Anticonceptivos orales para los quistes ováricos funcionales
Referencias
Referencias de los estudios incluidos en esta revisión
Referencias de los estudios excluidos de esta revisión
Referencias adicionales
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | Randomized controlled trial. Report notes women were "randomized into three groups consecutively". | |
| Participants | 186 women diagnosed with clomiphene citrate‐related ovarian cyst > 20 mm on third day of menstrual cycle. Diagnosis based on evaluation by transvaginal ultrasonography. No participant had a basal cyst early in cycle prior to clomiphene citrate treatment. No other inclusion or exclusion criteria were mentioned. | |
| Interventions | 1) levonorgestrel 100 μg plus EE 20 μg versus 3) placebo Duration: 4 weeks; women with persistent cysts at 4 weeks were called for a second visit 4 weeks later and assessed again at 12 weeks. | |
| Outcomes | Regressed cysts at 4 weeks and 12 weeks; evaluated with transvaginal ultrasonography | |
| Notes | No information on randomization method, blinding, or sample size estimation. Attempted to contact the researcher for more information on methods. Loss to follow up by 12 weeks: 6.5% overall; levonorgestrel group, 2/62; desogestrel group, 4/62; placebo group, 6/62. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | High risk | Women were randomized "consecutively. No information on concealment or blinding. |
| Methods | Randomized controlled trial | |
| Participants | 141 premenopausal women, < 50 years old, with low serum CA‐125 antigen and ovarian cyst detected by transvaginal ultrasonography in the first 5 days of the menstrual cycle. Simple cysts were defined as unilocular, smooth‐walled, from 3 to 10 cm in diameter, with or without internal echo. No exclusion criteria were reported. | |
| Interventions | Desogestrel 150 μg plus EE 20 μg (daily) versus expectant management; duration 24 months. | |
| Outcomes | Resolution of cyst by 6 months; also mean diameter of cyst at end of study, but laparoscopic intervention was performed if cyst persisted at 6 months | |
| Notes | No information on method of generating randomization, allocation concealment, blinding, or sample size estimation. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | no information |
| Methods | Randomized controlled trial without blinding | |
| Participants | 54 women in Israel found to have ovarian cysts with mean diameter larger than 2.0 cm after ovulation induction. Mean ages of 34 and 33 years in treatment and control groups, respectively. Mean cyst diameters 2.9 and 2.8 cm, respectively | |
| Interventions | Oral contraceptive containing levonorgestrel 125 μg and ethinyl estradiol 50 μg versus expectant management for one cycle, after which ultrasound examination was repeated. | |
| Outcomes | Resolution of cyst, defined as complete disappearance on ultrasound examination. | |
| Notes | Method of randomization and allocation concealment not described. Sample size calculation not provided. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | no information |
| Methods | Randomized controlled trial conducted in Turkey. | |
| Participants | 62 women referred to university clinic, with ovarian cyst > 20x20 mm on menstrual cycle day 3 via vaginal ultrasound. No other criteria were reported for inclusion or exclusion. | |
| Interventions | Three arms: 1) expectant management; 2) levonorgestrel 100 μg plus ethinyl estradiol 20 μg; 3) desogestrel 150 μg plus ethinyl estradiol 30 μg. | |
| Outcomes | Regression of cyst on vaginal ultrasound during follow‐up examinations at 4, 8, and 12 weeks. | |
| Notes | Abstract only; attempted to contact author regarding full report. No mention of method for randomization or blinding. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Methods | Randomized controlled trial; "block randomization"; open label, intent‐to‐treat analysis used. | |
| Participants | 70 women attending gynecologic clinic and found to have functional ovarian cyst (diameter 2 to 8 cm). | |
| Interventions | Oral contraceptive (OC) containing levonorgestrel 150 μg and ethinyl estradiol 30 μg versus expectant management. For OC group, 1 "package" was provided; if no remission at 1 month, the woman continued on same treatment for another month. Cyclical administration was presumed, although the report did not specify. | |
| Outcomes | Remission of cyst by 2 months (ultrasonographic exam unable to detect the cyst or cyst < 2 cm). Cyst was assessed at one‐month follow up and, if no remission at one month, assessed again at two months. | |
| Notes | No mention of block size for randomization or allocation concealment before assignment. Attempted to reach corresponding author regarding methodological issues and data presented in figures. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | no information |
| Methods | Randomized controlled trial | |
| Participants | 48 women in the U.S. who had an adnexal cyst 1.5 cm in diameter or larger document by vaginal ultrasound examination. All participants were having ovulation induction with clomiphene, human menopausal gonadotropin, or both. Mean ages of 33 and 32 years in treatment and control groups, respectively. Mean cyst diameters 3.0 and 2.9 cm, respectively | |
| Interventions | Oral contraceptive containing norethindrone 1 mg and mestranol 50 μg, taken daily for up to six weeks versus expectant management. | |
| Outcomes | Resolution of cyst on vaginal ultrasound follow‐up examinations at three, six, and nine weeks. | |
| Notes | Method of randomization and allocation concealment not specified. Sample size calculation not provided. One participant excluded from analysis because of noncompliance (treatment group unknown). An additional six women with persistent cysts were deleted from the analysis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | no information |
| Methods | Randomized controlled trial; table of random numbers used for sequence generation. | |
| Participants | 45 women aged 18 to 34 years in Turkey who had newly diagnosed "ovarian cysts" four to six cm in diameter. Exclusion criteria included prior surgery, endometriosis, pregnancy, masses not purely cystic, cysts more than six cm in diameter, and contraindications to oral contraceptives. | |
| Interventions | Oral contraceptive containing levonorgestrel 150 μg plus ethinyl estradiol 30 μg given cyclically for three months versus expectant management. | |
| Outcomes | Resolution of cyst on vaginal ultrasound examination "every four weeks and at the end of the second and third month just after menses." Cyst volumes were also measured, using the prolate ellipsoid formula. | |
| Notes | Allocation concealment not mentioned. Sample size not explained. A parallel trial was done in women without ovarian cysts to study cyst prevention. Those 50 women without cysts were not considered in this review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Methods | Randomized controlled trial, with randomization stratified by cyst diameter and participant age | |
| Participants | 80 women of reproductive age in Turkey with unilateral, mobile, unilocular, thin‐walled ovarian cysts without internal echoes and from three to six cm in diameter on ultrasound examination. Exclusion criteria were ovarian dysfunction, drug use that might interfere with hormone metabolism, and known contraindications to oral contraceptives. | |
| Interventions | 1) oral contraceptive containing desogestrel 150 μg plus ethinyl estradiol 30 μg versus 2) oral contraceptive containing levonorgestrel 250 μg plus ethinyl estradiol 50 μg versus 3) multiphasic oral contraceptive containing levonorgestrel 50/75/125 μg plus ethinyl estradiol 30/40/30 μg versus 4) expectant management. | |
| Outcomes | Resolution of cyst on vaginal ultrasound examination after 5 and 10 weeks of therapy. | |
| Notes | Method of randomization not specified, and allocation concealment not described. Sample size justification not provided. Blinding as to therapy not described. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | no information |
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| Not a randomized controlled trial; observational study of prevention, not treatment. | |
| Not a treatment trial of ovarian cysts. | |
| Treatment allocation was based on the preference of the participants. | |
| Participants allocated to three treatment groups based on birth dates. | |
| Not a treatment trial of ovarian cysts. | |
| Participants allocated to two treatments by alternate weeks of enrollment. | |
| Prevention, rather than treatment, trial. | |
| Participants chose OC or expectant management. | |
| Abstract described 95 participants with cysts, 29 of whom had a history of endometriosis. Potential overlap with participants reported in Nezhat 1996. Corresponding author did not reply to query. Authors have had two published papers retracted by another journal (Carlsen 2001; Editors 2001). | |
| Possible overlap of participants reported in Nezhat 1994. Corresponding author did not reply to query. Authors have had two published papers retracted by another journal (Carlsen 2001; Editors 2001). | |
| No mention of randomization in article. | |
| Not a treatment trial of ovarian cysts. | |
