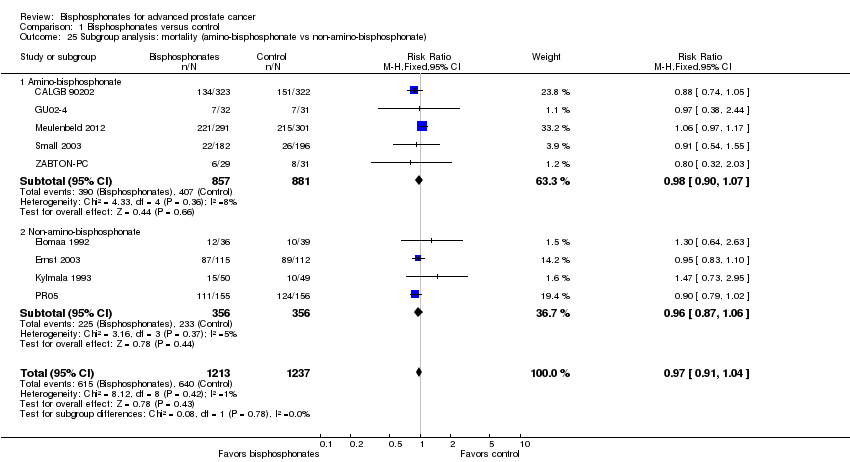
Comparison 1 Bisphosphonates versus control, Outcome 25 Subgroup analysis: mortality (amino‐bisphosphonate vs non‐amino‐bisphosphonate).
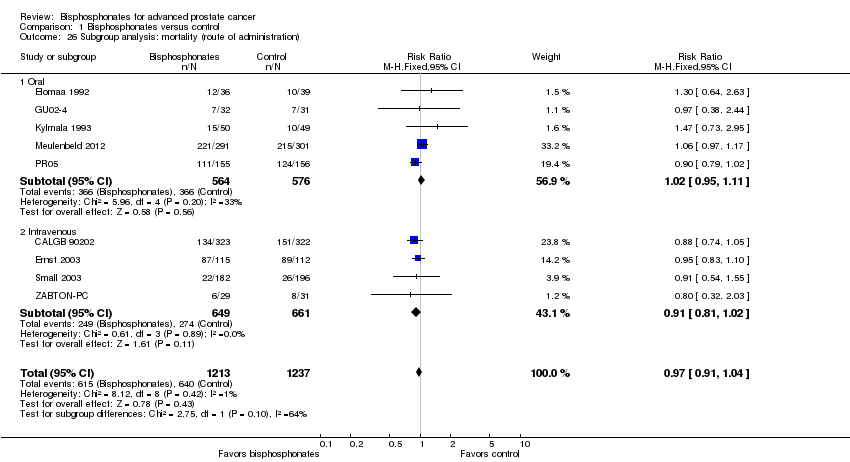
Comparison 1 Bisphosphonates versus control, Outcome 26 Subgroup analysis: mortality (route of administration).

Comparison 1 Bisphosphonates versus control, Outcome 27 Sensitivity analysis: adverse event: nausea (low risk of bias vs high risk of bias).
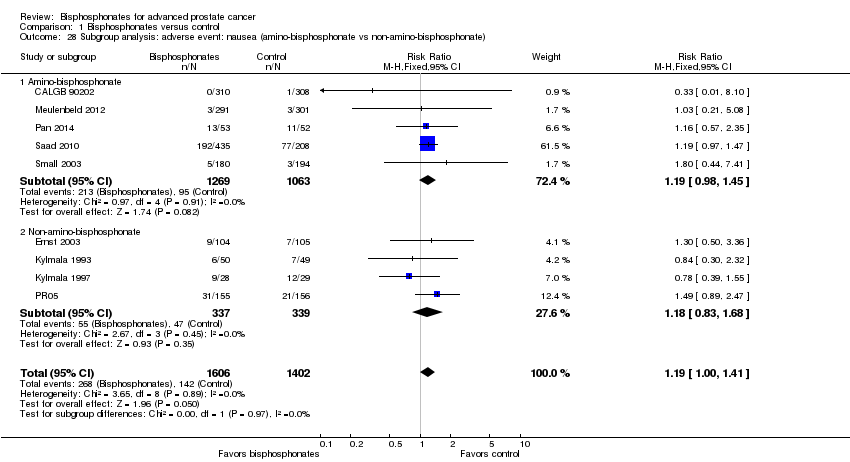
Comparison 1 Bisphosphonates versus control, Outcome 28 Subgroup analysis: adverse event: nausea (amino‐bisphosphonate vs non‐amino‐bisphosphonate).
