Inspiratory muscle training for cystic fibrosis
Referencias
References to studies included in this review
References to studies excluded from this review
Additional references
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | Parallel design over 12 weeks | |
| Participants | n = 27 | |
| Interventions | IMT: no details; plus, cycle ergometer training 3 times per week Control: cycle ergometer training 3 times per week | |
| Outcomes | FEV1, FVC, IMS, IME, MEC, perceived breathlessness, antibiotic use and ease or degree of expectoration | |
| Notes | IME protocol: abstract only, no details given | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised, no details given |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) | High risk | Performance bias: clear difference between the interventions received Dectection bias: No reference to any blinding |
| Incomplete outcome data (attrition bias) | Unclear risk | No information provided. Intention to treat: unclear |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information available to arrive at a conclusion |
| Other bias | Unclear risk | Insufficient information available to arrive at a conclusion |
| Methods | Parallel design over six weeks | |
| Participants | n = 20 Age range was not stated Gender mix: no information | |
| Interventions | Threshold loading device: Intervention group: 30% of PImax Control group: 7cm H2O Training regimen: 10 to 15 minutes bd for 6 weeks | |
| Outcomes | FEV1, FVC, PImax, IC, RMS, RME and exercise capacity | |
| Notes | Abstract only | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The authors only state that the allocation was random without explaining the process involved |
| Allocation concealment (selection bias) | Unclear risk | No details are provided |
| Blinding (performance bias and detection bias) | High risk | Performance bias: The comparison group are referred to only as the "control group" with no mention of the intensity of the training used; i.e. if it was at "sham" or sub‐maximal levels Dectection bias: No reference to any blinding |
| Incomplete outcome data (attrition bias) | High risk | No statistical data is presented for the control group. One subject from the intervention group did not complete the trial; it was not stated whether they were included or excluded from the final analysis |
| Selective reporting (reporting bias) | High risk | Two outcomes (respiratory muscle strength and dyspnoea) are mentioned as having been analysed, but no data are provided for them. |
| Other bias | Unclear risk | Insufficient information available to arrive at a conclusion |
| Methods | Consecutive, self‐control design over 8 weeks | |
| Participants | n = 11 | |
| Interventions | IMT: Inspiratory resistance, 15 minutes bd, no dosage Control: no details provided | |
| Outcomes | IMS, Wmax, VO2max, VE and heart rate | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised, no details given |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) | High risk | Performance bias: no details of the control training regimen are provided = high risk Dectection bias: observer blind = low risk |
| Incomplete outcome data (attrition bias) | High risk | Two participants were unable to satisfactorily perform the outcome measure PIMax, due to expiration up to residual volume resulting in coughing. The authors do not stipulate whether this occurred during the intervention or control phase of the trial. Intention to treat: unclear |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information available to arrive at a conclusion |
| Other bias | Unclear risk | Insufficient information available to arrive at a conclusion |
| Methods | Parallel design over 8 weeks | |
| Participants | Intervention: n = 9 No data was provided on the ages of the participants | |
| Interventions | Intervention: Computer‐generated through range inspiratory muscle training (TIRE) at 80% of individual capacity Control: Threshold loading device at 30% of peak; the measure used is not named | |
| Outcomes | Chronic Respiratory Disease Questionnaire ('mastery' and 'emotion' elements), RMS and RME | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised, no details given |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information available to arrive at a conclusion |
| Blinding (performance bias and detection bias) | High risk | Perfomance bias: the training intensities employed (80% and "threshold" 30% training) could, potentially, have led the participants to know which group they were in Dectection bias: no reference to any blinding |
| Incomplete outcome data (attrition bias) | Unclear risk | Insufficient information available to arrive at a conclusion; no statistical data is presented for the control group Intention to treat: 3 from 18 (17%) |
| Selective reporting (reporting bias) | Unclear risk | As this study (to date) is only published in abstract form it is unclear whether the reported outcomes are all that were analysed. |
| Other bias | Unclear risk | Insufficient information available to arrive at a conclusion |
| Methods | Parallel design over 6 weeks | |
| Participants | Intervention: n = 8; mean (SD) age = 17 (5.2) years Control: n = 8; mean (SD) age = 19 (5.5) years | |
| Interventions | IMT: Threshold loading; 20 minutes a day, 5 days per week. At 40% of PImax Control: Threshold loading; 20 minutes a day, 5 days per week. At 10% of PImax | |
| Outcomes | FEV1, FVC, Wmax, VO2max, VEmax, IME, perceived breathlessness, general fatigue, physical fatigue, reduced activity score, reduced motivation score, mental fatigue and dyspnoea | |
| Notes | IME protocol: a commercially‐available threshold‐loading device (Threshold, Healthscan Products, Inc. U.S.A.) was used during an incremental loading procedure. In order to obtain pressures over 41 cm H2O an additional spring was inserted with a double‐spring constant. Participants started inspiring from a threshold‐loading device set at 30% of PImax for 2 min. The threshold load was then increased every 2 min in increments of 10% | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Minimisation |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) | High risk | Performance bias: both training intensities were low; however, no attempt was made to ascertain whether the participants knew if the received the training intensity Detection bias: no reference to any blinding |
| Incomplete outcome data (attrition bias) | High risk | One participant in the intervention group was withdrawn due to earache experienced whilst training at 40% of PImax Intention to treat: 1 from 15 (6%) |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information available to arrive at a conclusion |
| Other bias | Unclear risk | Insufficient information available to arrive at a conclusion |
| Methods | Parallel design over 8 weeks | |
| Participants | All participants: n = 29, mean (SD) age = 22 (4.2) years | |
| Interventions | Intervention 1: IMT at 80% of "maximal inspiratory effort" Control: "No Training" | |
| Outcomes | FEV1(% predicted), FVC (% predicted), PImax, SPImax, heart rate, perceived exertion, dyspnoea and Chronic Respiratory Disease Questionnaire | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) | High risk | Performance bias: the comparison was "no training" making it clear to the participants which arm they were in Dectection bias: outcome assessors at the final data collection session, although they did not state whether this was the case at the initial assessment or even if the same assessors carried out all the assessments |
| Incomplete outcome data (attrition bias) | Unclear risk | No mention is made of whether all subjects completed the trial or not. Nor are there any statistical indications Intention to treat: unclear |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information available to arrive at a conclusion |
| Other bias | Unclear risk | Insufficient information available to arrive at a conclusion |