| Not a treatment trial of ovarian cysts. |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Resolution of cyst within nine weeks Show forest plot | 1 | 41 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Analysis 1.1  Comparison 1 Norethindrone 1 mg plus mestranol 50 μg daily versus expectant management, Outcome 1 Resolution of cyst within nine weeks. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Resolution of cyst by last follow up (10 or 12 weeks) Show forest plot | 2 | 76 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.52 [0.46, 5.00] |
| Analysis 2.1  Comparison 2 Desogestrel 150 μg plus ethinyl estradiol 30 μg taken cyclically versus expectant management, Outcome 1 Resolution of cyst by last follow up (10 or 12 weeks). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Resolution of cyst by six months Show forest plot | 1 | 141 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.27, 1.42] |
| Analysis 3.1  Comparison 3 Desogestrel 150 μg plus ethinyl estradiol 20 μg taken daily versus expectant management, Outcome 1 Resolution of cyst by six months. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Resolution of cyst by 12 weeks Show forest plot | 1 | 42 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.71 [0.45, 6.51] |
| Analysis 4.1  Comparison 4 Levonorgestrel 100 μg plus ethinyl estradiol 20 μg taken cyclically versus expectant management, Outcome 1 Resolution of cyst by 12 weeks. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Resolution of cyst within one menstrual cycle Show forest plot | 1 | 54 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.14, 3.57] |
| Analysis 5.1  Comparison 5 Levonorgestrel 125 μg plus ethinyl estradiol 50 μg taken cyclically versus expectant management, Outcome 1 Resolution of cyst within one menstrual cycle. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Resolution of cyst by last follow up (second or third month) Show forest plot | 2 | 112 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.54, 2.60] |
| Analysis 6.1  Comparison 6 Levonorgestrel 150 μg plus ethinyl estradiol 30 μg taken cyclically versus expectant management, Outcome 1 Resolution of cyst by last follow up (second or third month). | ||||
| 2 Cyst volume after third month Show forest plot | 1 | 45 | Mean Difference (IV, Fixed, 95% CI) | 3.20 [‐0.87, 7.27] |
| Analysis 6.2  Comparison 6 Levonorgestrel 150 μg plus ethinyl estradiol 30 μg taken cyclically versus expectant management, Outcome 2 Cyst volume after third month. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Resolution of cyst by 10 weeks Show forest plot | 1 | 34 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.18 [0.12, 83.76] |
| Analysis 7.1  Comparison 7 Levonorgestrel 250 μg plus ethinyl estradiol 50 μg taken cyclically versus expectant management, Outcome 1 Resolution of cyst by 10 weeks. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Resolution of cyst by 10 weeks Show forest plot | 1 | 35 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.36 [0.13, 88.39] |
| Analysis 8.1  Comparison 8 Levonorgestrel 50/75/125 μg plus ethinyl estradiol 30/40/30 μg taken cyclically versus expectant management, Outcome 1 Resolution of cyst by 10 weeks. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Regression of cyst by 4 weeks Show forest plot | 1 | 124 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.55, 2.38] |
| Analysis 9.1  Comparison 9 Levonorgestrel 100 μg plus ethinyl estradiol 20 μg versus desogestrel 150 μg plus ethinyl estradiol 30 μg, Outcome 1 Regression of cyst by 4 weeks. | ||||
| 2 Regression of cyst by 12 weeks Show forest plot | 1 | 124 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.41 [0.55, 3.64] |
| Analysis 9.2  Comparison 9 Levonorgestrel 100 μg plus ethinyl estradiol 20 μg versus desogestrel 150 μg plus ethinyl estradiol 30 μg, Outcome 2 Regression of cyst by 12 weeks. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Regression of cyst by 4 weeks Show forest plot | 1 | 124 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.44, 1.95] |
| Analysis 10.1  Comparison 10 Levonorgestrel 100 μg plus ethinyl estradiol 20 μg versus placebo, Outcome 1 Regression of cyst by 4 weeks. | ||||
| 2 Regression of cyst by 12 weeks Show forest plot | 1 | 124 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.27 [0.49, 3.32] |
| Analysis 10.2  Comparison 10 Levonorgestrel 100 μg plus ethinyl estradiol 20 μg versus placebo, Outcome 2 Regression of cyst by 12 weeks. | ||||

Comparison 1 Norethindrone 1 mg plus mestranol 50 μg daily versus expectant management, Outcome 1 Resolution of cyst within nine weeks.
