Entrenamiento de los músculos respiratorios para la fibrosis quística
Appendices
Appendix 1. Search strategy: clinicaltrials.gov
| Date of Search | 07/05/2018 |
| Years Covered | 1920 to 07/05/2018 |
| Complete Strategy | 1. inspir*mus*train AND cystic fibrosis 2. resp*mus*train AND cystic fibrosis 3. press*thresh*load AND cystic fibrosis |
| Language Restrictions | None |
Appendix 2. Search strategy: WHO ICTRP
| Date of Search | 07/05/2018 |
| Years Covered | 1920 to 07/05/2018 |
| Complete Strategy | 1. inspir*mus*train AND cystic fibrosis 2. resp*mus*train AND cystic fibrosis 3. press*thresh*load AND cystic fibrosis |
| Language Restrictions | None |
Appendix 3. Search strategy: MEDLINE, Embase, CINAHL, AMED, BIOSIS, EMB reviews (OVID‐WEB)
| Date of Search | 01/08/2013 |
| Years Covered | 1966 to present |
| Complete Strategy | 1. randomised controlled trial.pt. |
| Summary of Strategy | Lines 1 & 29 are the Cochrane RCT filter. |
| Language Restrictions | None |
Appendix 4. Search strategy: PEDro
| Date of search | 01/08/2013 |
| Years covered | 1929 to present |
| Complete strategy | 1. inspir* mus* train* |
| Summary of strategy | PEDro is a database of controlled clinical trials, therefore no specific design oriented search terms. |
| Language restrictions | None |
Appendix 5. Search strategy: Science Direct
| Date of search | 01/08/2013 |
| Years covered | 1823 to present |
| Complete strategy | 1: (Title‐Abstr‐Key (random*)) OR (Title‐Abstr‐Key (placebo*)) OR (Title‐Abstr‐Key ((singl* or doubl* or trebl* or tripl*) w/25 (blind* or mask*))) OR (Title‐Abstr‐Key (clin* w/25 trial*)) |
| Summary of strategy | Lines 1 & 2 were to isolate studies of appropriate design. |
| Language restrictions | None |
Appendix 6. Search strategy: SCOPUS
| Date of search | 01/08/2013 |
| Years covered | 1966 to present |
| Complete strategy | 1: TITLE‐ABS‐KEY(randomised controlled trial) |
| Summary of strategy | Lines 1 to 7 were to isolate appropriate study design. |
| Language restrictions | None |
Appendix 7. Search strategy: MEDLINE, CINHAL and AMED (EBSCOHost)
| Date of search | 01/08/2013 |
| Years covered | 1966 to present |
| Complete strategy | 1: TITLE‐ABS‐KEY(randomised controlled trial) |
| Summary of strategy | Lines 1 to 7 were to isolate appropriate study design. |
| Language restrictions | None |

Comparison 1 RMT (80% of maximal effort) versus control, Outcome 1 Forced expiratory volume at one second (L).

Comparison 1 RMT (80% of maximal effort) versus control, Outcome 2 Forced vital capacity (L).
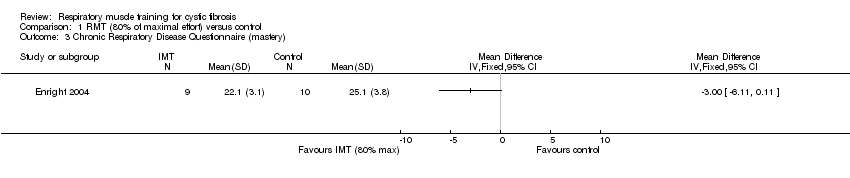
Comparison 1 RMT (80% of maximal effort) versus control, Outcome 3 Chronic Respiratory Disease Questionnaire (mastery).
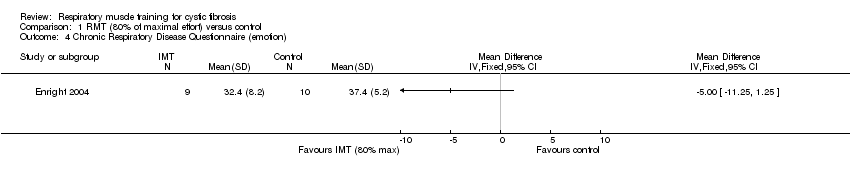
Comparison 1 RMT (80% of maximal effort) versus control, Outcome 4 Chronic Respiratory Disease Questionnaire (emotion).

Comparison 2 RMT (60% of maximal effort) versus control, Outcome 1 Forced expiratory volume at one second (L).

Comparison 2 RMT (60% of maximal effort) versus control, Outcome 2 PImax (cm H₂O).
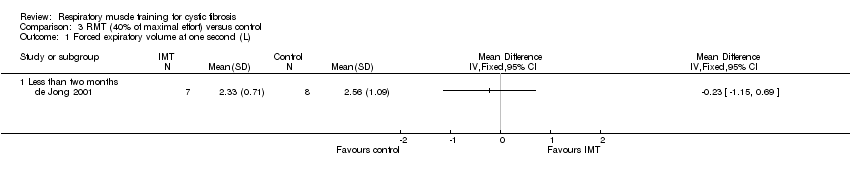
Comparison 3 RMT (40% of maximal effort) versus control, Outcome 1 Forced expiratory volume at one second (L).

Comparison 3 RMT (40% of maximal effort) versus control, Outcome 2 Forced expiratory volume at one second (% predicted).

