Anticonceptivos orales para los quistes ováricos funcionales
Resumen
Antecedentes
Los quistes ováricos funcionales son un problema ginecológico frecuente entre las mujeres en edad reproductiva en todo el mundo. Cuando son grandes, persistentes o dolorosos, estos quistes pueden requerir operaciones, que a veces resultan en la extirpación del ovario. Debido a que los primeros anticonceptivos orales se asociaron con una menor incidencia de quistes ováricos funcionales, muchos médicos dedujeron que las píldoras anticonceptivas también se podían utilizar para tratar los quistes. Esto se convirtió en una práctica clínica habitual a principios de la década de 1970.
Objetivos
Esta revisión examinó todos los ensayos controlados aleatorizados que estudiaron los anticonceptivos orales como tratamiento para los quistes ováricos funcionales.
Métodos de búsqueda
En marzo de 2014, se realizaron búsquedas en las bases de datos de CENTRAL, PubMed, EMBASE y POPLINE, así como en las bases de datos de ensayos clínicos (ClinicalTrials.gov y ICTRP). También se examinaron las listas de referencias de los artículos. Para la revisión inicial, se escribió a los autores de los ensayos identificados para buscar los artículos que se habían omitido.
Criterios de selección
Se incluyeron los ensayos controlados aleatorizados en cualquier idioma que incluyeran los anticonceptivos orales utilizados para el tratamiento y no la prevención de los quistes ováricos funcionales. Los criterios diagnósticos de los quistes fueron los utilizados por los autores de los ensayos.
Obtención y análisis de los datos
Dos autores de la revisión, de forma independiente, extrajeron los datos de los artículos. Uno introdujo los datos en RevMan y un segundo verificó la exactitud de la entrada de datos. Para los resultados dicotómicos se calculó el odds ratio (OR) de Mantel‐Haenszel con un intervalo de confianza (IC) del 95%. Para los resultados continuos se calculó la diferencia de medias (DM) con los IC del 95%.
Resultados principales
Se identificaron ocho ensayos controlados aleatorizados de cuatro países; los estudios incluyeron un total de 686 pacientes. El tratamiento con anticonceptivos orales combinados no aceleró la resolución de los quistes ováricos funcionales en los ensayos. Esto se aplicó tanto a los quistes que se produjeron espontáneamente como a los que se desarrollaron después de la inducción de la ovulación. La mayoría de los quistes se resolvieron sin tratamiento en unos pocos ciclos; los quistes persistentes tendían a ser patológicos (p.ej., endometrioma o quiste paraovárico) y no fisiológicos.
Conclusiones de los autores
Aunque se utilizan ampliamente para el tratamiento de los quistes ováricos funcionales, los anticonceptivos orales combinados no parecen tener efectos beneficiosos. La conducta expectante durante dos o tres ciclos es lo adecuado. En caso de que los quistes persistan, se suele indicar el tratamiento quirúrgico.
PICO
Resumen en términos sencillos
Anticonceptivos orales para tratar los quistes de ovario
Las mujeres en edad reproductiva suelen liberar un óvulo una vez al mes. En el ovario el óvulo se mueve desde el interior hasta la superficie y se crea una ampolla o un espacio lleno de líquido alrededor del óvulo en desarrollo. Cuando la ampolla (o quiste) alcanza la superficie del ovario, se rompe y libera el óvulo en la cavidad abdominal. Después de que esto ocurre, la ampolla se puede convertir en otro tipo de quiste, que produce una hormona (progesterona) que ayuda a que el embarazo crezca. La mayoría de estos quistes aparecen y desaparecen sin problemas. Sin embargo, a veces, los quistes se agrandan o son dolorosos; otros pueden permanecer durante meses. Hace varias décadas, los profesionales sanitarios se percataron de que las mujeres que tomaban píldoras anticonceptivas tenían menos quistes, ya que las píldoras generalmente impedían que se liberara el óvulo. Sobre la base de este hecho, muchos médicos comenzaron a tratar estos quistes con píldoras anticonceptivas para que desaparecieran más rápido.