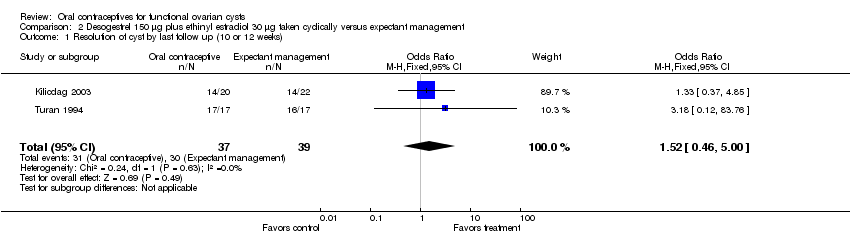
Comparison 2 Desogestrel 150 μg plus ethinyl estradiol 30 μg taken cyclically versus expectant management, Outcome 1 Resolution of cyst by last follow up (10 or 12 weeks).

Comparison 3 Desogestrel 150 μg plus ethinyl estradiol 20 μg taken daily versus expectant management, Outcome 1 Resolution of cyst by six months.

Comparison 4 Levonorgestrel 100 μg plus ethinyl estradiol 20 μg taken cyclically versus expectant management, Outcome 1 Resolution of cyst by 12 weeks.

Comparison 5 Levonorgestrel 125 μg plus ethinyl estradiol 50 μg taken cyclically versus expectant management, Outcome 1 Resolution of cyst within one menstrual cycle.

Comparison 6 Levonorgestrel 150 μg plus ethinyl estradiol 30 μg taken cyclically versus expectant management, Outcome 1 Resolution of cyst by last follow up (second or third month).
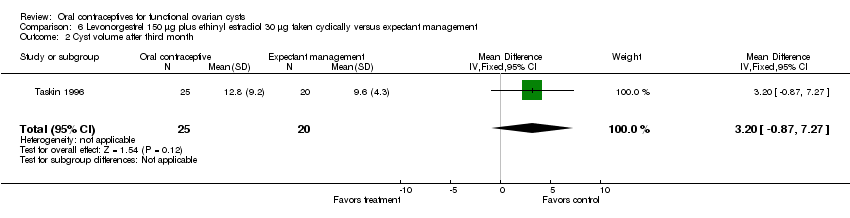
Comparison 6 Levonorgestrel 150 μg plus ethinyl estradiol 30 μg taken cyclically versus expectant management, Outcome 2 Cyst volume after third month.

Comparison 7 Levonorgestrel 250 μg plus ethinyl estradiol 50 μg taken cyclically versus expectant management, Outcome 1 Resolution of cyst by 10 weeks.

Comparison 8 Levonorgestrel 50/75/125 μg plus ethinyl estradiol 30/40/30 μg taken cyclically versus expectant management, Outcome 1 Resolution of cyst by 10 weeks.
