Inspiratory muscle training for cystic fibrosis
Appendices
Appendix 1. Search strategy: MEDLINE, EMBASE, CINAHL, AMED, BIOSIS, EMB reviews (OVID‐WEB)
| Date of Search | 09/12/05 |
| Years Covered | 1966 to present |
| Complete Strategy | 1. randomised controlled trial.pt. |
| Summary of Strategy | Lines 1 & 29 are the Cochrane RCT filter. |
| Language Restrictions | None |
Appendix 2. Search strategy: PEDro
| Date of search | 09/12/05 |
| Years covered | 1929 to present |
| Complete strategy | 1. inspir* mus* train* |
| Summary of strategy | PEDro is a database of controlled clinical trials, therefore no specific design oriented search terms. |
| Language restrictions | None |
Appendix 3. Search strategy: Science Direct
| Date of search | 08/08/2011 |
| Years covered | 1823 to present |
| Complete strategy | 1: (Title‐Abstr‐Key (random*)) OR (Title‐Abstr‐Key (placebo*)) OR (Title‐Abstr‐Key ((singl* or doubl* or trebl* or tripl*) w/25 (blind* or mask*))) OR (Title‐Abstr‐Key (clin* w/25 trial*)) |
| Summary of strategy | Lines 1 & 2 were to isolate studies of appropriate design. |
| Language restrictions | None |
Appendix 4. Search strategy: SCOPUS
| Date of search | 08/08/2011 |
| Years covered | 1966 to present |
| Complete strategy | 1: TITLE‐ABS‐KEY(randomised controlled trial) |
| Summary of strategy | Lines 1 to 7 were to isolate appropriate study design. |
| Language restrictions | None |
Appendix 5. Search strategy: MEDLINE, CINHAL and AMED (EBSCOHost)
| Date of search | 08/08/2011 |
| Years covered | 1966 to present |
| Complete strategy | 1: TITLE‐ABS‐KEY(randomised controlled trial) |
| Summary of strategy | Lines 1 to 7 were to isolate appropriate study design. |
| Language restrictions | None |
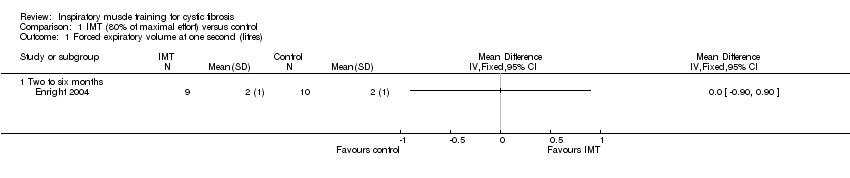
Comparison 1 IMT (80% of maximal effort) versus control, Outcome 1 Forced expiratory volume at one second (litres).

Comparison 1 IMT (80% of maximal effort) versus control, Outcome 2 Forced vital capacity (litres).

Comparison 1 IMT (80% of maximal effort) versus control, Outcome 3 Chronic Respiratory Disease Questionnaire (mastery).

Comparison 1 IMT (80% of maximal effort) versus control, Outcome 4 Chronic Respiratory Disease Questionnaire (emotion).

Comparison 2 IMT (60% of maximal effort) versus control, Outcome 1 Forced expiratory volume at one second (litres).
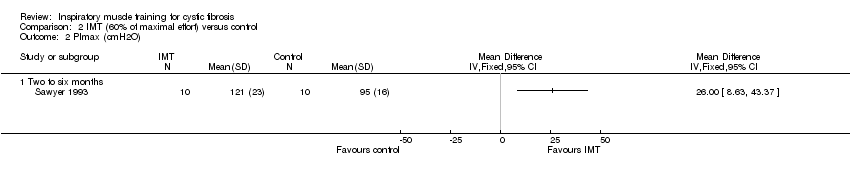
Comparison 2 IMT (60% of maximal effort) versus control, Outcome 2 PImax (cmH2O).

Comparison 3 IMT (40% of maximal effort) versus control, Outcome 1 Forced expiratory volume at one second (litres).

Comparison 3 IMT (40% of maximal effort) versus control, Outcome 2 Forced expiratory volume at one second (% predicted).
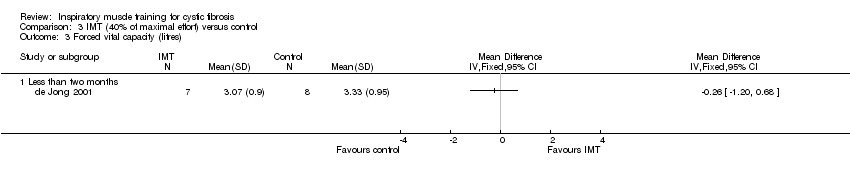
Comparison 3 IMT (40% of maximal effort) versus control, Outcome 3 Forced vital capacity (litres).

Comparison 3 IMT (40% of maximal effort) versus control, Outcome 4 Forced vital capacity (% predicted).

Comparison 3 IMT (40% of maximal effort) versus control, Outcome 5 Inspiratory muscle endurance (%PImax).

Comparison 4 IMT (20% of maximal effort) versus control, Outcome 1 Forced expiratory volume at one second (litres).

Comparison 4 IMT (20% of maximal effort) versus control, Outcome 2 Forced expiratory volume at one second (% predicted).

Comparison 4 IMT (20% of maximal effort) versus control, Outcome 3 Forced vital capacity (litres).
| Term | Explanation |
| Continuous training | Training at 70% to 80% of maximum effort for 30 to 45 minutes. The percentage of maximal effort and/or the duration of the training may be adjusted depending on the goal of the training. |
| Elastic load | Refers to the load imposed by the stiffness of the lung and chest wall that must be overcome by the inspiratory muscles in order to generate inspiratory flow. Elastic loads are greater when breathing from a higher lung volume as a consequence of the associated decrease in lung and chest wall compliance. Imposing elastic loads has not been used to train the inspiratory muscles most likely due to the need for complicated equipment and poor clinical utility. |
| Forced expiratory volume at 1 second (FEV1) | The volume of air expelled during the 1st second of forced exhalation from total lung capacity. |
| Forced vital capacity (FVC) | The total volume of air expelled during a forced exhalation from total lung capacity. |
| Inspiratory capacity (IC) | The maximum volume of air taken into the lungs during a maximal inhalation from functional residual capacity. |
| Forced expiratory flow 25‐75% (FEF 25‐75%) | The speed of the air leaving the lungs during the middle section of a forced exhalation. |
| Interval Training | Periods of intense training interspersed with periods of recuperation. As with continuous training, the level of effort required during the training period may be adjusted to suit the individual and the intended goal. The period of recuperation will be adjusted accordingly. |
| Maximal inspiratory pressure [PImax] | The maximum pressure generated by the inspiratory muscles against an occluded airway. |
| Resistive loading | Requires person to breathe through a narrow Inspiratory pathway/aperture. The load imposed is dependent on inspiratory flow, i.e. when using resistive training devices, participants can reduce the load imposed by manipulating their breathing pattern. Breathing pattern, specifically inspiratory flow, should be controlled when using resistive inspiratory muscle training devices. |
| Threshold loading | Requires the person to inspire through a device which imposes a threshold load via either a weighted plunger system or a spring‐loaded valve. The person needs to generate a critical inspiratory pressure, prior to the threshold valve opening and allowing inspiratory flow. Once the threshold valve is open, pressure and flow are largely independent and therefore the person is unable to reduce the load imposed by the device by manipulations in breathing pattern. |
| Total lung capacity (TLC) | The maximum amount of air the lungs can hold when they are fully inflated. |
| Voluntary isocapnic (normocapnic) hyperpnoea | Requires the person to maintain a high level of minute ventilation for a specified period. Imposes a high flow, low pressure load on the inspiratory muscles which is analogous to the loads borne by the inspiratory muscles during periods of increased minute ventilation (i.e. during exercise). Requires the use of complex equipment to ensure stable levels of carbon dioxide in the arterial blood (PaCO2), so is rarely used in the clinical setting. |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Forced expiratory volume at one second (litres) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 Two to six months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Forced vital capacity (litres) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Two to six months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Chronic Respiratory Disease Questionnaire (mastery) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4 Chronic Respiratory Disease Questionnaire (emotion) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Forced expiratory volume at one second (litres) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 Two to six months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 PImax (cmH2O) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Two to six months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Forced expiratory volume at one second (litres) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 Less than two months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Forced expiratory volume at one second (% predicted) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Less than two months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Forced vital capacity (litres) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 Less than two months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Forced vital capacity (% predicted) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 Less than two months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Inspiratory muscle endurance (%PImax) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.1 Less than two months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Forced expiratory volume at one second (litres) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 Two to six months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Forced expiratory volume at one second (% predicted) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Two to six months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Forced vital capacity (litres) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 Two to six months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |

