Programas de cribado y tratamiento de infecciones del aparato genital inferior para la prevención del parto prematuro
Resumen
Antecedentes
La infección del tracto genital se asocia con el parto prematuro (antes de las 37 semanas de gestación). Por lo tanto, el cribado de infecciones durante el embarazo puede reducir el número de fetos que nacen de manera prematura. Sin embargo, el cribado de infecciones puede tener algunos efectos adversos, como el aumento de la resistencia a los antibióticos y el aumento del coste del tratamiento.
Objetivos
Evaluar la efectividad y las complicaciones de los programas prenatales de cribado y tratamiento de infecciones del aparato genital inferior para reducir el parto prematuro y la morbilidad posterior.
Métodos de búsqueda
Se realizaron búsquedas en el Registro Especializado de Ensayos Controlados del Grupo Cochrane de Embarazo y Parto (Cochrane Pregnancy and Childbirth Group) (30 de noviembre 2014), en el Registro Cochrane Central de Ensayos Controlados (Cochrane Central Register of Controlled Trials, CENTRAL) (The Cochrane Library 2014, Número 7) y en las listas de referencias de los artículos identificados.
Criterios de selección
Se incluyeron todos los ensayos controlados aleatorizados en cualquier idioma, publicados y no publicados, que evaluaron cualquier método descrito de cribado prenatal de infecciones del aparato genital inferior, en comparación con ningún cribado.
Obtención y análisis de los datos
Dos autores de la revisión, de forma independiente, evaluaron los ensayos para inclusión y el riesgo de sesgo, extrajeron los datos y verificaron su exactitud.
Resultados principales
Un estudio (4155 mujeres con menos de 20 semanas de gestación) cumplió los criterios de inclusión. Al grupo de intervención (2058 mujeres) se le realizó cribado de infecciones y tratamiento para la vaginosis bacteriana, la tricomoniasis vaginal y la candidiasis; al grupo control (2097 mujeres) también se le realizó el cribado, pero los resultados del programa de cribado no se revelaron y las mujeres recibieron atención prenatal habitual. La tasa de partos prematuros antes de las 37 semanas de gestación fue significativamente menor en el grupo de intervención (3% versus 5% en el grupo control), con un riesgo relativo (RR) de 0,55; intervalo de confianza (IC) del 95%: 0,41 a 0,75; la evidencia de este resultado se calificó como de calidad moderada. La incidencia de parto prematuro en los fetos con un peso igual o inferior a 2500 g (bajo peso al nacer) y los fetos con un peso igual o inferior a 1500 g (muy bajo peso al nacer) fueron significativamente inferiores en el grupo de intervención en comparación con el grupo control (RR 0,48; IC del 95%: 0,34 a 0,66 y RR 0,34; IC del 95%: 0,15 a 0,75, respectivamente; ambos calificados como evidencia de calidad moderada). Según un subconjunto de costes de los partos prematuros de < 1900 g, los autores informaron que por cada uno de esos partos prematuros evitados, se ahorrarían 60 262 euros.
Conclusiones de los autores
Hay evidencia de que los programas de cribado y tratamiento de infecciones en las embarazadas antes de las 20 semanas de gestación reducen el parto prematuros y el bajo peso al nacer prematuro. Los programas de cribado y tratamiento de infecciones se asocian con un ahorro en los costes cuando se utilizan para la prevención del parto prematuro. Los ensayos futuros deberían evaluar los efectos de los diferentes tipos de programas de cribado de infecciones.
PICO
Resumen en términos sencillos
Programas de cribado y tratamiento de infecciones del aparato genital inferior para la prevención del parto prematuro
Una infección del aparato genital durante el embarazo puede pasar al líquido amniótico y dar lugar a la rotura prematura de las membranas y al trabajo de parto prematuro. El parto prematuro (antes de las 37 semanas de gestación) se asocia con salud infantil deficiente y muertes tempranas, ingreso del recién nacido en unidades de cuidados intensivos neonatales en las primeras semanas de vida, estancia hospitalaria prolongada y discapacidad neurológica a largo plazo, incluida la parálisis cerebral.
En esta revisión solo se incluyó un estudio con evidencia de calidad moderada. El estudio informó sobre 4155 mujeres asignadas al azar a un grupo de intervención (2058 mujeres recibieron cribado de infecciones y tratamiento de la vaginosis bacteriana, la tricomoniasis vaginal y la candidiasis) o a un grupo control (2097 mujeres se sometieron a cribado, pero no se revelaron los resultados del programa de cribado). A partir de un solo estudio controlado identificado, esta revisión sistemática encontró que un programa sencillo de cribado y tratamiento de infecciones durante la atención prenatal habitual puede reducir los partos prematuros y los recién nacidos prematuros con bajo peso (menos de 2500 g) y muy bajo peso (menos de 1500 g) al nacer. Un método sencillo de cribado de infecciones redujo los partos prematuros, del 5% en las mujeres del grupo control al 3% en el grupo de intervención. El número de recién nacidos prematuros con bajo peso al nacer y con muy bajo peso al nacer fue significativamente menor en el grupo de intervención, comparado con el grupo control. Además, un programa de cribado y tratamiento de infecciones durante la atención prenatal habitual probablemente ahorre más de 60 000 euros por cada parto prematuro evitado.
Authors' conclusions
Summary of findings
| Lower genital tract infection screening versus no screening for preventing preterm delivery | ||||||
| Patient or population: pregnant women presenting for routine prenatal care | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| No screening | Lower genital tract infection screening | |||||
| Preterm birth less than 37 weeks | Study population | RR 0.55 | 4155 | ⊕⊕⊕⊝ | ||
| 53 per 1000 | 29 per 1000 | |||||
| Preterm low birthweight (below or equal 2500 g) | Study population | RR 0.48 | 4155 | ⊕⊕⊕⊝ | ||
| 51 per 1000 | 24 per 1000 | |||||
| Preterm very low birthweight (below or equal 1500 g) | Study population | RR 0.34 | 4155 | ⊕⊕⊕⊝ | ||
| 11 per 1000 | 4 per 1000 | |||||
| *The basis for the assumed risk is the median control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 One study with design limitations. | ||||||
Background
Description of the condition
Preterm birth, defined as birth occurring prior to 37 weeks' gestation, occurs in 5% to 10% of all pregnancies and is the most common cause of perinatal morbidity and mortality in the world. Preterm birth is implicated in at least two‐thirds of early infant deaths (Cunningham 1997) and 60% of perinatal mortality including long‐term neurologic disability such as cerebral palsy. It is associated with admission to neonatal intensive care, severe morbidity in the first weeks of life, prolonged hospital stay after birth, and readmission to hospital in the first year of life (Cunningham 2001; Goldenberg 1998; Roberts 2000; Wood 2000). Surviving infants, especially those born before 32 weeks, have a substantially increased risk of chronic lung disease, and major and minor impairments (Doyle 1996; Saigal 2000). Whatever the result, the emotional impact on the family can be enormous.
A wide spectrum of causes and demographic factors have been implicated in preterm birth. These can be categorized into four groups.
-
Medical and obstetric complications: there are associations with placental hemorrhage and hypertensive disorders in about one‐third of cases (Meis 1995).
-
Lifestyle factors: there is an association with alcohol abuse, low maternal age, and occupational factors (Henriksen 1995; Holzman 1995; Satin 1994).
-
Amniotic fluid infection caused by a variety of micro‐organisms located in the genital tract: approximately one‐third of preterm births are associated with chorioamniotic infection (Lettieri 1993).
-
Asymptomatic cervical dilatation (Papiernik 1986).
Many micro‐organisms cause both symptomatic and asymptomatic infection and may result in preterm prelabour rupture of membranes, preterm labour, or both. For example, bacterial vaginosis (including Gardnerella vaginalis, Bacteroides species, Mobiluncus species, Ureaplasma urealyticum, and Mycoplasma hominis) (Hillier 1995; McDonald 1994; McGregor 1990; Meis 1995), Chlamydia trachomatis (Gravett 1986), Trichomonas vaginalis (Cotch 1997), Neisseria gonorrhoeae (Elliott 1990), Group B Streptococci (GBS; Regan 1981), Staphylococcus aureus (McGregor 1990), syphilis (McFarlin 1995), HIV (Temmerman 1994), enteropharyngeal bacteria and Peptostreptococcus species (McDonald 1994) have been associated with an increased risk of preterm birth. Candida species, however, have been associated with an unclear risk of preterm birth (Roberts 2011).
A possible mechanism for the link between infection and preterm birth is the bacterial stimulation of the biosynthesis of prostaglandins. This may occur either directly via phospholipase A2 and C (Bejar 1981) or as a result of bacterial endotoxin introduced into the amniotic fluid which stimulates decidual cells to produce cytokines and prostaglandins that initiate labour (Cox 1989). Indirect links via substances such as interleukin‐1, tumour necrosis factor and platelet activating factor, all of which may be found in infected amniotic fluid, have also been identified (Romero 1992; Yoon 2000).
Description of the intervention
By identifying and treating vaginal infections, screening programs may be able to reduce the rate of preterm birth. Different screening methods are used for different types of organisms, however there is scant evidence to inform the optimal screening regimen for detecting these organisms during pregnancy. Therefore, it is unclear whether all women should be routinely screened, how often the screening should occur, and which tests should be used.
How the intervention might work
Chlamydia trachomatis has been identified by multiple tests from different specimen sources. The samples may be analysed by three types of DNA‐based test: ligase chain reaction, polymerase chain reaction (PCR) and enzyme immuno‐assay (Watson 2002). DNA amplification techniques allow for highly sensitive and specific tests (Black 1997) that are more sensitive than cell culture (Jespersen 2005). These screening tests can detect Chlamydia in genital secretions, urine specimens, and endocervical, vaginal or urethral samples (Domeika 1999; Shrier 2004).
Trichomoniasis may be asymptomatic in up to 50% of infected women (Wolner‐Hanssen 1989). The diagnosis is usually made on clinical findings and laboratory procedures (Petrin 1998). Most frequently, the saline wet‐mount preparation is used for observation of motile organisms under the light microscope. Wet‐mount smear is a cheap and quick method but more sensitive techniques are culture, immunofluorescence and enzyme immunoassay (Lossick 1991; Borchardt 1991). Different staining techniques include Gram stain, Giemsa stain, Papanicolaou smear, acridine orange (Borchardt 1991; Rein 1990); diverse molecularly‐based diagnostic methods, such as hybridization assay and PCR, may also be used. These tests vary widely in sensitivities and specificities for screening trichomoniasis (DeMeo 1996; Madico 1998; Mayta 2000; Muresu 1994).
Bacterial vaginosis is a clinical syndrome; the microbiology of bacterial vaginosis is complex and is composed of Gardnerella vaginalis, Mycoplasma hominis and anaerobic bacteria (Amsel 1983). The diagnosis is usually made on clinical Amsel criteria findings (Amsel 1983) and laboratory tests. Vaginal pH testing may be a valuable screening tool as it is a quick and inexpensive test (Gjerdingen 2000). Vaginal swab Gram stain with quantification of the microbial flora has high sensitivity and specificity and is accepted as an alternative method (Nugent 1991).
Multiple screening tests exist for other organisms including syphilis. Screening tests such as Treponema pallidum hemagglutination assay, Treponema pallidum particle agglutination assay, and enzyme‐linked immunosorbent assays (ELISAs) are more reliable than Venereal Disease Research Laboratory testing, the fluorescent treponemal antibody absorption test, and immunoblot assays (Muller 2006). The screening test for Neisseria gonorrhoeae, usually from a culture, remains accurate when transport conditions are suitable; this tests can be used with cervical, urine and vaginal swabs. Diagnosis of HIV infection can be obtained from enzyme‐linked immunosorbent assay (ELISA), Western blot, and RNA PCR testing (Kleinman 1998). The HIV‐p24 Ag is effective for early diagnosis of an acute HIV infection (Thies 1994). Strategies for the diagnosis of GBS include obtaining vaginal or both vaginal and anorectal GBS cultures (Quinlan 2000) and a rapid enrichment cum antigen detection test (Das 2003).
Why it is important to do this review
Other Cochrane reviews have addressed a number of issues regarding treatment of infection in pregnancy. Antibiotic treatment of chlamydia, trichomoniasis, bacterial vaginosis and gonorrhoeal infection in pregnancy appear to be effective to clear organisms (Brocklehurst 1998; Brocklehurst 2002; Gülmezoglu 2002; Brocklehurst 2013) but it is not known whether treatment of trichomonas will have any effect on pregnancy outcomes (Gülmezoglu 2002). There is also little evidence to show that screening and treatment in all asymptomatic pregnant women for bacterial vaginosis can prevent preterm birth (Brocklehurst 2013), although antibiotic prophylaxis in pregnancies with a previous preterm birth associated with bacterial vaginosis can reduce preterm delivery (Thinkhamrop 2002). There is insufficient evidence regarding the treatment of ureaplasmas to reduce preterm birth (Raynes‐Greenow 2004), and there is no evidence that antiretrovirals and the treatment of syphilis influence the incidence of preterm birth (Volmink 2007; Walker 2001). None of the aforementioned reviews are concerned primarily with screening programs for antenatal lower genital tract infection, thus a review of the effects of screening programs for lower genital tract infection to prevent preterm birth is required.
Objectives
To assess the effects of antenatal lower genital tract infection screening and treatment programs in reducing preterm birth and subsequent morbidity.
Methods
Criteria for considering studies for this review
Types of studies
We included all published and unpublished randomised controlled trials evaluating any described method of antenatal lower genital tract infection screening in pregnancy.
Types of participants
Women, at 37 or fewer weeks' gestation, who are not in labour, have no vaginal bleeding and are without symptoms of lower genital tract infection.
Types of interventions
Any lower genital tract infection screening and treatment program compared with no screening. The infection screening programs are defined as screening tests (such as wet mount, Gram stain and culture of vaginal secretions) followed by appropriate treatment after a positive screening test, or no treatment after a negative screening test. No screening is defined as routine antenatal care without screening for lower genital tract infections.
Types of outcome measures
Primary outcomes
-
Preterm birth (less than 37 weeks' gestation)
Secondary outcomes
-
Low birthweight (LBW) less than 2500 g
-
Very LBW less than 1500 g (not prespecified)
-
Neonatal morbidity: sepsis, respiratory distress syndrome, intraventricular hemorrhage, necrotizing enterocolitis, seizures
-
Duration of admission to neonatal intensive care unit or hospital
-
Death: stillbirth, neonatal mortality, infant mortality
-
Side‐effects of treatment including drug resistance
-
Persistent infection
-
Recurrent infection
-
Failure of treatment
-
Economic analysis (cost effectiveness, cost utility)
-
False positive/negative result of the screening program
-
Women's satisfaction
Search methods for identification of studies
The following methods section of this review is based on a standard template used by the Cochrane Pregnancy and Childbirth Group.
Electronic searches
We searched the Cochrane Pregnancy and Childbirth Group’s Trials Register by contacting the Trials Search Co‐ordinator (30 November 2014).
The Cochrane Pregnancy and Childbirth Group’s Trials Register is maintained by the Trials Search Co‐ordinator and contains trials identified from:
-
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
-
weekly searches of MEDLINE (Ovid);
-
weekly searches of Embase (Ovid);
-
handsearches of 30 journals and the proceedings of major conferences;
-
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Details of the search strategies for CENTRAL, MEDLINE and Embase, the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service can be found in the ‘Specialized Register’ section within the editorial information about the Cochrane Pregnancy and Childbirth Group.
Trials identified through the searching activities described above are each assigned to a review topic (or topics). The Trials Search Co‐ordinator searches the register for each review using the topic list rather than keywords.
In addition, we searched CENTRAL (The Cochrane Library 2014, Issue 7) using the search strategy detailed in Appendix 1.
Searching other resources
We did not identify any additional or ongoing trials from personal communication. We searched the reference lists of trials and review articles identified.
We did not apply any language or date restrictions.
Data collection and analysis
For methods used in the previous version of this review, seeSangkomkamhang 2008.
For this update, the following methods were used for assessing the three reports that were identified as a result of the updated search.
The following methods section of this review is based on a standard template used by the Cochrane Pregnancy and Childbirth Group.
Selection of studies
Two review authors independently assessed for inclusion all the potential studies identified as a result of the search strategy. We resolved any disagreement through discussion or, if required, we consulted the third review author.
Data extraction and management
We designed a form to extract data. For eligible studies, two review authors extracted the data using the agreed form. We resolved discrepancies through discussion or, if required, we consulted the third review author. Data were entered into Review Manager software (RevMan 2014) and checked for accuracy.