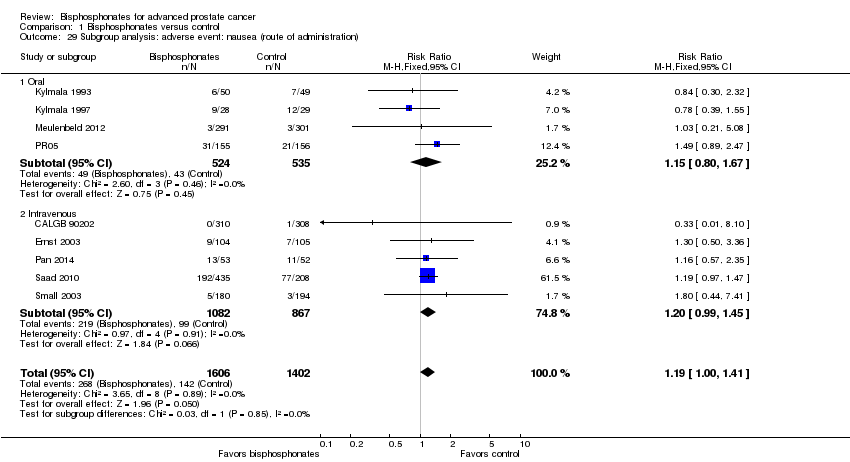
Comparison 1 Bisphosphonates versus control, Outcome 29 Subgroup analysis: adverse event: nausea (route of administration).

Comparison 1 Bisphosphonates versus control, Outcome 30 Sensitivity analysis: renal (low risk of bias vs high risk of bias).
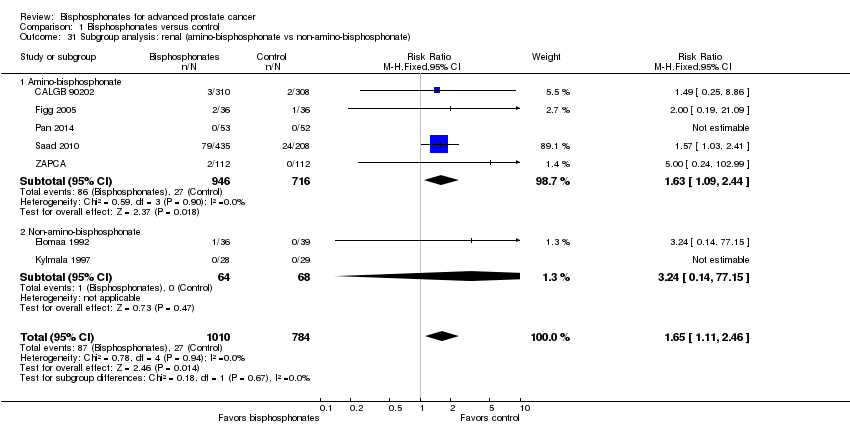
Comparison 1 Bisphosphonates versus control, Outcome 31 Subgroup analysis: renal (amino‐bisphosphonate vs non‐amino‐bisphosphonate).

Comparison 1 Bisphosphonates versus control, Outcome 32 Subgroup analysis: renal (route of administration).