En marzo de 2014, se realizó una búsqueda computarizada de todos los ensayos controlados aleatorizados que estudiaran el uso de píldoras anticonceptivas para tratar estos quistes benignos (también llamados funcionales). Se escribió a los investigadores para encontrar otros ensayos. Se encontraron ocho ensayos de cuatro países, que incluyeron 686 pacientes. Tres ensayos incluyeron a pacientes que recibían fármacos para ayudarlas a quedar embarazadas. Los otros cinco incluyeron a pacientes que desarrollaron quistes sin tratamiento de fertilidad. En ninguno de estos ensayos los anticonceptivos orales ayudaron a que los quistes desaparecieran más rápido. Por lo tanto, las píldoras anticonceptivas no se deben utilizar con este objetivo. Un mejor enfoque es esperar dos o tres meses para que los quistes desaparezcan por sí solos.
Authors' conclusions
Background
Description of the condition
Functional ovarian cysts (follicular and corpus luteum) are a common gynecological problem among women of reproductive age worldwide. Hospital‐based studies in the U.K. and USA have provided a range of reported incidences. In England and Wales from 1983 to 1985, the annual hospital discharge rate for women with a main diagnosis of ovarian cyst was 67 per 100,000 women (Westhoff 1992). From 1984 to 1986 in the USA, the comparable figure was 131 per 100,000 women. In recent U.S. reports, more than a quarter million women per year have been discharged from hospitals with a diagnosis of ovarian cysts (ICD‐9 codes 620.0 (follicular), 620.1 (corpus luteum), and 620.2 (other and unspecified) (Kozak 2005). In cross‐sectional studies using ultrasound evaluation of women, 4% to 7% had ovarian cysts greater than 30 mm in diameter (Teichmann 1995; Christensen 2002). While many of these physiological cysts will resolve spontaneously, some require surgical intervention, with its attendant discomfort, risk, and expense (Chiaffarino 1998).
How the intervention might work
Early epidemiological studies reported an inverse relationship between use of oral contraceptives (OCs) and surgically confirmed functional cysts (Ory 1974; Ramcharan 1981; Vessey 1987; Booth 1992). Based on the apparent strong protective effect against functional cysts, some clinicians inferred that oral contraceptives might be useful for treatment as well as prevention. Since pituitary gonadotropins promote follicular growth and since combined oral contraceptives suppress gonadotropins, pills might decrease cyst size.
An uncontrolled case‐series report popularized this approach. Spanos 1973 described 286 reproductive‐age women with adnexal masses ranging from 4 to 10 cm in diameter; he treated each with a combined oral contraceptive. Most cysts regressed, and those that did not were found at operation to be neoplasms. Despite the lack of a comparison group in this report, many clinicians (Starks 1984; Anderson 1990; Muram 1990) concluded that combined oral contraceptives hastened resolution of functional ovarian cysts, for example, claiming that "others have demonstrated that functional ovarian cysts regress more quickly when OCs are provided to women" (ContracTech 1982).
Why it is important to do this review
Despite the absence of an association between low‐dose oral contraceptive use and the occurrence of functional ovarian cysts in more recent studies (Holt 1992; Lanes 1992; Parazzini 1996), this treatment continues to be recommended: "...higher dose formulations should be considered for treatment purposes, although the overall risk/benefit ratio must be evaluated with caution" (Chiaffarino 1998). Others, however, caution that "The results of two small trials do not support the prescription of oral contraceptives to treat pre‐existing ovarian cysts" (ESHRE 2001).
Due to the frequency of ovarian cysts and the uncertainty concerning treatment with oral contraceptives, this review evaluated the randomized controlled trials addressing this question.
Objectives
To evaluate the usefulness of treating functional ovarian cysts with combined oral contraceptives.
Methods
Criteria for considering studies for this review
Types of studies
We included all randomized controlled trials in any language that include oral contraceptives as treatment of ovarian cysts. We excluded trials that focused on prevention of cysts. The definitions of functional ovarian cysts were those used in trial reports; the minimum cyst diameter varied across studies. Cysts associated with ovulation induction were also included.
Types of participants
All women of reproductive age enrolled in the randomized controlled trials were included; eligibility criteria were those used by the trial investigators.
Types of interventions
Any type of oral contraceptive (estrogen plus progestin or progestin alone) used in any regimen (cyclic or continuous) and for any duration was included. Comparisons could include no treatment, placebo treatment, or treatment with alternative drugs such as other oral contraceptives or danazol.
Types of outcome measures
Resolution of cysts at follow up, as judged by ultrasound or physical examination, was the principal outcome. Time to resolution was reported in some trials.
Search methods for identification of studies
Electronic searches
In March 2014, we searched the computerized databases of PubMed, POPLINE, EMBASE, and Cochrane Central Register of Controlled Trials (CENTRAL). In addition, we searched for recent clinical trials through ClinicalTrials.gov and the International Clinical Trials Registry Platform (ICTRP). The 2014 search strategies are shown in Appendix 1. Previous strategies can be found in Appendix 2.
Searching other resources
We examined the reference lists of reports found to seek other trials. For the initial review, we also wrote to authors of all included trial reports to solicit other published or unpublished trials that we may have missed; none responded.
Data collection and analysis
Data extraction and management
We assessed all titles and abstracts found for inclusion. Two reviewers independently abstracted data from the studies identified to improve accuracy. One reviewer entered data into RevMan, and a second confirmed correct data entry. We attempted to contact authors of trial reports to supplement published information but without success.
Assessment of risk of bias in included studies
We evaluated the methodological quality of the trials for potential bias by qualitatively assessing the study design, randomization method, allocation concealment, blinding, premature discontinuation rates, and loss to follow‐up rates. The principles used in the initial review and 2009 update were consistent with those recommended in Higgins 2008. For the 2011 update, we followed the guidelines in Higgins 2011.
Data synthesis
The Mantel‐Haenszel ratio (OR) with 95% confidence interval (CI) was used for dichotomous outcomes, such as resolution of cysts. For continuous variables, such as cyst diameter or days to resolution, we computed the mean difference with 95% CI using a fixed‐effect model. RevMan uses the inverse variance approach (Higgins 2011). Subgroup analyses were not done. Sensitivity analyses were not done to examine the impact of including trials with weaker methods.
Results
Description of studies
Results of the search
2014
The most recent search produced 118 unduplicated references from the main databases. In addition, 12 duplicates were removed electronically and by hand. We did not find any new RCTs that met our eligibility criteria. Two studies were excluded; the full text verified that the participants chose their contraceptive methods (Ferrero 2014; Naz 2011). Searches for current clinical trials produced four unduplicated listings but none were relevant.
2006 to 2011
We found eight randomized controlled trials including a total of 686 participants. Four trials were from Turkey (Turan 1994; Taskin 1996; Kilicdag 2003; Bayar 2005; Altinkaya 2009) and one each was from the United States (Steinkampf 1990), Israel (Ben‐Ami 1993), and Thailand (Sanersak 2006). Three trials included participants receiving ovulation induction treatment (Steinkampf 1990; Ben‐Ami 1993; Altinkaya 2009), while the other five had women with cysts unrelated to fertility treatment (Turan 1994; Taskin 1996; Kilicdag 2003; Bayar 2005; Sanersak 2006).
Included studies
Cysts related to ovulation induction
Steinkampf 1990 randomized 48 women with ovarian cysts after ovulation induction to either a combined oral contraceptive or to expectant management. The ovulation‐induction regimen included clomiphene, human menopausal gonadotropin, or both. Eligibility criteria included an adnexal cyst of 1.5 cm diameter or greater on vaginal ultrasound examination. Women allocated to oral contraceptives received a monophasic pill containing norethindrone 1 mg and mestranol 50 μg daily for up to six weeks. Repeat ultrasound examinations took place at three, six, and nine weeks after beginning therapy. Those with a persistent cyst at nine weeks were referred for an operation.
Ben‐Ami 1993 randomized 54 women with ovarian cysts after ovulation induction to either a combined oral contraceptive or to expectant management. The ovulation‐induction techniques included clomiphene citrate and human chorionic gonadotropins or human menopausal gonadotropins and human chorionic gonadotropin. All women were confirmed by ultrasound examination to have ovarian cysts at least 2.0 cm in diameter. Women were randomized to a pill containing levonorgestrel 125 μg plus ethinyl estradiol (EE) 50 μg or to expectant management. Participants had a repeat ultrasound examination after one cycle. If the cyst had not resolved, oral contraceptive treatment continued for the OC group or was begun for the expectant‐management group. However, this review includes only data from the first cycle comparing the two approaches.
In Altinkaya 2009, 186 women were diagnosed as having a clomiphene citrate‐related ovarian cyst greater than 20 mm. Cysts were diagnosed and later assessed with transvaginal ultrasonography. The women were randomized to 1) levonorgestrel (LNG) 100 μg plus EE 20 μg, 2) desogestrel (DSG) 150 μg plus EE 30 μg, or 3) placebo. Assessment occurred at four weeks. Those with persistent cysts were called for another visit 4 weeks later, treated again, and assessed at 12 weeks.
Spontaneously‐occurring cysts
In Turan 1994, 80 women with simple cysts were randomly assigned to one of four treatment arms. The three oral contraceptives were as follows: 1) monophasic containing desogestrel 150 μg plus EE 30 μg; 2) monophasic containing levonorgestrel 250 μg plus EE 50 μg; and 3) multiphasic containing levonorgestrel 50/75/125 μg plus EE 30/40/30 μg. The fourth treatment group was expectant management. Women had to have unilateral, mobile, unilocular, thin‐walled cysts without internal echoes; the size had to be from three to six cm in diameter. Women were randomized into four groups "by stratification according to cyst diameter and patient age." Repeat ultrasound examinations were done at 5 and 10 weeks after starting treatment. Because of the similarity of results in the three oral‐contraceptive groups, in this review each oral contraceptive has been compared to the expectant management arm rather than with other oral contraceptives.
Kilicdag 2003 examined low‐dose oral contraceptives, and randomized 62 women with ovarian cysts to three treatment arms. Like Turan 1994, one of the active treatments was desogestrel 150 μg plus EE 30 μg. The other oral contraceptive was levonorgestrel 100 μg plus EE 20 μg, and the third arm received expectant management. Ultrasound assessments were conducted at 4, 8, and 12 weeks.
In Bayar 2005, 141 women were randomly assigned to daily administration of an oral contraceptive containing desogestrel or to expectant management. The OC contained desogestrel 150 μg plus ethinyl estradiol 20 μg. The simple cysts were defined as unilocular, smooth‐walled, and from 3 to 10 cm in diameter; they could be with or without internal echo. Laparoscopic intervention was conducted if the cyst persistent at six months.
Taskin 1996 randomized 45 women, who had ovarian cysts of four to six cm in diameter, to either a combined oral contraceptive or to expectant management. Women allocated to pills received a preparation containing levonorgestrel 150 μg plus EE 30 μg in cyclical fashion for three cycles. Treatment began with the first cycle after ultrasound evaluation. All participants were followed with repeat ultrasound examinations "every four weeks and at the end of the second and third month just after menses." Outcomes included cyst resolution and cyst volume, measured by ultrasound.
In Sanersak 2006, 70 women with ovarian cysts were randomly assigned to a combined oral contraceptive or expectant management with 35 women in each arm. Women allocated to pills initially received "one package" of levonorgestrel 150 μg plus EE 30 μg. If no remission was noted by ultrasound at the one‐month follow up, the woman continued on same treatment for another month and had another ultrasound assessment at two months.
Excluded studies
Two reports that claimed to be randomized trials were not; one allocated participants by birth date (Graf 1995) and the other by alternate weeks (MacKenna 2000). Two other reports (Nezhat 1994; Nezhat 1996) were excluded because of possible overlap of participants and methodological concerns (Carlsen 2001; Editors 2001).
Risk of bias in included studies
Allocation
The method of sequence generation was not described in five trials (Ben‐Ami 1993; Steinkampf 1990; Kilicdag 2003; Bayar 2005; Altinkaya 2009). Further, Altinkaya 2009 reported that women were randomized "consecutively", making randomization suspect. Taskin 1996 reported using a table of random numbers for sequence generation. Turan 1994 reported a randomization stratified by cyst size and age, but further details were not provided. Sanersak 2006 reportedly used "block randomization" but provided no detail. Allocation concealment was not mentioned in any of the trials.
Blinding
Three trials did not use any blinding (Ben‐Ami 1993; Steinkampf 1990; Sanersak 2006), and the other five did not mention blinding.
Incomplete outcome data
No participants appeared to have been lost to follow up in five trials (Ben‐Ami 1993; Taskin 1996; Kilicdag 2003; Bayar 2005; Sanersak 2006). However, Kilicdag 2003 did not use intent‐to‐treat analysis. Two women assigned to the oral contraceptive groups could not tolerate the medication, and were included in the expectant management group.
In Steinkampf 1990, one participant was noncompliant with the protocol and was dropped from analysis; her treatment group was not stated. In addition, the analysis was restricted to women with cysts that resolved within nine weeks, so six women who had persistent cysts at nine weeks and had an operation were removed from the analysis. Thus, 7 of 48 participants (15%) were excluded from analysis.
The report of Turan 1994 was unclear regarding losses to follow up. Of 80 enrolled, 1 woman assigned to expectant management was lost to follow up, and 3 other women whose cysts resolved by 5 weeks did not return as requested at 10 weeks. Nevertheless, follow‐up information was provided for only 72 women at 5 weeks and 69 women at 10 weeks. The authors may have dropped from analysis the eight women (10%) with persistent ovarian cysts who were referred for surgical evaluation.
For Altinkaya 2009, loss to follow up at four weeks was 2% overall; the groups were similar. By 12 weeks, the overall loss to follow up was 6.5%.
Other potential sources of bias
Sanersak 2006 was the only report with an a priori sample size calculation. Six trials did not explain the sample size used (Steinkampf 1990; Ben‐Ami 1993; Turan 1994; Kilicdag 2003; Bayar 2005). In Taskin 1996, power was only addressed in the discussion section of the report.
Effects of interventions
The resolution of functional cysts was not hastened by use of oral contraceptives; this held true for spontaneously‐occurring cysts and those related to ovulation induction.
Cysts related to ovulation induction
Ben‐Ami 1993 found administration of oral contraceptives of no value in the setting of ovulation induction. Of 27 women (85%) given oral contraceptives, 23 (85%) had resolution of the cyst within one menstrual cycle, in contrast to 24 of 27 (89%) allocated to expectant management (Analysis 5.1: OR 0.72; 95% CI 0.14 to 3.57). No cysts persistent at the first observation period subsequently resolved with a second cycle of treatment. Of the seven women with persistent cysts, all had laparoscopy done. Two had dermoid cysts, two had para‐ovarian cysts, two had hydrosalpinges, and one had a follicular (functional) cyst.
In another population having ovulation induction, Steinkampf 1990 found no benefit of oral contraceptives. The analysis was restricted to participants with cysts that resolved. At three weeks, 20 of 22 (91%) participants given oral contraceptives had resolution, in contrast to 16 of 19 (84%) assigned to expectant management. At six weeks, 21 of 22 (95%) and 18 of 19 (95%) had cyst resolution, respectively. By the final observation at nine weeks, all participants in both groups had cyst resolution (Analysis 1.1) Six participants had persistent cysts; three had endometriomas and three had hydrosalpinges found at operation.
Altinkaya 2009 compared resolution of cysts related to ovulation induction in three groups. At four weeks, the levonorgestrel COC did not appear to provide any advantage over the desogestrel COC (Analysis 9.1) nor over the placebo (Analysis 10.1). The groups also had similar proportions with resolution at 12 weeks, which includes those treated again for cysts persisting at 4 weeks (Analysis 9.2; Analysis 10.2).
Spontaneously‐occurring cysts
OCs containing desogestrel and EE
In Turan 1994 and Kilicdag 2003, an oral contraceptive with desogestrel 150 μg plus EE 30 μg was studied in another population not undergoing ovulation induction. Oral contraceptives did not appear to hasten cyst resolution compared to expectant management (Analysis 2.1). With the two trials combined, cysts had disappeared by the second follow up (at 10 or 12 weeks) in 31 of 37 participants (84%) assigned to the desogestrel monophasic pill and 30 of 39 (77%) assigned to expectant management. In Bayar 2005, the OC preparation was desogestrel 150 μg plus EE 20 μg. The proportion of cysts that persisted at six months was similar for the OC group and the expectant management group (Analysis 3.1).
OCs containing levonorgestrel and EE
Taskin 1996 and Sanersak 2006 examined the oral contraceptive with levonorgestrel 150 μg plus EE 30 μg in a population not undergoing ovulation induction. No benefit was found for oral contraceptives on cyst resolution compared to expectant management (Analysis 6.1). With the two trials combined, 58 women were allocated to the oral contraceptive, of which 37 (64%) had resolution of the cyst by the last follow up (at two or three months). Among 54 assigned to expectant management, the corresponding figure was 33 (61%). In Taskin 1996, no significant difference was seen in the mean cyst volume at the end of treatment (Analysis 6.2). The mean volume in the group assigned to the oral contraceptive was 12.8 mL (standard deviation (SD) 9.2), while that in the expectant management group was 9.6 mL (SD 4.3). Eight women with cysts three to four cm in diameter that failed to regress underwent further treatment: three had percutaneous cyst aspiration under ultrasound guidance, and five had operations. Two of the five women having operations were found to have endometriomas, and the remainder had persistent corpus luteum cysts.
Turan 1994 studied an OC containing levonorgestrel 250 μg plus EE 50 μg (Analysis 7.1) and a multiphasic levonorgestrel pill (Analysis 8.1). Again, the groups were similar for cyst resolution. Eight participants with persistent cysts had surgical evaluation; three were found to have endometriomas, three had para‐ovarian cysts, one had a hydrosalpinx, and one had a simple cyst that was not further characterized. In addition, Kilicdag 2003 examined a low‐dose pill containing levonorgestrel 100 μg plus EE 20 μg. No advantage was found for the oral contraceptive versus expectant management (Analysis 4.1). The 19 women with persistent cysts underwent laparoscopy (Kilicdag 2003): serous cystadenoma was found in six, endometrioma in four, mucinous cystadenoma in two, mucinous cystadenofibroma in one, and follicular cysts in six women.
Discussion
Summary of main results
Treatment of functional ovarian cysts with oral contraceptives appears no better than watchful waiting. Most such cysts resolve spontaneously with or without treatment. The observation that cysts resolved after oral contraceptive administration (Spanos 1973) led to the clinical impression that oral contraceptives had benefit (ContracTech 1982), an example of post hoc ergo propter hoc reasoning (after the fact, therefore on account of the fact). In this common error in logic, a temporal association is inappropriately considered a causal association. Only with contemporaneous control groups could the putative effect of the contraceptives have been assessed. In none of these small trials was any important difference found. This held true for cysts discovered during ovulation induction and for those unrelated to fertility drugs.
Cystic masses that did not resolve within several months were unlikely to be functional cysts. Endometriomas, para‐ovarian cysts, and other pathology accounted for most of these. In these circumstances, surgical evaluation of persistent or painful adnexal masses is appropriate (Stein 1990).
Quality of the evidence
Limitations of these studies include incomplete or no description of sequence generation and allocation concealment. Bias may result from non‐random methods of generating the allocation sequence or inadequate allocation concealment (Schulz 1995; Schulz 2002a; Schulz 2002b).
Because of the small sample sizes and, in three reports (Turan 1994; Kilicdag 2003; Altinkaya 2009) multiple treatment arms, the power of these trials to detect important differences was limited. For meta‐analysis, we only aggregated trials that used the same oral contraceptives and had similar outcome measures. Nevertheless, taken as a whole, these trials do not suggest an important benefit of oral contraceptives in treating functional ovarian cysts.

Comparison 1 Norethindrone 1 mg plus mestranol 50 μg daily versus expectant management, Outcome 1 Resolution of cyst within nine weeks.
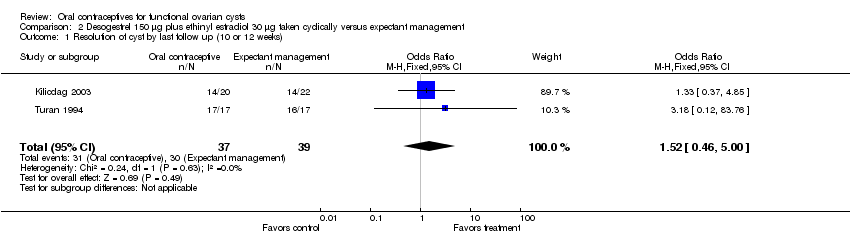
Comparison 2 Desogestrel 150 μg plus ethinyl estradiol 30 μg taken cyclically versus expectant management, Outcome 1 Resolution of cyst by last follow up (10 or 12 weeks).

Comparison 3 Desogestrel 150 μg plus ethinyl estradiol 20 μg taken daily versus expectant management, Outcome 1 Resolution of cyst by six months.

Comparison 4 Levonorgestrel 100 μg plus ethinyl estradiol 20 μg taken cyclically versus expectant management, Outcome 1 Resolution of cyst by 12 weeks.

Comparison 5 Levonorgestrel 125 μg plus ethinyl estradiol 50 μg taken cyclically versus expectant management, Outcome 1 Resolution of cyst within one menstrual cycle.

Comparison 6 Levonorgestrel 150 μg plus ethinyl estradiol 30 μg taken cyclically versus expectant management, Outcome 1 Resolution of cyst by last follow up (second or third month).
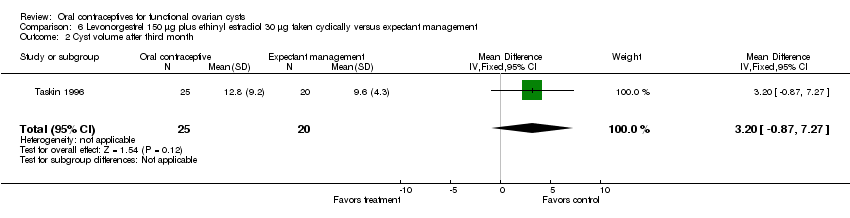
Comparison 6 Levonorgestrel 150 μg plus ethinyl estradiol 30 μg taken cyclically versus expectant management, Outcome 2 Cyst volume after third month.

Comparison 7 Levonorgestrel 250 μg plus ethinyl estradiol 50 μg taken cyclically versus expectant management, Outcome 1 Resolution of cyst by 10 weeks.

Comparison 8 Levonorgestrel 50/75/125 μg plus ethinyl estradiol 30/40/30 μg taken cyclically versus expectant management, Outcome 1 Resolution of cyst by 10 weeks.