| Methods | Parallel design over 8 weeks | |
| Participants | Experimental: n = 19, mean (SD) age = 22.5 (3.5) years | |
| Interventions | IMT: IMT at 80% of "maximal effort". No dosage stated 3 control groups: healthy participants: IMT at 80% of "maximal effort"; healthy participants: "No Training" and CF participants: "No Training" | |
| Outcomes | VC, TLC | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised, no details given |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) | High risk | Performance bias: the comparison was "no training" making it clear to the participants which arm they were in Detection bias: no reference to any blinding |
| Incomplete outcome data (attrition bias) | Unclear risk | No information provided Intention to treat: unclear |
| Selective reporting (reporting bias) | High risk | The post‐training pulmonary function results were not presented |
| Other bias | Unclear risk | Insufficient information available to arrive at a conclusion |
| Methods | Parallel design over 10 weeks | |
| Participants | Experimental: n = 10, mean (SD) age = 11.46 (2.45) Sham: n = 10, mean (SD) age = 9.76 (2.57) | |
| Interventions | IMT: IMT at 60% PImax Control: IMT at 10% PImax | |
| Outcomes | FEV1, VC, FRC, IC, RV, TLC, RV/TLC, FEV1/FVC, MVV, exercise time | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised, no details given |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) | High risk | Performance bias: there was a clear difference in the intensity of training although no attempt was made to ascertain whether the participants in the training groups knew if they received the training intensity Dectection bias: outcome assessors at the final data collection session, although they did not state whether this was the case at the initial assessment or even if the same assessors carried out all the assessments |
| Incomplete outcome data (attrition bias) | High risk | 2 participants removed from analysis and the reasons for this were explained; however, it is unclear which group(s) they were in |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information available to arrive at a conclusion |
| Other bias | Unclear risk | Insufficient information available to arrive at a conclusion |
% predicted: the volume of air exhaled expressed as a percentage of the expected volume based on the physical attributes of the individual
bd: twice a day
FEV1: volume of air exhaled over the first second of a forced exhalation
FEV1/FVC = the ratio of FEV1 to FVC
FRC: functional residual capacity
FVC: total volume of air forcibly exhaled
FEF 25‐75%: forced expiratory flow 25‐75%
IC: inspiratory capacity
IME: inspiratory muscle endurance
IMF: inspiratory muscle function
IMS: inspiratory muscle strength
IMT: inspiratory muscle training
MEC: maximal exercise capacity
MVV: maximum voluntary ventilation
RME: respiratory muscle endurance
RMS: respiratory muscle strength
n: number of participants
PImax: maximal inspiratory pressure
RV: residual volume; i.e. the volume of air retained in the lungs following a maximal, voluntary exhalation (FVC)
RV/TLC: the ratio of residual volume to total lung capacity
SD: standard deviation
SPImax: sustained maximal inspiratory pressure
TLC: total lung capacity; i.e. the calculated maximum potential volume of an individual's lungs
VC: the total volume of air that can be exhaled in any one breath
VE(max): peak expired ventilation
VO2max: peak oxygen consumption
Wmax: maximum work load
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| Study excluded as the intervention was not inspiratory muscle training | |
| Study excluded as allocation not randomised | |
| Observational study, no randomisation |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
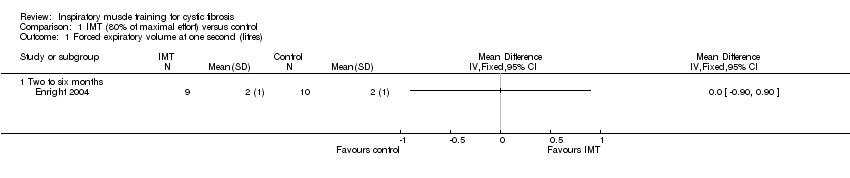
| 1 Forced expiratory volume at one second (litres) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.1  Comparison 1 IMT (80% of maximal effort) versus control, Outcome 1 Forced expiratory volume at one second (litres). | ||||