Comparison 3 RMT (40% of maximal effort) versus control, Outcome 3 Forced vital capacity (L).
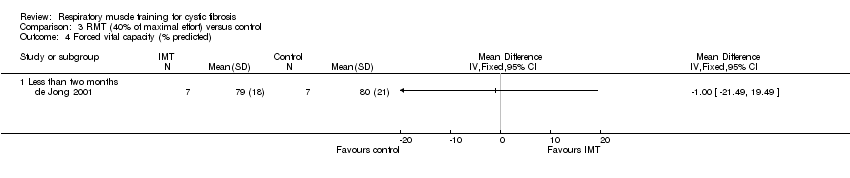
Comparison 3 RMT (40% of maximal effort) versus control, Outcome 4 Forced vital capacity (% predicted).
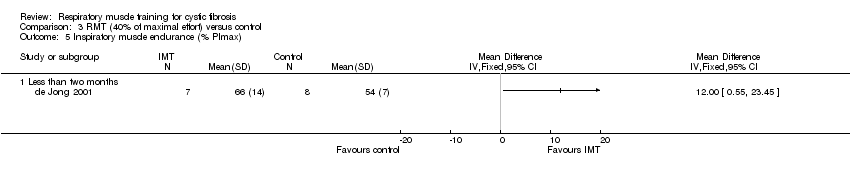
Comparison 3 RMT (40% of maximal effort) versus control, Outcome 5 Inspiratory muscle endurance (% PImax).

Comparison 4 RMT (20% of maximal effort) versus control, Outcome 1 Forced expiratory volume at one second (L).

Comparison 4 RMT (20% of maximal effort) versus control, Outcome 2 Forced vital capacity (L).
| Respiratory muscle training compared with control for cystic fibrosis | ||||||
| Patient or population: adults and children with cystic fibrosis Settings: outpatients Intervention: respiratory muscle trainingₑ Comparison: controlₑ | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Controlₑ | Respiratory muscle trainingₑ | |||||
| FEV1: % predicted Follow‐up: 6‐12 weeks | No significant differences between the respiratory muscle training group and the control group were reported in any study. | NA | 145 (7 studies including 2 cross‐over studies) | ⊕⊝⊝⊝ | Studies reported FEV1 as % predicted, litres or z score. One study with respiratory muscle training level 30% of maximal effort reported a significant improvement within the training group. | |
| FVC: % predicted Follow‐up: 6‐12 weeks | No significant differences between the respiratory muscle training group and the control group were reported in any study. | NA | 114 (5 studies including 1 cross‐over study) | ⊕⊝⊝⊝ | Studies reported FVC as % predicted, litres or z score. One study with respiratory muscle training level 30% of maximal effort reported a significant improvement within the training group. | |
| Exercise capacity: VO2max (mL/kg/min) Follow‐up: 6‐12 weeks | No significant differences between the respiratory muscle training group and the control group were reported in any study. | NA | 54 (3 studies including 1 cross‐over study) | ⊕⊝⊝⊝ | One study with an unspecified level of resistance reported a significant improvement within the respiratory muscle training group. | |
| HRQoL: total score Follow‐up: 8 weeks | Two studies reported no significant differences between the respiratory muscle training group and the control group. One study reported significant improvements in the parameters of mastery and emotion in the respiratory muscle training group compared to the control group. | NA | 69 (3 studies including 1 cross‐over study) | ⊕⊝⊝⊝ | Two studies used the Chronic Respiratory Disease Questionnaire (CRDQ) and one study used the cystic fibrosis questionnaire (CFQ). | |
| Respiratory muscle function: maximal inspiratory pressure (PImax) Follow‐up: 6‐10 weeks | Significant improvements were observed in all respiratory muscle training groups. Two studies reported no significant differences between the respiratory muscle training group and the control group. | NA | 51 (3 studies including 1 cross‐over study) | ⊕⊕⊝⊝ | ||
| Respiratory muscle function: inspiratory capacity Follow‐up: NA | NA | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1. The resistance level of the respiratory muscle training intervention was variable; three studies used 80% of maximal effort, one study used 60% of maximal effort, one study used 40% of maximal effort, one study used 30% of maximal effort and three studies did not specify the level of resistance. Control groups were also variable; cycle ergometer, H20, treatment as usual, standard chest physiotherapy, low resistance threshold loading device, no training or sham training. 2. Downgraded twice due to serious risk of bias: the included studies lacked methodological detail relating to methods of randomisation, allocation concealment and blinding. Most of the studies were at high risk of bias due to lack of blinding, incomplete outcome data or selective reporting, or both. 3. Downgraded due to imprecision: studies included a small number of participants and numerical results were not available for some of the studies. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Forced expiratory volume at one second (L) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 Two to six months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Forced vital capacity (L) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Two to six months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Chronic Respiratory Disease Questionnaire (mastery) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4 Chronic Respiratory Disease Questionnaire (emotion) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Forced expiratory volume at one second (L) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 Two to six months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 PImax (cm H₂O) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Two to six months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Forced expiratory volume at one second (L) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 Less than two months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Forced expiratory volume at one second (% predicted) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Less than two months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Forced vital capacity (L) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 Less than two months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Forced vital capacity (% predicted) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 Less than two months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Inspiratory muscle endurance (% PImax) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.1 Less than two months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Forced expiratory volume at one second (L) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 Two to six months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Forced vital capacity (L) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Two to six months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |