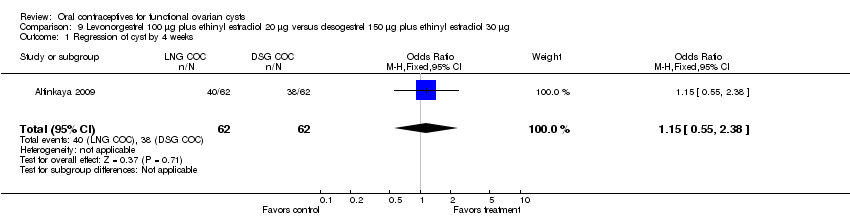
Comparison 9 Levonorgestrel 100 μg plus ethinyl estradiol 20 μg versus desogestrel 150 μg plus ethinyl estradiol 30 μg, Outcome 1 Regression of cyst by 4 weeks.
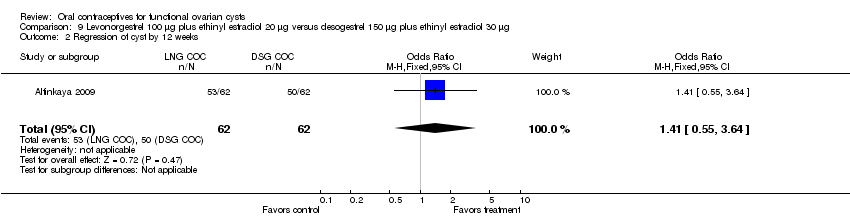
Comparison 9 Levonorgestrel 100 μg plus ethinyl estradiol 20 μg versus desogestrel 150 μg plus ethinyl estradiol 30 μg, Outcome 2 Regression of cyst by 12 weeks.
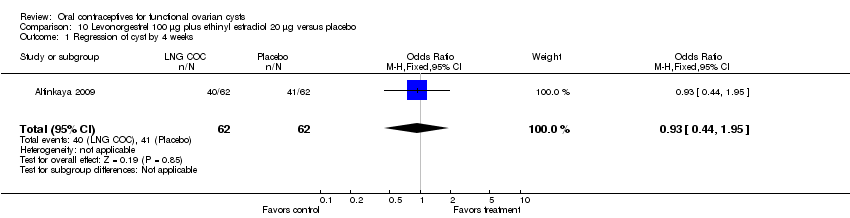
Comparison 10 Levonorgestrel 100 μg plus ethinyl estradiol 20 μg versus placebo, Outcome 1 Regression of cyst by 4 weeks.

Comparison 10 Levonorgestrel 100 μg plus ethinyl estradiol 20 μg versus placebo, Outcome 2 Regression of cyst by 12 weeks.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Resolution of cyst within nine weeks Show forest plot | 1 | 41 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Resolution of cyst by last follow up (10 or 12 weeks) Show forest plot | 2 | 76 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.52 [0.46, 5.00] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Resolution of cyst by six months Show forest plot | 1 | 141 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.27, 1.42] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Resolution of cyst by 12 weeks Show forest plot | 1 | 42 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.71 [0.45, 6.51] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Resolution of cyst within one menstrual cycle Show forest plot | 1 | 54 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.14, 3.57] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Resolution of cyst by last follow up (second or third month) Show forest plot | 2 | 112 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.54, 2.60] |
| 2 Cyst volume after third month Show forest plot | 1 | 45 | Mean Difference (IV, Fixed, 95% CI) | 3.20 [‐0.87, 7.27] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Resolution of cyst by 10 weeks Show forest plot | 1 | 34 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.18 [0.12, 83.76] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Resolution of cyst by 10 weeks Show forest plot | 1 | 35 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.36 [0.13, 88.39] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Regression of cyst by 4 weeks Show forest plot | 1 | 124 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.55, 2.38] |
| 2 Regression of cyst by 12 weeks Show forest plot | 1 | 124 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.41 [0.55, 3.64] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Regression of cyst by 4 weeks Show forest plot | 1 | 124 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.44, 1.95] |
| 2 Regression of cyst by 12 weeks Show forest plot | 1 | 124 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.27 [0.49, 3.32] |