| 1.1 Two to six months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Forced vital capacity (litres) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.2  Comparison 1 IMT (80% of maximal effort) versus control, Outcome 2 Forced vital capacity (litres). | ||||
| 2.1 Two to six months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Chronic Respiratory Disease Questionnaire (mastery) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.3  Comparison 1 IMT (80% of maximal effort) versus control, Outcome 3 Chronic Respiratory Disease Questionnaire (mastery). | ||||
| 4 Chronic Respiratory Disease Questionnaire (emotion) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.4  Comparison 1 IMT (80% of maximal effort) versus control, Outcome 4 Chronic Respiratory Disease Questionnaire (emotion). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Forced expiratory volume at one second (litres) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.1  Comparison 2 IMT (60% of maximal effort) versus control, Outcome 1 Forced expiratory volume at one second (litres). | ||||
| 1.1 Two to six months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
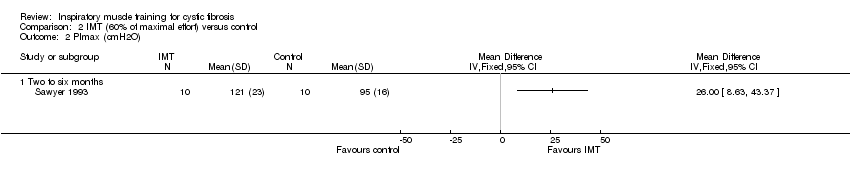
| 2 PImax (cmH2O) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.2  Comparison 2 IMT (60% of maximal effort) versus control, Outcome 2 PImax (cmH2O). | ||||
| 2.1 Two to six months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Forced expiratory volume at one second (litres) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 3.1  Comparison 3 IMT (40% of maximal effort) versus control, Outcome 1 Forced expiratory volume at one second (litres). | ||||
| 1.1 Less than two months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Forced expiratory volume at one second (% predicted) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 3.2  Comparison 3 IMT (40% of maximal effort) versus control, Outcome 2 Forced expiratory volume at one second (% predicted). | ||||
| 2.1 Less than two months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
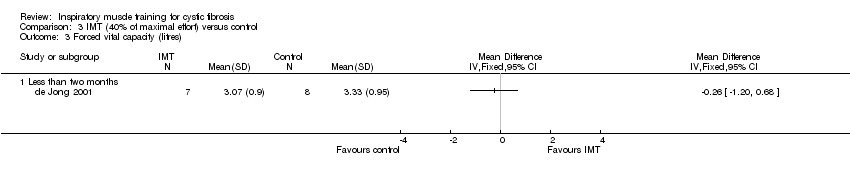
| 3 Forced vital capacity (litres) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 3.3  Comparison 3 IMT (40% of maximal effort) versus control, Outcome 3 Forced vital capacity (litres). | ||||
| 3.1 Less than two months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Forced vital capacity (% predicted) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 3.4  Comparison 3 IMT (40% of maximal effort) versus control, Outcome 4 Forced vital capacity (% predicted). | ||||
| 4.1 Less than two months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Inspiratory muscle endurance (%PImax) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 3.5  Comparison 3 IMT (40% of maximal effort) versus control, Outcome 5 Inspiratory muscle endurance (%PImax). | ||||
| 5.1 Less than two months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Forced expiratory volume at one second (litres) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 4.1  Comparison 4 IMT (20% of maximal effort) versus control, Outcome 1 Forced expiratory volume at one second (litres). | ||||
| 1.1 Two to six months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Forced expiratory volume at one second (% predicted) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 4.2  Comparison 4 IMT (20% of maximal effort) versus control, Outcome 2 Forced expiratory volume at one second (% predicted). | ||||
| 2.1 Two to six months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Forced vital capacity (litres) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 4.3  Comparison 4 IMT (20% of maximal effort) versus control, Outcome 3 Forced vital capacity (litres). | ||||
| 3.1 Two to six months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
Comparison 1 IMT (80% of maximal effort) versus control, Outcome 1 Forced expiratory volume at one second (litres).

Comparison 1 IMT (80% of maximal effort) versus control, Outcome 2 Forced vital capacity (litres).

Comparison 1 IMT (80% of maximal effort) versus control, Outcome 3 Chronic Respiratory Disease Questionnaire (mastery).

Comparison 1 IMT (80% of maximal effort) versus control, Outcome 4 Chronic Respiratory Disease Questionnaire (emotion).

Comparison 2 IMT (60% of maximal effort) versus control, Outcome 1 Forced expiratory volume at one second (litres).
Comparison 2 IMT (60% of maximal effort) versus control, Outcome 2 PImax (cmH2O).

Comparison 3 IMT (40% of maximal effort) versus control, Outcome 1 Forced expiratory volume at one second (litres).

Comparison 3 IMT (40% of maximal effort) versus control, Outcome 2 Forced expiratory volume at one second (% predicted).
Comparison 3 IMT (40% of maximal effort) versus control, Outcome 3 Forced vital capacity (litres).

Comparison 3 IMT (40% of maximal effort) versus control, Outcome 4 Forced vital capacity (% predicted).

Comparison 3 IMT (40% of maximal effort) versus control, Outcome 5 Inspiratory muscle endurance (%PImax).

Comparison 4 IMT (20% of maximal effort) versus control, Outcome 1 Forced expiratory volume at one second (litres).

Comparison 4 IMT (20% of maximal effort) versus control, Outcome 2 Forced expiratory volume at one second (% predicted).

Comparison 4 IMT (20% of maximal effort) versus control, Outcome 3 Forced vital capacity (litres).
| Term | Explanation |
| Continuous training | Training at 70% to 80% of maximum effort for 30 to 45 minutes. The percentage of maximal effort and/or the duration of the training may be adjusted depending on the goal of the training. |
| Elastic load | Refers to the load imposed by the stiffness of the lung and chest wall that must be overcome by the inspiratory muscles in order to generate inspiratory flow. Elastic loads are greater when breathing from a higher lung volume as a consequence of the associated decrease in lung and chest wall compliance. Imposing elastic loads has not been used to train the inspiratory muscles most likely due to the need for complicated equipment and poor clinical utility. |
| Forced expiratory volume at 1 second (FEV1) | The volume of air expelled during the 1st second of forced exhalation from total lung capacity. |
| Forced vital capacity (FVC) | The total volume of air expelled during a forced exhalation from total lung capacity. |
| Inspiratory capacity (IC) | The maximum volume of air taken into the lungs during a maximal inhalation from functional residual capacity. |
| Forced expiratory flow 25‐75% (FEF 25‐75%) | The speed of the air leaving the lungs during the middle section of a forced exhalation. |
| Interval Training | Periods of intense training interspersed with periods of recuperation. As with continuous training, the level of effort required during the training period may be adjusted to suit the individual and the intended goal. The period of recuperation will be adjusted accordingly. |
| Maximal inspiratory pressure [PImax] | The maximum pressure generated by the inspiratory muscles against an occluded airway. |
| Resistive loading | Requires person to breathe through a narrow Inspiratory pathway/aperture. The load imposed is dependent on inspiratory flow, i.e. when using resistive training devices, participants can reduce the load imposed by manipulating their breathing pattern. Breathing pattern, specifically inspiratory flow, should be controlled when using resistive inspiratory muscle training devices. |
| Threshold loading | Requires the person to inspire through a device which imposes a threshold load via either a weighted plunger system or a spring‐loaded valve. The person needs to generate a critical inspiratory pressure, prior to the threshold valve opening and allowing inspiratory flow. Once the threshold valve is open, pressure and flow are largely independent and therefore the person is unable to reduce the load imposed by the device by manipulations in breathing pattern. |
| Total lung capacity (TLC) | The maximum amount of air the lungs can hold when they are fully inflated. |
| Voluntary isocapnic (normocapnic) hyperpnoea | Requires the person to maintain a high level of minute ventilation for a specified period. Imposes a high flow, low pressure load on the inspiratory muscles which is analogous to the loads borne by the inspiratory muscles during periods of increased minute ventilation (i.e. during exercise). Requires the use of complex equipment to ensure stable levels of carbon dioxide in the arterial blood (PaCO2), so is rarely used in the clinical setting. |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Forced expiratory volume at one second (litres) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 Two to six months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Forced vital capacity (litres) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Two to six months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Chronic Respiratory Disease Questionnaire (mastery) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4 Chronic Respiratory Disease Questionnaire (emotion) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Forced expiratory volume at one second (litres) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 Two to six months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 PImax (cmH2O) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Two to six months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Forced expiratory volume at one second (litres) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 Less than two months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Forced expiratory volume at one second (% predicted) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Less than two months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Forced vital capacity (litres) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 Less than two months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Forced vital capacity (% predicted) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 Less than two months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Inspiratory muscle endurance (%PImax) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.1 Less than two months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Forced expiratory volume at one second (litres) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 Two to six months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Forced expiratory volume at one second (% predicted) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Two to six months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Forced vital capacity (litres) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 Two to six months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |

