Probióticos para el tratamiento del eccema
Referencias
Referencias de los estudios incluidos en esta revisión
Referencias de los estudios excluidos de esta revisión
Referencias de los estudios en espera de evaluación
Referencias de los estudios en curso
Referencias adicionales
Referencias de otras versiones publicadas de esta revisión
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Three‐month parallel‐group randomised controlled trial | |
| Participants | Fifty‐one infants under 5 months age with mild/moderate eczema diagnosed using Hanifin and Rajka criteria, and a clinical history suggestive of cow's milk allergy. Randomisation was done at a 1:1 ratio: 17 participants were randomised in each arm (L rhamnosus, L GG, placebo). All participants were exclusively formula fed and received an extensively hydrolysed formula for 3 to 5 weeks before receiving the study intervention. Infants receiving antihistamines, oral corticosteroids, or any probiotic/antibiotic/antimycotic in the preceding 4 weeks were excluded, as were those with a congenital gastrointestinal malformation Setting: primary care in the Netherlands One participant lost to follow‐up | |
| Interventions | Extensively hydrolysed whey‐based formula given alone, with Lactobacillus rhamnosus at 5 × 10⁹ CFUs/100 mL or with Lactobacillus GG at 5 × 10⁹ CFUs/100 mL. The study formula was offered at all feeds during the intervention period | |
| Outcomes | SCORAD assessed at baseline, and at 1, 2, and 3 months* Total IgE, specific IgE to food mix (cow's milk, egg white, soy, peanut, cod, and wheat) and cow's milk, and skin prick test for cow's milk *Denotes outcomes prespecified for this review | |
| Notes | Study was funded by unrestricted grant from Numico Research, Wageningen, the Netherlands ‐ not related to probiotic. No information on conflicts of interest | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "all patients were randomised to either one of the study formulas..." Comment: no other information provided; method of randomisation not known |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: "We conducted a randomised, double‐blind, placebo‐controlled study..." Comment: no further information provided; unclear whether blinding was adequate |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: "We conducted a randomised, double‐blind, placebo‐controlled study..." Comment: no further information provided; unclear whether blinding was adequate |
| Incomplete outcome data (attrition bias) | Low risk | Only 1 participant lost to follow‐up after randomisation; available case analysis without exclusions or imputation used |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting found: all outcomes reported |
| Other bias | Low risk | No other bias found |
| Methods | Eight‐month (3‐month intervention and 5‐month observation period) parallel‐group randomised double‐blind controlled trial | |
| Participants | Sixty children aged up to 24 months with atopic dermatitis diagnosed according to the criteria of Hanifin and Rajka (3 out of 4 major criteria had to be met) and with symptoms of cow's milk allergy were recruited. Randomisation was done at a 1:1 ratio: 29 participants were randomised in the intervention arm and 31 in the placebo arm. Participants and mothers of breast‐fed children had to be on a non‐dairy diet. Participants who had used antibiotics and probiotics within 6 months before recruitment were excluded from the trial. Recruitment took place at a secondary paediatric centre in Poland | |
| Interventions | Probiotic mixture: Lactobacillus casei LOCK 0900, Lactobacillus casei LOCK 08, Lactobacillus paracasei LOCK 0919 at a total daily dose of 10⁹ CFUs/d given orally for 3 months Placebo: hydrolysed casein given orally for 3 months | |
| Outcomes | Improvement vs exacerbation/no improvement based on SCORAD* • If SCORAD reduction > 2: improvement • If SCORAD reduction 0 to 2: lack of improvement • If SCORAD increased: deterioration *Denotes outcomes prespecified for this review | |
| Notes | Study financed by Ministry of Education grant | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote from translation from Polish: "The study carried out was randomised, double‐blinded..." Comment: no information provided on the random sequence generation method |
| Allocation concealment (selection bias) | Unclear risk | Quote from translation from Polish: "The study carried out was randomised, double‐blinded..." Comment: no information given on allocation concealment |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote from translation from Polish: "The study carried out was randomised, double‐blinded..." Comment: no information given on blinding method |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote from translation from Polish: "The study carried out was randomised, double‐blinded..." Comment: no information given on blinding method |
| Incomplete outcome data (attrition bias) | High risk | 29% of participants in probiotic group and 32% in placebo group lost to follow‐up at the end of the intervention Comment: losses to follow‐up similar in both groups but high in both and may have influenced the outcome of short‐term change in global eczema severity; no information provided on whether available case analysis was used. No reasons for losses to follow‐up given |
| Selective reporting (reporting bias) | Low risk | All outcomes reported as predefined |
| Other bias | Low risk | No other bias found |
| Methods | Twenty‐week parallel‐group double‐blind placebo‐controlled randomised trial | |
| Participants | Thiirty‐eight adult participants between 18 and 46 years with moderate to severe atopic dermatitis diagnosed according to "Consensus guidelines in diagnosis and treatment of atopic dermatitis" (Eichenfield 2004). Randomisation was done at a 1:1 ratio: 19 participants were randomised in each arm. Patients who had received probiotics or antibiotics or who had used immunomodulators (tacrolimus or pimecrolimus) within 6 months from enrolment were excluded from the trial. Also excluded were patients with active allergic disease of the skin or the respiratory tract or chronic infectious disease, and pregnant or lactating patients. All participants completed the study Secondary care setting; recruiting from an Allergy and Immunology Unit in Italy | |
| Interventions | Probiotic: Lactobacillus salivarius LDR0723 in maltodextrin, given in sachets dissolved in water or other cold liquid of preference twice daily at a dose of 1 × 10⁹ CFUs/g for 16 weeks. Placebo: maltodextrin alone given twice daily for 16 weeks | |
| Outcomes | • SCORAD at baseline and at end of treatment at 16 weeks* • DLQI at baseline, and at 4, 8, 16, and 20 weeks* *Denotes outcomes prespecified for this review | |
| Notes | Probiotics supplied by Probiotic Company. No information about conflicts of interest | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A computerised randomisation schedule was prepared..." Comment: judged as low risk |
| Allocation concealment (selection bias) | Low risk | Quote: "...allocation and dispensing by a blind clinical investigator..." ‐ "the probiotic and placebo sachets were matched for size, shape and volume of contents" Comment: judged as adequate allocation concealment and hence at low risk of bias |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "...allocation and dispensing by a blind clinical investigator..." ‐ "the probiotic and placebo sachets were matched for size, shape and volume of contents" Comment: judged as adequate and hence at low risk of bias. |
| Blinding of outcome assessment (detection bias) | Low risk | Quote for participants who completion of the DLQI questionnaires: "the probiotic and placebo sachets were matched for size, shape and volume of contents" Quote for clinical assessor: "a single investigator who was blind to the treatment intervention performed all SCORAD assessment at the beginning.... and at the end of treatment..." Comment: judged as adequate and hence at low risk of bias |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "All patients completed the study" Comment: no losses to follow‐up; all participants analysed in the group to they were randomised; therefore, no incomplete data |
| Selective reporting (reporting bias) | High risk | All outcomes reported with numerical data or narratively Comment on SCORAD reporting: significance or non‐significance of difference in global eczema severity (SCORAD) between probiotic and placebo groups not reported. Only scores at baseline and end of treatment and at baseline and 4 weeks after treatment reported and commented on Quote: "the mean SCORAD score in the probiotic group was 27.57±3.4 versus 24.28±3.8 in the placebo group. After 4 months we observed a significant reduction in the SCORAD score in the probiotic‐treated group only (T0: 27.57±3.4 vs T16: 13.14±0.27, P<0.001) whereas no changes were reported in the placebo group (T0: 24.28±2.15 vs T16: 20.14±0.27, NS)" Comment on DLQI reporting: data presented at end of treatment and 4 weeks after end of treatment for the probiotic group only. Significant difference from baseline stated. Data for the placebo group not reported and given only narratively; no difference from baseline Quote: "DLQI progressively decreased in probiotic patients during treatment. This significant modification was observed after 8 weeks of treatment (T8) and was also maintained after 4 weeks after the end of the treatment (T20) (T0: 8.28±1.79 vs T8: 4.57±1.11, P=0.02; T0: 8.28±1.79 vs T16: 4.42±0.27, P=0.04; T0: 8.28±1.79 vs T20: 3.71±0.27, P=0.02). No differences were reported in the placebo group" Comment: only outcome data that were significant for the probiotic group reported. No comparison between probiotics and placebo. Report judged to be at high risk of reporting bias |
| Other bias | High risk | Commercial bias: probiotics supplied by Probiotic Company. Study double‐blind and randomised; unlikely that the commercial bias had an effect on the outcome. However, it had an impact on reporting because only positive outcomes for the probiotic group were reported Study assessed as having high risk of other bias |
| Methods | Thirty‐day randomised placebo‐controlled parallel pilot trial | |
| Participants | Twenty‐five adults 25 to 63 years of age with diagnosis of atopic dermatitis according to Hanifin and Rajka, with predominant rough fissured skin as well as pruritus for at least 2 months were recruited. Randomisation was done at a 1:2 ratio: 13 participants were randomised in the intervention arm and 12 in the control arm Pregnant and lactating women were excluded. Also excluded were patients with chronic dermatoses such as seborrhoeic dermatitis, contact dermatitis, nummular eczema, psoriasis, ichthyosis, immunodeficiency or any immunological disorder, scabies, cutaneous fungal infection, HIV‐associated skin disorders, malignant disease, T‐cell lymphoma, Letterer‐Siwe disease, progressive systemic disease, serious internal disease (e.g. serious decompensated diseases of the heart, liver, and/or kidneys, diabetes mellitus), or hypersensitivity toward one of the ingredients in the investigational product. Excluded were patients who had been taking part in another study or had taken an investigational product during the last 4 weeks before the start of treatment, or who had been receiving treatment with physical ultraviolet therapy, anti‐inflammatory medications used to treat atopic dermatitis, or immunomodulating medications 30 days before the start of the study, or non‐steroidal antirheumatic drugs, systemic glucocorticosteroids, tranquillisers, or antiemetic agents from the phenothiazine group 14 days before the start of the study, or antidepressants 7 days before the study All participants completed the study Country: Italy Setting: secondary Dermatology Unit | |
| Interventions | Participants in the probiotic group (n = 13) received freeze‐dried mixture of 5 × 10⁹ CFUs/sachet of Lactobacillus salivarius LS01 (DSM 227775), 2 × 10⁹ CFUs/sachet of Streptococcus thermophilus ST10 (DSM 25246), and tara gum (125 mg) Participants in the placebo group (n = 12) received treatment with sachets containing gluten‐free maltodextrins Sachets were dissolved in water and were taken once daily for 30 days Study participants were allowed to continue to use any medication that they had been taking before the study at the same dose, unless the medication could be discontinued | |
| Outcomes | Objective SCORAD index before treatment and after 30 days of treatment* *Denotes outcomes prespecified for this review | |
| Notes | The study has not been registered Probiotics were supplied by the manufacturer. Investigators declared no conflicts of interest | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants randomised at a 1:1 ratio to receive treatment or placebo Quote from communication with study author: "Which was the method of randomisation? Patients were randomised to either probiotic or placebo groups with a 1:1 allocation according to computer‐generated random numbers" Judgement: probably low risk of bias |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) | Low risk | Quote from correspondence with study author: "Was there any blinding? Clinicians, microbiologists and participants were blinded" Judgement: probably adequate, i.e. low risk of bias |
| Blinding of outcome assessment (detection bias) | Low risk | Quote from correspondence with study author: "Was there any blinding? Clinicians, microbiologists and participants were blinded" Judgement: probably adequate, i.e. low risk of bias |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "All of the 25 patients who agreed to commence the protocol completed the study" Judgement: low risk of bias |
| Selective reporting (reporting bias) | Low risk | Quote: "The SCORAD index significantly diminished in the active group from T0 to T1 (P<0.0001, Fig. 1), whereas no variations were observed in the placebo group (P=0.274, Fig. 1). After 1 month of treatment, the SCORAD index in group A was significantly lower than in group B (P=0.015)" Judgement: SCORAD changes reported narratively and not numerically (P value only) between baseline and end of treatment for each group only and between the 2 groups at end of treatment All other outcomes reported |
| Other bias | Unclear risk | Probiotics supplied by the manufacturer. Investigators declared no conflicts of interest but did not report numerical results. Also inadequate information on allocation concealment, and not clear whether the manufacturer had any influence on this. Study not registered For these reasons, study judged to be at unclear risk of commercial bias |
| Methods | Eight‐week parallel‐group double‐blind placebo‐controlled randomised trial conducted between November 2007 and March 2009 | |
| Participants | Fifty‐two children from 3 months to 6 years of age with mild to severe atopic dermatitis. Randomisation was done at a 1:1 ratio, but no exact numbers were given for randomised participants in each arm. Patients who had prior exposure to probiotics, or who were at the time taking antibiotics, or who had major medical problems, were excluded from the trial. Twelve patients were lost to follow‐up Setting: secondary care, paediatric Allergy and Immunology Department in Iran | |
| Interventions | Synbiotic mixture: Lactobacillus casei, Lactobacillus rhamnosus, Streptococcus thermophilus, Bifidobacterium breve, Lactobacillus acidophilus, Bifidobacterium infantis, Lactobacillus bulgaricus, and fructo‐oligo‐saccharide in 1‐gram sachets dissolved in water or breast milk, at a dose of 1 × 10⁹ CFUs/g twice daily for 8 weeks. Placebo not specified | |
| Outcomes | SCORAD change from baseline to 4 and 8 weeks and from 4 to 8 weeks* *Denotes outcomes prespecified for this review | |
| Notes | "No conflicts of interest" declared but funding not declared; probiotic provided by the manufacturer | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Participants were randomly divided into two groups..." Comment: no other information provided; method of randomisation unknown; therefore unclear risk of bias |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Participants were randomly divided into two groups..." Comment: unclear whether allocation was concealed; no more information provided |
| Blinding of participants and personnel (performance bias) | Unclear risk | Participant: "placebo powder was matched for size, shape and volume of contents" Clinicians: "this randomised, double blind, placebo controlled trial..." ‐ "SCORAD index assessment was performed by a single clinician who was blinded to intervention" Comment: risk of bias unclear regarding blinding of personnel allocating participants to treatment; no relevant information |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "SCORAD index assessment was performed by a single clinician who was blinded to intervention" ‐ "placebo powder was matched for size, shape and volume of contents" ‐ "placebo powder was matched for size, shape and volume of contents" Comment: blinding of participants and investigators who assess the global eczema severity score (the only clinical outcome of this study) judged to be adequate; therefore study judged to be of low risk of detection bias |
| Incomplete outcome data (attrition bias) | High risk | High rate of losses to follow‐up: 12 participants (23%) lost to follow‐up and excluded from analysis. We judged this to lead to high risk of attrition bias. Not clear whether rates of loss to follow‐up were similar in both groups. Reasons for loss to follow‐up given, but treatment group from which losses occurred not specified |
| Selective reporting (reporting bias) | Unclear risk | Twelve participants (23%) lost to follow‐up and excluded from analysis. Baseline characteristics and outcomes reported only for participants completing the trial. Unclear whether reporting only characteristics of participants can lead to reporting bias. Trial registered in the Iranian Registry for Clinical Trials retrospectively, and number of participants registered pertains to those who completed the study only. Unclear risk of bias, as not clear whether this could have influenced the outcome for global eczema severity |
| Other bias | Unclear risk | "No conflicts of interest" declared, but not the funding. Probiotic provided by the manufacturer |
| Methods | Three‐month parallel‐group randomised double‐blind placebo‐controlled trial | |
| Participants | Thirteen children from 0 to 3 years of age with atopic dermatitis, skin prick test ≥ 2+ for at least 2 food allergens, strong clinical history suggestive of food allergy, or positive placebo‐controlled challenge and IgE RAST. Randomisation was done at a 1:2 ratio: 7 participants were randomised in the intervention arm, and 6 in the control arm ≥ 0.7 KU/L for at least 2 food allergens were recruited in the trial. Patients on systemic immunomodulating drugs and those with other systemic diseases or immunodeficiency were excluded from the trial Recruitment took place at a secondary care setting in the Netherlands | |
| Interventions | Probiotic mixture: Lactobacillus acidophilus W55, Lactobacillus casei W56, Lactobacillus salivarius W57, Lactobacillus lactis W58, Bifidobacterium infantis W52, Bifidobacterium lactis W18, Bifidobacterium longum W51 In rice starch and maltodextrin powder dissolved in warm water or infant formula before administration given once daily at a dose of 1 × 10⁹ CFUs for 3 months Placebo: rice starch and maltodextrin powder dissolved in warm water or infant formula before administration given once daily for 3 months | |
| Outcomes | • Total SCORAD: secondary outcome for the trial ‐ not reported in relevant publication* • Allergen‐specific T‐ and B‐cell response in vivo and ex vivo • RAST (IgE levels) • Skin prick test *Denotes outcomes prespecified for this review | |
| Notes | Study sponsored by probiotic manufacturer; no information on conflicts of interest | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote from communication with author: "Randomization was performed by the sponsor (Winclove Bio Industries BV), by using a randomisation table. They provided us with boxes with blinded sachets, with only study numbers on it. We anticipated to include 12 children (6 in each group), so Winclove made sure that the randomisation was performed as such that the first 12 numbers included 6 verum and 6 placebo" ‐ "We had 20 sets of blinded sachets at start of the study, to be able to include more children if there would be dropouts during the study" Comment: judged as adequate for low risk of bias |
| Allocation concealment (selection bias) | Low risk | See above quote. Study author confirmed that the allocation sequence was concealed Comment: judged as adequate for low risk of bias |
| Blinding of participants and personnel (performance bias) | Low risk | See above quote and: "The participants, clinician and outcome assessor were blinded until after the analysis of the results had been performed" Comment: judged as adequate for low risk of bias |
| Blinding of outcome assessment (detection bias) | Low risk | See above quote and: "The participants, clinician and outcome assessor were blinded until after the analysis of the results had been performed" Comment: judged as adequate for low risk of bias |
| Incomplete outcome data (attrition bias) | Low risk | 1/13 participants lost to follow‐up but no exclusions from analysis. Reason for exclusion given by study author: "one child had to stop during the study because of the use of antibiotics and high fever" Judged as low attrition bias |
| Selective reporting (reporting bias) | Low risk | All primary outcomes reported SCORAD ‐ secondary outcome ‐ not reported in the publication but provided by the study author |
| Other bias | Unclear risk | Study sponsored by probiotic manufacturer. Role of the sponsor in data analysis and publication unclear; hence study was judged to be at unclear risk of other bias |
| Methods | Eight‐week parallel‐group randomised controlled trial from 2001 until 2002 | |
| Participants | Fifty‐four children 1 to 55 months of age with eczema diagnosed using Hanifin and Rajka criteria Setting: German Dermatology Centre Randomisation was done at a 1:1 ratio: 27 participants were randomised in each arm. Two‐centre trial. Six participants were lost to follow‐up, and 1 participant dropped out post randomisation but before treatment started | |
| Interventions | Lactobacillus GG at 10¹º CFU/d as a twice‐daily dose, or microcrystalline cellulose placebo. Interventions given as capsules, which were mixed with milk if bottle fed, or mixed with water if not bottle fed | |
| Outcomes | • Parent global assessment of disease severity* *Denotes outcomes prespecified for this review | |
| Notes | Study was supported by InfectoPharm GmbH (Heppenheim, Germany) and Pharmacia GmbH (Freiburg, Germany) ‐ not related to probiotic "No conflicts of interest" declared | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "...randomly allocated" Comment: no concrete information on randomisation method ‐ inadequate for a judgement |
| Allocation concealment (selection bias) | Unclear risk | No information provided. Unable to assess the risk of bias |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quotes: "double blind..." ‐ "...placebo preparation (microcrystalline cellulose) with identical appearance" Comment: inadequate information for a judgement on risk of bias. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quotes: "double blind..." ‐ "...placebo preparation (microcrystalline cellulose) with identical appearance" Comment: inadequate information for a judgement on risk of bias |
| Incomplete outcome data (attrition bias) | Low risk | Seven participants lost to follow‐up post randomisation (13%); of those, 1 after randomisation (not clear from which group) but before treatment started. Low rates of loss to follow‐up in both groups (15.4% in probiotic group and 7.4% in placebo). Available case analysis used without exclusions Comment: low incomplete data and judged as low risk for attrition bias for all outcomes |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting found. All outcomes reported |
| Other bias | Low risk | No other bias found |
| Methods | Eight‐week parallel‐group randomised double‐blind placebo‐controlled trial held between June 2007 and June 2008 | |
| Participants | Ninety‐six children between 1 and 3 years of age with moderate to severe atopic dermatitis diagnosed according to the criteria of Hanifin and Rajka were recruited Randomisation was done at a 1:1 ratio: 48 participants were randomised in each arm. Only patients whose parents or legal guardians had the ability to comprehend the study requirements and to provide informed consent and those with direct telephone access were recruited Patients with clinically evident bacterial skin lesions, chronic concomitant disease that would likely require use of immunosuppression or antihistamines during research period, presence of severe systemic disease or cancer at any site and stage, suspected or established primary/secondary immune deficiency, and food allergy other than egg or cow’s milk were excluded from the study. Also excluded were patients with mild disease and those currently taking systemic corticosteroids Six participants were lost to follow‐up Recruitment took place at a paediatric secondary care unit in Ukraine | |
| Interventions | Synbiotic: mixture of Lactobacillus acidophilus DDS‐1 and Bifidobacterium lactis UABLA‐12 with fructo‐oligosaccharide in a rice maltodextrin powder given twice daily reconstituted in tepid water or juice or baby food and immediately fed for 8 weeks. Dose given: 5 × 10⁹ CFU/gr for the 2 probiotics and 50 mgr/gr of fructo‐oligosaccharide Placebo: rice maltodextrin powder only given twice daily reconstituted in tepid water or juice or baby food and immediately fed for 8 weeks Parents were given 140 doses of the intervention and were asked to give 112 doses in total Treatment of atopic dermatitis during intervention: skin hydration, emollients, avoidance of allergens and irritants according to PRACTALL (Practical Allergology) recommendations. Hydrocortisone 1% or Mometasone 0.1% ointment was allowed as rescue medication. Elimination diet for 2 months before and during trial period. Diet was cow's milk or egg free, depending on which food allergy the participant had. No elimination diet for participants without food allergies | |
| Outcomes | • IDQoL changes at 4 and 8 weeks* • DFI at 4 and 8 weeks* • SCORAD parts A, B, and C at 2, 4, and 8 weeks* • Frequency of topical corticosteroid use (days per week) at 8 weeks* • Cumulative use of topical corticosteroids during intervention period* *Denotes outcomes prespecified for this review | |
| Notes | Study was funded by the Lviv National Medical University of Ukraine. "No conflicts of interest" declared Twenty‐six participants in the probiotic group (60.5%) and 24 in the placebo group (51.1%) developed adverse effects: upper respiratory tract infection, lower respiratory tract infection, herpetic stomatitis, diarrhoea, constipation, abdominal colic. Two children in the probiotic group (4.7%) and 3 in the placebo group (6.4%) experienced severe adverse events (head injury and food poisoning) that were reported to be unrelated to the intervention under investigation | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "patients were randomised using computer‐generated random codes to receive either probiotic or placebo treatment" Comment: judged as adequate for low risk of bias |
| Allocation concealment (selection bias) | Unclear risk | Inadequate information; study judged to be at unclear risk of bias |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "probiotic and placebo were identical in appearance, taste, smell, packing and manner of administration. All formulations were dispensed by a technician with investigator and patient blinded regarding the identity of the treatment" Comment: judged as adequate for low risk of performance bias |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: "probiotic and placebo were identical in appearance, taste, smell, packing and manner of administration. All formulations were dispensed by a technician with investigator and patient blinded regarding the identity of the treatment" Comment: judged as having unclear risk of bias; no information on blinding of outcome assessor |
| Incomplete outcome data (attrition bias) | Low risk | Six participants lost to follow‐up (6.25%): 5/48 (10%) in the probiotic group and 1/48 (2%) in the placebo group. Reasons for losses to follow‐up given: intercurrent illness (2 in probiotic and 1 in placebo group, 1 protocol violation in probiotic group and 1 diet deviation in probiotic group). Participants analysed in the group to which they were randomised Comment: judged as low risk, as rates for follow up are low and were unlikely to have influenced outcomes |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | No other bias found |
| Methods | Eight‐week 3‐arm parallel‐group randomised double‐blind placebo‐controlled trial | |
| Participants | Fifty children from 7 to 24 months of age with atopic dermatitis diagnosed by general practitioner or dermatologist were recruited. Randomisation was done at a 1:1 ratio: 17 participants were randomised in the 2 intervention arms and 16 in the control arm. All participants completed the trial Setting: Danish primary care centre | |
| Interventions | First arm/probiotic: Lactobacillus acidophilus NCFM in cellulose, silicon dioxide, and rice maltodextrin in a capsule given daily at a dose of 10¹º CFUs for 8 weeks Second arm/probiotic: Bifidobacterium animalis subsp lactis (B lactis Bi‐07) in cellulose, silicon dioxide, and rice maltodextrin in a capsule given daily at a dose of 10¹º CFUs for 8 weeks Third arm/placebo: cellulose, silicon dioxide, and rice maltodextrin in a capsule given daily | |
| Outcomes | Total and subjective SCORAD at baseline and end of treatment at 8 weeks* *Denotes outcomes prespecified for this review | |
| Notes | Funding was by Danish Directorate of Food Fisheries and Agri Business Danish Dairy Research Foundation. "No conflicts of interest" declared | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote from communication with author: "Randomization sequence was generated by the online available program http://www.randomizer.org/" Comment: judged as adequate for low risk |
| Allocation concealment (selection bias) | Low risk | Quote from communication with author: "The producer of the intervention product blinded the capsules as A, B and C before shipment" Comment: allocation sequence judged as adequate for low risk of bias after the study author's assurance |
| Blinding of participants and personnel (performance bias) | Low risk | Quote from communication with author: "The producer of the intervention product blinded the capsules as A, B and C before shipment, and blinding was maintained for participants, clinician and outcome assessor until finalized data analysis" Comment: judged as adequate for low risk |
| Blinding of outcome assessment (detection bias) | Low risk | Quote from communication with author: "The producer of the intervention product blinded the capsules as A, B and C before shipment, and blinding was maintained for participants, clinician and outcome assessor until finalized data analysis" Comment: judged as adequate for low risk. |
| Incomplete outcome data (attrition bias) | Low risk | No losses to follow‐up. All participants analysed in the group were randomised |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | No other bias found |
| Methods | Twelve‐week 3‐arm parallel‐group randomised double‐blind placebo‐controlled trial with follow‐up up to 36 months | |
| Participants | One hundred thirty‐seven infants between 3 and 6 months of age with physician‐diagnosed atopic dermatitis, in good general health, with normal growth, and consuming > 200 mL standard formula/d were recruited. Randomisation was done at a 1:2 ratio: 45 participants were randomised in the 2 intervention arms, and 45 in the control arm. SCORAD score at recruitment had to be ≥ 10 after standardised skin treatment for 2‐week run‐in period: 1% hydrocortisone ointment twice daily and emollients 2 to 4 times daily. Infants who were taking antibiotics or were on soya or extensively hydrolysed formula, those with congenital abnormalities or chronic disease, and those at less than 34 weeks' gestation were excluded from the trial Participants who were exclusively breastfed and those whose parents declined use of extensively hydrolysed formula were followed up as an open observational group Four participants were lost to follow‐up at 12 weeks Participants were recruited from primary care community clinics in the United Kingdom | |
| Interventions | First probiotic arm: Bifidobacterium lactis in powder sachets at a dose of 10¹º CFUs/d given with meals for 12 weeks Second probiotic arm: Lactobacillus paracasei in powder sachets at a dose of 10¹º CFUs/d given with meals for 12 weeks Placebo arm: maltodextrin in powder sachets given with meals for 12 weeks All arms followed a dairy elimination diet and used an extensively hydrolysed whey formula | |
| Outcomes | • Total SCORAD before 2‐week run‐in period, at baseline, at 4 and 12 weeks, and at 12, 18, and 36 months* • IDQoL before 2‐week run‐in period, at baseline, at 12 weeks, and at 12, 18, and 36 months* • Use of other eczema treatment at 12 weeks (end of intervention period)* • Number of infants receiving standard skin care: combination of topical steroids (TSs), emollients (≥ twice/d), and bath emollient* • Potency of TS* *Denotes outcomes prespecified for this review | |
| Notes | Funding and "no conflict of interest" declared Forty‐two out of 137 (30.7%) parents reported some difficulties, e.g. green loose stools, increased vomiting, feed refusal, or colic thought to be related to change in formula), and 24 of 137 (17.5%) stopped the formula | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Minimization method was applied to allocate subjects to study groups applying breast feeding, family history and initial SCORAD as stratification factors (TrialBalance randomisation programme; Nestec, Lausanne, Switzerland)" Comment: judged as adequate for low risk |
| Allocation concealment (selection bias) | Unclear risk | No information to confirm whether treatment allocation could be predicted |
| Blinding of participants and personnel (performance bias) | Low risk | All interventions identical Blinding of clinicians confirmed by study author |
| Blinding of outcome assessment (detection bias) | Low risk | Confirmed by study author |
| Incomplete outcome data (attrition bias) | Low risk | 2/45 (4.4%) participants inL paracasei group, 1/45 (2.2%) in B lactis group, and 1/47 (2.1%) in placebo group lost to follow‐up. 10/45 (22.2%) participants in L paracasei group, 9/45 in B lactis group, and 9/47 (19.1%) in placebo group stopped the study diet as per protocol but continued the intervention. Available case analysis used without exclusions, with imputation for missing data Comment: judged as unlikely to have influenced outcomes on eczema severity and quality of life, as similar and low rates of loss to follow‐up in all groups Also similar rates of stopping study formula in all groups |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | No other bias found |
| Methods | Twelve‐month parallel‐group double‐blind placebo‐controlled randomised trial | |
| Participants | Fifty children from 3 months to 4 years of age with moderate atopic dermatitis. No additional information provided on randomised numbers of participants in each arm | |
| Interventions | Probiotic:Lactobacillus reuteri at a dose of 1 × 10⁸ CFUs/d taken orally suspended in 5 drops of food oil for 12 months Placebo: not specified but also given for 12 months | |
| Outcomes | • SCORAD* • Subjective symptoms of atopic dermatitis: itching and loss of sleep* • Use of steroid treatment* *Denotes outcomes prespecified for this review | |
| Notes | All available information taken from a conference abstract. No publication found, and study authors could not be contacted | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "In this prospective, double blind, randomised study..." Inadequate information: information on this study taken only from a conference abstract |
| Allocation concealment (selection bias) | Unclear risk | Inadequate information: information on this study taken only from a conference abstract |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: "In this prospective, double blind, randomised study..." Inadequate information: information on this study taken only from a conference abstract |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: "In this prospective, double blind, randomised study..." Inadequate information: information on this study taken only from a conference abstract |
| Incomplete outcome data (attrition bias) | Unclear risk | Inadequate information: information on this study taken only from a conference abstract |
| Selective reporting (reporting bias) | Unclear risk | Inadequate information: information on this study taken only from a conference abstract |
| Other bias | Unclear risk | Inadequate information: information on this study taken only from a conference abstract |
| Methods | Twelve‐week parallel‐group randomised controlled trial Last observation carried forward approach used for missing continuous data | |
| Participants | 106 children 3 to 12 months of age with mild/moderate eczema and SCORAD 15 to 40, not receiving anti‐inflammatory treatment. Randomisation was done at a 1:1 ratio: 56 participants randomised in the intervention arm and 50 in the control arm. Four participants excluded from analysis after randomisation due to protocol breaches | |
| Interventions | Lactobacillus GG at 10¹º CFUs/d as a twice‐daily dose, or placebo | |
| Outcomes | • SCORAD* *Denotes outcomes prespecified for this review | |
| Notes | Funding declared and provided by InfectoPharm Arzneimittel und Consilium GmbH, Heppenheim, Germany (not linked with probiotics). No information on conflicts of interest | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Inadequate information for a judgement |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) | Unclear risk | Described as double‐blind but no details given |
| Blinding of outcome assessment (detection bias) | Unclear risk | Described as double‐blind but no details given |
| Incomplete outcome data (attrition bias) | Low risk | No losses to follow‐up. Four participants (3.9%) excluded from analysis after randomisation because of protocol breaches. Unlikely to have an impact on effect estimate |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting found |
| Other bias | Low risk | No other bias found |
| Methods | Randomised trial | |
| Participants | 180 children with eczema, from 2 months to 3 years of age. No additional information provided on exact ratio of randomisation into 2 arms. Patients with other medical conditions excluded | |
| Interventions | Active: routine symptomatic treatment and combination of 4 living bacterium tablets (Bifidobacterium, Lactobacillus, Enterococcus, and Bacilus cereus) taken orally at a dose of 1 tablet twice a day for 1 month Control: routine symptomatic treatment Routine symptomatic treatment: mild disease – calamine lotion and zinc oxide ointment, 3 times daily for 2 weeks Severe disease – loratadine syrup, topical mometasone and topical mupirocin to stop after disease control All participants advised to avoid washing with hot water/spa products and scratching, and to look for potential allergens and avoidance advice | |
| Outcomes | IL‐4, IL‐10, IgE, IFN‐γ, and Th1:Th2 ratio Relapse rate of the 2 groups in the 3‐month follow‐up visit. Relapse is defined as recurrence of rash within the 3 months Eczema improvement: • Complete resolution: ‐ complete clearance of rash and itch; and normal eating and sleeping patterns • Good response: > 70% eczema clearance, no obvious lichenification, almost complete resolution of itch/intermittent itch, eating and daily activities not affected • Partial response: 30% to 70% clearance, symptom improvement • No response: < 30% rash clearance, no obvious reduction in itch Complete resolution, good response, and partial response make up total percentage of responding participants | |
| Notes | Contact: [email protected] Trial was conducted in a secondary care setting at a paediatric clinic No information provided on sponsorship/conflicts of interest | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Inadequate information for a judgement |
| Allocation concealment (selection bias) | Unclear risk | Inadequate information for a judgement |
| Blinding of participants and personnel (performance bias) | Unclear risk | Inadequate information for a judgement |
| Blinding of outcome assessment (detection bias) | Unclear risk | Inadequate information for a judgement |
| Incomplete outcome data (attrition bias) | Unclear risk | Inadequate information for a judgement |
| Selective reporting (reporting bias) | Unclear risk | All outcomes reported; however dose of probiotics not given, and results reported only narratively |
| Other bias | Unclear risk | No information on trial registration and sponsorship |
| Methods | Sixteen‐week parallel‐group double‐blind placebo‐controlled randomised trial | |
| Participants | One hundred eighteen children between 1 and 13 years of age with atopic dermatitis diagnosed according to Hanifin and Rajka criteria with SCORAD between 20 and 50 Randomisation was done at a 1:1 ratio: 58 participants were randomised in the intervention arm, and 60 in the control arm. After selection, participants went through a 2‐week washout period, when both groups were administered placebo only. Patients who had taken systemic corticosteroids, probiotics, or phototherapy within a month before enrolment, with systemic immunosuppression within 3 months before enrolment, with SCORAD < 20 after a 2‐week washout period, or with other concomitant skin disease or systemic illness were excluded from the study. One recruiting centre was located in Korea. Thirty‐five participants were lost to follow‐up | |
| Interventions | Probiotic: Lactobacillus plantarum CJLP 133 given orally twice daily at a dose of 0.5 × 10¹º CFUs for 12 weeks Placebo: maltodextrin and anhydrous glucose twice daily for 2 weeks as a washout period by both groups, and then for 12 weeks by the control group | |
| Outcomes | • Total SCORAD at baseline, after 2‐week washout period, and at 8, 14, and 16 weeks, and changes from week 2 (start of intervention) to week 14 (end of intervention) and week 16 (2‐week follow‐up after end of intervention)* • Amount in weight of topical corticosteroids used during the whole study and during intervention only* • Number of participants using topical corticosteroids during the study* *Denotes outcomes prespecified for this review | |
| Notes | Trial sponsored by probiotic supplier; 2 investigators are employed by this company. No other information on conflicts of interest | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "...using a computer‐generated list of random numbers" Comment: judged as adequate for low risk |
| Allocation concealment (selection bias) | Low risk | Quote: "The random number list was prepared and posted on the fronts of the bags containing the materials by an investigator who was not involved clinically in the trial", "...placebo preparation with identical appearance and taste" Comment: probably done and judged as adequate for low risk. |
| Blinding of participants and personnel (performance bias) | Low risk | Intervention and placebo identical in appearance and taste Also quote from correspondence with study author: "clinicians and outcome assessors had been blinded during the study period" Comment: judged as adequate for low risk |
| Blinding of outcome assessment (detection bias) | Low risk | Quote from correspondence with study author: "clinicians and outcome assessors had been blinded during the study period" Comment: judged as adequate for low risk |
| Incomplete outcome data (attrition bias) | High risk | Intention‐to‐treat analysis used but high rates of losses to follow‐up (30%). 14/58 (24%) participants from probiotic group and 21/60 (35%) from placebo group lost to follow‐up. Reasons for losses to follow‐up given and similar in both groups Incomplete data likely to have an impact on the effect estimate; judged as high risk |
| Selective reporting (reporting bias) | High risk | Symptom scores (i.e. for pruritus, sleep loss, and SCORAD part C) after intervention not reported |
| Other bias | High risk | Commercial bias: trial sponsored by probiotic supplier, and 2 of the investigators employed by the company. It is likely that the sponsor had an influence on the outcome and on selective reporting Selection bias: power calculation and final numbers of participants suggest that study did not meet the target According to publication: "Study discontinued after the second interim analysis showed statistically significant differences between the groups" Study assessed to be at high risk of bias |
| Methods | Eighteen‐month multi‐centre double‐blind randomised controlled parallel‐group trial conducted from March 2004 until May 2007 | |
| Participants | One hundred nineteen infants younger than 6 months of age with documented cow's milk allergy, judged by an elimination challenge test (open) and re‐elimination, in conformity with guidelines of the European Society for Pediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN). Randomisation was done at a 1:1 ratio. Excluded from the study were infants breastfeeding during the study, infants older than 6 months of age, patients with chronic disease that may be relevant to this study, such as pre‐existing chest abnormalities (e.g. bronchopulmonary dysplasia (BPD), relevant congenital abnormalities), gastrointestinal disease (coeliac disease, enzyme disorders), and metabolic disease, premature infants at less than 32 weeks, infants with congenital abnormalities that may be relevant to this study, and infants using systemic drugs for allergy (corticosteroids and antihistamines) Multi‐centre trial recruiting in a paediatric secondary care setting in the Netherlands One hundred six infants completed the study (89%) | |
| Interventions | Probiotic group: Lactobacillus casei CRL431 (Lactobacillus paracasei, subspparacasei) and Bifidobacterium lactis Bb‐12 (B animalis subsp lactis). For each probiotic, 10⁷ CFUs/gr of formula was provided. This was given for 6 months in extensively hydrolysed formula and was continued in standard formula if infants became cow's milk tolerant, or in the same extensively hydrolysed formula if not Control group: extensively hydrolysed formula for 6 months and continued in standard formula if infants became cow's milk tolerant, or in the same extensively hydrolysed formula if not | |
| Outcomes | • Development of tolerance for cow's milk, observed by a challenge test (double‐blind) at 6 months • Eczema severity by SCORAD index at 6 and 12 months* • T‐ and B‐lymphocyte subsets at 12 months *Denotes outcomes prespecified for this review | |
| Notes | Study co‐sponsored by probiotic/formula supplier. No information on conflicts of interest | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A computerized randomisation schedule was prepared by a statistician" ‐ "the groups were stratified and block randomised according to age at inclusion (<20 weeks and >20 weeks), birth weight (<2500gr or >2500gr) and (reported) atopic diseases in first‐degree relatives (yes or no)" Comment: judged as adequate for low risk |
| Allocation concealment (selection bias) | Unclear risk | No information available to allow a judgement |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "Probiotics and formula were image and taste matched, and participants and researchers remained blinded to group assignment for the duration of the study" Comment: probably done for all parties |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "Probiotics and formula were image and taste matched, and participants and researchers remained blinded to group assignment for the duration of the study" Comment: probably done for all parties |
| Incomplete outcome data (attrition bias) | Unclear risk | Overall losses to follow‐up 11%. No data available for the 2 groups separately, and no data available for the subgroup of participants who had eczema Inadequate for a judgement for that subgroup only Low risk of attrition bias for the primary outcome of the study (development of tolerance to cow's milk) |
| Selective reporting (reporting bias) | Low risk | All outcomes reported. No detailed data reported on all eczema participants (only moderate to severe), but significance of the difference noted Quote: "The probiotic group (n=51) showed improvement at 6 and 12 months, and the placebo group (n=54) showed improvement only at 6 months. However, after adjusting for the baseline values there were no significant differences in the change from baseline between probiotics and placebo treatment at 6 months (P=.9) and at 12 months (P=.14)" |
| Other bias | Unclear risk | Commercial bias: formula and probiotics supplied by producer company. The role of the manufacturer in data analysis and publication is unknown; therefore we assessed this study to be at unclear risk of other bias |
| Methods | Twenty‐week parallel‐group double‐blind placebo‐controlled randomised trial from April until September 2010 | |
| Participants | 48 adults between 18 and 55 years of age with moderate to severe atopic dermatitis diagnosed according to "Consensus guidelines in diagnosis and treatment of atopic dermatitis" (Eichenfield 2004). Participants were randomised at a 2:1 intervention:control ratio (32 participants in intervention arm/16 participants in control arm). Patients who had taken probiotics in the 6 months before enrolment, or topical immunomodulators, corticosteroids, or antihistamines; who had chronic disease, congenital or acquired immunosuppression, acute or chronic infection, or allergic contact dermatitis; and pregnant or lactating patients were excluded from the trial. Also excluded were patients who were on elimination diets without a known food allergy and those with hypersensitivity to the components of the probiotics Participants were recruited at a secondary Immunology centre in Italy Two participants were lost to follow‐up | |
| Interventions | Mixture of probiotics in maltodextrin: Lactobacillus salivarius LS01 DSM 2275, Bifidobacterium breve BR03 DSM 16604, given twice daily at a dose of 1 × 10⁹ CFUs/gr for each probiotic for 12 weeks Placebo: maltodextrin given only twice daily for 12 weeks | |
| Outcomes | • DLQI at baseline and at 12 and 20 weeks* • SCORAD at baseline and at 12 and 20 weeks* *Denotes outcomes prespecified for this review | |
| Notes | "No conflicts of interest" declared. No information on funding. Probiotics provided by the manufacturer | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "patients were randomised with a 2:1 ratio..." Quote from communication with study author: "A computerized randomisation schedule was prepared..." Comment: judged as adequate for low risk of bias |
| Allocation concealment (selection bias) | Low risk | Quote from communication with study author: "allocation and dispensing by blind clinical investigator" Comment: judged as adequate for low risk of bias |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "Probiotic and placebo sachets were matched for size, shape and volume of contents" Quote from communication with study author: "allocation and dispensing by blind clinical investigator" Comment: judged as adequate for low risk of bias |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "A single investigator blinded to the treatment arm, performed all SCORAD assessments..." Comment: judged as adequate for low risk of bias |
| Incomplete outcome data (attrition bias) | Low risk | Low rates of loss to follow‐up (4%) overall. 1 participant lost to follow‐up in each arm: 3% in probiotic group and 6% in placebo group. Different reasons for losses to follow‐up given but overall low rates unlikely to have a significant influence on effect estimates for all outcomes |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Unclear risk | "No conflicts of interest" declared. Unavailable information on funding. Probiotics provided by the manufacturer |
| Methods | Parallel‐group three‐arm randomised controlled trial. Duration of treatment unclear | |
| Participants | Twenty‐seven infants ‐ ages not stated ‐ with eczema diagnosed using the Hanifin and Rajka criteria. Randomisation was done at a 1:1 ratio: 9 participants were randomised in each arm. All infants were exclusively breastfed and were tolerant of the study formula without added probiotic Setting: paediatric service in Finland Unclear how many participants lost to follow‐up | |
| Interventions | Extensively hydrolysed whey‐dominant cow's milk formula with no probiotic added, with Lactobacillus GG added at 3 × 10⁸ CFUs/g, or with Bifidobacterium lactis Bb‐12 added at 1 × 10⁹ CFUs/g | |
| Outcomes | SCORAD ‐ interval of assessment unclear* *Denotes outcomes prespecified for this review | |
| Notes | Study funded by the Academy of Finland, European Union (FAIR CT96‐1028), and the Medical Research Funds of Tampere and Turku University Hospital "No conflict of interest" declared | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "In this randomised, double blind study, the patients were divided into three groups..." Comment: inadequate information to assess risk of bias |
| Allocation concealment (selection bias) | Unclear risk | No information given. No assessment of risk of bias can be done |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: "In this randomised, double blind study, the patients were divided into three groups..." Comment: inadequate information for a judgement on risk of bias |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: "In this randomised, double blind study, the patients were divided into three groups..." Comment: inadequate information for a judgement on risk of bias |
| Incomplete outcome data (attrition bias) | Unclear risk | Information on losses to follow‐up not given. Inadequate information for a judgement on risk of bias |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting found |
| Other bias | Low risk | No other bias found |
| Methods | Eight‐week parallel‐group open‐label randomised placebo‐controlled trial | |
| Participants | Sixty full‐term infants 3 to 12 months of age with clinically diagnosed eczema and challenge proven cow's milk allergy, breast and formula fed. Randomisation was done at a 1:1 ratio: 30 participants were randomised in each arm. Excluded were patients who had received any probiotics within a month before recruitment and those with other allergies, severe comorbidities, and malformations Country: Ukraine 5 participants lost to follow‐up | |
| Interventions | Probiotic: Bifidobacterium lactis BB‐12 and Streptococcus thermophilus TH‐4 for 4 weeks No information on placebo Daily dose of probiotics: 1 × 10⁹ CFUs for BB‐12 and 1 × 10⁸ CFUs for TH‐4. Both groups on cow's milk elimination diet | |
| Outcomes | SCORAD at 4 and 8 weeks* *Denotes outcomes prespecified for this review | |
| Notes | No mention of sponsor. No declaration of conflicts of interest | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote from translated Russian article: "In this open label randomised prospective clinical study..." Comment: inadequate information for a judgement on risk of bias |
| Allocation concealment (selection bias) | Unclear risk | Inadequate information for assessment, so study judged as having unclear risk of bias |
| Blinding of participants and personnel (performance bias) | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) | High risk | Open‐label study |
| Incomplete outcome data (attrition bias) | Low risk | Five participants lost to follow‐up (8%), with 2 participants from probiotic group (6.6%) and 3 participants (10%) from placebo group. Reasons for losses to follow‐up not given for each group. Not clear whether losses to follow‐up were excluded from analysis. However overall rates of loss to follow‐up were low and were unlikely to have a significant influence on effect estimates for study outcomes |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Unclear risk | No mention of sponsor. No declaration of conflicts of interest |
| Methods | Parallel‐group randomised controlled trial. Intended duration of treatment not clear | |
| Participants | Twenty‐seven infants (mean age 5.5 months) with eczema and suspected cow's milk allergy. Method for diagnosing eczema not described Setting: hospital paediatric department in Finland Unclear how many participants lost to follow‐up Participants randomised 2:1, probiotic:control | |
| Interventions | Lactobacillus GG at 3 × 10¹º CFUs/kg/d, mixed with extensively hydrolysed whey formula, or the same formula without probiotic. A third treatment arm (excluded from this review) used heat‐inactivatedLGG at 3 × 10¹º CFUs/kg/d, mixed with extensively hydrolysed whey formula | |
| Outcomes | SCORAD* *Denotes outcomes prespecified for this review | |
| Notes | Study terminated early due to adverse effects in a third treatment arm. Third treatment arm not included in this systematic review because it involved the use of killed bacteria Study funded by the Academy of Finland | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "These infants were randomly assigned into placebo, viable LGG..." Comment: inadequate information for a judgement on risk of bias |
| Allocation concealment (selection bias) | Unclear risk | No information reported. Inadequate information for a judgement on risk of bias |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: "These infants were randomly assigned into placebo, viable LGG or heat‐inactivated LGG groups and accordingly given in a double blind manner..." Comment: inadequate information for a judgement on risk of bias |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: "These infants were randomly assigned into placebo, viable LGG or heat‐inactivated LGG groups and accordingly given in a double blind manner..." Comment: inadequate information for a judgement on risk of bias |
| Incomplete outcome data (attrition bias) | Unclear risk | No data given on losses to follow‐up. Inadequate information for a judgement on risk of bias |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | Low risk | No other bias found |
| Methods | Four‐week randomised parallel controlled trial | |
| Participants | Forty‐four infants of both genders with newly diagnosed eczema based on the diagnostic criteria for eczema up to 3 years of age were recruited. Randomisation was done at 1:2 ratio: 22 participants were randomised in each group. Infants who had been treated with antibiotics, probiotics, or other drugs and food at least 2 weeks before the start of the study were excluded. Also excluded were children suffering from pneumonia, capillary bronchitis, and other diseases, or who had been treated with antibiotics or hormones during the experimental process Infants were treated with antiallergic therapy and dietary guidance Recruitment took place in a secondary paediatric setting in China between December 2010 and March 2011 | |
| Interventions | Intervention group received triple viable capsules containing Bifidobacterium bifidum 3 times daily for 4 weeks Control group received no treatment | |
| Outcomes | • Total SCORAD index at baseline and after 4 weeks of intervention • Bifidobacterium bifidum stool levels at baseline and after 4 weeks of treatment | |
| Notes | Sponsorship of the trial not declared. Not clear what role the supplier of the probiotic played in the study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The 40 infants were randomly divided into treatment and control groups" Judgement: inadequate information on randomisation method; risk of bias judged as unclear |
| Allocation concealment (selection bias) | Unclear risk | Inadequate information for a judgement |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "The control group … did not receive any special treatment and were not administered a placebo drug" Judgement: unlikely to be done adequately with no treatment and no placebo in the control group |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: "The control group … did not receive any special treatment and were not administered a placebo drug" Otherwise no information on blinding of outcome assessors; hence risk of detection bias judged to be unclear |
| Incomplete outcome data (attrition bias) | Unclear risk | No information provided on losses to follow‐up and missing outcome data Judgement: unclear risk of bias |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Unclear risk | Not clear what role the supplier of the probiotic played in the study. Sponsorship of the trial not declared Judgement: unclear risk of commercial bias |
| Methods | One‐month parallel‐group randomised controlled trial | |
| Participants | Thirty‐one children 2 to 16 months of age with eczema diagnosed using the Hanifin and Rajka criteria, and a history suggestive of cow's milk allergy. Randomisation was done at a 1:1 ratio. Children currently receiving systemic corticosteroid treatment were excluded Setting: paediatric clinic in Finland Unclear how many participants were lost to follow‐up | |
| Interventions | Cow's milk elimination diet, topical eczema treatment, and extensively hydrolysed cow's milk formula with or without addition of probiotic Lactobacillus GG. Probiotic given at 5 × 10⁸ CFUs/g formula | |
| Outcomes | SCORAD assessed at 1 and 2 months* *Denotes outcomes prespecified for this review | |
| Notes | No information available on funding or conflicts of interest | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The patients....participated in a randomised double blind study" Comment: inadequate information for a judgement on risk of bias |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: "The patients....participated in a randomised double blind study" Comment: inadequate information for a judgement on risk of bias |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: "The patients....participated in a randomised double blind study" Comment: inadequate information for a judgement on risk of bias |
| Incomplete outcome data (attrition bias) | Unclear risk | No data on losses to follow‐up. Inadequate information for a judgement on risk of bias |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | Unclear risk | No information on funding available |
| Methods | Eight‐week multi‐centre randomised double‐blind placebo‐controlled parallel trial | |
| Participants | Forty‐four adults with moderate to severe atopic dermatitis diagnosed according to the criteria of Hanifin and Rajka were recruited. Randomisation was done at a 1:1 ratio: 22 participants were randomised in each arm. Participants continued to use their medications as usual and did not change quantities or levels of corticosteroid medicine during the experimental period. Participants were asked to avoid probiotic supplements, fermented milk, lactic acid bacterial drinks, and fermented soybean (natto) during the experimental period Recruitment took place at 8 dermatology clinics in Japan | |
| Interventions | Participants in the intervention group received capsules containing approximately 6 × 10⁹ CFUs of Bifidobacterium animalis subsp lactis and an excipient that consisted of skim milk, glucose, inulin, dextrin, and silicone dioxide for 8 weeks Control group received capsules containing the excipient only for 8 weeks | |
| Outcomes | • Itch score by behavioural rating scales and by 100‐mm visual analogue scale (VAS) at baseline and at 4 and 8 weeks • Skin severity score using the reference proposed by the Research Group granted by the Japanese Ministry of Health, Labor and Welfare at baseline and at 4 and 8 weeks • Quality of life using Skindex‐29 (Japanese version) at baseline and at 4 and 8 weeks • Faecal levels of B lactis at baseline and at 4 and 8 weeks | |
| Notes | Trial registration: UMIN000005695 Four of the investigators/study authors are employed by the supplier | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The 44 participants eligible for the study were randomly assigned to receive LKM512 capsule or a placebo by the masked quota director" Judgement: inadequate information on randomisation method; hence judgement of unclear risk of selection bias |
| Allocation concealment (selection bias) | Unclear risk | Inadequate information for a judgement |
| Blinding of participants and personnel (performance bias) | Unclear risk | "Double‐blind" mentioned, but trial registration shows open‐label |
| Blinding of outcome assessment (detection bias) | Unclear risk | "Double‐blind" mentioned, but trial registration shows open‐label |
| Incomplete outcome data (attrition bias) | Unclear risk | No information provided |
| Selective reporting (reporting bias) | Unclear risk | All outcomes reported but not numerically, and any favourable information/outcome for probiotics particularly analysed, even if not statistically significant |
| Other bias | High risk | Commercial bias: 4 investigators/study authors employed by supplier; likely that this has influenced the design and outcome of the trial |
| Methods | Three‐month parallel‐group randomised placebo‐controlled trial | |
| Participants | Thirty‐nine full‐term and otherwise healthy infants with atopic dermatitis diagnosed according to the Hanifin and Rajka criteria were recruited. Randomisation was done at a 1:1 ratio: 19 participants were randomised in the intervention arm, and 20 in the control arm. Patients with skin or other severe infections were excluded Recruitment was done at a paediatric secondary care setting in Finland Two participants were lost to follow‐up | |
| Interventions | Probiotic: Lactobacillus rhamnosus GG in extensively hydrolysed casein formula given at a dose of 5.0 × 10⁷ CFUs/gr, achieving a daily dose of 3.4 × 10⁹ CFUs/d for 3 months Placebo: extensively hydrolysed casein formula for 3 months | |
| Outcomes | • Total SCORAD* *Denotes outcomes prespecified for this review | |
| Notes | Study was partially funded by the formula manufacturer and a grant from the Academy of Finland. No conflicts of interest declared | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote from correspondence with study author: "the randomisation was carried out by the formula supplier (Mead Johnson, Evansville, IN, USA). The method used was block randomisation..." Comment: probably done; judged as adequate for low risk of bias |
| Allocation concealment (selection bias) | Unclear risk | No information given; inadequate information for a judgement |
| Blinding of participants and personnel (performance bias) | Low risk | Quote from correspondence with study author: "The study was a double‐blind, placebo controlled intervention study" ‐ "The information disclosing the codes of the study products was kept by a person not involved in the study and opened after the study was completed" Comment: probably done; judged as adequate for low risk of bias |
| Blinding of outcome assessment (detection bias) | Low risk | Quote from correspondence with study author: "The study was a double‐blind, placebo controlled intervention study" ‐ "The information disclosing the codes of the study products was kept by a person not involved in the study and opened after the study was completed" Comment: probably done; judged as adequate for low risk of bias |
| Incomplete outcome data (attrition bias) | Low risk | Two participants lost to follow‐up, all from placebo group. Available case analysis unclear but very low rates of loss to follow‐up (5% in total and 10% in placebo group) unlikely to change the effect estimate. Reasons for losses to follow‐up given |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | No other bias found |
| Methods | Three‐month parallel‐group randomised controlled trial | |
| Participants | Forty‐eight children 2 to 12 years of age with moderate/severe eczema diagnosed by UK Working Party Criteria and total SCORAD over 14. Randomisation was done at a 1:1 ratio: 24 participants were randomised in each arm. Exclusion criteria included current flare of eczema, exposure to systemic corticosteroids or immunosuppressants in the previous 3 months, and other known immune deficiency Setting: hospital dermatology clinic in France Nine participants lost to follow‐up | |
| Interventions | Skim milk powder, potato starch, and lactose‐containing prebiotic, with or without Lactobacillus rhamnosus Lcr35 at 3.6 × 10⁹ CFUs/d, given as a 3‐times‐daily dose mixed with cold water or other liquid | |
| Outcomes | • Parent or participant global assessment of eczema severity* *Denotes outcomes prespecified for this review | |
| Notes | Three episodes of mild abdominal pain reported ‐ 2 in probiotic group, 1 in placebo (prebiotic alone) group Funding and conflict of interest not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation sequence as confirmed by study authors. Judged as having low risk of bias |
| Allocation concealment (selection bias) | Low risk | Adequate as confirmed by study authors and judged as having low risk |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "The patients, their parents and the dermatologists were blinded to the treatment the patient was receiving" ‐ "Each patient was examined by the same dermatologist at each visit" Study authors confirmed blinding of all parties in the trial Comment: probably done and judged as adequate for low risk of bias |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "The patients, their parents and the dermatologists were blinded to the treatment the patient was receiving" "Each patient was examined by the same dermatologist at each visit" Study authors confirmed blinding of all parties in the trial Comment: probably done and judged as adequate for low risk of bias |
| Incomplete outcome data (attrition bias) | High risk | Available case analysis used without exclusions after randomisation with low total rates of loss to follow‐up (18.7%). Losses to follow‐up per group: 29% in synbiotic group and 8% in placebo group. Reasons for losses in the synbiotic group were non‐attendance at follow‐up visits (5/24 participants) and withdrawal of consent (2/24). In the prebiotic group, 2/24 participants did not attend for follow‐up Significant differences in rates of loss to follow‐up in the 2 groups, which probably had an impact on all outcomes |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | Unclear risk | Funding and conflict of interest not reported |
| Methods | Twenty‐week cross‐over randomised double‐blind placebo‐controlled trial | |
| Participants | Sixteen adults between 18 and 40 years of age with eczema diagnosed based on Erlangen score > 10 (atopic score of Diepgen) and SCORAD severity score 5 to 30 were recruited. Only patients who were willing to apply cosmetic products and the Class II topical corticosteroid Advantan were recruited. Exclusion criteria were disease necessitating medication with systemic corticosteroids, immunosuppression, or cytostatics within 4 weeks before the start of the study; phototherapy or systemic treatments within 4 weeks before the start of the study; long‐acting antihistamines, antibiotics, long‐term systemic corticosteroids, depot steroids, tranquillisers, and psychopharmaceuticals with antihistamine effect or within 7 days from skin prick test; or astemizole intake within 4 weeks before prick testing. Also excluded were patients with active skin infection; asthma needing treatment with corticosteroids; indigestibility/allergy to milk components (including skin prick test); lactose intolerance; acute or chronic symptomatic heart disease or severe internistic disease; autoimmune disease; immune deficiency (including immune suppressive treatment); immune complex‐induced immunopathy or malignant tumour; or abuse of alcohol, drugs, or medicaments, as well as pregnant and breastfeeding women Recruitment was done at a secondary care centre in Germany One participant withdrew after randomisation and before treatment started | |
| Interventions | Probiotic yoghurt drink containing Streptococcus thermophilus enriched with Lactobacillus paracasei Lpc‐37 (3.9 × 10⁸ CFUs/g), Lactobacillus acidophilus 74‐2 (2.9 × 10⁴ CFUs/g), Bifidobacterium lactis DGCC 420 (5.9 × 10⁴ CFUs/g) taken as 100 mL twice daily for 8 weeks Total daily dose was Lpc‐37: 7.8 × 10¹º CFUs/d, 74‐2: 5.8 × 10⁶ CFUs/d, and DGCC 420: 1.2 × 10⁷ CFUs/d Placebo drink not otherwise specified given as 100 mL twice daily Crossing over from one to the other intervention involved a washout period of 2 weeks Instructions were given for elimination of other probiotic and prebiotic products for 3 weeks before the start of treatment and during the 20 weeks of intervention | |
| Outcomes | Total SCORAD and SCORAD part C at baseline and after 8 weeks of each intervention* *Denotes outcomes prespecified for this review | |
| Notes | Study was sponsored by a grant from Zott Dairy GmbH (not the probiotic supplier). It was declared that Zott had no involvement in study design, data analysis, and publication, and study authors declared no conflict of interest | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "all subjects were randomly assigned to 2 treatment groups (1:1) according to a computer generated blocked randomisation list (blocked randomisation)" Comment: judged as adequate for low risk of bias |
| Allocation concealment (selection bias) | Unclear risk | Comment: not clear whether treatment allocation was concealed |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "The placebo drink had an identical composition except for the probiotic cultures and had the same appearance, taste and smell as the probiotic drink" ‐ "Enrollment and assignment to interventions were performed by the trial physician and AR. All involved persons (trial physician and scientific staff) were blinded. In addition study products were blinded and labelled with a numerical code by the production dairy" Comment: judged as adequate for low risk of bias |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "The placebo drink had an identical composition except for the probiotic cultures and had the same appearance, taste and smell as the probiotic drink" ‐ "Enrollment and assignment to interventions were performed by the trial physician and AR. All involved persons (trial physician and scientific staff) were blinded. In addition study products were blinded and labelled with a numerical code by the production dairy" Comment: judged as adequate for low risk of bias |
| Incomplete outcome data (attrition bias) | Low risk | One participant (6.6%) withdrew after randomisation and before the start of treatment and was excluded from analysis. All other participants were analysed in the group to which they had been randomised. Low rates of loss to follow‐up were unlikely to have a significant influence on effect estimates |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | No other bias found |
| Methods | Six‐week randomised controlled cross‐over trial | |
| Participants | Fifty‐eight children 1 to 13 years of age with eczema diagnosed using the UK Working Party Criteria. Children who had received systemic corticosteroids at any time were excluded Setting: hospital paediatric and dermatology departments in Denmark 15 participants lost to follow‐up | |
| Interventions | Skimmed milk powder with dextrose anhydrate 2 g/d or a mix of Lactobacillus rhamnosus 19070‐2 and Lactobacillus reuteri DSM12246 at 2 × 10¹º CFUs/d of each strain. Both placebo and probiotic preparations administered twice daily with 2.5 to 5 mL water | |
| Outcomes | • Global self‐assessment by participant or parent* *Denotes outcomes prespecified for this review | |
| Notes | Study was supported by Danish Research and Development Programme for Food Technology | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The patients were randomised in a double‐blind crossover design...." ‐ "Blocked randomisation with 4 patients in each block was applied" Comment: inadequate information for a judgement on risk of bias |
| Allocation concealment (selection bias) | Unclear risk | No information provided. Inadequate information for a judgement |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: "The patients were randomised in a double‐blind crossover design...." Comment: inadequate information for a judgement on risk of bias |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: "The patients were randomised in a double‐blind crossover design...." Comment: inadequate information for a judgement on risk of bias |
| Incomplete outcome data (attrition bias) | High risk | Fifteen participants (25.9%) excluded from analysis. Five of these excluded during active treatment and 9 during placebo. Reasons for exclusion reported Comment: high rates of exclusion probably affecting all outcomes |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | Low risk | No other bias found |
| Methods | Parallel‐group double‐blind placebo‐controlled randomised trial conducted between December 2008 and November 2009 | |
| Participants | Forty‐one children from 1 to 36 months of age with moderate to severe (SCORAD > 25) atopic dermatitis diagnosed according to Hanifin and Rajka criteria were recruited. Randomisation was done at a 1:1 ratio: 20 participants were randomised in the intervention arm, and 21 in the control arm. Exclusion criteria were administration of systemic steroids, recurrent infection, evidence of immunodeficiency, congenital abnormality, chronic disease, and problems in eating. Five participants were lost to follow‐up Setting: Iranian Paediatric Allergy and Immunology Department | |
| Interventions | Synbiotic: 7‐strain probiotic and synbiotic in a sachet given daily at a dose of 1 × 10⁹ CFUs of probiotic and 990 mgr of fructo‐oligosaccharide for 2 months (59 days) Placebo: sucrose in a 1000‐mgr sachet given daily for 2 months (58 days) Both interventions were prepared by mixing with water, breast milk, formula, or solid food | |
| Outcomes | Total and objective SCORAD changes from baseline to end of intervention after 2 months* *Denotes outcomes prespecified for this review | |
| Notes | Sponsorship is unclear. Conflicts of interest are not declared | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomisation was performed according to a computer‐generated balanced block randomisation to synbiotic and placebo groups" Comment: judged as adequate for low risk of bias |
| Allocation concealment (selection bias) | Unclear risk | Quote: "At enrolment we assigned the study number and provided the participants with the appropriate sachet" Comment: not clear whether allocation to a treatment could have been predicted |
| Blinding of participants and personnel (performance bias) | Low risk | It is stated: "Partcipants and investigators blinded for the duration of the trial" ‐ "Placebo and intervention image‐matched and identical in appearance, taste and smell" Comment: judged as adequate for low risk of bias |
| Blinding of outcome assessment (detection bias) | Unclear risk | It is stated: "Partcipants and investigators blinded for the duration of the trial", but no specific information provided on outcome assessor Comment: judged as unclear risk due to lack of specific information |
| Incomplete outcome data (attrition bias) | Low risk | Five participants out of 41 (12%) were lost to follow‐up: 2 participants were from the probiotic group (10%) and 3 from the placebo group (14%). Reasons for loss to follow‐up were given and were similar in both groups. Case analysis was available. Low rates of loss to follow‐up unlikely to have a significant impact on the effect estimate |
| Selective reporting (reporting bias) | Unclear risk | Baseline characteristics and outcomes reported only for participants who completed the trial. Unclear whether groups were matched at baseline |
| Other bias | Unclear risk | Sponsorship unclear |
| Methods | Twelve‐week parallel‐group randomised controlled trial | |
| Participants | Sixty children 1 to 10 years of age with eczema diagnosed by UK Working Party criteria, SCORAD of at least 10 at recruitment, and a positive skin prick or RAST test to at least 1 common environmental or food allergen. Randomisation was done at a 1:1 ratio: 30 participants were randomised in each arm. Exclusion criteria were oral corticosteroid, immunosuppressant, or antibiotic in the previous month; previous immune deficiency or malignancy; and greater than 10‐point improvement in SCORAD during 2 weeks before the start of study treatment Setting: hospital clinic in New Zealand One participant lost to follow‐up | |
| Interventions | Microcrystalline cellulose placebo or Lactobacillus rhamnosus and Bifidobacterium lactis given together once daily at a combined total dose of 2 × 10¹º CFUs/d. Treatment capsules administered as a powder mixed with food or drink, or taken in capsule form | |
| Outcomes | SCORAD assessed at 2 weeks before treatment, at the start of treatment, and 2, 12, and 16 weeks later* *Denotes outcomes prespecified for this review | |
| Notes | One participant noted to be taking another non‐investigational probiotic Study was was funded by New Zealand Research Council. No conflicts of interest were declared | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Children were randomly assigned to treatment or placebo groups, using computer‐generated random numbers" Comment: judged as adequate for low risk of bias |
| Allocation concealment (selection bias) | Low risk | Adequate: treatment allocated by third party as confirmed by study authors |
| Blinding of participants and personnel (performance bias) | Low risk | Quotes: "The control group received a placebo....that looked and tasted the same as the probiotic" ‐ "Both subjects and investigators were blind to the treatment groups" Comment: judged as adequate for low risk of bias |
| Blinding of outcome assessment (detection bias) | Low risk | Quotes: "The control group received a placebo....that looked and tasted the same as the probiotic" ‐ "Both subjects and investigators were blind to the treatment groups" Comment: judged as adequate for low risk of bias |
| Incomplete outcome data (attrition bias) | Low risk | Available case analysis used without exclusions after randomisation and very low rates of loss to follow‐up (1/60 participants, 1.7%) Comment: low risk of attrition bias |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | Unclear risk | Significant differences in baseline SCORAD in the 2 groups, with more severe mean SCORAD in the placebo group. Study authors state that this could have been avoided if randomisation had been blocked Comment: uncertain how this difference could have influenced the effect estimate |
| Methods | Three‐month parallel‐group randomised controlled trial | |
| Participants | Seventeen children 3 to 18 months of age with eczema diagnosed by Hanifin and Rajka criteria, and with cow's milk hypersensitivity diagnosed by suggestive history plus evidence of cow's milk‐specific IgE. No information on the randomisation ratio was given. All participants had reduced levels of Bifidobacteria in their faeces (< 30% of total bacteria) and were receiving extensively hydrolysed cow's milk formula for at least 2 weeks before randomisation Setting unclear Unclear how many participants were lost to follow‐up | |
| Interventions | Raffinose prebiotic containing extensively hydrolysed cow's milk formula with or without Bifidobacterium breve M‐16V at 5 to 15 × 10⁹ CFUs/d | |
| Outcomes | Investigator‐rated eczema scoring system* *Denotes outcomes prespecified for this review | |
| Notes | No numerical outcome data available Study was supported by Grants‐in‐Aid from the Morianga Houshikai and the Mami Mitzutani Foundation. No conflicts of interest reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The 17 infants were divided into 2 groups at random" Comment: inadequate information; inadequate information for a judgement on risk of bias |
| Allocation concealment (selection bias) | Unclear risk | No information; inadequate information for a judgement on risk of bias |
| Blinding of participants and personnel (performance bias) | Unclear risk | No information available |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information available |
| Incomplete outcome data (attrition bias) | Unclear risk | No information on losses to follow‐up or data analyses |
| Selective reporting (reporting bias) | Low risk | No risk found |
| Other bias | Low risk | No other bias found |
| Methods | Twelve‐week multi‐centre parallel‐group double‐blind placebo‐controlled randomised trial | |
| Participants | Ninety infants 0 to 7 months of age, exclusively formula fed, with a diagnosis of atopic dermatitis based on Hanifin and Rajka diagnostic criteria and SCORAD > 15 were recruited in the study. Randomisation was done at a 1:1 ratio: 46 participants were randomised in the intervention arm, and 44 in the control arm Infants who had received systemic corticosteroids, antibiotics, antimycotics, calcineurin inhibitors, or probiotics during 4 weeks or antihistamines during 2 weeks before enrolment were excluded from the study. Also excluded were infants needing systemic treatments other than antibiotics during the study and infants with major medical problems or GI or skin conditions other than atopic dermatitis Setting: participants recruited at 7 paediatric and dermatology secondary care centres in the Netherlands Eight participants were lost to follow‐up. Five participants (4 in the symbiotic group and 1 in the placebo group) were excluded from analysis because no SCORAD assessment was undertaken after baseline assessment | |
| Interventions | Synbiotic: extensively hydrolysed whey‐based formula with Bifidobacterium breve M‐16V with 90% scGOS and 10% lcFOS given on demand at a dose of 1.3 × 10⁹ CFUs/100 mL of probiotic and 0.8 gr/100 mL of prebiotic for 12 weeks Placebo: formula given only on demand for 12 weeks | |
| Outcomes | • Changes in total SCORAD from baseline to 4, 8, and 12 (end of treatment) weeks* • Total SCORAD in 1 year after the intervention • Frequency and mean class of topical steroids used at baseline and at end of treatment* *Denotes outcomes prespecified for this review | |
| Notes | Two participants experienced severe adverse events: RSV bronchiolitis and severe cow's milk allergy. These were not related to the intervention Sponsored by a probiotic/formula company | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Participants were randomised, using computer‐generated 4‐blocked design lists, drawn up by a statistician with stratification according to recruiting hospital and current use of topical steroids..." Comment: judged as adequate for low risk of bias |
| Allocation concealment (selection bias) | Low risk | Quote: "Formulas were prepared and coded by Danone Research and dispensed by the pharmacy of the Academic Medical Centre", "both formulas were identical with respect to smell, taste, texture, colour and packaging" Comment: third party involved; judged as adequate for low risk of bias |
| Blinding of participants and personnel (performance bias) | Low risk | Quotes: "both formulas were identical with respect to smell, taste, texture, colour and packaging. The investigator, participants' own physicians and parents were all blind to the treatment groups" Comment: judged as adequate for low risk of bias |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "The investigator, participants' own physicians and parents were all blind to the treatment groups. Participants were clinically assessed by one investigator (L.B.A.), at weeks 0, 4, 8 and 12" Comment: judged as adequate for low risk of bias |
| Incomplete outcome data (attrition bias) | Low risk | 6/46 (13%) participants from the probiotic group and 2/44 (4.5%) from the placebo group discontinued treatment but were not excluded from analysis. Five participants (5.5%) were excluded post randomisation because of unavailable assessment data after baseline. Four were from the probiotic group, and one from the placebo group. Losses to follow‐up included in analysis but not certain how missing data were handled Comment: overall low rates of missing data; judged to have low risk of attrition bias |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Unclear risk | Sponsored by a probiotic/formula company. The role of the sponsor in data analysis is unclear; therefore the study was judged to be at unclear risk of bias |
| Methods | Four‐week parallel‐group randomised controlled trial | |
| Participants | 252 infants younger than 12 months of age with a clinical diagnosis of eczema and a clinical history suggestive of cow's milk allergy. Randomisation was done at a 1:1 ratio: 84 participants were randomised in each arm. Infants who had received a probiotic preparation for over a week in the preceding 6 weeks were excluded Participants were selected from primary care referrals to a hospital clinic in Finland Twenty‐two participants were lost to follow‐up | |
| Interventions | Cow's milk elimination diet, extensively hydrolysed formula, and capsules of microcrystalline cellulose placebo, Lactobacillus GG (10¹º CFUs/d), or probiotic mix (Lactobacillus GG 10¹º CFUs/d, Bifidobacterium breve Bbi 99 4 × 10⁸ CFUs/d,Lactobacillus rhamnosus LC705 10¹º CFUs/d, and Propionibacterium JS 4 × 10⁹ CFUs/d). Capsules were mixed with food twice daily | |
| Outcomes | SCORAD assessed at end of treatment and 4 weeks later* *Denotes outcomes prespecified for this review | |
| Notes | Study was supported by Finnish Research foundations and the probiotic supplier. No information on conflicts of interest | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Infants were randomised at the first visit according to computer‐generated block randomisation..." Comment: judged as adequate for low risk of bias |
| Allocation concealment (selection bias) | Low risk | Adequate: treatment allocated by third party as confirmed by study author |
| Blinding of participants and personnel (performance bias) | Low risk | Participants and clinician blinded as confirmed by study author |
| Blinding of outcome assessment (detection bias) | Low risk | Outcome assessor blinded as confirmed by study author |
| Incomplete outcome data (attrition bias) | Low risk | Analysis was by "treatment received", and 4 participants (1.6%) were excluded from analysis because they did not tolerate the study formula. Twenty‐two (8.7% to 5.9% in LGG group, 10.6% in probiotic mix group, and 9.75% in placebo group) losses to follow‐up occurred. Unlikely to have an impact on effect estimate and judged as having low risk of attrition bias |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | Unclear risk | Study was supported by Finnish Research foundations and the probiotic supplier. Not clear whether the probiotic supplier had any influence on data analysis |
| Methods | Four‐arm parallel‐group double‐blind randomised placebo‐controlled trial conducted from December 2011 until September 2013 | |
| Participants | 220 children and adolescents from 1 to 18 years of age with moderate to severe atopic dermatitis (SCORAD > 15) diagnosed according to the criteria of Hanifin and Rajka with symptoms present for at least 6 months before the study and with atopy as shown by at least 1 positive skin test (weal size ≧ 3 mm) or 1 positive RAST (IgE ≥ 0.7 kU/L) test to any common food or environmental allergens. Randomisation was done at a 1:1 ratio: 55 participants were randomised in each arm Excluded were patients on systemic corticosteroids, immunosuppressive therapy, or antibiotic or antimycotic treatment 4 weeks before the study, or using antihistamine 3 days before enrolment; and those who used probiotic preparations within 4 weeks before the study. Also excluded were patients with immune deficiency or other major medical problems Setting: secondary paediatric centre Country: Taiwan | |
| Interventions | Four intervention arms: first arm received Lactobacillus paracasei GMNL‐133 (LP) at a dose of 2 × 10⁹ CFUs/d. Second arm received Lactobacillus fermentum GM‐090 (LF) at a dose of 2 × 10⁹ CFUs/d. Third arm received Lactobacillus paracasei GMNL‐133 (LP) and Lactobacillus fermentum GM‐090 (LF) at a dose of 4 × 10⁹ CFUs/d. Fourth arm received placebo. Interventions were given orally in capsule form for 3 months During the study, corticosteroids, antibiotics, calcineurin inhibitors, antihistamines, and other probiotics were not permitted, with the exception of topical corticosteroids (fluticasone propionate) in case of severe flares and itching. All patients applied emollients and were educated on skin care | |
| Outcomes | For AD: SCORAD, Children's Dermatology Life Quality Index (CDLQI) and Family Dermatology Life Quality Index (FDLQI) at baseline and at 1, 2, 3, and 4 months Changes in total serum IgE and skin prick test reactivity, serum and urine biomarkers, and faecal probiotic species composition at baseline and at 3 months For asthma: GINA guideline asthma severity | |
| Notes | Trial registration: NCT01635738. Approval by the Ethics Committee of the Taipei Hospital Ministry of Health and Welfare GenMont Biotec Inc.: probiotic manufacturer that provided study costs for probiotics. No information on conflicts of interest | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Participants were randomised, using computer‐generated 4‐block design lists, drawn up by a statistician, with stratification according to age, gender, AD severity, and current use of topical steroids" |
| Allocation concealment (selection bias) | Low risk | Comment: probably done and judged as having low risk of bias |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "Patients were enrolled by the investigator and sequentially assigned a patient number connected to a code. Capsules were prepared and coded by GenMont Biotec Inc, with cGMP facilities and dispensed by the study nurse" ‐ "All investigators, study nurses and participants were blind to treatment assignment for the duration of the study" Judgement: probably done |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "Patients were enrolled by the investigator and sequentially assigned a patient number connected to a code. Capsules were prepared and coded by GenMont Biotec Inc, with cGMP facilities and dispensed by the study nurse" ‐ "All investigators, study nurses and participants were blind to treatment assignment for the duration of the study" Judgement: probably done |
| Incomplete outcome data (attrition bias) | Low risk | Quotes: "Finally analysed 100% of LP, 96% of LF, 98% of LP + LF group, and 96% of placebo" ‐ "Intention‐to‐treat analysis regardless of compliance" Judgement: low risk of attrition bias |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Unclear risk | GenMont Biotec Inc.: probiotic manufacturer that provided study costs for probiotics. It is not clear what the role of the sponsor was in data analysis and publication; hence the study was judged to be at unclear risk of bias |
| Methods | Eight‐week parallel‐group block randomised controlled trial | |
| Participants | Fifty‐six children 6 to 18 months of age with moderate/severe eczema diagnosed by Hanifin and Rajka criteria and modified SCORAD score of at least 25 at enrolment Randomisation was done at a 1:1 ratio: 28 participants were randomised in each arm. Those previously exposed to probiotics, currently receiving antibiotics, or with other major medical problems were excluded Setting: community and hospital outpatient clinic in Australia | |
| Interventions | Lactobacillus fermentum VR1‐003PCC 2 × 10⁹ CFUs/d as a sachet reconstituted by parents with 5 to 10 mL water twice daily, or maltodextrin placebo | |
| Outcomes | • Global self‐assessment by parent* *Denotes outcomes prespecified for this review | |
| Notes | One probiotic‐treated participant withdrew due to gastrointestinal illness (vomiting) Funding for principal investigator was provided by Research Fellowship by television channel and funding for IgE assay by VRI Biomedical. Probiotics and placebo supplied by manufacturer. No conflicts of interest declared | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A computerised randomisation schedule was prepared by the hospital biostatistician with allocation and dispensing of sachets by the pharmacy department" ‐ "The groups were stratified and block randomised according to following criteria: (a) modified SCORAD (25‐50; 50 and over), (b) current topical corticosteroid potency.... and (c) age..." Comment: judged as adequate for low risk of bias |
| Allocation concealment (selection bias) | Low risk | Quote: "A computerised randomisation schedule was prepared by the hospital biostatistician with allocation and dispensing of sachets by the pharmacy department. The probiotic and placebo sachets were matched for size, shape and volume of contents" Comment: third party involved in the allocation process and interventions were identical. Judged as adequate for low risk of bias |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quotes: "The probiotic and placebo sachets were matched for size, shape and volume of contents" ‐ "A SCORAD assessment was also performed by a clinician blind to the intervention" ‐ "...a single investigator performed all SCORAD assessments at week 0, 8, and 16" Comment: outcome assessor clearly stated as blinded and interventions probably identical. However no other information provided to clarify whether other personnel and the parents of infants were blinded |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quotes: "The probiotic and placebo sachets were matched for size, shape and volume of contents" ‐ "A SCORAD assessment was also performed by a clinician blind to the intervention" ‐ "...a single investigator performed all SCORAD assessments at week 0, 8, and 16" Comment: outcome assessor clearly stated as blinded and interventions probably identical. However no other information provided to clarify whether other personnel and the parents of infants were blinded, which may have affected patient/parent‐reported outcomes (DFI and global self‐assessment) |
| Incomplete outcome data (attrition bias) | Low risk | Available case analysis was used without exclusions after randomisation. Low rates of loss to follow‐up (5.4% overall; 7.1% in probiotic group and 3.6% in placebo group). Reasons for losses to follow‐up presented Low risk of attrition bias for all outcomes |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | Low risk | No other bias found |
| Methods | Twelve‐week parallel‐group randomised double‐blind placebo‐controlled trial conducted between January 2007 and August 2008 | |
| Participants | Eighty‐eight children between 2 and 10 years of age with atopic eczema‐dermatitis syndrome (AEDS) defined as pruritic chronic or chronically relapsing non‐infectious dermatitis with features and distribution as suggested by Hanifin. Randomisation was done at a 1:1 ratio: 45 participants were randomised in the intervention arm, and 43 in the control arm. Participants had to have the disease for at least 6 months, with SCORAD ≥ 25 and change in SCORAD not more than 10% within the first 2 weeks Excluded from the trial were patients who had used cyclosporine, systemic corticosteroids, topical calcineurin inhibitors, or Chinese herbal medicine during the 3 months before recruitment Recruitment was carried out at a secondary care paediatric unit in Korea Thirteen participants were lost to follow‐up | |
| Interventions | Probiotic: freeze‐driedLactobacillus sakei KCTC 10755BP in microcrystalline cellulose dissolved in 2.5 to 5 mL of water or any liquid preferred by the participant at a dose of 5 × 10⁹ CFUs not otherwise specified for 12 weeks Placebo: microcrystalline cellulose dissolved in 2.5 to 5 mL of water or any liquid preferred by the participant for 12 weeks Standardised topical treatment for all participants: take a bath once daily with warm water for 5 to 10 minutes and apply an emollient immediately after bathing. Permitted to use topical corticosteroids as required but only 0.1% prednicarbate | |
| Outcomes | • Total SCORAD and SCORAD part C* • Amount of topical corticosteroid (TCS) used during the study, change in TCS use from baseline, and number of participants using TCS during the intervention* *Denotes outcomes prespecified for this review | |
| Notes | Research supported by the Basic Science Research Program, National Research Foundation of Korea Funded by the Ministry of Education, Science and Technology, Korea. No conflicts of interest reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "patients were randomised in a double‐blind design..." Comment: inadequate information for a judgement on risk of bias |
| Allocation concealment (selection bias) | Unclear risk | No information given |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: "patients were randomised in a double‐blind design..." Comment: inadequate information on which part was blinded and the method of blinding interventions; inadequate information for a judgement on risk of bias for blinding |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: "patients were randomised in a double‐blind design..." Comment: inadequate information on which part was blinded and the method of blinding interventions; inadequate information for a judgement on risk of bias for blinding |
| Incomplete outcome data (attrition bias) | Low risk | All participants were analysed in the group to which they were initially randomised. 4/45 participants (8.9%) from the probiotic group and 9/43 (21%) from the placebo group were lost to follow‐up and were excluded from analysis. The difference in losses to follow‐up in the 2 groups was found to be statistically insignificant (P = 0.11). Overall losses to follow up were low (14%) Comment: judged as low risk for attrition bias |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | Baseline characteristics presented only for participants who completed the study, but low rates of loss to follow‐up; hence the study was judged to be at low risk of bias |
| Methods | Ten‐week parallel‐group double‐blind randomised placebo‐controlled trial | |
| Participants | Sixty children from 2 to 14 years of age with eczema diagnosed by Hanifin and Rajka criteria and moderate to severe disease (SCORAD > 25) who had eczema symptoms for at least 4 days before diagnosis. Randomisation was done at a 1:1 ratio: 30 children were randomised in each arm Setting: secondary care with 1 recruiting centre in Taiwan Six participants were excluded post randomisation but before treatment started | |
| Interventions | Synbiotic: Lactobacillus salivarius PM‐A0006 2 × 10⁹ CFUs/25 mgr and fructo‐oligosaccharide 475 mgr in a capsule preparation twice daily Control: corn starch 25 mgr and fructo‐oligosaccharide 475 mgr in a capsule twice daily | |
| Outcomes | • SCORAD* • 13‐item quality of life daily diary • Global eczema severity • SCORAD parameters for pruritus and sleep loss* • Frequency of use of topical corticosteroid or calcineurin inhibitor (times/month)* *Denotes outcomes prespecified for this review | |
| Notes | Two probiotic‐treated participants initially had mild diarrhoea but overall tolerated treatment well Sponsorship declared and no conflicts of interest reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The children were randomised into two groups...in a double‐blind manner using a computer‐generated blocked randomisation list provided by ProMD Biotech Co., Taiwan. A block size of four was used and stratified according to sex, age and diagnosis of moderate to severe AD" Comment: judged as adequate for low risk of bias |
| Allocation concealment (selection bias) | Low risk | Quote: "The code was opened only after all data were analysed." Comment: probably done, so judged as adequate for low risk of bias |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "The synbiotic and prebiotic products, which were identical in appearance, odour and taste, were delivered in numbered packages directly to the parents according to randomisation list. The code was opened only after all data were analysed" ‐ "the same investigator (KGW), who was blinded to group assignment, enrolled patients and performed all SCORAD assessments at weeks 0, 4, 8 and 10" Comment: judged as adequate for low risk of bias |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "The synbiotic and prebiotic products, which were identical in appearance, odour and taste, were delivered in numbered packages directly to the parents according to randomisation list. The code was opened only after all data were analysed" ‐ "the same investigator (KGW), who was blinded to group assignment, enrolled patients and performed all SCORAD assessments at weeks 0, 4, 8 and 10" Comment: judged as adequate for low risk of bias |
| Incomplete outcome data (attrition bias) | Low risk | Six participants (10% in total and in each group) withdrew after randomisation but before treatment initiation and were excluded from analysis. Reasons for withdrawal given and similar in both groups Comment: low rates of excluded patients unlikely to have a significant impact on effect estimate; hence low risk of attrition bias |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | Low risk | No other bias found |
| Methods | Eight‐week parallel‐group 2‐centre randomised double‐blind placebo‐controlled trial | |
| Participants | Sixty‐seven children 4 to 48 months of age with atopic dermatitis diagnosed using Hanifin‐Rajka criteria with SCORAD > 15 at enrolment. Randomisation was done at a 1:1 ratio: 34 participants were randomised in the intervention arm, and 33 in the control arm Exclusion criteria: clinically evident infection in skin lesions, severe asthma or acute asthma attack within 3 months, autoimmune disease, immunodeficiency, exposure to phototherapy, use of systemic corticosteroids within 1 month | |
| Interventions | Active: 1 capsule of ComProbi containing 350 mg ofLactobacillus rhamnosus (MP108) and maltodextrin per day Control: 1 capsule of maltodextrin per day If capsule could not be swallowed, parents were instructed to mix the powder in water, breast milk, milk, or food heated to < 40°C Rescue medication: Cutivate cream (GlaxoSmithKline, Duhram, UK) in cases of uncontrolled symptoms | |
| Outcomes | Primary • Change in SCORAD after 8‐week treatment Secondary • Change in SCORAD at post‐baseline visits • Frequency and total quantity of corticosteroids used during 8‐week treatment • Comparison of frequency of atopic dermatitis and symptom‐free duration • Comparisong of mean changes from baseline in IDQoL at weeks 4 and 8 • Comparisong of mean changes from baseline on Dermatitis Family Impact Questionnaire at weeks 4 and 8 | |
| Notes | Contact: [email protected] Country: Taiwan Study registration not given CY Biotech provided probiotic, but it is not clear whether the company had a role in study design, data analysis, or interpretation or other aspects of the study. However, study authors declared no conflicts of interest | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Inadequate information provided; stated that the study was "randomised" |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) | Unclear risk | Study stated as "double‐blind", but no information provided to explain who was blinded and how blinding was done |
| Blinding of outcome assessment (detection bias) | Unclear risk | Study stated as "double‐blind", but no information provided to explain who was blinded and how blinding was done |
| Incomplete outcome data (attrition bias) | Low risk | 3 (9%) from the probiotic arm and 1 (3%) from the control arm Study authors present data derived from intention‐to‐treat analysis |
| Selective reporting (reporting bias) | Unclear risk | All predefined outcomes were at least described, but not all data were given. No clinical trial registration was given |
| Other bias | Unclear risk | CY Biotech provided probiotic, but it is not clear whether the company had a role in study design, data analysis or interpretation, or other aspects of the study. However study authors declared no conflict of interest Hence we judged the study to be at unclear risk of commercial bias |
| Methods | Six‐week parallel‐group double‐blind placebo‐controlled randomised trial conducted between November 2010 and October 2011 | |
| Participants | One hundred children from 2 to 9 years of age with mild to moderate (SCORAD < 40) atopic dermatitis diagnosed according to the criteria of Hanifin and Rajka were recruited. Randomisation was done at a 1:1 ratio: 50 participants were randomised in each arm Exposure to commercial probiotic products during the 4 weeks before the study Premature children and those receiving antibiotic, systemic corticosteroid, immunosuppressive, or Chinese herbal therapies within 4 weeks before enrolment were excluded form the study. Also excluded were patients with acute gastrointestinal infection, chronic underlying disease, or baseline factors predisposing to infection (e.g. neurological disease; metabolic disease; chronic respiratory disease; congenital anomaly of the heart, gastrointestinal system, or lung; known or suspected immunodeficiency) Recruitment took place at a tertiary paediatric centre in Korea Twenty‐nine participants were lost to follow‐up | |
| Interventions | Probiotic mixture: Lactobacillus casei, Lactobacillus rhamnosus, Lactobacillus plantarum, Bifidobacterium lactis in glucose anhydrous crystalline powder derived from cornstarch prepared in warm water or juice given orally immediately after meals twice daily at a dose of 1 × 10⁹ CFUs of each probiotic strain for 6 weeks Control (placebo): glucose anhydrous crystalline powder prepared in warm water or juice given orally immediately after meals twice daily for 6 weeks Instructions given to stop topical corticosteroids and calcineurin inhibitors, oral antihistamines, and any commercial probiotic‐containing products 2 weeks before study initiation Parents were trained in appropriate bathing and skin care practices and were given instructions on application of emollients | |
| Outcomes | • EASI score at baseline and end of treatment and change from baseline* • VASP score at baseline and end of treatment and change from baseline* *Denotes outcomes prespecified for this review | |
| Notes | Study sponsored by the probiotic supplier; the supplier's role in data analysis and publication is unclear | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomisation software was used to randomly allocate children..." Comment: computer‐generated and so judged as adequate for low risk of bias |
| Allocation concealment (selection bias) | Low risk | Comment: third party ‐ not involved in the trial ‐ allocated treatment. Study judged as adequate for low risk of bias |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: "The probiotics mixture and placebo controls were identical in colour, taste, smell, packing and manner of administration. All formulations were dispensed by a pharmacist not associated with the study.", "both investigators and study subjects were blinded to the identity of the intervention" Comment: not clear if clinicians were blinded; patients were blinded; judged as having unclear risk of bias |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: "The probiotics mixture and placebo controls were identical in colour, taste, smell, packing and manner of administration. All formulations were dispensed by a pharmacist not associated with the study" ‐ "both investigators and study subjects were blinded to the identity of the intervention" Comment: not clear if outcome assessor was blinded; judged as having unclear risk of bias |
| Incomplete outcome data (attrition bias) | High risk | Available case analysis but high rates of loss to follow‐up (29%: 26% in probiotic group and 32% in placebo group), which were excluded from analysis Comment: effect estimate for all outcomes may have been affected; judged as having high risk of attrition bias |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | Unclear risk | Study sponsored by the probiotic supplier, and the supplier's role in data analysis and publication is unclear. Therefore the study was judged to be at unclear risk of bias |
| Methods | Eight‐week parallel‐group double‐blind randomised placebo‐controlled trial | |
| Participants | Forty children 1 to 13 years of age with atopic dermatitis diagnosed according to Hanifin and Rajka criteria and with moderate to severe disease. Randomisation was done at a 1:1 ratio: 20 participants were randomised in each arm. Excluded were patients on medication including antihistamines and corticosteroids for 14 days before recruitment, as well as those with malabsorption. One participant was lost to follow‐up | |
| Interventions | Probiotic mixture:Bifidobacterium bifidum,Lactobacillus acidophilus,Lactobacillus casei,Lactobacillus salivarius given orally at a dose of 2 × 10⁹ CFUs and a total daily dose of 4 × 10⁹ CFUs for 8 weeks Placebo: skim milk powder and dextrose | |
| Outcomes | SCORAD at baseline and at 8 weeks* *Denotes outcomes prespecified for this review | |
| Notes | Conflicts of interest/sponsorship not declared | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The patients were referred to a nurse who was involved in the study to receive either probiotic or placebo. The nurse randomised each patient to two different treatment groups using the closed‐envelope method" Comment: not clear how the "closed envelopes" had been created and whether the sequence generation had been random. Judged as having unclear risk of bias |
| Allocation concealment (selection bias) | Low risk | Quote: "Closed envelope method" Comment: probably done; judged as adequate for low risk of bias |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: "The patients were referred to a nurse who was involved in the study to receive either probiotic or placebo. The nurse randomised each patient to two different treatment groups using the closed‐envelope method. The authors had no role in the treatment decision and were blinded to the treatment groups" Comment: inadequate information provided on which parts were blinded and whether the interventions were identical. Judged as having unclear risk of bias |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: "The patients were referred to a nurse who was involved in the study to receive either probiotic or placebo. The nurse randomised each patient to two different treatment groups using the closed‐envelope method. The authors had no role in the treatment decision and were blinded to the treatment groups" Comment: inadequate information on which parts were blinded and whether interventions were identical. Judged as having unclear risk of bias |
| Incomplete outcome data (attrition bias) | Low risk | One participant from the placebo group was lost to follow‐up (2.5%). Available case analysis was used. Very few losses to follow‐up were unlikely to affect the effect estimate for any outcome |
| Selective reporting (reporting bias) | Low risk | All outcomes reported. Differences between probiotic and placebo not given but reported narratively: "Our results demonstrated an improved SCORAD index in both groups, but with higher levels in the probiotic group (65%) than in the placebo group (46%). In the probiotic group, a greater decrease of SCORAD index scores was shown after treatment in patients with high SCORAD index scores. However this difference did not reach a statistically significant level" |
| Other bias | Unclear risk | Conflicts of interest/sponsorship not declared Baseline characteristics of 2 groups not given. Uncertain if they were matched Judged to be at uncertain risk of other bias |
| Methods | Eight‐week parallel‐group placebo‐controlled trial | |
| Participants | Twenty‐four adults with atopic dermatitis diagnosed according to the Guideline for Management of AD by the Japanese Dermatological Association were recruited and randomised at a 2:1 ratio of intervention:control (16 participants in intervention arm/8 participants in control arm) Recruitment took place in a centre in Japan No participants were lost to follow‐up | |
| Interventions | Probiotic: Bifidobacterium breve (lyophilised powder of live B breve YY) in a capsule preparation at a dose of 1.0 × 10¹º CFUs/capsule taken twice daily after breakfast and after dinner for 8 weeks Placebo not described | |
| Outcomes | • SCORAD: total, objective, subjective* • Japanese version of Skindex‐29* *Denotes outcomes prespecified for this review | |
| Notes | Sponsorship was not declared, but 2 of the authors of the report are linked to the supplier of the probiotic | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Test meals for 24 subjects were randomly allocated..." Comment: no other information provided; judged as having unclear risk |
| Allocation concealment (selection bias) | Unclear risk | No information given |
| Blinding of participants and personnel (performance bias) | Unclear risk | No information given |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information given |
| Incomplete outcome data (attrition bias) | Low risk | No losses to follow‐up. All participants analysed |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | High risk | Selection bias: baseline SCORAD scores not matched between the 2 groups Commercial bias: sponsorship not declared but 2 of the authors of the report linked to the supplier of the probiotic; this may have affected the outcome Study was judged to have high risk of other bias |
AD: atopic dermatitis.
AEDS: atopic eczema‐dermatitis syndrome.
BPD: bronchopulmonary dysplasia.
CDLQI: Children's Dermatology Life Quality Index.
CFU: colony‐forming unit.
cGMP: cyclic guanosine monophosphate.
DFI: Dermatology Family Impact.
DLQI: Dermatology Life Quality Index.
EASI: Eczema Area Severity Index.
FDLQI: Family Dermatology Life Quality Index.
IDQoL: Infant Dermatitis Quality of Life.
IFN: interferon.
IgE: immunoglobulin E.
IL: interleukin.
LF: Lactobacillus fermentum.
LP: Lactobacillus paracasei.
RAST: radioallergosorbent test.
RSV: respiratory syncytial virus.
SCORAD: Severity Scoring of Atopic Dermatitis.
TCS: topical corticosteroid.
TS: topical steroid.
VAS: visual analogue scale.
VASP: Visual Analogue Scale of Pruritus.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Intradermally given probiotic | |
| Participants did not have eczema | |
| Not an RCT. Only a quasi‐RCT | |
| Not all participants had eczema | |
| Not an RCT. Quasi‐RCT | |
| Not a trial on probiotics. Unpasteurised mare's milk trial | |
| Topical intervention | |
| Intervention was not a probiotic | |
| Participants did not have eczema | |
| Not a study of changes in eczema symptoms or severity. Follow‐up of a study on probiotics for prevention, not treatment, of allergies | |
| Intervention was not a probiotic | |
| Control is also a probiotic. Exclusion criteria in protocol | |
| Heat‐killed bacteria. Not live micro‐organisms; exclusion criteria in protocol | |
| Heat‐killed bacteria. Not live micro‐organisms; exclusion criteria in protocol | |
| Trial studied effects of probiotics in mice only. Human participants were healthy volunteers without eczema | |
| Not all participants had eczema | |
| Not all participants had eczema | |
| Not a probiotic ‐ a prebiotic only | |
| Heat‐killed bacteria. Not live micro‐organisms; exclusion criteria in protocol |
RCT: randomised controlled trial.
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | Eight‐week parallel‐group randomised placebo‐controlled trial |
| Participants | Children from 6 months to 12 years of age with eczema and SCORAD > 15 Inclusion criteria: guardians proficient in English. Not previously actively treated with probiotics for eczema. No current chronic illness other than asthma, allergic rhinitis, or food allergy Exclusion criteria: none Country: Australia |
| Interventions | Probiotic: Lactobillus GG 3 × 10⁸ to 1 × 10⁹ CFUs twice daily for 8 weeks vs placebo |
| Outcomes | SCORAD at 0, 8, and 16 weeks. |
| Notes | ACTRN12605000615684. Unknown current status |
| Methods | Eight‐week prospective randomised multi‐centre double‐blind controlled study |
| Participants | Seventy‐one full‐term infants with suspected non‐IgE‐mediated cow's milk allergy (36 infants in control group, 35 in active group) Cow's milk allergy participants presented predominantly with gastrointestinal symptoms, and 10% with dermatological symptoms Country: Netherlands |
| Interventions | Active: amino acid‐based formula (AAF) with synbiotics designed for dietary management of cow's milk allergy (prebiotic: chicory‐derived neutral oligofructose, long‐chain inulin 9:1 ratio, and concentration 0.63 g/100 mL; probiotic: Bifidobacterium breve M‐16V at a concentration 1.47 × 10⁹ CFUs/100 mL formula) Control: commercially available formula with AAF only Intake/instructions: participants were instructed to consume a minimum, age‐specific, daily formula intake from the end of week 2 (infants 0 to 6 months of age, 500 mL; 6 to 8 months of age, 450 mL; and 49 months of age, 350 mL) Duration of intervention: 8 weeks |
| Outcomes | Primary
Secondary
|
| Notes | Trial acronym: ASSIGN‐1 Register: NTR3979 (Netherlands Trial Register) Funding/Sponsor: Nutricia Research BV Contact: Willemien Sinke; [email protected] |
| Methods | Randomised double‐blind controlled trial |
| Participants | 31 infants with objective SCORAD (score > 20; moderate to severe atopic dermatitis) up to and including 11 months of age, with elevated total IgE or specific IgE levels, or both, were included (n = 31) Country: Netherlands |
| Interventions | Synbiotic: extensively hydrolysed whey‐based formula with mixture of short‐chain galacto‐, long‐chain fructo‐oligosaccharides (scGOS/lcFOS, ratio 9:1) and Bifidobacterium breve M‐16V (active) at a dose of 1.0 × 10⁹ CFUs/g Control: extensively hydrolysed whey‐based formula Duration of intervention: 4 months |
| Outcomes | Severity of atopic dermatitis and correlation to serum chemokines
|
| Notes | Study presented at Conference Registered in the Dutch Trial Register: NTR3447 |
| Methods | Randomised double‐blind placebo‐controlled clinical trial |
| Participants | Children of both genders between 4 and 17 years of age. Estimated enrolment: 50. Country: Spain Inclusion criteria:
Exclusion criteria
|
| Interventions |
Duration of intervention: 12 weeks |
| Outcomes | Primary outcome measures
Secondary outcome measures
|
| Notes | Current status: results published Registration: NCT02585986 (ClinicalTrials.gov). Sponsor: Biopolis SL |
| Methods | Randomised double‐blind placebo‐controlled trial |
| Participants | 22 children 0 to 14 years of age (12 probiotic and 10 placebo) with mild to moderate atopic dermatitis meeting the Hanifin‐Rajka diagnostic criteria. New outpatients from the clinic at the Allergy Immunology Division of the Dermatology and Venereology Department, Faculty of Medicine, Indonesia. Participants had to have age‐related total serum IgE levels: 10 to 15 years > 200 IU/mL, 6 to 9 years > 90 IU/mL, 1 to 5 years > 60 IU/mL, < 1 year > 1.5 IU/mL. Participants had to be in apparent good health and willing to participate in the study, and had to sign informed consent Exclusion criteria: use of systemic corticosteroids or phototherapy in the previous month, systemic immunosuppressive drugs in the previous 3 months, probiotic use in the previous 4 weeks, use of topical medications such as corticosteroids or calcineurin inhibitors in the previous week, immunosuppressive conditions or other serious disease, clinical skin disease, and other systemic disease Country: Indonesia |
| Interventions | Intervention: microencapsulated L plantarum IS‐10506 at a dose of 10¹º CFUs/d Control: placebo; skim milk ‐ Avicel Duration: 12 weeks |
| Outcomes | SCORAD score at 0, 2, 8, and 14 weeks Total IgE, IL‐4, IFN‐γ, Foxp3=/IL‐10, IL‐17 at 0 and 14 weeks |
| Notes | ‐ |
AAF: amino acid‐based formula.
CC: Clostridium coccidioides.
CFU: colony‐forming unit.
CGI: Clinical Global Impression.
ER: Eubacterium rectale.
IFN: interferon.
IgE: immunoglobulin E.
IL: interleukin.
SCFA: short‐chain fatty acid.
SCORAD: Severity Scoring of Atopic Dermatitis.
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Study on the effect and mechanism of probiotics on patients with atopic dermatitis |
| Methods | Randomised parallel controlled trial with 4 intervention groups |
| Participants | Participants randomised in 4 groups. Aim is to recruit 30 participants in each group Inclusion criteria: meet the diagnostic criteria of Hanifin and Rajka; from 7 to 60 years old; have not taken probiotics (such as lactic acid bacteria, bifidobacteria, etc.) yoghourt, beverages, etc. (in the past 2 months, adherence to requirement of taking probiotic productions for 2 months, co‐operation with survey and collection of blood samples, faeces, and other biological samples); volunteers who sign informed consent Exclusion criteria: pregnant or lactating women; short‐term or long‐term use of antibiotics, accompanied by diabetes, cardiovascular disease, history of malignant disease complications, and any other interference with results of tests evaluating skin disease, enteritis, etc.; accompanied by mental illness |
| Interventions |
|
| Outcomes | Primary
Secondary
|
| Starting date | Date of registration: 23/03/2018. Prospective registration |
| Contact information | Wenwei Lu; Tel: +8618762691080; email: [email protected] 1800 Lihu Avenue, Binhu District, Wuxi, Jiangsu, China |
| Notes | Open for recruitment Study sponsors: People's Hospital of Tinghu District of Yancheng, China, and Jiangnan University |
| Trial name or title | A study to observe if probiotics supplementation is helpful in atopic dermatitis in children |
| Methods | Phase III randomised placebo‐controlled parallel‐group trial. Blinded investigator. Randomisation computer generated |
| Participants | Children of both genders, from 6 months to 12 years of age who have atopic dermatitis of any severity diagnosed based on the Hanifin and Rajka criteria Exclusion criteria: immunocompromised children; children with severe kidney, liver, or systemic disease; other ages Target sample size = 114 |
| Interventions | Probiotics and placebo. No other information available |
| Outcomes | Primary outcomes
|
| Starting date | Date of first enrolment: 02/07/2016 |
| Contact information | Professor Sanjeev Handa, Professor of Dermatology, Venereology, and Leprology Post Graduate Institute of Medical Education and Research (PGIMER), Chandigarh 160012, India |
| Notes | Study sponsored by PGIMER Currently recruiting |
| Trial name or title | Probiotics in treatment of atopic dermatitis in children |
| Methods | Double‐blind randomised placebo‐controlled parallel‐group trial. Computer‐generated randomisation Method of allocation concealment: sequentially numbered, sealed, opaque envelopes |
| Participants | Children with atopic dermatitis of both genders, from 6 months to 18 years of age, attending JIPMER Paediatrics or Dermatology. Target sample size = 108 Exclusion criteria: children with acute gastrointestinal infection; children with chronic underlying disease on immunosuppressive therapy; children with known or suspected immunodeficiency; children on prolonged antibiotic and antituberculous therapy; children who are included in other studies |
| Interventions | Probiotic: Lactobacillus rhamnosus GG. Dose: 10 billion CFUs/capsule or sachet/d for 3 months Control: placebo: anhydrous glucose powder Both groups receive also standard treatment |
| Outcomes | Primary outcome
Secondary outcomes
Time point for secondary outcomes: 1 year |
| Starting date | 01/11/2017. Registered as not recruiting yet |
| Contact information | Dhayalini RK; [email protected] Department of Pediatrics (OPD 165) and Department of Dermatology (OPD 72), JIPMER Hospital, Dhauranthri Nagar, Gorimedu, Pondicherry 605006, PONDICHERRY |
| Notes | Country: India Registered prospectively Ethics approval received Sponsorship: JIPMER Hospital and Research Institution |
| Trial name or title | IRT5 probiotics atopic dermatitis |
| Methods | Phase III 8‐week parallel‐group randomised double‐blind placebo‐controlled clinical trial |
| Participants | Children 5 to 12 years of age with atopic dermatitis, SCORAD score between 25 and 50, and continuous or intermittent symptoms of atopic dermatitis over 6 months. Target sample size = 110 participants; 55 in each group Other inclusion criterion: signs informed consent form with a legal representative before participating in the study Exclusion criteria
|
| Interventions | Probiotic: IRT5 probiotics, 1 × 10¹º CFUs/sachet, to be taken orally 1 sachet per day for 8 weeks |
| Outcomes | Primary
Secondary
|
| Starting date | Date of first enrolment: 06/11/2013 |
| Contact information | Professor Kim Beom Jun, CHUNG‐ANG University, Korea |
| Notes | Registration: KCT0000914. Current status: recruiting. No updates to the registry since 2013 Primary sponsor: KOREA YAKULT CORPORATION. Affiliation: CHUNG‐ANG University |
| Trial name or title | Trial on effectiveness of combined probiotics in atopic dermatitis in children |
| Methods | Phase IV randomised parallel‐group double‐blind placebo‐controlled clinical trial |
| Participants | Children of both genders older than 6 months up to 19 years of age Inclusion criteria
Exclusion criteria
|
| Interventions | Probiotic: probiotic comprising the mixture of strains: Lactobacillus rhamnosus, Lactobacillus acidophilus, Lactobacillus paracasei, and Bifidobacterium lactis, at a dose of 1 gram sachet, once a day for 6 months (drug: Probiatop) Control: placebo or maltodextrin in sachet once a day for 6 months |
| Outcomes | Primary outcome measures
Secondary outcome measures
|
| Starting date | August 2015, with provisional completion date July 2017 |
| Contact information | Contact: Paula Albuquerque, MD; 5516981329192; [email protected] |
| Notes | Registration: NCT02519556 (ClinicalTrials.gov) Country: Brazil. Sponsor: Casa Espirita Terra de Ismael. Estimated primary completion date: July 2017. Currently enrolling by participant invitation only |
| Trial name or title | ATOPIA‐D3: effects of Lactobacillus reuteri plus vitamin D3 in children with atopic dermatitis |
| Methods | Randomised double‐blind placebo‐controlled clinical trial |
| Participants | Children of both genders, 1 to 4 years of age with diagnosis of atopic dermatitis. Recruitment target: 88 participants Inclusion criteria
Exclusion criteria
|
| Interventions |
|
| Outcomes | Primary outcome
Secondary outcomes
|
| Starting date | February 2015 |
| Contact information | Attilio Boner, Professor +390458124615 |
| Notes | Study currently recruiting. Estimated completion date February 2018 |
AD: atopic dermatitis.
CDLQI: Children's Dermatology Life Quality Index.
CFU: colony‐forming unit.
ELISA: enzyme‐linked immunosorbent assay.
IDLQI: Infant's Dermatitis Life Quality Index.
IFN: interferon.
IgE: immunoglobulin E.
IL: interleukin.
QoL: quality of life.
SCORAD: Severity Scoring of Atopic Dermatitis.
TGF: transforming growth factor.
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Participant‐ or parent‐rated symptoms of eczema (SCORAD part C) at the end of treatment Show forest plot | 13 | Mean difference (Random, 95% CI) | ‐0.44 [‐1.22, 0.33] | |
| Analysis 1.1  Comparison 1 Probiotic vs placebo, Outcome 1 Participant‐ or parent‐rated symptoms of eczema (SCORAD part C) at the end of treatment. | ||||
| 1.1 Parallel‐group trials | 11 | Mean difference (Random, 95% CI) | ‐0.42 [‐1.27, 0.43] | |
| 1.2 Cross‐over trials | 2 | Mean difference (Random, 95% CI) | ‐0.52 [‐3.16, 2.12] | |
| 2 Participant‐ or parent‐rated global change in eczema symptoms at the end of treatment (binary outcome) Show forest plot | 3 | Odds ratio (Random, 95% CI) | 0.40 [0.14, 1.15] | |
| Analysis 1.2  Comparison 1 Probiotic vs placebo, Outcome 2 Participant‐ or parent‐rated global change in eczema symptoms at the end of treatment (binary outcome). | ||||
| 2.1 Parallel‐group trials | 2 | Odds ratio (Random, 95% CI) | 0.70 [0.27, 1.77] | |
| 2.2 Cross‐over trials | 1 | Odds ratio (Random, 95% CI) | 0.18 [0.05, 0.60] | |
| 3 Change in participant‐ or parent‐rated symptoms of eczema (SCORAD part C) at the end treatment (continuous outcome) Show forest plot | 9 | Mean Difference (Random, 95% CI) | ‐0.70 [‐1.47, 0.06] | |
| Analysis 1.3  Comparison 1 Probiotic vs placebo, Outcome 3 Change in participant‐ or parent‐rated symptoms of eczema (SCORAD part C) at the end treatment (continuous outcome). | ||||
| 3.1 Parallel‐group trials | 8 | Mean Difference (Random, 95% CI) | ‐0.82 [‐1.62, ‐0.02] | |
| 3.2 Cross‐over studies | 1 | Mean Difference (Random, 95% CI) | 0.66 [‐1.79, 3.11] | |
| 4 Participant‐ or patient‐related quality of life score at the end of treatment Show forest plot | 6 | Std. Mean Difference (Random, 95% CI) | 0.03 [‐0.36, 0.42] | |
| Analysis 1.4  Comparison 1 Probiotic vs placebo, Outcome 4 Participant‐ or patient‐related quality of life score at the end of treatment. | ||||
| 4.1 Infant's Dermatitis Quality of Life Index (IDQoL) | 2 | Std. Mean Difference (Random, 95% CI) | 0.35 [0.08, 0.62] | |
| 4.2 Dermatology Life Quality Index | 1 | Std. Mean Difference (Random, 95% CI) | ‐0.33 [‐0.95, 0.29] | |
| 4.3 Skindex‐29 Questionnaire | 2 | Std. Mean Difference (Random, 95% CI) | ‐0.66 [‐4.24, 2.92] | |
| 4.4 Children's Dermatology Quality of Life Index | 1 | Std. Mean Difference (Random, 95% CI) | ‐0.39 [‐0.70, ‐0.08] | |
| 5 Participant‐ or patient‐related quality of life score at the end of treatment Show forest plot | 3 | Std. Mean Difference (Random, 95% CI) | ‐0.19 [‐0.56, 0.18] | |
| Analysis 1.5  Comparison 1 Probiotic vs placebo, Outcome 5 Participant‐ or patient‐related quality of life score at the end of treatment. | ||||
| 5.1 Dermatitis Family Impact Questionnaire (DFI) | 2 | Std. Mean Difference (Random, 95% CI) | ‐0.31 [‐0.86, 0.24] | |
| 5.2 Family Dermatology Dermatology Life Quality Index | 1 | Std. Mean Difference (Random, 95% CI) | ‐0.01 [‐0.32, 0.30] | |
| 6 Parent‐ or participant‐rated eczema severity (SCORAD part C) within 6 months after treatment has ceased Show forest plot | 3 | 185 | Mean Difference (IV, Random, 95% CI) | ‐1.81 [‐3.13, ‐0.49] |
| Analysis 1.6  Comparison 1 Probiotic vs placebo, Outcome 6 Parent‐ or participant‐rated eczema severity (SCORAD part C) within 6 months after treatment has ceased. | ||||
| 7 Participant‐ or parent‐related quality of life within 6 months after treatment has ceased Show forest plot | 2 | 261 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.08 [‐0.35, 0.20] |
| Analysis 1.7  Comparison 1 Probiotic vs placebo, Outcome 7 Participant‐ or parent‐related quality of life within 6 months after treatment has ceased. | ||||
| 7.1 Dermatology Life Quality Index | 1 | 46 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.14 [‐0.75, 0.48] |
| 7.2 Child Dermatology Life Quality Index | 1 | 215 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.06 [‐0.37, 0.25] |
| 8 Global eczema severity score (total SCORAD) at the end of treatment Show forest plot | 24 | Mean difference (Random, 95% CI) | ‐3.91 [‐5.86, ‐1.96] | |
| Analysis 1.8  Comparison 1 Probiotic vs placebo, Outcome 8 Global eczema severity score (total SCORAD) at the end of treatment. | ||||
| 8.1 Parallel‐group studies | 22 | Mean difference (Random, 95% CI) | ‐3.84 [‐5.95, ‐1.72] | |
| 8.2 Cross‐over studies | 2 | Mean difference (Random, 95% CI) | ‐4.14 [‐7.68, ‐0.59] | |
| 9 Global eczema severity score (total SCORAD) at the end of treatment ‐ sensitivity analysis ‐ change score Show forest plot | 14 | Mean difference (Random, 95% CI) | ‐4.46 [‐6.49, ‐2.43] | |
| Analysis 1.9  Comparison 1 Probiotic vs placebo, Outcome 9 Global eczema severity score (total SCORAD) at the end of treatment ‐ sensitivity analysis ‐ change score. | ||||
| 9.1 Parallel‐group trial | 13 | Mean difference (Random, 95% CI) | ‐4.53 [‐6.72, ‐2.33] | |
| 9.2 Cross‐over trial | 1 | Mean difference (Random, 95% CI) | ‐3.93 [‐9.25, 1.40] | |
| 10 Global eczema severity score (total SCORAD) at the end of treatment ‐ low risk of bias studies only Show forest plot | 8 | Mean difference (Random, 95% CI) | Totals not selected | |
| Analysis 1.10  Comparison 1 Probiotic vs placebo, Outcome 10 Global eczema severity score (total SCORAD) at the end of treatment ‐ low risk of bias studies only. | ||||
| 10.1 Parallel‐group studies | 8 | Mean difference (Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 11 Investigator‐rated eczema severity (SCORAD parts A/B) at the end of treatment ‐ continuous outcome Show forest plot | 10 | 529 | Mean Difference (IV, Random, 95% CI) | ‐2.24 [‐4.69, 0.20] |
| Analysis 1.11  Comparison 1 Probiotic vs placebo, Outcome 11 Investigator‐rated eczema severity (SCORAD parts A/B) at the end of treatment ‐ continuous outcome. | ||||
| 12 Global eczema severity score (total SCORAD) within 6 months after treatment has ceased Show forest plot | 7 | Mean difference (Random, 95% CI) | ‐7.72 [‐11.85, ‐3.59] | |
| Analysis 1.12  Comparison 1 Probiotic vs placebo, Outcome 12 Global eczema severity score (total SCORAD) within 6 months after treatment has ceased. | ||||
| 12.1 Parallel‐group studies | 6 | Mean difference (Random, 95% CI) | ‐9.27 [‐13.88, ‐4.65] | |
| 12.2 Cross‐over studies | 1 | Mean difference (Random, 95% CI) | 0.2 [‐3.86, 4.26] | |
| 13 Investigator‐rated eczema severity (SCORAD parts A/B) within 6 months after treatment has ceased Show forest plot | 2 | 102 | Mean Difference (IV, Random, 95% CI) | ‐8.11 [‐13.14, ‐3.09] |
| Analysis 1.13  Comparison 1 Probiotic vs placebo, Outcome 13 Investigator‐rated eczema severity (SCORAD parts A/B) within 6 months after treatment has ceased. | ||||
| 14 Adverse events (short term) Show forest plot | 7 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.14  Comparison 1 Probiotic vs placebo, Outcome 14 Adverse events (short term). | ||||
| 14.1 Gastrointestinal symptoms | 7 | 402 | Risk Ratio (M‐H, Random, 95% CI) | 1.54 [0.90, 2.63] |
| 15 Participant/parent‐rated global change in symptoms of eczema at the end of treatment ‐ stratified by age groups Show forest plot | 3 | Odds ratio (Random, 95% CI) | Totals not selected | |
| Analysis 1.15  Comparison 1 Probiotic vs placebo, Outcome 15 Participant/parent‐rated global change in symptoms of eczema at the end of treatment ‐ stratified by age groups. | ||||
| 15.1 Age under 2 years | 1 | Odds ratio (Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 15.2 Age 2 to 12 years | 1 | Odds ratio (Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 15.3 Age not categorised | 1 | Odds ratio (Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 16 Participant/parent‐rated symptoms of eczema (SCORAD part C) at the end of treatment ‐ stratified by age groups Show forest plot | 12 | Mean difference (Random, 95% CI) | Subtotals only | |
| Analysis 1.16  Comparison 1 Probiotic vs placebo, Outcome 16 Participant/parent‐rated symptoms of eczema (SCORAD part C) at the end of treatment ‐ stratified by age groups. | ||||
| 16.1 Age under 2 years | 5 | Mean difference (Random, 95% CI) | ‐0.39 [‐2.20, 1.42] | |
| 16.2 Age 2 to 12 years | 4 | Mean difference (Random, 95% CI) | ‐0.63 [‐2.04, 0.78] | |
| 16.3 Age not categorised | 2 | Mean difference (Random, 95% CI) | ‐2.23 [‐3.71, ‐0.74] | |
| 16.4 Adults only | 2 | Mean difference (Random, 95% CI) | 1.01 [‐0.82, 2.84] | |
| 17 Global eczema severity score (total SCORAD) at the end of treatment ‐ stratified by age groups Show forest plot | 24 | Mean difference (Random, 95% CI) | Subtotals only | |
| Analysis 1.17  Comparison 1 Probiotic vs placebo, Outcome 17 Global eczema severity score (total SCORAD) at the end of treatment ‐ stratified by age groups. | ||||
| 17.1 Age under 2 years | 10 | Mean difference (Random, 95% CI) | ‐0.99 [‐3.97, 1.99] | |
| 17.2 Age 2 to 12 years | 3 | Mean difference (Random, 95% CI) | ‐6.08 [‐9.68, ‐2.48] | |
| 17.3 Age not categorised | 7 | Mean difference (Random, 95% CI) | ‐5.25 [‐10.43, ‐0.07] | |
| 17.4 Adults only | 5 | Mean difference (Random, 95% CI) | ‐6.51 [‐10.09, ‐2.93] | |
| 18 Global eczema severity score (total SCORAD) at the end of treatment ‐ stratified by presence of atopy Show forest plot | 23 | Mean difference (Random, 95% CI) | Subtotals only | |
| Analysis 1.18  Comparison 1 Probiotic vs placebo, Outcome 18 Global eczema severity score (total SCORAD) at the end of treatment ‐ stratified by presence of atopy. | ||||
| 18.1 Participants with atopy | 4 | Mean difference (Random, 95% CI) | ‐3.90 [‐15.52, 7.73] | |
| 18.2 Participants with unknown atopic status | 19 | Mean difference (Random, 95% CI) | ‐4.15 [‐6.02, ‐2.27] | |
| 19 Global eczema severity score (total SCORAD) at the end of treatment ‐ stratified by challenge‐proven food allergy Show forest plot | 21 | Mean difference (Random, 95% CI) | Subtotals only | |
| Analysis 1.19  Comparison 1 Probiotic vs placebo, Outcome 19 Global eczema severity score (total SCORAD) at the end of treatment ‐ stratified by challenge‐proven food allergy. | ||||
| 19.1 Food allergy present | 3 | Mean difference (Random, 95% CI) | ‐1.84 [‐6.22, 2.54] | |
| 19.2 Unknown food allergic status | 18 | Mean difference (Random, 95% CI) | ‐3.21 [‐5.63, ‐0.79] | |
| 20 Global eczema severity score (total SCORAD) at the end of treatment ‐ stratified by eczema severity Show forest plot | 6 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 1.20  Comparison 1 Probiotic vs placebo, Outcome 20 Global eczema severity score (total SCORAD) at the end of treatment ‐ stratified by eczema severity. | ||||
| 20.1 Severe eczema (SCORAD over 40) | 5 | 95 | Mean Difference (IV, Random, 95% CI) | ‐3.71 [‐10.05, 2.64] |
| 20.2 Moderate eczema (SCORAD 15 to 40) | 6 | 279 | Mean Difference (IV, Random, 95% CI) | ‐2.95 [‐7.65, 1.74] |
| 20.3 Mild eczema (SCORAD under 15) | 1 | 8 | Mean Difference (IV, Random, 95% CI) | ‐5.53 [‐15.29, 4.23] |
| 21 Participant‐ or parent‐rated symptoms of eczema (SCORAD part C) at the end of treatment ‐ stratified by probiotic ‐ Lactobacillus species Show forest plot | 12 | Mean difference (Random, 95% CI) | Subtotals only | |
| Analysis 1.21  Comparison 1 Probiotic vs placebo, Outcome 21 Participant‐ or parent‐rated symptoms of eczema (SCORAD part C) at the end of treatment ‐ stratified by probiotic ‐ Lactobacillus species. | ||||
| 21.1 Lactobacillus GG alone or in combination with or without prebiotic | 1 | Mean difference (Random, 95% CI) | 1.79 [0.29, 3.29] | |
| 21.2 Lactobacillus rhamnosus alone or in combination with or without prebiotic | 5 | Mean difference (Random, 95% CI) | ‐0.82 [‐2.24, 0.60] | |
| 21.3 Lactobacillus salivarius alone or in combination with or without prebiotic | 1 | Mean difference (Random, 95% CI) | 0.0 [‐0.88, 0.88] | |
| 21.4 Lactobacillus casei/paracasei alone or in combination with or without prebiotics | 2 | Mean difference (Random, 95% CI) | 0.56 [‐0.29, 1.41] | |
| 21.5 Any Lactobacillus species alone or in combination with or without prebiotics | 12 | Mean difference (Random, 95% CI) | ‐0.50 [‐1.31, 0.31] | |
| 22 Participant‐ or parent‐rated symptoms of eczema (SCORAD part C) at the end of treatment ‐ stratified by probiotic ‐ Bifidobacterium species Show forest plot | 5 | Mean difference (Random, 95% CI) | Subtotals only | |
| Analysis 1.22  Comparison 1 Probiotic vs placebo, Outcome 22 Participant‐ or parent‐rated symptoms of eczema (SCORAD part C) at the end of treatment ‐ stratified by probiotic ‐ Bifidobacterium species. | ||||
| 22.1 Bifidobacterium lactis alone or in combination with or without prebiotics | 4 | Mean difference (Random, 95% CI) | ‐0.34 [‐1.92, 1.24] | |
| 22.2 Bifidobacterium breve alone or in combination with or without prebiotics | 1 | Mean difference (Random, 95% CI) | 1.3 [‐2.15, 4.75] | |
| 22.3 Any Bifidobacteria species alone or in combination with or without prebiotic | 5 | Mean difference (Random, 95% CI) | ‐0.11 [‐1.47, 1.25] | |
| 23 Participant‐ or parent‐rated symptoms of eczema (SCORAD part C) at the end of treatment ‐ number of probiotics Show forest plot | 13 | Mean difference (Random, 95% CI) | Subtotals only | |
| Analysis 1.23  Comparison 1 Probiotic vs placebo, Outcome 23 Participant‐ or parent‐rated symptoms of eczema (SCORAD part C) at the end of treatment ‐ number of probiotics. | ||||
| 23.1 Studies using single probiotic with or without prebiotics | 8 | Mean difference (Random, 95% CI) | ‐0.40 [‐1.45, 0.66] | |
| 23.2 Studies using multiple probiotics with or without prebiotics | 5 | Mean difference (Random, 95% CI) | ‐0.58 [‐1.98, 0.81] | |
| 24 Participant‐ or parent‐rated symptoms of eczema (SCORAD part C) at the end of treatment ‐ probiotics with no prebiotics Show forest plot | 13 | Mean difference (Random, 95% CI) | ‐0.44 [‐1.22, 0.33] | |
| Analysis 1.24  Comparison 1 Probiotic vs placebo, Outcome 24 Participant‐ or parent‐rated symptoms of eczema (SCORAD part C) at the end of treatment ‐ probiotics with no prebiotics. | ||||
| 25 Global eczema severity score (total SCORAD) at the end of treatment ‐ stratified by probiotic ‐ Lactobacillus species Show forest plot | 21 | Mean difference (Random, 95% CI) | Subtotals only | |
| Analysis 1.25  Comparison 1 Probiotic vs placebo, Outcome 25 Global eczema severity score (total SCORAD) at the end of treatment ‐ stratified by probiotic ‐ Lactobacillus species. | ||||
| 25.1 Lactobacillus GG alone or in combination with or without prebiotic | 3 | Mean difference (Random, 95% CI) | 3.37 [0.55, 6.20] | |
| 25.2 Lactobacillus rhamnosus alone or in combination with or without prebiotic | 5 | Mean difference (Random, 95% CI) | ‐3.49 [‐9.81, 2.83] | |
| 25.3 Lactobacillus salivarius alone or in combination with or without prebiotic | 6 | Mean difference (Random, 95% CI) | ‐6.86 [‐10.08, ‐3.63] | |
| 25.4 Lactobacillus casei/paracasei alone or in combination with or without prebiotics | 6 | Mean difference (Random, 95% CI) | ‐2.58 [‐7.21, 2.05] | |
| 25.5 Any Lactobacillus species alone or in combination with or without prebiotics | 21 | Mean difference (Random, 95% CI) | ‐3.80 [‐6.06, ‐1.54] | |
| 26 Global eczema severity score (total SCORAD) at the end of treatment ‐ stratified by probiotic ‐ Bifidobacterium species Show forest plot | 12 | Mean difference (Random, 95% CI) | Subtotals only | |
| Analysis 1.26  Comparison 1 Probiotic vs placebo, Outcome 26 Global eczema severity score (total SCORAD) at the end of treatment ‐ stratified by probiotic ‐ Bifidobacterium species. | ||||
| 26.1 Bifidobacterium lactis alone or in combination with or without prebiotics | 7 | Mean difference (Random, 95% CI) | ‐1.90 [‐5.42, 1.63] | |
| 26.2 Bifidobacterium breve alone or in combination with or without prebiotics | 3 | Mean difference (Random, 95% CI) | ‐0.36 [‐11.39, 10.67] | |
| 26.3 Any Bifidobacteria species alone or in combination with or without prebiotic | 12 | Mean difference (Random, 95% CI) | ‐2.26 [‐5.14, 0.63] | |
| 27 Global eczema severity score (total SCORAD) at the end of treatment ‐ stratified by probiotic ‐ number of probiotics Show forest plot | 24 | Mean difference (Random, 95% CI) | Subtotals only | |
| Analysis 1.27  Comparison 1 Probiotic vs placebo, Outcome 27 Global eczema severity score (total SCORAD) at the end of treatment ‐ stratified by probiotic ‐ number of probiotics. | ||||
| 27.1 Studies using single probiotic with or without prebiotics | 13 | Mean difference (Random, 95% CI) | ‐4.90 [‐7.66, ‐2.15] | |
| 27.2 Studies using multiple probiotics with or without prebiotics | 12 | Mean difference (Random, 95% CI) | ‐3.54 [‐6.50, ‐0.58] | |
| 28 Global eczema severity score (total SCORAD) at the end of treatment ‐ stratified by probiotic ‐ probiotics with no prebiotics Show forest plot | 24 | Mean difference (Random, 95% CI) | ‐3.83 [‐5.81, ‐1.86] | |
| Analysis 1.28  Comparison 1 Probiotic vs placebo, Outcome 28 Global eczema severity score (total SCORAD) at the end of treatment ‐ stratified by probiotic ‐ probiotics with no prebiotics. | ||||

Study flow diagram.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
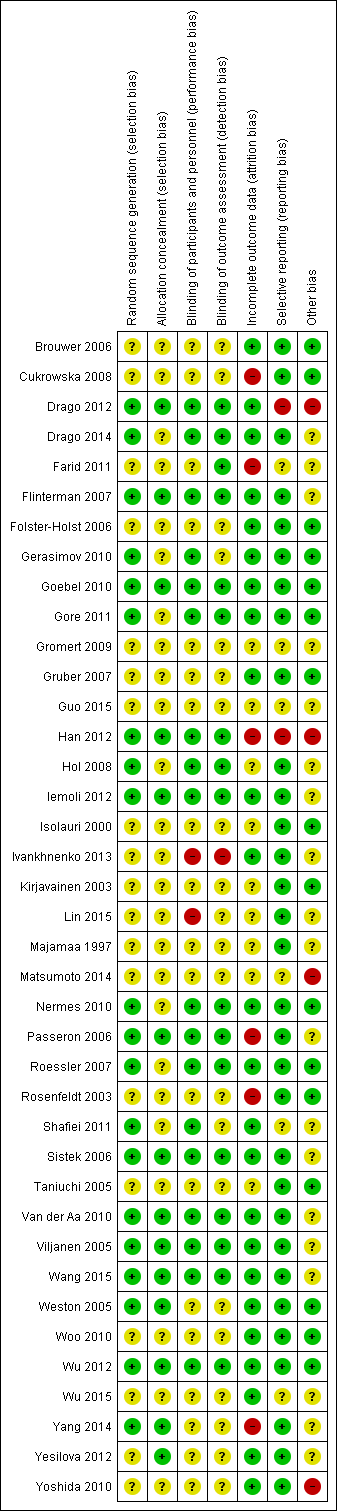
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Trial sequential analysis for a minimum difference of ‐2 points difference in eczema symptoms (SCORAD part C; range 0 to 20) between probiotic and no probiotics at 90% power. The blue z‐curve of the meta‐analysis shows that the optimal heterogeneity‐adjusted information size of 258 has been reached. This suggests that future trials of similar interventions are unlikely to change the findings of no significant difference between probiotic and control for detection of at least a 2‐point difference.
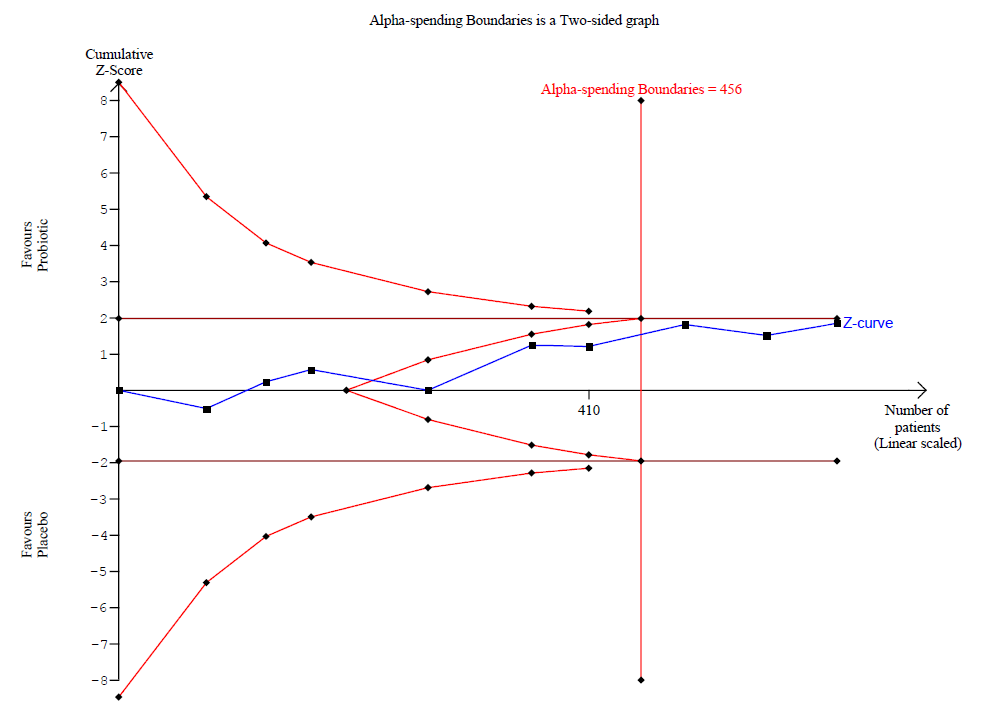
Trial sequential analysis for a minimum difference of ‐1.5 points difference in eczema symptoms (SCORAD part C; range 0 to 20) between probiotics and no probiotics at 90% power. The blue z‐curve of the meta‐analysis has crossed the red v‐shaped line of futility and has reached the optimal heterogeneity‐adjusted information size of 456. This suggests that future trials of similar interventions are unlikely to change the findings of no significant difference between probiotic and control for detection of at least a 1.5‐point difference.

Trial sequential analysis for a minimum difference of ‐1 point difference in eczema symptoms (SCORAD part C; range 0 to 20) between probiotics and no probiotics at 90% power. The blue z‐curve of the meta‐analysis has not crossed the red v‐shaped line of futility and has not yet reached the optimal heterogeneity‐adjusted information size of 1026. This suggests that future trials of similar interventions may change the findings of no significant difference between probiotic and control for detection of at least a 1‐point difference.

Egger's plot for Analysis 1.1: probiotic vs placebo for participant‐ or parent‐rated symptoms of eczema (SCORAD part C) at the end of treatment.

Egger's plot for Analysis 1.8: probiotic vs placebo for global eczema severity score (total SCORAD) at the end of treatment.

Egger's plot for Analysis 1.9: probiotic vs placebo for global eczema severity score (total SCORAD) at the end of treatment ‐ sensitivity analysis ‐ change score.

Comparison 1 Probiotic vs placebo, Outcome 1 Participant‐ or parent‐rated symptoms of eczema (SCORAD part C) at the end of treatment.

Comparison 1 Probiotic vs placebo, Outcome 2 Participant‐ or parent‐rated global change in eczema symptoms at the end of treatment (binary outcome).
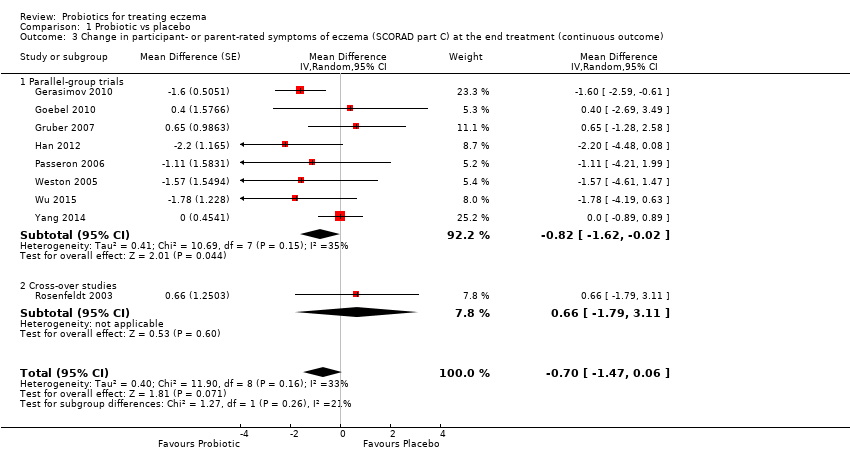
Comparison 1 Probiotic vs placebo, Outcome 3 Change in participant‐ or parent‐rated symptoms of eczema (SCORAD part C) at the end treatment (continuous outcome).

Comparison 1 Probiotic vs placebo, Outcome 4 Participant‐ or patient‐related quality of life score at the end of treatment.

Comparison 1 Probiotic vs placebo, Outcome 5 Participant‐ or patient‐related quality of life score at the end of treatment.

Comparison 1 Probiotic vs placebo, Outcome 6 Parent‐ or participant‐rated eczema severity (SCORAD part C) within 6 months after treatment has ceased.

Comparison 1 Probiotic vs placebo, Outcome 7 Participant‐ or parent‐related quality of life within 6 months after treatment has ceased.

Comparison 1 Probiotic vs placebo, Outcome 8 Global eczema severity score (total SCORAD) at the end of treatment.
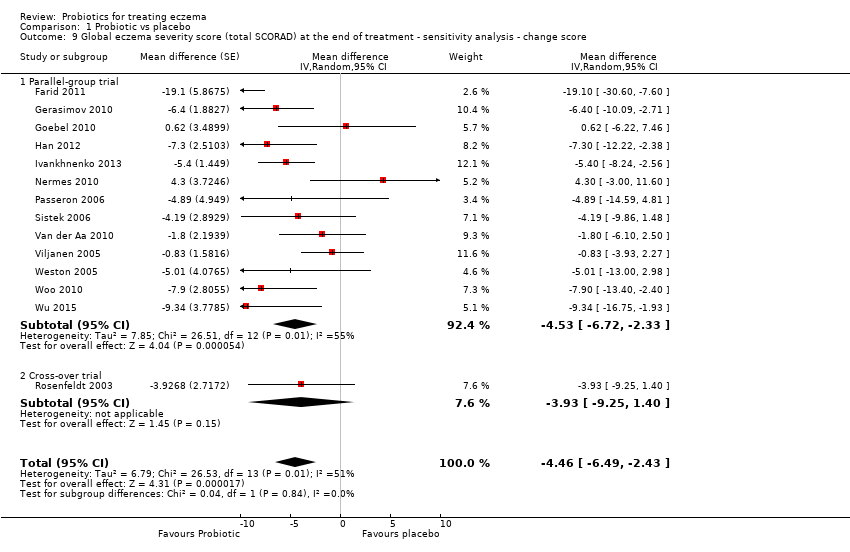
Comparison 1 Probiotic vs placebo, Outcome 9 Global eczema severity score (total SCORAD) at the end of treatment ‐ sensitivity analysis ‐ change score.

Comparison 1 Probiotic vs placebo, Outcome 10 Global eczema severity score (total SCORAD) at the end of treatment ‐ low risk of bias studies only.

Comparison 1 Probiotic vs placebo, Outcome 11 Investigator‐rated eczema severity (SCORAD parts A/B) at the end of treatment ‐ continuous outcome.
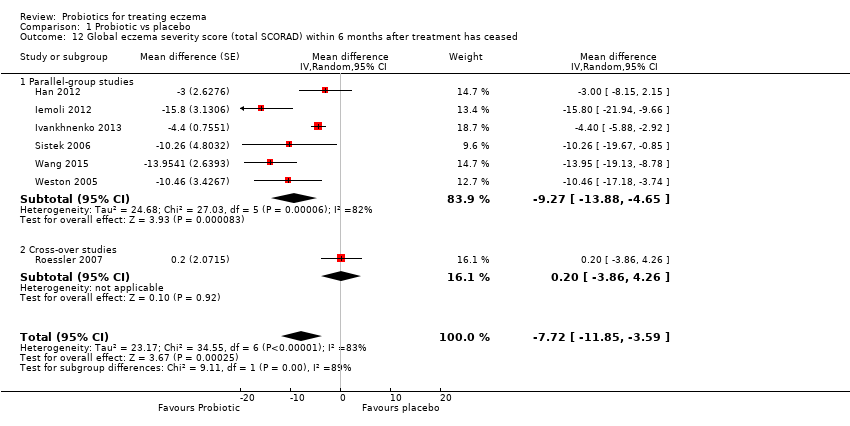
Comparison 1 Probiotic vs placebo, Outcome 12 Global eczema severity score (total SCORAD) within 6 months after treatment has ceased.
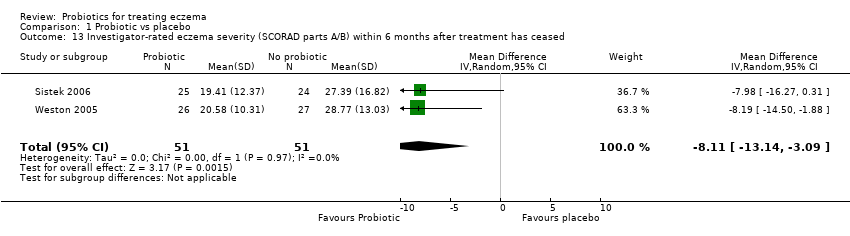
Comparison 1 Probiotic vs placebo, Outcome 13 Investigator‐rated eczema severity (SCORAD parts A/B) within 6 months after treatment has ceased.

Comparison 1 Probiotic vs placebo, Outcome 14 Adverse events (short term).

Comparison 1 Probiotic vs placebo, Outcome 15 Participant/parent‐rated global change in symptoms of eczema at the end of treatment ‐ stratified by age groups.

Comparison 1 Probiotic vs placebo, Outcome 16 Participant/parent‐rated symptoms of eczema (SCORAD part C) at the end of treatment ‐ stratified by age groups.

Comparison 1 Probiotic vs placebo, Outcome 17 Global eczema severity score (total SCORAD) at the end of treatment ‐ stratified by age groups.

Comparison 1 Probiotic vs placebo, Outcome 18 Global eczema severity score (total SCORAD) at the end of treatment ‐ stratified by presence of atopy.

Comparison 1 Probiotic vs placebo, Outcome 19 Global eczema severity score (total SCORAD) at the end of treatment ‐ stratified by challenge‐proven food allergy.

Comparison 1 Probiotic vs placebo, Outcome 20 Global eczema severity score (total SCORAD) at the end of treatment ‐ stratified by eczema severity.
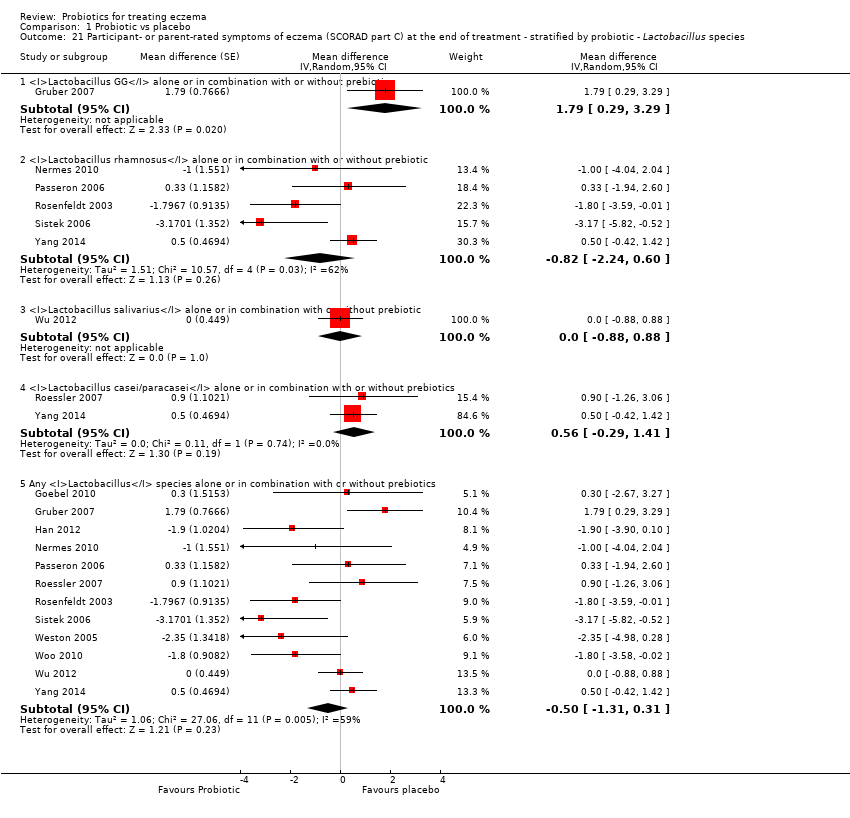
Comparison 1 Probiotic vs placebo, Outcome 21 Participant‐ or parent‐rated symptoms of eczema (SCORAD part C) at the end of treatment ‐ stratified by probiotic ‐ Lactobacillus species.

Comparison 1 Probiotic vs placebo, Outcome 22 Participant‐ or parent‐rated symptoms of eczema (SCORAD part C) at the end of treatment ‐ stratified by probiotic ‐ Bifidobacterium species.
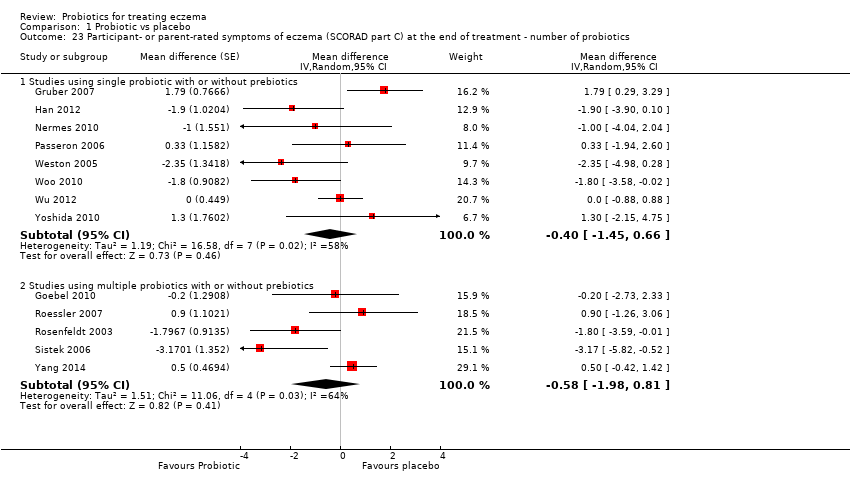
Comparison 1 Probiotic vs placebo, Outcome 23 Participant‐ or parent‐rated symptoms of eczema (SCORAD part C) at the end of treatment ‐ number of probiotics.

Comparison 1 Probiotic vs placebo, Outcome 24 Participant‐ or parent‐rated symptoms of eczema (SCORAD part C) at the end of treatment ‐ probiotics with no prebiotics.

Comparison 1 Probiotic vs placebo, Outcome 25 Global eczema severity score (total SCORAD) at the end of treatment ‐ stratified by probiotic ‐ Lactobacillus species.
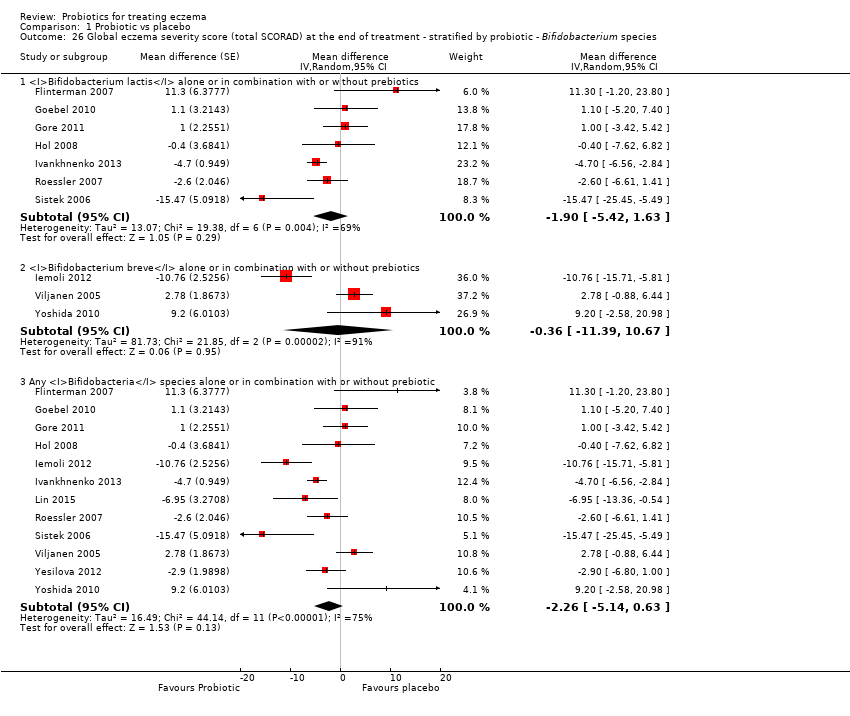
Comparison 1 Probiotic vs placebo, Outcome 26 Global eczema severity score (total SCORAD) at the end of treatment ‐ stratified by probiotic ‐ Bifidobacterium species.

Comparison 1 Probiotic vs placebo, Outcome 27 Global eczema severity score (total SCORAD) at the end of treatment ‐ stratified by probiotic ‐ number of probiotics.

Comparison 1 Probiotic vs placebo, Outcome 28 Global eczema severity score (total SCORAD) at the end of treatment ‐ stratified by probiotic ‐ probiotics with no prebiotics.
| Comparison: probiotics vs no probiotics for treating eczema | ||||||
| Patient or population: male and female patients 0 to 55 years of age with physician‐diagnosed eczema Settings: primary or secondary care. Europe: 22 studies with 1390 participants. Asia: 8 studies with 500 participants. Australasia: 2 studies with 116 participants Intervention: probiotics ± prebiotics Comparison: no probiotics | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| No probiotics | Probiotics | |||||
| Primary outcome 1: participant‐ or parent‐rated symptoms of eczema (SCORAD part C) at the end of active treatment Visual analogue scale for itch and sleep disturbance ranging from 0 to 10 for each symptom and combined ranging from 0 to 20. The higher the score, the more severe the symptoms Duration of follow‐up from baseline until end of active treatment from 6 weeks to 3 months | Mean SCORAD part C score ranged across control groups from 2 to 7.9 | Mean SCORAD part C score in the intervention groups was 0.44 points lower (1.22 lower to 0.33 higher) | ‐ | 754 | ⊕⊕⊕⊝ | Two cross‐over studies included. Significant heterogeneity between studies Post hoc trial sequential analysis showed no effects of probiotics over control and suggests that further studies of currently available probiotic strains for this outcome may be futile |
| Primary outcome 1: participant‐ or parent‐rated global change in eczema symptoms at the end of active treatment (binary outcome) Change in risk for worsened/unchanged eczema Duration of follow‐up from baseline until end of active treatment from 6 weeks to 3 months | Low‐risk population | OR 0.40 (0.14 to 1.15) | 135 | ⊕⊕⊝⊝ | One cross‐over study included. Number of studies for this outcome was small. Moderate heterogeneity between studies | |
| 300 per 1000 | 146 per 1000 | |||||
| Medium‐risk population | ||||||
| 400 per 1000 | 210 per 1000 | |||||
| High‐risk population | ||||||
| 500 per 1000 | 286 per 1000 | |||||
| Primary outcome 2: participant‐ or parent‐rated participant quality of life score at the end of active treatment Scales used: DLQI, IDQoL, Skindex‐29, CDLQI. On those scales, the higher the score, the more severely the quality of life is affected Duration of follow‐up from baseline until end of active treatment from 8 weeks to 3 months | Mean DLQI score ranged across control groups from | Mean participant quality of life score in the intervention groups was | ‐ | 552 (6) | ⊕⊕⊝⊝ | Small number of studies for this outcome. Significant heterogeneity |
| Primary outcome 2: participant‐ or parent‐rated family quality of life score at the end of active treatment Scale used: DFI, FDLQI. On those scales, the higher the score, the more severely the quality of life is affected Duration of follow‐up from baseline until end of active treatment from 8 weeks to 3 months | Mean change in DFI score during treatment ranged across control groups from ‐2 points to ‐3 points | Mean family quality of life score in the intervention groups was 0.19 standard deviations lower (0.56 lower to 0.18 higher) | ‐ | 358 | ⊕⊝⊝⊝ | Very small number of studies for this outcome. Significant heterogeneity |
| Secondary outcome 4: global eczema severity score (total SCORAD) at the end of active treatment (Investigator‐rated eczema severity) Scale used: total SCORAD ranging from 0 to 103. The higher the score, the more severe the disease Duration of follow‐up from baseline until end of active treatment from 8 weeks to 16 weeks | Mean total SCORAD ranged across control groups from | Mean total SCORAD score in the intervention groups was 3.91 points lower (5.86 to 1.96 points lower) | ‐ | 1596 | ⊕⊕⊝⊝ | Two cross‐over studies included. Extreme levels of heterogeneity for this outcome. Evidence of reporting bias |
| Secondary outcome 6: adverse events (gastrointestinal symptoms) during active treatment Duration of follow‐up from baseline until end of active treatment from 8 weeks to 3 months | Low‐risk population | RR 1.54 (0.90 to 2.63) | 402 (7) | ⊕⊕⊝⊝ | Small number of studies reported adverse events. Small number of events were included in this analysis | |
| 0 per 1000 | 0 per 1000 | |||||
| Medium‐risk population | ||||||
| 100 per 1000 | 154 per 1000 | |||||
| High‐risk population | ||||||
| 200 per 1000 | 308 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence. | ||||||
| aDowngraded by one level due to inconsistency as there was significant heterogeneity among studies (I² = 57%). bDowngraded by two levels due to small number of studies for this outcome (imprecision) and because of moderate levels of heterogeneity among studies (I² = 48%). cDowngraded by two levels due to small number of studies for this outcome (imprecision) and because of significant levels of heterogeneity among studies (I² = 68%). dDowngraded by three levels due to inconsistency (one level) as there was significant heterogeneity among studies (I² = 57%) and because of very small number of studies (imprecision) for this outcome (two levels). eDowngraded by two levels because of extreme levels of heterogeneity among studies (I² = 79%) and because of evidence of reporting bias. fDowngraded by two levels because of small number of studies reporting adverse events and small number of events in the meta‐analysis for this outcome (imprecision). | ||||||
| Forms of eczema included | Forms of eczema excluded |
| Atopic eczema | Seborrhoeic eczema |
| Atopic dermatitis | Contact eczema |
| Besnier's prurigo | Allergic contact eczema |
| Neurodermatitis atopica (German) | Irritant contact eczema |
| Flexural eczema/dermatitis | Discoid/nummular eczema |
| Periorbital eczema | Asteatotic eczema |
| Childhood eczema | Varicose/stasis eczema |
| Infantile eczema | Photo‐/light‐sensitive eczema |
| 'Eczema' unspecified | Chronic actinic dermatitis |
| Constitutional eczema | Dyshidrotic eczema |
| Endogenous eczema | Pompholyx eczema |
| Chronic eczema | Hand eczema |
| Neurodermatitis | Frictional lichenoid dermatitis |
| Neurodermatitis (German) | Lichen simplex |
| Occupational dermatitis | |
| Prurigo |
| Isolauri 2mo LGG | Isolauri 2mo Bb12 | Isolauri 2mo placebo | |
| N | 9 | 9 | 9 |
| Median | 1 | 0 | 13.4 |
| IQR | 0.1 to 8.7 | 0 to 3.8 | 4.5 to 18.2 |
| IQR: interquartile range. | |||
| Study | Clarity of methods | Compliance | Dietary management |
| Clear | No compliance measures described | Adequate exclusion of other probiotics during the study | |
| Total daily dose of intervention clear, but individual dose, frequency, and mode of administration not given | No compliance measures reported | Not stated | |
| Clear | Dose count (returned sachet packets counted by clinical investigator). Compliance measured for the 2 groups: 84.5% and 84.7%. No significant difference | Clear instructions given: no change in usual diet but avoid any type of fermented food containing live micro‐organisms | |
| Clear | No information provided | No information provided | |
| Aims and Interventions clear. Outcomes not clear and baseline severity (SCORAD) not given | No information given | Inadequate information | |
| Aims, interventions clear | Inadequate information | Inadequate information | |
| Clear | No compliance measures reported | Not stated | |
| Total daily dose of probiotics not clear. Remaining methods clear | Compliance checked from the parental report and the weight of remaining powder. Reported that there were no differences in compliance between the 2 groups | No information on adequate exclusion of other probiotics from the diet. Participants with challenge proved milk or egg allergy followed milk or egg elimination diet, respectively | |
| Clear | Compliance based on count of remaining capsules: average 94% for all groups and 93.6%, 95%, and 93.3% for Bifidobacterium, Lactobacillus, and placebo groups, respectively. No significant difference in compliance between the 3 groups (P = 0.6). No participating child had compliance lower than 72% | No information given | |
| All methods clear Reporting of adverse events suggests that these were the result of the change in formula but the numbers are totals from intervention and control groups, and it is not certain whether the AEs are associated with the formula or the probiotics | No compliance measures reported | Instructions given that other fermented or probiotic‐containing products were to be avoided | |
| Inadequate information available | No information | No information | |
| Unclear what the placebo was; otherwise clear | 92.5% of doses taken by probiotic group; 94.4% by placebo group | Not stated, other than encouragement to avoid allergens | |
| Dose and exact consistency of probiotics unclear | No information | No information | |
| Preparation of the intervention not clear. Otherwise clear | No compliance measure described | Clear instructions not to consume fermented food and products containing live micro‐organisms | |
| Trial designed to study effects of probiotics in participants with cow's milk allergy. Effects of probiotics on eczema ‐ secondary outcome. Aims, interventions, and outcome measures clear | Compliance measure not presented. "To optimise compliance, participants were supplied with study formula through the study team and batches were delivered at home" | No information provided | |
| Clear | Method: count of return sachets. Reported that compliance was similar in the 2 groups but no measures reported | Instructions given so that participants do not change their diet during trial but should avoid fermented food products containing live micro‐organisms | |
| Unclear ‐ dose and duration of probiotic treatment received not clearly described. Severity of participant eczema at baseline not described | No compliance measures reported | Not stated | |
| Placebo not described. Otherwise methods clear | No compliance measures reported | Not stated | |
| Unclear ‐ intended duration of study treatment not stated | No compliance measures reported | Not stated | |
| Exact dose of probiotics not given | No information provided | No information provided | |
| Unclear ‐ precise dose of probiotic received by participants not stated | No compliance measures described | Not stated | |
| Clear | No information provided | Clearly stated: "All patients were asked to avoid probiotic supplements, fermented milk, lactic acid bacterial drinks and fermented soybean (natto) during the experimental period…" | |
| Clear | No compliance measures reported | Not stated | |
| Clear | No compliance measures described | Not stated | |
| Clear | No compliance measures described | Adequate exclusion of prebiotics and probiotics 3 weeks before the start and during the 20 weeks of the intervention | |
| Clear | No compliance measures described | Adequate exclusion of other probiotics during study | |
| Intervention type not clear: synbiotic mixture of 7 strains of probiotics and fructo‐oligosaccharide. Dose, frequency of intake, and preparation clear Baseline characteristics given only for participants who completed the trial | No compliance measures reported | Unclear. Stated that participants did not change diet before or during the trial | |
| Clear | Assessed by 2 telephone calls | One participant noted to have taken non‐study probiotic | |
| Clear | No compliance measures described | Not stated | |
| Clear | No compliance measures reported. Participants' parents were keeping diary for formula intake and adverse events. Formula with intervention was given on demand and at the end of intervention. No significant differences in formula intake were noted between the 2 groups | Unclear | |
| Method for diagnosing eczema not described | No compliance measures described | Not stated | |
| All clear. In the publication, not clear what the placebo was, but this was clarified by the study author | Yes: capsule count performed | Yes Stated: "During the study…and other probiotics were not permitted" | |
| Clear | Sachet counts and parent‐completed sachet administration chart. Good compliance (94%) ‐ no differences between the 2 groups | Adequate exclusion of other probiotics during study | |
| Clear | No measure of compliance was reported, but it was stated that the 2 groups had no difference in compliance | No information provided | |
| Aims, interventions, and outcome measures clear. Exclusion criteria not given | Patients and parents were to return to investigators all unused intervention. No measure was reported | Instruction given to parents not to feed their children other probiotic preparations during the intervention | |
| Aims, interventions, and outcome measures clear. Dose of probiotic not given in colony‐forming units, or similar measure of bacterial numbers | Compliance recorded: assessed based on a count of returned medication | No information provided | |
| All clear | No information given | Instructions given to avoid any commercial probiotic‐containing products 2 weeks before study initiation. No comment about diet during the trial | |
| All clear | No information provided | No information provided | |
| Placebo not described. Otherwise clear | No compliance measures reported | No information given | |
| SCORAD: Severity Scoring of Atopic Dermatitis. | |||
| Rosenfeldt 2003 | Gruber 2007 | Weston 2005 | Folster‐Holst 2006 | Gerasimov 2010 | Han 2012 | Wu 2012 | Woo 2010 | Van der Aa 2010 | Gore 2011 | Wu 2015 | |
| Median grams hydrocortisone butyrate applied (range) | Probiotic: 7.8 (0 to 67) Placebo: 6.0 (0 to 59) | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Mean grams 1% hydrocortisone applied (SD) | ‐ | Probiotic: 0.8 (45.0) Placebo: 3.5 (29.8) | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Median change in topical corticosteroid use score (IQR) | ‐ | ‐ | Probiotic: 0.25 (‐6.7 to 7.0) Placebo: ‐1.0 (‐8.0 to 0.7) | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Mean applications per week (SD) | ‐ | ‐ | ‐ | Probiotic: 3.0 (0.6) Placebo: 3.2 (0.9) | Probiotic: 0.8 (0.9) Placebo: 1.2 (1.4) | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Number of participants using topical CS during study (%) | ‐ | ‐ | ‐ | ‐ | ‐ | Baseline Probiotics: 13/58 (22.4%) Placebo: 14/60 (23.3%) At end of treatment Probiotics: 13/44 (29.5%) Placebo: 14/39 (36%) | ‐ | Probiotic: 22/45 (49%) Placebo: 20/43 (46%) | Baseline Synbiotic: 25/45 (55.6%) Placebo: 22/44 (50%) At end of treatment Synbiotic: 22/41 (53.7%) Placebo: 24/42 (57.1%) | ‐ | ‐ |
| Mean grams 0.25% prednicarbate applied during study (SD) | ‐ | ‐ | ‐ | ‐ | ‐ | Probiotic: Placebo: | ‐ | ‐ | ‐ | ‐ | ‐ |
| Mean applications of CS or calcineurin inhibitor per month (SD) | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | Probiotic: 23.5 (19.1) Placebo: 19.1 (19) | ‐ | ‐ | ‐ | ‐ |
| Median grams of 0.1% prednicarbate during Intervention (range) | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | Probiotic: Placebo: | ‐ | ‐ | ‐ |
| Median change in grams of 0.1% prednicarbate use during intervention (range) | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | Probiotic: ‐0.5 (‐2.7 to 1.3) Placebo: ‐0.3 | ‐ | ‐ | ‐ |
| Number of participants using standard skin care at end of intervention | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | Probiotic: 29/88 (33%) Placebo: 21/47 (45%) | ‐ |
| Number of participants using different potencies of TCS at end of intervention | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | Emollients only Probiotic: 31/88 (35%) Placebo: 18/47 (38%) Mild Probiotic: 54/88 (61%) Placebo: 29/47 (62%) ≥ moderate/potent Probiotic: 3/88 (3%) Placebo: 0 | ‐ |
| Mean grams of TCS (hydrocortisone 1% or mometasone 0.1%) used during study (SD) | ‐ | ‐ | ‐ | ‐ | Probiotic: Placebo: | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Median grams of TCS (hydrocortisone 1% or mometasone 0.1%) used during study (range) | ‐ | ‐ | ‐ | ‐ | Probiotic: 25.0 (0.0 to 45.0) Placebo: 35.0 (15.0 to 50.0) | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Mean total amount (gr) of corticosteroid used during treatment period ± SD | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | Probiotic: 5.87 ± 7.48 Placebo: 4.73 ± 5.48 |
| CS: corticosteroids. | |||||||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Participant‐ or parent‐rated symptoms of eczema (SCORAD part C) at the end of treatment Show forest plot | 13 | Mean difference (Random, 95% CI) | ‐0.44 [‐1.22, 0.33] | |
| 1.1 Parallel‐group trials | 11 | Mean difference (Random, 95% CI) | ‐0.42 [‐1.27, 0.43] | |
| 1.2 Cross‐over trials | 2 | Mean difference (Random, 95% CI) | ‐0.52 [‐3.16, 2.12] | |
| 2 Participant‐ or parent‐rated global change in eczema symptoms at the end of treatment (binary outcome) Show forest plot | 3 | Odds ratio (Random, 95% CI) | 0.40 [0.14, 1.15] | |
| 2.1 Parallel‐group trials | 2 | Odds ratio (Random, 95% CI) | 0.70 [0.27, 1.77] | |
| 2.2 Cross‐over trials | 1 | Odds ratio (Random, 95% CI) | 0.18 [0.05, 0.60] | |
| 3 Change in participant‐ or parent‐rated symptoms of eczema (SCORAD part C) at the end treatment (continuous outcome) Show forest plot | 9 | Mean Difference (Random, 95% CI) | ‐0.70 [‐1.47, 0.06] | |
| 3.1 Parallel‐group trials | 8 | Mean Difference (Random, 95% CI) | ‐0.82 [‐1.62, ‐0.02] | |
| 3.2 Cross‐over studies | 1 | Mean Difference (Random, 95% CI) | 0.66 [‐1.79, 3.11] | |
| 4 Participant‐ or patient‐related quality of life score at the end of treatment Show forest plot | 6 | Std. Mean Difference (Random, 95% CI) | 0.03 [‐0.36, 0.42] | |
| 4.1 Infant's Dermatitis Quality of Life Index (IDQoL) | 2 | Std. Mean Difference (Random, 95% CI) | 0.35 [0.08, 0.62] | |
| 4.2 Dermatology Life Quality Index | 1 | Std. Mean Difference (Random, 95% CI) | ‐0.33 [‐0.95, 0.29] | |
| 4.3 Skindex‐29 Questionnaire | 2 | Std. Mean Difference (Random, 95% CI) | ‐0.66 [‐4.24, 2.92] | |
| 4.4 Children's Dermatology Quality of Life Index | 1 | Std. Mean Difference (Random, 95% CI) | ‐0.39 [‐0.70, ‐0.08] | |
| 5 Participant‐ or patient‐related quality of life score at the end of treatment Show forest plot | 3 | Std. Mean Difference (Random, 95% CI) | ‐0.19 [‐0.56, 0.18] | |
| 5.1 Dermatitis Family Impact Questionnaire (DFI) | 2 | Std. Mean Difference (Random, 95% CI) | ‐0.31 [‐0.86, 0.24] | |
| 5.2 Family Dermatology Dermatology Life Quality Index | 1 | Std. Mean Difference (Random, 95% CI) | ‐0.01 [‐0.32, 0.30] | |
| 6 Parent‐ or participant‐rated eczema severity (SCORAD part C) within 6 months after treatment has ceased Show forest plot | 3 | 185 | Mean Difference (IV, Random, 95% CI) | ‐1.81 [‐3.13, ‐0.49] |
| 7 Participant‐ or parent‐related quality of life within 6 months after treatment has ceased Show forest plot | 2 | 261 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.08 [‐0.35, 0.20] |
| 7.1 Dermatology Life Quality Index | 1 | 46 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.14 [‐0.75, 0.48] |
| 7.2 Child Dermatology Life Quality Index | 1 | 215 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.06 [‐0.37, 0.25] |
| 8 Global eczema severity score (total SCORAD) at the end of treatment Show forest plot | 24 | Mean difference (Random, 95% CI) | ‐3.91 [‐5.86, ‐1.96] | |
| 8.1 Parallel‐group studies | 22 | Mean difference (Random, 95% CI) | ‐3.84 [‐5.95, ‐1.72] | |
| 8.2 Cross‐over studies | 2 | Mean difference (Random, 95% CI) | ‐4.14 [‐7.68, ‐0.59] | |
| 9 Global eczema severity score (total SCORAD) at the end of treatment ‐ sensitivity analysis ‐ change score Show forest plot | 14 | Mean difference (Random, 95% CI) | ‐4.46 [‐6.49, ‐2.43] | |
| 9.1 Parallel‐group trial | 13 | Mean difference (Random, 95% CI) | ‐4.53 [‐6.72, ‐2.33] | |
| 9.2 Cross‐over trial | 1 | Mean difference (Random, 95% CI) | ‐3.93 [‐9.25, 1.40] | |
| 10 Global eczema severity score (total SCORAD) at the end of treatment ‐ low risk of bias studies only Show forest plot | 8 | Mean difference (Random, 95% CI) | Totals not selected | |
| 10.1 Parallel‐group studies | 8 | Mean difference (Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 11 Investigator‐rated eczema severity (SCORAD parts A/B) at the end of treatment ‐ continuous outcome Show forest plot | 10 | 529 | Mean Difference (IV, Random, 95% CI) | ‐2.24 [‐4.69, 0.20] |
| 12 Global eczema severity score (total SCORAD) within 6 months after treatment has ceased Show forest plot | 7 | Mean difference (Random, 95% CI) | ‐7.72 [‐11.85, ‐3.59] | |
| 12.1 Parallel‐group studies | 6 | Mean difference (Random, 95% CI) | ‐9.27 [‐13.88, ‐4.65] | |
| 12.2 Cross‐over studies | 1 | Mean difference (Random, 95% CI) | 0.2 [‐3.86, 4.26] | |
| 13 Investigator‐rated eczema severity (SCORAD parts A/B) within 6 months after treatment has ceased Show forest plot | 2 | 102 | Mean Difference (IV, Random, 95% CI) | ‐8.11 [‐13.14, ‐3.09] |
| 14 Adverse events (short term) Show forest plot | 7 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 14.1 Gastrointestinal symptoms | 7 | 402 | Risk Ratio (M‐H, Random, 95% CI) | 1.54 [0.90, 2.63] |
| 15 Participant/parent‐rated global change in symptoms of eczema at the end of treatment ‐ stratified by age groups Show forest plot | 3 | Odds ratio (Random, 95% CI) | Totals not selected | |
| 15.1 Age under 2 years | 1 | Odds ratio (Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 15.2 Age 2 to 12 years | 1 | Odds ratio (Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 15.3 Age not categorised | 1 | Odds ratio (Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 16 Participant/parent‐rated symptoms of eczema (SCORAD part C) at the end of treatment ‐ stratified by age groups Show forest plot | 12 | Mean difference (Random, 95% CI) | Subtotals only | |
| 16.1 Age under 2 years | 5 | Mean difference (Random, 95% CI) | ‐0.39 [‐2.20, 1.42] | |
| 16.2 Age 2 to 12 years | 4 | Mean difference (Random, 95% CI) | ‐0.63 [‐2.04, 0.78] | |
| 16.3 Age not categorised | 2 | Mean difference (Random, 95% CI) | ‐2.23 [‐3.71, ‐0.74] | |
| 16.4 Adults only | 2 | Mean difference (Random, 95% CI) | 1.01 [‐0.82, 2.84] | |
| 17 Global eczema severity score (total SCORAD) at the end of treatment ‐ stratified by age groups Show forest plot | 24 | Mean difference (Random, 95% CI) | Subtotals only | |
| 17.1 Age under 2 years | 10 | Mean difference (Random, 95% CI) | ‐0.99 [‐3.97, 1.99] | |
| 17.2 Age 2 to 12 years | 3 | Mean difference (Random, 95% CI) | ‐6.08 [‐9.68, ‐2.48] | |
| 17.3 Age not categorised | 7 | Mean difference (Random, 95% CI) | ‐5.25 [‐10.43, ‐0.07] | |
| 17.4 Adults only | 5 | Mean difference (Random, 95% CI) | ‐6.51 [‐10.09, ‐2.93] | |
| 18 Global eczema severity score (total SCORAD) at the end of treatment ‐ stratified by presence of atopy Show forest plot | 23 | Mean difference (Random, 95% CI) | Subtotals only | |
| 18.1 Participants with atopy | 4 | Mean difference (Random, 95% CI) | ‐3.90 [‐15.52, 7.73] | |
| 18.2 Participants with unknown atopic status | 19 | Mean difference (Random, 95% CI) | ‐4.15 [‐6.02, ‐2.27] | |
| 19 Global eczema severity score (total SCORAD) at the end of treatment ‐ stratified by challenge‐proven food allergy Show forest plot | 21 | Mean difference (Random, 95% CI) | Subtotals only | |
| 19.1 Food allergy present | 3 | Mean difference (Random, 95% CI) | ‐1.84 [‐6.22, 2.54] | |
| 19.2 Unknown food allergic status | 18 | Mean difference (Random, 95% CI) | ‐3.21 [‐5.63, ‐0.79] | |
| 20 Global eczema severity score (total SCORAD) at the end of treatment ‐ stratified by eczema severity Show forest plot | 6 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 20.1 Severe eczema (SCORAD over 40) | 5 | 95 | Mean Difference (IV, Random, 95% CI) | ‐3.71 [‐10.05, 2.64] |
| 20.2 Moderate eczema (SCORAD 15 to 40) | 6 | 279 | Mean Difference (IV, Random, 95% CI) | ‐2.95 [‐7.65, 1.74] |
| 20.3 Mild eczema (SCORAD under 15) | 1 | 8 | Mean Difference (IV, Random, 95% CI) | ‐5.53 [‐15.29, 4.23] |
| 21 Participant‐ or parent‐rated symptoms of eczema (SCORAD part C) at the end of treatment ‐ stratified by probiotic ‐ Lactobacillus species Show forest plot | 12 | Mean difference (Random, 95% CI) | Subtotals only | |
| 21.1 Lactobacillus GG alone or in combination with or without prebiotic | 1 | Mean difference (Random, 95% CI) | 1.79 [0.29, 3.29] | |
| 21.2 Lactobacillus rhamnosus alone or in combination with or without prebiotic | 5 | Mean difference (Random, 95% CI) | ‐0.82 [‐2.24, 0.60] | |
| 21.3 Lactobacillus salivarius alone or in combination with or without prebiotic | 1 | Mean difference (Random, 95% CI) | 0.0 [‐0.88, 0.88] | |
| 21.4 Lactobacillus casei/paracasei alone or in combination with or without prebiotics | 2 | Mean difference (Random, 95% CI) | 0.56 [‐0.29, 1.41] | |
| 21.5 Any Lactobacillus species alone or in combination with or without prebiotics | 12 | Mean difference (Random, 95% CI) | ‐0.50 [‐1.31, 0.31] | |
| 22 Participant‐ or parent‐rated symptoms of eczema (SCORAD part C) at the end of treatment ‐ stratified by probiotic ‐ Bifidobacterium species Show forest plot | 5 | Mean difference (Random, 95% CI) | Subtotals only | |
| 22.1 Bifidobacterium lactis alone or in combination with or without prebiotics | 4 | Mean difference (Random, 95% CI) | ‐0.34 [‐1.92, 1.24] | |
| 22.2 Bifidobacterium breve alone or in combination with or without prebiotics | 1 | Mean difference (Random, 95% CI) | 1.3 [‐2.15, 4.75] | |
| 22.3 Any Bifidobacteria species alone or in combination with or without prebiotic | 5 | Mean difference (Random, 95% CI) | ‐0.11 [‐1.47, 1.25] | |
| 23 Participant‐ or parent‐rated symptoms of eczema (SCORAD part C) at the end of treatment ‐ number of probiotics Show forest plot | 13 | Mean difference (Random, 95% CI) | Subtotals only | |
| 23.1 Studies using single probiotic with or without prebiotics | 8 | Mean difference (Random, 95% CI) | ‐0.40 [‐1.45, 0.66] | |
| 23.2 Studies using multiple probiotics with or without prebiotics | 5 | Mean difference (Random, 95% CI) | ‐0.58 [‐1.98, 0.81] | |
| 24 Participant‐ or parent‐rated symptoms of eczema (SCORAD part C) at the end of treatment ‐ probiotics with no prebiotics Show forest plot | 13 | Mean difference (Random, 95% CI) | ‐0.44 [‐1.22, 0.33] | |
| 25 Global eczema severity score (total SCORAD) at the end of treatment ‐ stratified by probiotic ‐ Lactobacillus species Show forest plot | 21 | Mean difference (Random, 95% CI) | Subtotals only | |
| 25.1 Lactobacillus GG alone or in combination with or without prebiotic | 3 | Mean difference (Random, 95% CI) | 3.37 [0.55, 6.20] | |
| 25.2 Lactobacillus rhamnosus alone or in combination with or without prebiotic | 5 | Mean difference (Random, 95% CI) | ‐3.49 [‐9.81, 2.83] | |
| 25.3 Lactobacillus salivarius alone or in combination with or without prebiotic | 6 | Mean difference (Random, 95% CI) | ‐6.86 [‐10.08, ‐3.63] | |
| 25.4 Lactobacillus casei/paracasei alone or in combination with or without prebiotics | 6 | Mean difference (Random, 95% CI) | ‐2.58 [‐7.21, 2.05] | |
| 25.5 Any Lactobacillus species alone or in combination with or without prebiotics | 21 | Mean difference (Random, 95% CI) | ‐3.80 [‐6.06, ‐1.54] | |
| 26 Global eczema severity score (total SCORAD) at the end of treatment ‐ stratified by probiotic ‐ Bifidobacterium species Show forest plot | 12 | Mean difference (Random, 95% CI) | Subtotals only | |
| 26.1 Bifidobacterium lactis alone or in combination with or without prebiotics | 7 | Mean difference (Random, 95% CI) | ‐1.90 [‐5.42, 1.63] | |
| 26.2 Bifidobacterium breve alone or in combination with or without prebiotics | 3 | Mean difference (Random, 95% CI) | ‐0.36 [‐11.39, 10.67] | |
| 26.3 Any Bifidobacteria species alone or in combination with or without prebiotic | 12 | Mean difference (Random, 95% CI) | ‐2.26 [‐5.14, 0.63] | |
| 27 Global eczema severity score (total SCORAD) at the end of treatment ‐ stratified by probiotic ‐ number of probiotics Show forest plot | 24 | Mean difference (Random, 95% CI) | Subtotals only | |
| 27.1 Studies using single probiotic with or without prebiotics | 13 | Mean difference (Random, 95% CI) | ‐4.90 [‐7.66, ‐2.15] | |
| 27.2 Studies using multiple probiotics with or without prebiotics | 12 | Mean difference (Random, 95% CI) | ‐3.54 [‐6.50, ‐0.58] | |
| 28 Global eczema severity score (total SCORAD) at the end of treatment ‐ stratified by probiotic ‐ probiotics with no prebiotics Show forest plot | 24 | Mean difference (Random, 95% CI) | ‐3.83 [‐5.81, ‐1.86] | |