| Bisphosphonates compared to control for advanced prostate cancer | |||||
| Patient or population: men with advanced prostate cancer Settings: ‐ Intervention: bisphosphonate Comparison: control | |||||
| Outcomes | No of participants | Quality of the evidence | Relative effect | Anticipated absolute effects* (95% CI) | |
| Risk with control | Risk difference with bisphosphonates | ||||
| Proportion of participants with pain response Follow‐up: 5‐12 months | 876 | ⊕⊕⊝⊝ | RR 1.15 | Study population | |
| 265 per 1000 | 40 more per 1000 | ||||
| Skeletal‐related events: any, composite outcome | 3153 | ⊕⊕⊕⊝ | RR 0.87 | Study population | |
| 448 per 1000 | 58 fewer per 1000 | ||||
| Mortality | 2450 | ⊕⊕⊕⊝ | RR 0.97 | Study population | |
| 517 per 1000 | 16 fewer per 1000 | ||||
| Quality of life | ‐ | ‐ | Not estimable | ‐ | |
| Adverse events: nausea | 3008 | ⊕⊕⊕⊝ | RR 1.19 | Study population | |
| 35 per 1000 | 7 more per 1000 | ||||
| Adverse events: renal Follow‐up: 5‐36 months | 1794 | ⊕⊕⊕⊝ | RR 1.65 | Study population | |
| 34 per 1000 | 22 more per 1000 | ||||
| Adverse events: osteonecrosis of the jaw | 1626 | ⊕⊝⊝⊝ | RR 1.92 | Study population | |
| 7 per 1000 | 7 more per 1000 | ||||
| Proportion of participants with disease progression | 2115 | ⊕⊕⊕⊝ | RR 0.95 | Study population | |
| 710 per 1000 | 36 fewer per 1000 | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomized controlled trial; RR: risk ratio. | |||||
| GRADE Working Group grades of evidence | |||||
| 1Potential risk of performance, detection and attrition bias leading to downgrading (one point). 2Small number of events leading to downgrading (one point). 3Potential risk of performance and attrition bias leading to downgrading (one point). 4Very small number of events leading to downgrading (two points). | |||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Proportion of participants with pain response Show forest plot | 3 | 876 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.93, 1.43] |
| 2 Skeletal‐related events: any Show forest plot | 9 | 3153 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.81, 0.94] |
| 3 Skeletal‐related events: pathologic fracture Show forest plot | 6 | 2226 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.53, 0.87] |
| 4 Skeletal‐related events: pathologic fractures: vertebral fracture Show forest plot | 2 | 993 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.52, 1.36] |
| 5 Skeletal‐related events: pathologic fractures: non‐vertebral fracture Show forest plot | 2 | 993 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.53, 1.10] |
| 6 Skeletal‐related events: spinal cord compression Show forest plot | 6 | 2226 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.50, 0.89] |
| 7 Skeletal‐related events: bone radiation therapy Show forest plot | 6 | 1696 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.77, 1.06] |
| 8 Skeletal‐related events: bone surgery Show forest plot | 5 | 1915 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.29, 0.86] |
| 9 Mortality Show forest plot | 9 | 2450 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.91, 1.04] |
| 10 Adverse events: nausea Show forest plot | 9 | 3008 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [1.00, 1.41] |
| 11 Adverse events: renal Show forest plot | 7 | 1794 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.65 [1.11, 2.46] |
| 12 Adverse events: bone pain Show forest plot | 5 | 1445 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.81, 1.06] |
| 13 Adverse events: osteonecrosis of the jaw Show forest plot | 5 | 1626 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.92 [0.75, 4.90] |
| 14 Proportion of participants with decreased analgesic consumption Show forest plot | 4 | 416 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.87, 1.63] |
| 15 Proportion of participants with disease progression Show forest plot | 7 | 2115 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.90, 0.98] |
| 16 Sensitivity analysis: pain response (low risk of bias vs high risk of bias) Show forest plot | 3 | 876 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.93, 1.43] |
| 16.1 Low risk of bias | 1 | 227 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.80, 1.89] |
| 16.2 High risk of bias | 2 | 649 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.88, 1.44] |
| 17 Subgroup analysis: pain response (amino‐bisphosphonate vs non‐amino‐bisphosphonate) Show forest plot | 3 | 876 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.93, 1.43] |
| 17.1 Amino‐bisphosphonate | 1 | 592 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.87, 1.44] |
| 17.2 Non‐amino‐bisphosphonate | 2 | 284 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.81, 1.87] |
| 18 Subgroup analysis: pain response (route of administration) Show forest plot | 3 | 876 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.93, 1.43] |
| 18.1 Oral | 1 | 592 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.87, 1.44] |
| 18.2 Intravenous | 2 | 284 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.81, 1.87] |
| 19 Sensitivity analysis: skeletal‐related events (low risk of bias vs high risk of bias) Show forest plot | 9 | 3153 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.81, 0.94] |
| 19.1 Low risk of bias | 5 | 1767 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.81, 0.99] |
| 19.2 High risk of bias | 4 | 1386 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.75, 0.94] |
| 20 Sensitivity analysis: skeletal‐related events (full‐text vs abstract publication) Show forest plot | 9 | 3153 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.81, 0.94] |
| 20.1 Full‐text publication | 8 | 3093 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.81, 0.95] |
| 20.2 Abstract publication | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.40 [0.12, 1.37] |
| 21 Subgroup analysis: skeletal‐related events (amino‐bisphosphonate versus non‐amino‐bisphosphonate) Show forest plot | 9 | 3153 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.81, 0.94] |
| 21.1 Amino‐bisphosphonate | 8 | 2842 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.79, 0.94] |
| 21.2 Non‐amino‐bisphosphonate | 1 | 311 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.78, 1.09] |
| 22 Subgroup analysis: skeletal‐related events (route of administration) Show forest plot | 9 | 3153 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.81, 0.94] |
| 22.1 Oral | 2 | 374 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.78, 1.09] |
| 22.2 Intravenous | 7 | 2779 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.79, 0.94] |
| 23 Sensitivity analysis: mortality (low risk of bias vs high risk of bias)) Show forest plot | 9 | 2450 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.91, 1.04] |
| 23.1 Low risk of bias | 6 | 1420 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.85, 1.02] |
| 23.2 High risk of bias | 3 | 1030 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.94, 1.15] |
| 24 Sensitivity analysis: mortality (full‐text vs abstract publication) Show forest plot | 9 | 2450 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.91, 1.04] |
| 24.1 Full‐text publication | 8 | 2390 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.91, 1.04] |
| 24.2 Abstract publication | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.32, 2.03] |
| 25 Subgroup analysis: mortality (amino‐bisphosphonate vs non‐amino‐bisphosphonate) Show forest plot | 9 | 2450 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.91, 1.04] |
| 25.1 Amino‐bisphosphonate | 5 | 1738 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.90, 1.07] |
| 25.2 Non‐amino‐bisphosphonate | 4 | 712 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.87, 1.06] |
| 26 Subgroup analysis: mortality (route of administration) Show forest plot | 9 | 2450 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.91, 1.04] |
| 26.1 Oral | 5 | 1140 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.95, 1.11] |
| 26.2 Intravenous | 4 | 1310 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.81, 1.02] |
| 27 Sensitivity analysis: adverse event: nausea (low risk of bias vs high risk of bias) Show forest plot | 9 | 3008 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [1.00, 1.41] |
| 27.1 Low risk of bias | 7 | 2042 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.99, 1.40] |
| 27.2 High risk of bias | 2 | 966 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.41 [0.49, 4.03] |
| 28 Subgroup analysis: adverse event: nausea (amino‐bisphosphonate vs non‐amino‐bisphosphonate) Show forest plot | 9 | 3008 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [1.00, 1.41] |
| 28.1 Amino‐bisphosphonate | 5 | 2332 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.98, 1.45] |
| 28.2 Non‐amino‐bisphosphonate | 4 | 676 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.83, 1.68] |
| 29 Subgroup analysis: adverse event: nausea (route of administration) Show forest plot | 9 | 3008 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [1.00, 1.41] |
| 29.1 Oral | 4 | 1059 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.80, 1.67] |
| 29.2 Intravenous | 5 | 1949 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.20 [0.99, 1.45] |
| 30 Sensitivity analysis: renal (low risk of bias vs high risk of bias) Show forest plot | 7 | 1794 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.65 [1.11, 2.46] |
| 30.1 Low risk of bias | 5 | 1498 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.59 [1.06, 2.40] |
| 30.2 High risk of bias | 2 | 296 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.48, 18.65] |
| 31 Subgroup analysis: renal (amino‐bisphosphonate vs non‐amino‐bisphosphonate) Show forest plot | 7 | 1794 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.65 [1.11, 2.46] |
| 31.1 Amino‐bisphosphonate | 5 | 1662 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.63 [1.09, 2.44] |
| 31.2 Non‐amino‐bisphosphonate | 2 | 132 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.24 [0.14, 77.15] |
| 32 Subgroup analysis: renal (route of administration) Show forest plot | 7 | 1794 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.65 [1.11, 2.46] |
| 32.1 Oral | 3 | 204 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.40 [0.37, 15.75] |
| 32.2 Intravenous | 4 | 1590 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.62 [1.07, 2.44] |