When information regarding any of the above was unclear, we planned to contact authors of the original reports to provide further details.
Assessment of risk of bias in included studies
Two review authors independently assessed the risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Any disagreement was resolved by discussion or by involving a third assessor.
(1) Random sequence generation (checking for possible selection bias)
We described for each included study the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the method as having a(n):
-
low risk of bias (any truly random process, for example random number table or computer random number generator);
-
high risk of bias (any non‐random process, for example odd or even date of birth or hospital or clinic record number);
-
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
We described for each included study the method used to conceal allocation to interventions prior to assignment and assessed whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the methods as having a(n):
-
low risk of bias (for example telephone or central randomisation or consecutively numbered sealed opaque envelopes);
-
high risk of bias (for example open random allocation, unsealed or non‐opaque envelopes, alternation, based on date of birth);
-
unclear risk of bias.
(3.1) Blinding of participants and personnel (checking for possible performance bias)
We described for each included study the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We considered that studies were at low risk of bias if they were blinded, or if we judged that the lack of blinding was unlikely to affect results. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed the methods as having a(n):
-
low, high or unclear risk of bias for participants;
-
low, high or unclear risk of bias for personnel.
(3.2) Blinding of outcome assessment (checking for possible detection bias)
We described for each included study the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed the methods used to blind outcome assessment as having a(n):
-
low, high or unclear risk of bias.
(4) Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data)
We described for each included study, and for each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported and the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported, or could be supplied by the trial authors, we planned to re‐include missing data in the analyses which we undertook.
We assessed the methods as having a(n):
-
low risk of bias (for example no missing outcome data or missing outcome data balanced across groups);
-
high risk of bias (for example numbers or reasons for missing data imbalanced across groups or ‘as treated’ analysis done with substantial departure from the treatment assigned at randomisation);
-
unclear risk of bias.
(5) Selective reporting (checking for reporting bias)
We described for each included study how we investigated the possibility of selective outcome reporting bias and what we found.
We assessed the methods as having a(n):
-
low risk of bias (where it was clear that all of the study’s pre‐specified outcomes and all expected outcomes of interest to the review have been reported);
-
high risk of bias (all of the study’s pre‐specified outcomes have been reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported);
-
unclear risk of bias.
(6) Other bias (checking for bias due to problems not covered by (1) to (5) above)
We described for each included study any important concerns we had about other possible sources of bias.
(7) Overall risk of bias
We made explicit judgements about whether studies were at a high risk of bias, according to the criteria given in the Handbook (Higgins 2011). With reference to (1) to (6) above, we planned to assess the likely magnitude and direction of the bias and whether we considered it likely to impact on the findings. In future updates, we will explore the impact of the level of bias through undertaking sensitivity analyses (see Sensitivity analysis).
For this update the quality of the evidence was assessed using the GRADE approach (Schunemann 2009). We assessed the quality of the body of evidence relating to the following outcomes for the comparison of lower genital tract infection screening versus no screening.
-
Preterm birth (less than 37 weeks).
-
Preterm low birthweight (below or equal to 2500 g).
-
Preterm very low birthweight (below or equal to 1500 g).
Outcomes number two and three are subsets of outcome number one.
GRADEprofiler (GRADE 2014) was used to import data from Review Manager 5.3 (RevMan 2014) in order to create ’Summary of findings’ tables. A summary of the intervention effect and a measure of quality for each of the above outcomes was produced using the GRADE approach. The GRADE approach uses five considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the quality of the body of evidence for each outcome. The evidence can be downgraded from 'high quality' by one level for serious (or by two levels for very serious) limitations, depending on assessments for risk of bias, indirectness of evidence, serious inconsistency, imprecision of effect estimates or potential publication bias.
Measures of treatment effect
Dichotomous data
For dichotomous data, we presented results as summary risk ratio with 95% confidence intervals.
Continuous data
We used the mean difference if outcomes were measured in the same way between trials (and in this case, since there was only one included trial). We would have used the standardised mean difference to combine trials that measured the same outcome but used different methods.
Unit of analysis issues
Cluster‐randomised trials
For future updates, we will include cluster‐randomised trials in the analyses along with individually randomised trials. We will adjust their sample sizes or standard errors using the methods described in the Handbook [Section 16.3.4 or 16.3.6] using an estimate of the intracluster correlation co‐efficient (ICC) derived from the trial (if possible), from a similar trial or from a study of a similar population. If we use ICCs from other sources, we will report this and conduct sensitivity analyses to investigate the effect of variation in the ICC. If we identify both cluster‐randomised trials and individually‐randomised trials, we plan to synthesise the relevant information. We will consider it reasonable to combine the results from both if there is little heterogeneity between the study designs and the interaction between the effect of the intervention and the choice of the randomisation unit is considered to be unlikely.
We will also acknowledge heterogeneity in the randomisation unit and perform sensitivity or subgroup analysis to investigate the effects of the randomisation unit.
Dealing with missing data
For the included study, levels of attrition were noted. In future updates, if more eligible studies are included, the impact of including studies with high levels of missing data in the overall assessment of treatment effect will be explored by using sensitivity analysis.
For all outcomes, analyses were carried out, as much as possible, on an intention‐to‐treat basis. In other words, we attempted to include all participants randomised to each group in the analyses. The denominator for each outcome was the number randomised minus any participants whose outcomes were known to be missing.
Assessment of heterogeneity
We assessed statistical heterogeneity in each meta‐analysis using the Tau², I² and Chi² statistics. We regarded heterogeneity as substantial if I² was greater than 30% and either Tau² was greater than zero, or there was a low P value (less than 0.10) in the Chi² test for heterogeneity. If we identified substantial heterogeneity (above 30%), we planned to explore it by pre‐specified subgroup analysis.
Assessment of reporting biases
In future updates, if there are 10 or more studies in the meta‐analysis we will investigate reporting biases (such as publication bias) using funnel plots. We will assess funnel plot asymmetry visually. If asymmetry is suggested by a visual assessment, we will perform exploratory analyses to investigate it.
Data synthesis
We carried out statistical analysis using the Review Manager software (RevMan 2014). We used a fixed‐effect meta‐analysis in this case, since only one study was included. In future updates, we will also used a fixed‐effect meta‐analysis to combine data where it is reasonable to assume that studies are estimating the same underlying treatment effect, i.e. where trials are examining the same intervention, and the trials’ populations and methods are judged to be sufficiently similar.
In future updates, ff there is clinical heterogeneity sufficient to expect that the underlying treatment effects differ between trials, or if substantial statistical heterogeneity was detected, we will use a random‐effects meta‐analysis to produce an overall summary (if an average treatment effect across trials is considered clinically meaningful). The random‐effects summary will be treated as the average range of possible treatment effects and we will discuss the clinical implications of treatment effects differing between trials. If the average treatment effect is not clinically meaningful, we will not combine trials. If we use random‐effects analyses, the results will be presented as the average treatment effect with 95% confidence intervals, and the estimates of Tau² and I².
Subgroup analysis and investigation of heterogeneity
For future updates if we identify substantial heterogeneity, we will investigate it using subgroup analyses and sensitivity analyses. We will consider whether an overall summary is meaningful, and if it is, we will use a random‐effects analysis to produce it.
We will carry out the following subgroup analyses.
-
Early versus late trimester at screening (defined by author).
-
Low risk versus high risk of preterm birth, for example multiple pregnancy or previous history of preterm birth.
We plan to restrict subgroup analyses to the primary outcome.
We will assess subgroup differences by interaction tests available within RevMan (RevMan 2014). We will report the results of subgroup analyses quoting the Chi² statistic and P value, and the interaction test I² value.
Sensitivity analysis
We planned to carry out sensitivity analyses to explore the effect of trial quality assessed by concealment of allocation, high attrition rates, or both, with poor quality studies being excluded from the analyses in order to assess whether this makes any difference to the overall result.
Results
Description of studies
Also see Characteristics of included studies table.
Results of the search
A new search identified three reports assessed for possible inclusion in the review. Two reports related to the same trial; this trial was excluded (Sungkar 2012). The third report was an additional report for the included trial Kiss 2004, with additional outcome data and economic analysis.
Included studies
One included article (Kiss 2004) reported a randomised controlled trial designed to evaluate a vaginal infection screening strategy for the prevention of preterm delivery in a general population of pregnant women. A total of 4155 pregnant women presenting for a routine prenatal visit without subjective complaints were randomised to either the intervention group (n = 2058) or the control group (n = 2097). All women were screened by Gram stain for asymptomatic vaginal infection. For the intervention group, women found to have vaginal infection received standard treatment. For the control group, vaginal smear test results were not revealed so the standard antenatal care program could not be influenced.
Additionally, cost effectiveness of a screen‐and‐treat program for asymptomatic vaginal infections in pregnancy (the direct medical costs of preterm delivery of infants with a birthweight below 1900 g and the costs of the screen‐and‐treat program) was reported.
Excluded studies
Three trials (Gjerdingen 2000; McGregor 1995; Sungkar 2012) were excluded because the participants did not meet the inclusion criteria or the study was not a randomised controlled trial. For further details, please see the Characteristics of excluded studies table.
Risk of bias in included studies
For Kiss 2004, sequence generation was by computer which was judged to be at low risk of bias. See 'Risk of bias' table in Characteristics of included studies and Figure 1.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
There were no details about the random assignment process, therefore the risk of selection bias was unclear.
Blinding
Blinding of the intervention was not possible. In the intervention group, women and their obstetricians were aware of the results of the screening tests, as women were treated for any detected infections. Thus, the risk of detection bias was present; the obstetricians may have provided a different level of care to women in the intervention group in whom an infection had been identified. Blinding of outcome assessors was not described, however this is unlikely to affect the outcome of birthweight.
Incomplete outcome data
Of the 4429 pregnant women who were randomised, 274 were excluded (140 lost to follow up; 68 did not fulfill all the inclusion criteria; 66 had multiple pregnancies). The overall attrition was less than 10%, however, it was not specified how many patients were lost to follow‐up in each arm.
Selective reporting
We did not have the protocol for the included study, therefore we assessed the risk of bias for selective reporting as unclear. We have requested that the authors provide us with additional data but have received no reply.
Other potential sources of bias
None identified.
Effects of interventions
Lower genital tract infection screening versus no screening
Primary outcomes
We identified a single randomised controlled trial (Kiss 2004) comparing antenatal lower genital tract infection screening and treatment programs for preventing preterm delivery with no screening program. A total of 4429 women were randomised with 274 women excluded from the analysis. In the intervention group (2058 women), the results of infection screening and treatment for bacterial vaginosis, Trichomonas vaginalis and candidiasis were reported; in the control group (2097 women), the results of the screening tests for the women allocated to receive routine antenatal care were not reported. There was a statistically significant difference in number of preterm births before 37 weeks between the two groups (risk ratio (RR) 0.55, 95% confidence interval (CI) 0.41 to 0.75, Analysis 1.1).
Secondary outcomes
Numbers of preterm low birthweight infants (weight equal to or below 2500 g) and preterm very low birthweight infants (weight equal to or below 1500 g) were significantly lower in the intervention group than in the control group (RR 0.48, 95% CI 0.34 to 0.66 and RR 0.34, 95% CI 0.15 to 0.75, respectively) (Kiss 2004). None of the women reported adverse effects during the treatment period. Neonatal morbidity and mortality were not reported.
For a subset of preterm infants with a birthweight less than 1900 g in the Kiss 2004 trial, hospital costs for mother and baby and the costs of the screening program were assessed to be EUR 60,692 (with screening and treatment contributing only 7% of costs). Overall cost savings per prevented preterm birth for this subset therefore amounted to EUR 60,692. In this trial, the screening program halved the number of preterm infants with a birthweight < 1900 g. This threshold was chosen as these babies were all transferred to the neonatal intensive care unit.
Discussion
Summary of main results
In a single trial, an antenatal lower genital tract infection screening and treatment program was shown to significantly reduce preterm birth, low birthweight preterm births (below 2500 g) and very low birthweight preterm births (below 1500 g). This intervention led to savings in direct costs associated with prematurity. The quality of the evidence was rated as moderate.
Overall completeness and applicability of evidence
The included trial was conducted in a developed country (Austria) where characteristics of the population, such as incidence and pattern of lower genital tract infections and socioeconomic status, might differ compared to other countries. Therefore, the results of this review might not be globally generalisable. There was also economic evaluation of this intervention.
Quality of the evidence
Using the Cochrane Grades of Recommendation, Assessment, Development, and Evaluation (GRADE) approach, the evidence for preterm birth outcomes was graded as moderate.The strength of this review was that the included trial was a large multi‐centre prospective, randomised controlled trial. There was a clear sample‐size calculation and an adequate number of participants were available for the analysis. However, around 3.2% of all randomised women (140/4429) were lost to follow up, and the study authors did not report whether the loss rate was balance between the two groups. Further, there was no blinding of group assignment or of screening results in the intervention group. The differences between the care received in the treatment and control arms may have introduced bias, depending upon the outcome measure in question.
The cost‐benefit analysis showed a substantial savings of more than EUR 60,000 per preterm birth averted for a subset of low birthweight babies routinely admitted to the neonatal intensive care unit. Costs for the 75% of preterm babies with birthweights > 1900 g in this trial were not reported but are assumed to be lower due to lower rates of hospitalisation.
Potential biases in the review process
We followed the methods set out in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). A comprehensive search was performed, all studies were examined, and the data were independently extracted by at least two review authors. We restricted the included studies to RCTs as they provide the strongest level of evidence. Therefore, we have attempted to reduce bias in the review process.
Agreements and disagreements with other studies or reviews
The results agree with another Cochrane systematic review (Brocklehurst 2013), which reported that antibiotic treatment of bacterial vaginosis in pregnant women with abnormal vaginal flora may reduce preterm birth at less than 37 weeks' gestation. Our results differed from Gülmezoglu 2011, which reported that metronidazole for the treatment of asymptomatic pregnant women with trichomoniasis led to an increase in preterm birth at less than 37 weeks' gestation compared with no treatment.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
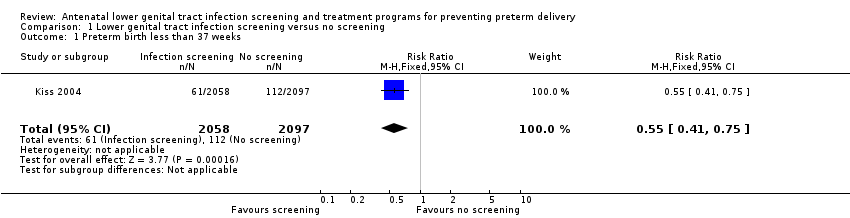
Comparison 1 Lower genital tract infection screening versus no screening, Outcome 1 Preterm birth less than 37 weeks.

Comparison 1 Lower genital tract infection screening versus no screening, Outcome 2 Preterm very low birthweight (below or equal 1500 g).

Comparison 1 Lower genital tract infection screening versus no screening, Outcome 3 Preterm low birthweight (below or equal 2500 g).
| Lower genital tract infection screening versus no screening for preventing preterm delivery | ||||||
| Patient or population: pregnant women presenting for routine prenatal care | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| No screening | Lower genital tract infection screening | |||||
| Preterm birth less than 37 weeks | Study population | RR 0.55 | 4155 | ⊕⊕⊕⊝ | ||
| 53 per 1000 | 29 per 1000 | |||||
| Preterm low birthweight (below or equal 2500 g) | Study population | RR 0.48 | 4155 | ⊕⊕⊕⊝ | ||
| 51 per 1000 | 24 per 1000 | |||||
| Preterm very low birthweight (below or equal 1500 g) | Study population | RR 0.34 | 4155 | ⊕⊕⊕⊝ | ||
| 11 per 1000 | 4 per 1000 | |||||
| *The basis for the assumed risk is the median control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 One study with design limitations. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Preterm birth less than 37 weeks Show forest plot | 1 | 4155 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.41, 0.75] |
| 2 Preterm very low birthweight (below or equal 1500 g) Show forest plot | 1 | 4155 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.15, 0.75] |
| 3 Preterm low birthweight (below or equal 2500 g) Show forest plot | 1 | 4155 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.34, 0.66] |
| 4 Neonatal morbidity | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Duration of admission to neonatal intensive care unit/hospital | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Neonatal death | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Side‐effects of treatment (including drug resistance) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Persistent infection | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Recurrent infection | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Women's satisfaction | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |