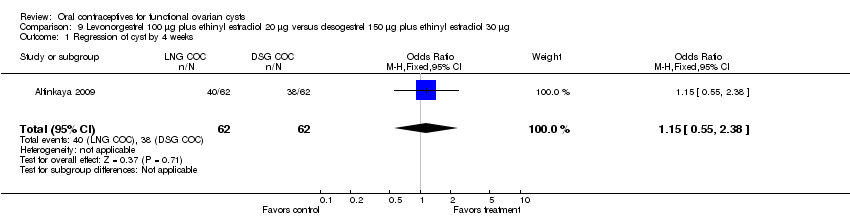
Comparison 9 Levonorgestrel 100 μg plus ethinyl estradiol 20 μg versus desogestrel 150 μg plus ethinyl estradiol 30 μg, Outcome 1 Regression of cyst by 4 weeks.
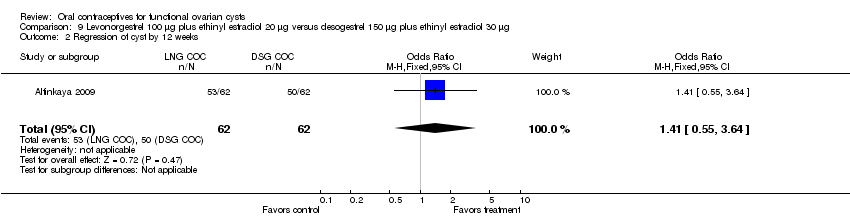
Comparison 9 Levonorgestrel 100 μg plus ethinyl estradiol 20 μg versus desogestrel 150 μg plus ethinyl estradiol 30 μg, Outcome 2 Regression of cyst by 12 weeks.
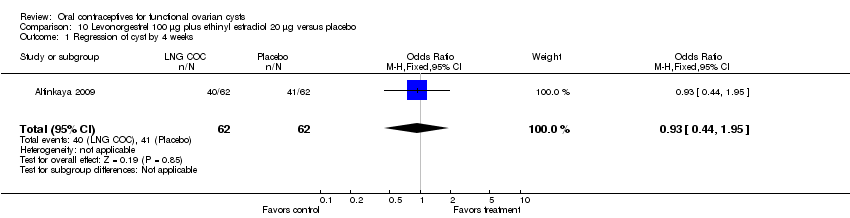
Comparison 10 Levonorgestrel 100 μg plus ethinyl estradiol 20 μg versus placebo, Outcome 1 Regression of cyst by 4 weeks.

Comparison 10 Levonorgestrel 100 μg plus ethinyl estradiol 20 μg versus placebo, Outcome 2 Regression of cyst by 12 weeks.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Resolution of cyst within nine weeks Show forest plot | 1 | 41 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Resolution of cyst by last follow up (10 or 12 weeks) Show forest plot | 2 | 76 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.52 [0.46, 5.00] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Resolution of cyst by six months Show forest plot | 1 | 141 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.27, 1.42] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Resolution of cyst by 12 weeks Show forest plot | 1 | 42 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.71 [0.45, 6.51] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Resolution of cyst within one menstrual cycle Show forest plot | 1 | 54 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.14, 3.57] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Resolution of cyst by last follow up (second or third month) Show forest plot | 2 | 112 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.54, 2.60] |
| 2 Cyst volume after third month Show forest plot | 1 | 45 | Mean Difference (IV, Fixed, 95% CI) | 3.20 [‐0.87, 7.27] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Resolution of cyst by 10 weeks Show forest plot | 1 | 34 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.18 [0.12, 83.76] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Resolution of cyst by 10 weeks Show forest plot | 1 | 35 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.36 [0.13, 88.39] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Regression of cyst by 4 weeks Show forest plot | 1 | 124 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.55, 2.38] |
| 2 Regression of cyst by 12 weeks Show forest plot | 1 | 124 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.41 [0.55, 3.64] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Regression of cyst by 4 weeks Show forest plot | 1 | 124 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.44, 1.95] |
| 2 Regression of cyst by 12 weeks Show forest plot | 1 | 124 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.27 [0.49, 3.32] |

