Programas de cribado y tratamiento de infecciones del aparato genital inferior para la prevención del parto prematuro
Referencias
Referencias de los estudios incluidos en esta revisión
Referencias de los estudios excluidos de esta revisión
Referencias adicionales
Referencias de otras versiones publicadas de esta revisión
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | Randomised trial with computer‐generated randomisation sequence. All pregnant women presenting for antenatal care were screened, with smear samples sent to the central laboratory where they were randomly assigned to the intervention or control group. Women in the intervention arm with a positive screening result received treatment. Women in the control group were blinded to screening results and received routine antenatal care. Description of withdrawals: yes. Intention‐to‐treat analysis: not used. | |
| Participants | 4429 pregnant women (mean age 28.9, SD 5.6) presenting for routine prenatal visits between 15 and 19 weeks' gestation (mean 17, SD 1.6). Intervention group: n = 2058 ; control group: n = 2097. Inclusion criteria: gestational age 15‐19 weeks without subjective complaints (e.g. contractions and vaginal bleeding). Exclusion criteria: clinical symptoms of vaginal infection, multiple pregnancies. Location: Vienna, Austria. | |
| Interventions | Intervention group: vaginal smears (Gram stain and evaluated by the scoring criteria proposed by Nugent 1991) screening for bacterial vaginosis, Trichomonas vaginalis and Candida species and received standard antibiotic treatment if positive screening test, i.e. 2% for 6 days local clindamycin for bacterial vaginosis, 300 mg twice daily for seven days oral clindamycin for recurrent bacterial vaginosis, 0.1 g for 6 days local clotrimazole for candidiasis, and 500 mg for 7 days local metronidazole for trichomoniasis (including treatment of the partner). Control group: were smeared, but the results of testing were not made available to the women's care providers and did not have any effect on the standard clinical antenatal care program routine antenatal examination. | |
| Outcomes | Primary outcome: spontaneous preterm delivery at less than 37 weeks' gestation. Secondary outcomes:
| |
| Notes | 4429 randomised, 274 excluded from analysis, 140 lost to follow up, 68 did not fulfill all inclusion criteria, 66 multiple pregnancies. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A pre‐established computer generated randomisation list was used to allocate patients to the treatment groups. |
| Allocation concealment (selection bias) | Unclear risk | No information on allocation concealment. |
| Blinding of participants and personnel (performance bias) | High risk | All obstetricians and women in the intervention group received their smear results and different treatment regimens. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not described, but assessors would not have influenced the objective outcome of birthweight. |
| Incomplete outcome data (attrition bias) | Unclear risk | 4429 pregnant women: 2058 in the treatment group, 2097 in the control group. There were 274 patients excluded from the study with group allocation not stated (140 lost to follow up, 68 did not fulfill all inclusion criteria, 66 multiple pregnancies). Intention to treat analysis was not described. |
| Selective reporting (reporting bias) | Unclear risk | No information available because protocol is not accessible; we have contacted authors for additional outcome data. |
| Other bias | Low risk | The study seems to be free of other types of bias. |
NICU: neonatal intensive care unit
SD: standard deviation
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| Participants did not meet inclusion criteria. | |
| Participants did not meet inclusion criteria. Study compared screening for and treatment of abnormal vaginal flora versus no treatment. | |
| Methods not clearly described, but seems likely that this was not a randomised controlled trial. Described as a prospective observational trial. | |
| Participants did not meet inclusion criteria. Study compared self‐examination of vaginal acidity, and microbiologic testing for BV (Gram staining) versus usual prenatal care standard care. |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Preterm birth less than 37 weeks Show forest plot | 1 | 4155 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.41, 0.75] |
| Analysis 1.1  Comparison 1 Lower genital tract infection screening versus no screening, Outcome 1 Preterm birth less than 37 weeks. | ||||
| 2 Preterm very low birthweight (below or equal 1500 g) Show forest plot | 1 | 4155 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.15, 0.75] |
| Analysis 1.2  Comparison 1 Lower genital tract infection screening versus no screening, Outcome 2 Preterm very low birthweight (below or equal 1500 g). | ||||
| 3 Preterm low birthweight (below or equal 2500 g) Show forest plot | 1 | 4155 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.34, 0.66] |
| Analysis 1.3  Comparison 1 Lower genital tract infection screening versus no screening, Outcome 3 Preterm low birthweight (below or equal 2500 g). | ||||
| 4 Neonatal morbidity | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Duration of admission to neonatal intensive care unit/hospital | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Neonatal death | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Side‐effects of treatment (including drug resistance) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Persistent infection | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Recurrent infection | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Women's satisfaction | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
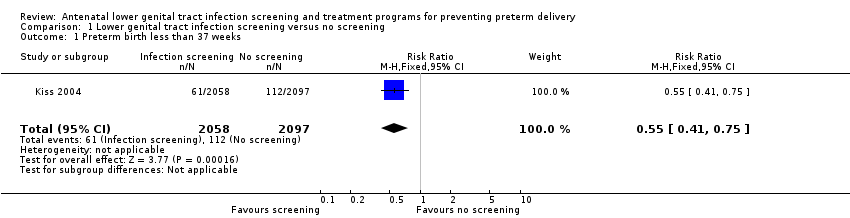
Comparison 1 Lower genital tract infection screening versus no screening, Outcome 1 Preterm birth less than 37 weeks.

Comparison 1 Lower genital tract infection screening versus no screening, Outcome 2 Preterm very low birthweight (below or equal 1500 g).

Comparison 1 Lower genital tract infection screening versus no screening, Outcome 3 Preterm low birthweight (below or equal 2500 g).
| Lower genital tract infection screening versus no screening for preventing preterm delivery | ||||||
| Patient or population: pregnant women presenting for routine prenatal care | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| No screening | Lower genital tract infection screening | |||||
| Preterm birth less than 37 weeks | Study population | RR 0.55 | 4155 | ⊕⊕⊕⊝ | ||
| 53 per 1000 | 29 per 1000 | |||||
| Preterm low birthweight (below or equal 2500 g) | Study population | RR 0.48 | 4155 | ⊕⊕⊕⊝ | ||
| 51 per 1000 | 24 per 1000 | |||||
| Preterm very low birthweight (below or equal 1500 g) | Study population | RR 0.34 | 4155 | ⊕⊕⊕⊝ | ||
| 11 per 1000 | 4 per 1000 | |||||
| *The basis for the assumed risk is the median control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 One study with design limitations. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Preterm birth less than 37 weeks Show forest plot | 1 | 4155 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.41, 0.75] |
| 2 Preterm very low birthweight (below or equal 1500 g) Show forest plot | 1 | 4155 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.15, 0.75] |
| 3 Preterm low birthweight (below or equal 2500 g) Show forest plot | 1 | 4155 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.34, 0.66] |
| 4 Neonatal morbidity | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Duration of admission to neonatal intensive care unit/hospital | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Neonatal death | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Side‐effects of treatment (including drug resistance) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Persistent infection | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Recurrent infection | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Women's satisfaction | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |

