Pretratamiento con píldora anticonceptiva oral, progestágeno o estrógeno para los protocolos de estimulación ovárica en pacientes sometidas a técnicas de reproducción asistida
Appendices
Appendix 1. Glossary
Embryo The product of conception from the time of fertilisation to the end of the embryonic stage eight weeks after fertilisation.
Embryo transfer (ET) Procedure of which embryos are placed in the uterus or fallopian tube.
Endogenous Developed or originated inside the organism. For example, hormones produced by the pituitary gland would be an endogenous supply, but hormones produced in the laboratory and then given to the body is called an exogenous supply.
Fertilisation The penetration of the ovum by the sperm cell and fusion of genetic materials, resulting in the development of an embryo.
Follicle The sac in which an egg develops in the ovary.
Follicle cohort synchronisation In the ovaries, a few eggs are maturing at the same time. These eggs are all in a different stage of maturation. If one egg reaches a threshold at the right time in the menstrual cycle, the final maturation process will start and this egg will reach ovulation. For in vitro fertilisation/intra‐cytoplasmic sperm injection cycles it is important that more than one egg reaches this threshold at the same time, so they can be retrieved at once before spontaneous ovulation occurs. This is called synchronisation of the follicle cohort.
Follicle‐stimulating hormone (FSH) A hormone produced and released from the pituitary gland. In women it stimulates the production of oestrogen and follicles in the ovary ready for ovulation.
Gestational sac A fluid‐filled structure containing an embryo that develops early in pregnancy, usually within the uterus.
Gonadotrophin releasing hormone (GnRH) A substance produced by the hypothalamus (part of the brain) to enable the pituitary gland to secrete LH and FSH.
Gonadotrophins Pituitary hormones FSH and LH which stimulate the ovaries in women.
Human menopausal gonadotrophin (hMG) An injectable preparation that is obtained from the urine of menopausal women and has biological activity similar to that of FSH.
Luteal phase The last 14 days of the menstrual cycle.
Luteinising Hormone (LH) A hormone produced and released by the pituitary gland. In women it is responsible for ovulation and progestogen production.
Negative feedback A common regulation mechanism to stabilise the body's internal environment. An example is the temperature control of the human body. When body temperature is too high, the body will react in such a way that it cools down, by opening pores and sweating. In this way the body's temperature will not fluctuate too much. The same type of mechanism is used to keep hormone values stable. An increase in gonadotrophin values will (through negative feedback) result in fewer GnRH receptors. The binding of GnRH to a GnRH receptor in the pituitary gland will result in the release of gonadotrophins, but with fewer GnRH receptors, the releasing process will be lowered and the gonadotrophin levels in the body will drop.
Oocyte The egg from a woman's ovary.
Ova A woman's reproductive cell, also known as egg or oocyte.
Ovarian hyperstimulation syndrome (OHSS) A condition that occurs from fertility drugs when a large number of follicles in the ovary are stimulated to develop and ovulate. This stimulation causes an enlargement of the ovaries.
Ovulation The release of an egg/ova from an ovarian follicle.
Ovulation induction Medical procedure to produce ovulation.
Polycystic ovary syndrome (PCOS) When a woman has enlarged ovaries with multiple cysts and the surface of the ovary is thickened. The woman may ovulate infrequently or not at all.
Premature LH‐surge In a normal menstrual cycle an increase in LH levels (LH‐surge) is needed to start ovulation. In in vitro fertilisation (IVF)/intra‐cytoplasmic sperm injection (ISCI) cycles it is important that the ovulation does not start before the oocytes are mature enough to be retrieved. A LH‐surge that occurs too early is called premature and is an unwanted event in IVF/ICSI cycles.
Recombinant (as in recombinant FSH or rFSH) A naturally occurring hormone which has been made in the laboratory with the use of DNA technology.
Subfertility Failure to achieve pregnancy after at least one year of unprotected coitus.
Ultrasound Radiology sounds waves of a high frequency used to visualise the developing foetus in the uterus to check size, growth and the presence of abnormalities.
All these definitions (except for follicle cohort synchronisation, negative feedback and premature LH‐surge) were obtained from the glossary of the MDSG Module 2008.
Appendix 2. Cochrane Gynaecology and Fertility (CGFG) specialised register search strategy
Searched 17 January 2017
Procite platform
Keywords CONTAINS "IVF" or "in vitro fertilization" or "in‐vitro fertilisation" or "ICSI" or"intracytoplasmic sperm injection" or "Embryo" or "in‐vitro fertilization" or "ART" or "controlled ovarian " or "COH" or Title CONTAINS "IVF" or "in vitro fertilization" or "in‐vitro fertilisation" or "ICSI" or"intracytoplasmic sperm injection" or "Embryo" or "in‐vitro fertilization" or "ART" or "controlled ovarian " or "COH"
AND
Keywords CONTAINS "oral contraceptive" or "Oral Contraceptive Agent" or "oral contraceptives" or "combined oral contraceptives" or "Pretreatment" or "OCP" or "Oral Contraception" or "oral contraceptive pill" or "oral conjugated estrogen" or "OCP pretreatment" or "progestagen" or progestin"or"progestins"or"progestogen"or"progestogens"or"Progesterone"or"Norgestrel"or"Norethindrone"or "Norethisterone"or"desogestral"or"desogestrel"or"Gestagen"or"Gestodene"or"gestrinone"or"Estradiol"or"estradiol acetate" or "estradiol valerate"or"Estriol"or"oestrodiol"or"oestrogen"or"estrogen"or"Estrogens"or Title CONTAINS "oral contraceptive" or "Oral Contraceptive Agent" or "oral contraceptives" or "combined oral contraceptives" or "Pretreatment" or "OCP" or "Oral Contraception" or "oral contraceptive pill" or "oral congugated estrogen" or "OCP pretreatment" or "progestagen" or "progestin"or"progestins"or"progestogen"or"progestogens"or"Progesterone"or"Norgestrel"or"Norethindrone"or "Norethisterone" or "Estradiol" (888 hits)
Appendix 3. CENTRAL Register of Studies Online (CRSO) search strategy
Searched 17 January 2017
Web platform
#1 MESH DESCRIPTOR Reproductive Techniques, Assisted EXPLODE ALL TREES 2792
#2 (IVF or ICSI):TI,AB,KY 3537
#3 (embryo* transfer*):TI,AB,KY 2049
#4 (vitro fertili?ation ):TI,AB,KY 1954
#5 (intracytoplasmic sperm injection*):TI,AB,KY 1076
#6 COH:TI,AB,KY 212
#7 (ovar* adj2 stimulat*):TI,AB,KY 1197
#8 (assisted reproduct*):TI,AB,KY 694
#9 (ovarian hyperstimulation):TI,AB,KY 849
#10 (pituitary desensiti?ation):TI,AB,KY 82
#11 (pituitary adj2 suppression):TI,AB,KY 170
#12 (poor responder*):TI,AB,KY 391
#13 (poor ovar* response):TI,AB,KY 84
#14 (low responder*):TI,AB,KY 137
#15 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 6479
#16 MESH DESCRIPTOR Contraceptives, Oral EXPLODE ALL TREES 3215
#17 (oral contracepti*):TI,AB,KY 2278
#18 (pretreatment* or pre‐treatment*):TI,AB,KY 14480
#19 (gestrinone or estradiol):TI,AB,KY 7388
#20 (norgestrel or desogestrel):TI,AB,KY 893
#21 (dimethisterone or levonorgestrel):TI,AB,KY 1169
#22 (norethindrone or gestodene):TI,AB,KY 1021
#23 (norgestimate or dienogest):TI,AB,KY 233
#24 (progestogen* or progestagen*):TI,AB,KY 839
#25 (progestin* or gestagen*):TI,AB,KY 1576
#26 (Medroxyprogesterone or Diane‐35):TI,AB,KY 1851
#27 (norethisterone or ?estrogen):TI,AB,KY 9170
#28 E2:TI,AB,KY 3182
#29 #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 32193
#30 #15 AND #29 1355
Appendix 4. MEDLINE search strategy
From 1946 to 17 January 2017
Ovid MEDLINE(R) Epub Ahead of Print, In Process & Other Non‐Indexed Citations, Ovid MEDLINE (R) Daily, and Ovid MEDLINE
1 reproductive techniques/ or exp reproductive techniques, assisted/ or exp embryo transfer/ or exp fertilization in vitro/ or exp sperm injections, intracytoplasmic/ or exp gamete intrafallopian transfer/ (65927)
2 (IVF or ICSI).tw. (24183)
3 embryo transfer.tw. (9681)
4 (in vitro fertilisation or in vitro fertilization).tw. (20881)
5 intracytoplasmic sperm injection$.tw. (6213)
6 COH.tw. (1533)
7 ovar$ stimulat$.tw. (5130)
8 assisted reproduct$.tw. (12517)
9 ovarian hyperstimulation.tw. (4687)
10 pituitary desensitisation.tw. (21)
11 pituitary desensitization.tw. (242)
12 pituitary suppression.tw. (327)
13 poor responder$.tw. (2039)
14 poor ovar$ response.tw. (327)
15 low responder$.tw. (2153)
16 or/1‐15 (84391)
17 oral contracepti$.tw. (26678)
18 (OC or OCP$).tw. (22318)
19 (pretreatment$ or pre‐treatment$).tw. (202425)
20 gestrinone$.tw. (199)
21 estradiol.tw. (81366)
22 desogestrel.tw. (1096)
23 dimethisterone.tw. (25)
24 levonorgestrel.tw. (4400)
25 norethindrone.tw. (1328)
26 norgestrel.tw. (1071)
27 norgestrienone.tw. (33)
28 gestodene.tw. (767)
29 norgestimate.tw. (363)
30 dienogest.tw. (376)
31 progestogen$.tw. (5441)
32 progestagen$.tw. (2202)
33 progestin$.tw. (11982)
34 gestagen$.tw. (1640)
35 Medroxyprogesterone.tw. (6404)
36 Diane‐35.tw. (87)
37 norethisterone.tw. (2044)
38 estrogen.tw. (120036)
39 E2.tw. (67681)
40 oestrogen.tw. (17720)
41 exp Contraceptives, Oral/ (47656)
42 or/17‐41 (500004)
43 randomized controlled trial.pt. (508490)
44 controlled clinical trial.pt. (98223)
45 randomized.ab. (438894)
46 randomised.ab. (87375)
47 placebo.tw. (210117)
48 clinical trials as topic.sh. (197894)
49 randomly.ab. (299032)
50 trial.ti. (202001)
51 (crossover or cross‐over or cross over).tw. (79992)
52 or/43‐51 (1282048)
53 exp animals/ not humans.sh. (4854866)
54 52 not 53 (1184243)
55 16 and 42 and 54 (977)
Appendix 5. Embase search strategy
From 1980 to 17 January 2017
Ovid platform
1 (IVF or ICSI).tw. (31186)
2 embryo transfer.tw. (11462)
3 (in vitro fertilisation or in vitro fertilization).tw. (21776)
4 intracytoplasmic sperm injection$.tw. (6743)
5 COH.tw. (1621)
6 ovar$ stimulat$.tw. (6451)
7 assisted reproduct$.tw. (14279)
8 ovarian hyperstimulation.tw. (5543)
9 pituitary desensitisation.tw. (21)
10 pituitary desensitization.tw. (263)
11 pituitary suppression.tw. (364)
12 poor responder$.tw. (2466)
13 poor ovar$ response.tw. (383)
14 low responder$.tw. (2074)
15 exp infertility therapy/ (81759)
16 exp embryo transfer/ or exp fertilization in vitro/ or exp intracytoplasmic sperm injection/ (55779)
17 or/1‐16 (97457)
18 exp oral contraceptive agent/ (53046)
19 oral contracepti$.tw. (23779)
20 (OC or OCP$).tw. (21319)
21 (pretreatment$ or pre‐treatment$).tw. (195469)
22 gestrinone$.tw. (187)
23 estradiol.tw. (74210)
24 norgestrel.tw. (706)
25 desogestrel.tw. (1122)
26 dimethisterone.tw. (11)
27 levonorgestrel.tw. (4344)
28 norethindrone.tw. (954)
29 norgestrel.tw. (706)
30 norgestrienone.tw. (15)
31 gestodene.tw. (758)
32 norgestimate.tw. (348)
33 dienogest.tw. (500)
34 progestogen$.tw. (5070)
35 progestagen$.tw. (2022)
36 progestin$.tw. (10993)
37 gestagen$.tw. (1564)
38 Medroxyprogesterone.tw. (5918)
39 Diane‐35.tw. (334)
40 norethisterone.tw. (1847)
41 estrogen.tw. (111911)
42 E2.tw. (68712)
43 oestrogen.tw. (16612)
44 or/18‐43 (485744)
45 Clinical Trial/ (845200)
46 Randomized Controlled Trial/ (372794)
47 exp randomization/ (66611)
48 Single Blind Procedure/ (20323)
49 Double Blind Procedure/ (120763)
50 Crossover Procedure/ (43072)
51 Placebo/ (257085)
52 Randomi?ed controlled trial$.tw. (117242)
53 Rct.tw. (17142)
54 random allocation.tw. (1418)
55 randomly allocated.tw. (22469)
56 allocated randomly.tw. (2023)
57 (allocated adj2 random).tw. (728)
58 Single blind$.tw. (15832)
59 Double blind$.tw. (150922)
60 ((treble or triple) adj blind$).tw. (452)
61 placebo$.tw. (214589)
62 prospective study/ (292450)
63 or/45‐62 (1465071)
64 case study/ (31878)
65 case report.tw. (282490)
66 abstract report/ or letter/ (925645)
67 or/64‐66 (1233732)
68 63 not 67 (1425818)
69 (exp animal/ or animal.hw. or nonhuman/) not (exp human/ or human cell/ or (human or humans).ti.) (5289150)
70 68 not 69 (1369914)
71 17 and 44 and 70 (1757)
Appendix 6. PsycINFO search strategy
From 1806 to 17 January 2017
Ovid platform
1 exp Infertility/ or exp Reproductive Technology/ (3107)
2 (IVF or ICSI).tw. (512)
3 (Reproducti$ adj2 Technolog$).tw. (980)
4 embryo transfer.tw. (104)
5 (in vitro fertilisation or in vitro fertilization).tw. (648)
6 intracytoplasmic sperm injection$.tw. (49)
7 or/1‐6 (3603)
8 exp Oral Contraceptives/ (844)
9 oral contracepti$.tw. (1413)
10 (OC or OCP$).tw. (1978)
11 estradiol.tw. (5475)
12 (progestogen$ or progestin$).tw. (741)
13 (progestagen$ or gestagen$).tw. (50)
14 (norethisterone or norethindrone).tw. (41)
15 (estrogen or oestrogen).tw. (6861)
16 (pretreatment$ or pre‐treatment$).tw. (16904)
17 or/8‐16 (30114)
18 7 and 17 (58)
19 random.tw. (48701)
20 control.tw. (377497)
21 double‐blind.tw. (20370)
22 clinical trials/ (10102)
23 placebo/ (4773)
24 exp Treatment/ (671953)
25 or/19‐24 (1038320)
26 18 and 25 (23)
Appendix 7. CINAHL Plus search strategy
From 1961 to 17 January 2017
Ebsco platform
| # | Query | Results |
| S40 | S27 AND S39 | 111 |
| S39 | S28 OR S29 OR S30 OR S31 OR S32 OR S33 OR S34 OR S35 OR S36 OR S37 OR S38 | 1,105,194 |
| S38 | TX allocat* random* | 5,945 |
| S37 | (MH "Quantitative Studies") | 15,237 |
| S36 | (MH "Placebos") | 9,967 |
| S35 | TX placebo* | 43,010 |
| S34 | TX random* allocat* | 5,945 |
| S33 | (MH "Random Assignment") | 42,264 |
| S32 | TX randomi* control* trial* | 117,316 |
| S31 | TX ( (singl* n1 blind*) or (singl* n1 mask*) ) or TX ( (doubl* n1 blind*) or (doubl* n1 mask*) ) or TX ( (tripl* n1 blind*) or (tripl* n1 mask*) ) or TX ( (trebl* n1 blind*) or (trebl* n1 mask*) ) | 870,796 |
| S30 | TX clinic* n1 trial* | 197,987 |
| S29 | PT Clinical trial | 79,975 |
| S28 | (MH "Clinical Trials+") | 208,852 |
| S27 | S18 AND S26 | 364 |
| S26 | S19 OR S20 OR S21 OR S22 OR S23 OR S24 OR S25 | 30,510 |
| S25 | TX Estradiol or TX oestrogen or TX estrogen or TX oestradiol | 17,624 |
| S24 | TX desogestral or TX desogestrel or TX Gestagen | 76 |
| S23 | TX Norgestrel or TX Norethindrone TX Norethisterone | 19 |
| S22 | TX progestagen* or TX progestin* or TX progestogen* | 1,722 |
| S21 | TX Pretreatment | 7,437 |
| S20 | TX oral contraceptive* | 6,329 |
| S19 | (MH "Contraceptives, Oral Combined") OR (MM "Contraceptives, Oral") | 3,014 |
| S18 | S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17 | 7,460 |
| S17 | TX (ovari* N2 induction) | 15 |
| S16 | TX COH | 119 |
| S15 | TX ovarian hyperstimulation | 403 |
| S14 | TX superovulat* | 24 |
| S13 | TX ovulation induc* | 660 |
| S12 | TX intrauterine insemination | 185 |
| S11 | TX IUI | 117 |
| S10 | TX assisted reproduct* | 1,678 |
| S9 | (MM "Reproduction Techniques+") | 4,518 |
| S8 | TX intracytoplasmic sperm injection* | 308 |
| S7 | TX embryo* N3 transfer* | 985 |
| S6 | TX ovar* N3 hyperstimulat* | 406 |
| S5 | TX ovari* N3 stimulat* | 335 |
| S4 | TX IVF or TX ICSI | 1,757 |
| S3 | (MM "Fertilization in Vitro") | 1,659 |
| S2 | TX vitro fertilization | 3,491 |
| S1 | TX vitro fertilisation | 3,491 |
Appendix 8. Virtual health library, clinicaltrials.gov and PubMed search strategies
Searched on 13 January 2017
Web platform
Clinicaltrials.gov
1. ivf and pretreatment (27 hits)
2. ivf and contraceptive* (53 hits)
3. ivf and estradiol (109 hits)
4. ivf and progestogen* (53 hits)
Virtual health library (LILACS)
1. ivf and pretreatment (1hit, limited to LILACS)
2. ivf and contraceptive* (4 hits, limited to LILACS and IBECS)
3. ivf and estradiol (4 hits, limited to LILACS and MEDCARIB)
4. ivf and progestogen* (35 hits no limit)
Pubmed
1. ivf and pretreatment (21 hits, limited from 01.01.14 to 11.06.15)
2. ivf and contraceptive* (23 hits, limited from 01.01.14 to 11.06.15)
3. ivf and estradiol (47 hits, limited from 01.12.14 to 11.06.15)
4. ivf and progestogen* (0 hits, limited from 01.01.14 to 11.06.15)
Appendix 9. Data extraction form (part 1)
| Assessment | |
| Assessor | SvO / BS |
| Date |
|
| Final conclusion |
| Inclusion |
| Exclusion |
| A. Study information | |
| 1. Title |
|
| 2. First author |
|
| 3. Year |
|
| 4. Published | Yes / No |
| 5. Journal |
|
| B. Criteria for eligibility | YES | NO | |
| Design | Described as randomised? | ||
| Patients | Women with subfertility, regardless of any cause, undergoing ART | ||
| Intervention | · OCP prior to gonadotrophins | ||
| B. Criteria for eligibility (continued) | YES | NO | |
| Comparison | · Placebo prior to gonadotrophins | ||
| Outcome | Primary: · number of live births Secondary: · no. of ongoing pregnancies Adverse: · no. of pregnancy loss | ||
| Remarks: |
| ||
| C. Characteristics | ||||||
| C1. Trial characteristics | ||||||
| Country of investigation |
| |||||
| Setting | Single Multicentre Unclear | |||||
|
| Academic Non‐academic Unclear | |||||
| Duration of trial | Y = M = D = | |||||
| Design | Parallel Crossover | |||||
| Number of participants | Intervention group: |
| ||||
| Remarks: |
| |||||
| C2. Participants characteristics | ||||||
|
| Intervention group | Comparison group | ||||
| Age | Mean: | Mean: |
| |||
| BMI | Mean: | Mean: | ||||
| Duration of subfertility | Mean: | Mean: | ||||
| No. of previous IVF trials | Mean: |
| Mean: |
| ||
| Subfertility | Primary: | N = | Primary: | N = | ||
| Causes of subfertility | Tubal: | N = | Tubal: | N = | ||
| Poor response | YES NO Defined as: * Mature ovarian follicles: * Other: | |||||
| C2. Flowchart of participants | ||||||
|
| ||||||
| Remarks: | ||||||
| C3. Protocol characteristics | ||||||
| Pretreatment | Combined OCP Oestrogen Progestogen Name of preparation: Dosage: Start: Stop: | |||||
| Ovarian stimulation | hMG rFSH Name of preparation: Dosage: Start: Stop: | |||||
| Pituitary desensitization | GnRH agonist GnRH antagonist Name of preparation: Dosage: Start: Stop: Protocol: | |||||
| Treatment schedule | ||||||
| C4. Follow‐up | |
| Duration of follow‐up |
|
| Analysis of loss to follow‐up | Per protocol Intention‐to‐treat |
| Remarks: | |
| D. Risk of bias assessment | ||||
|
|
| YES | NO | Unclear |
| Study size | Was a power calculation performed and adhered? | |||
| Selection bias | Was the allocation sequence adequately generated? | |||
| Was the patient allocation concealment adequate? | ||||
| Detection bias | Was the length of follow‐up long enough to detect stated outcomes? | |||
| Was the investigator (performer of hormone administration) blinded? | ||||
| Was the outcome assessor blinded? | ||||
| Were the participants blinded? | ||||
| Attrition bias | Was loss to follow‐up accounted for? | |||
| Was an intention‐to‐treat analysis performed? | ||||
| Reporting bias | Where there any suggestions of selective report of outcome? | |||
| Source of funding | Is the source of funding stated? | |||
| Remarks: |
| |||
Appendix 10. Data extraction form (part 2)
| D. Risk of bias assessment | ||||
|
|
| YES | NO | Unclear |
| Study size | Was a power calculation performed and adhered? | |||
| Selection bias | Was the allocation sequence adequately generated? | |||
| Was the patient allocation concealment adequate? | ||||
| Detection bias | Was the length of follow‐up long enough to detect stated outcomes? | |||
| Was the investigator (performer of hormone administration) blinded? | ||||
| Was the outcome assessor blinded? | ||||
| Were the participants blinded? | ||||
| Attrition bias | Was loss to follow‐up accounted for? | |||
| Was an intention‐to‐treat analysis performed? | ||||
| Reporting bias | Where there any suggestions of selective report of outcome? | |||
| Source of funding | Is the source of funding stated? | |||
| Remarks: |
| |||
| E. Outcomes | ||||||||
| Comparison | a. Define treatment: b. Define control:
| |||||||
| E1. Primary outcomes | ||||||||
| Live births Defined: YES NO |
| No. of live birth | No. of no live birth | Total | ||||
| Treatment group |
|
|
| |||||
| Control group |
|
|
| |||||
| Total |
|
|
| |||||
| Remarks: | ||||||||
| E2. Secondary outcomes |
| Ongoing pregnancy Defined: YES NO |
| No. of ongoing pregnancy | No. of no ongoing pregnancy | Total | ||||
| Treatment group |
|
|
| |||||
| Control group |
|
|
| |||||
| Total |
|
|
| |||||
| Remarks: | ||||||||
| Clinical pregnancy Defined: YES NO |
| No. of clinical pregnancy | No. of no clinical pregnancy | Total | ||||
| Treatment group |
|
|
| |||||
| Control group |
|
|
| |||||
| Total |
|
|
| |||||
| Remarks: | ||||||||
| Oocytes retrieved Defined: YES NO
|
| Mean no. of oocytes retrieved | SD | |||||
| Treatment group |
|
| ||||||
| Control group |
|
| ||||||
| Remarks: | ||||||||
| Days of gonadotrophins treatment Defined: YES NO |
| Mean no. of days of | SD | |||||
| Treatment group |
|
| ||||||
| Control group |
|
| ||||||
| Remarks: | ||||||||
| Total days of treatment Defined: YES NO
|
| Mean no. of days of pituitary suppression | SD | |||||
| Treatment group |
|
| ||||||
| Control group |
|
| ||||||
| Remarks: | ||||||||
| E3. Adverse outcomes |
| Pregnancy loss Defined: YES NO |
| No. of pregnancy loss | No. of no pregnancy loss | Total |
| Treatment group |
|
|
| |
| Control group |
|
|
| |
| Total |
|
|
| |
| Remarks: | ||||
| Ovarian cyst formation Defined: YES NO |
| No. of ovarian | No. of no ovarian | Total |
| Treatment group |
|
|
| |
| Control group |
|
|
| |
| Total |
|
|
| |
| Remarks: | ||||
| Multiple pregnancy Defined: YES NO |
| No. of multiple | No. of no multiple | Total |
| Treatment group |
|
|
| |
| Control group |
|
|
| |
| Total |
|
|
| |
| Remarks: | ||||
| Ovarian hyperstimulation Defined: YES NO |
| No. of OHS syndrome | No. of no OHS syndrome | Total |
| Treatment group |
|
|
| |
| Control group |
|
|
| |
| Total |
|
|
| |
| Remarks: | ||||
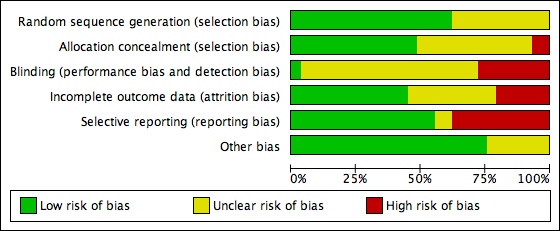
Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
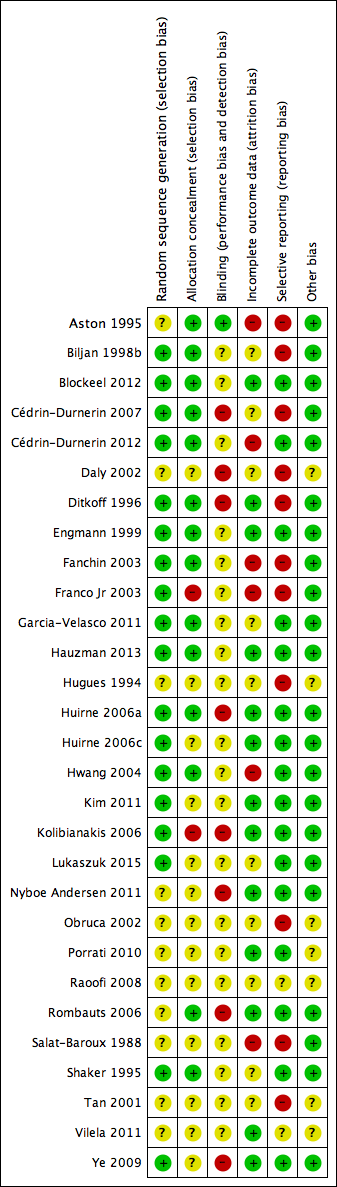
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.

Study PRISMA flow chart

Forest plot of comparison: 1 Combined oral contraceptive pill (OCP) versus no pretreatment (Rx), outcome: 1.1 Live birth or ongoing pregnancy.

Forest plot of comparison: 1 Combined oral contraceptive pill (COCP) versus no pretreatment (Rx), outcome: 1.2 Pregnancy loss.

Forest plot of comparison: 3 Oestrogen versus no pretreatment (Rx), outcome: 3.1 Live birth or ongoing pregnancy.
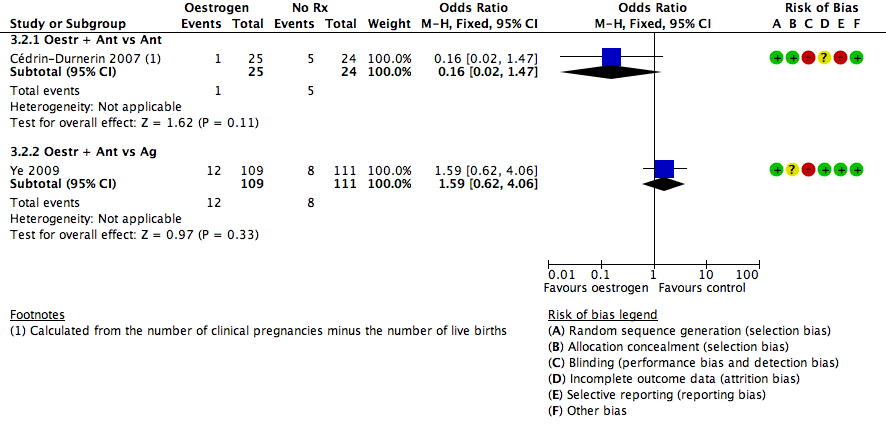
Forest plot of comparison: 3 Oestrogen versus no pretreatment (Rx), outcome: 3.2 Pregnancy loss.

Comparison 1 Combined oral contraceptive pill (COCP) versus no pretreatment (Rx), Outcome 1 Live birth or ongoing pregnancy.
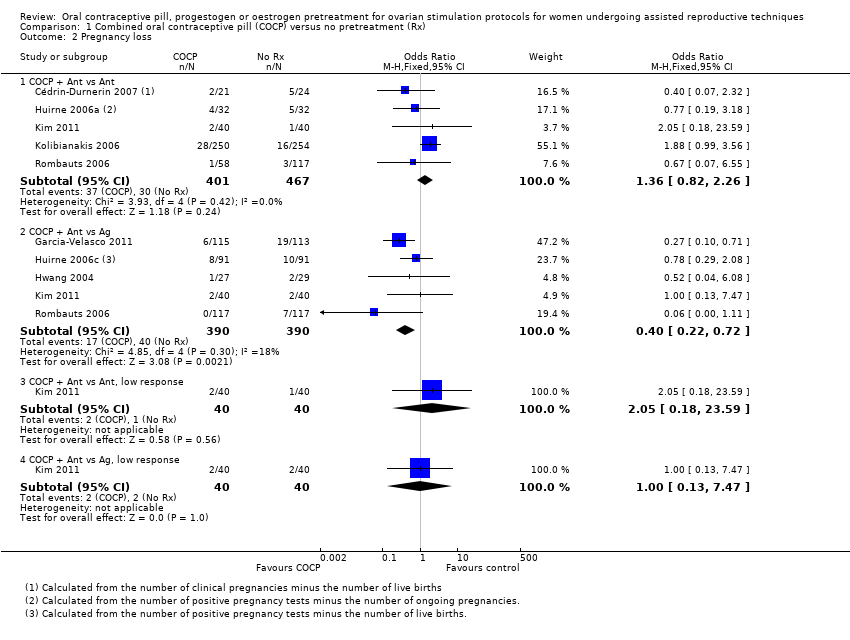
Comparison 1 Combined oral contraceptive pill (COCP) versus no pretreatment (Rx), Outcome 2 Pregnancy loss.

Comparison 1 Combined oral contraceptive pill (COCP) versus no pretreatment (Rx), Outcome 3 Clinical pregnancy rate.

Comparison 1 Combined oral contraceptive pill (COCP) versus no pretreatment (Rx), Outcome 4 Multiple pregnancy rate.
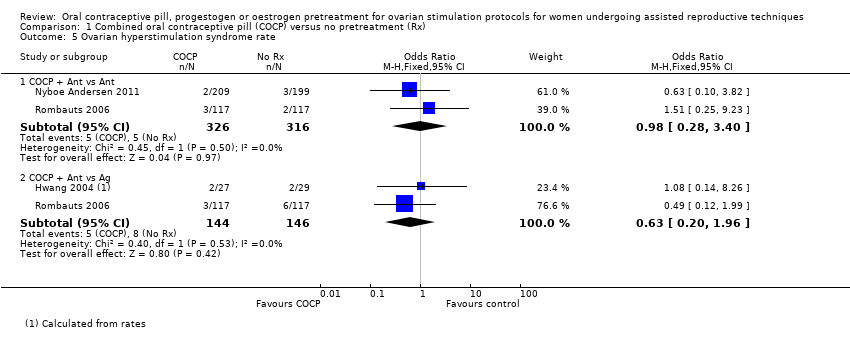
Comparison 1 Combined oral contraceptive pill (COCP) versus no pretreatment (Rx), Outcome 5 Ovarian hyperstimulation syndrome rate.

Comparison 1 Combined oral contraceptive pill (COCP) versus no pretreatment (Rx), Outcome 6 Number of oocytes retrieved.
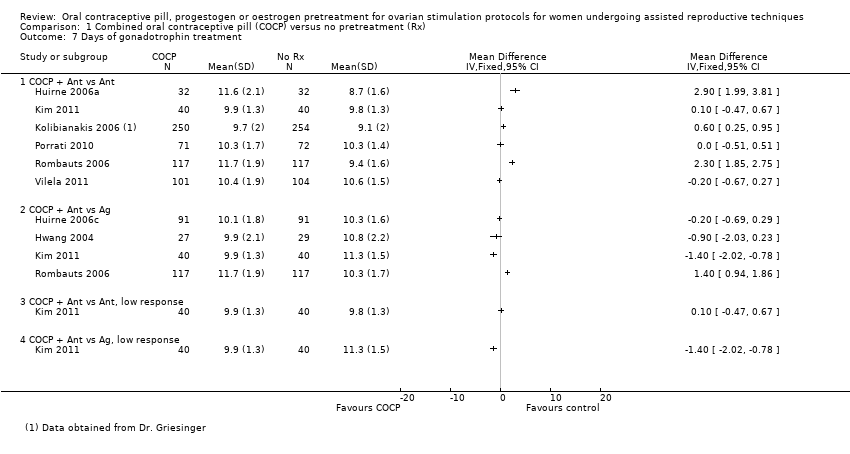
Comparison 1 Combined oral contraceptive pill (COCP) versus no pretreatment (Rx), Outcome 7 Days of gonadotrophin treatment.
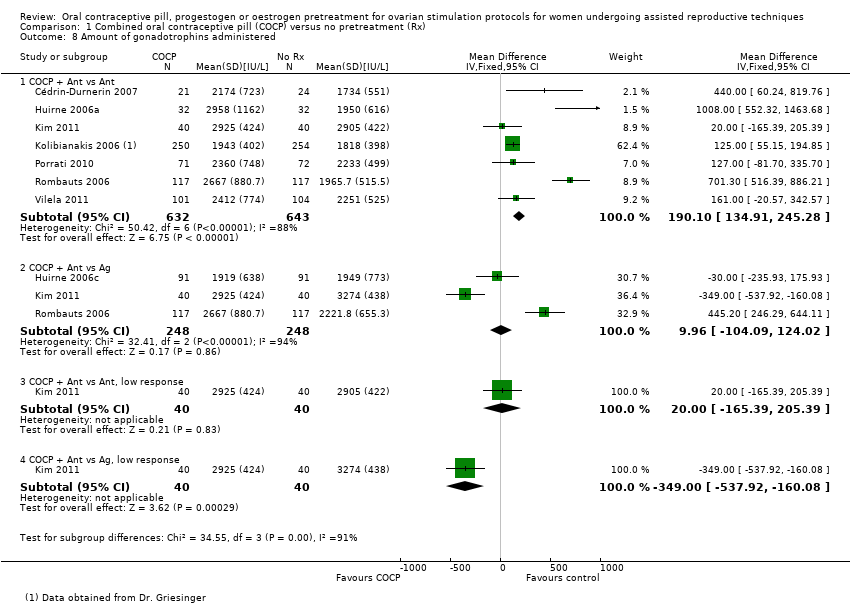
Comparison 1 Combined oral contraceptive pill (COCP) versus no pretreatment (Rx), Outcome 8 Amount of gonadotrophins administered.
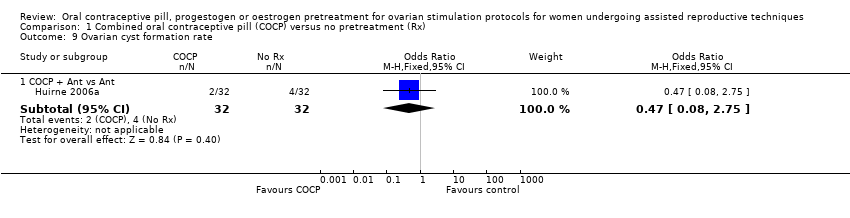
Comparison 1 Combined oral contraceptive pill (COCP) versus no pretreatment (Rx), Outcome 9 Ovarian cyst formation rate.
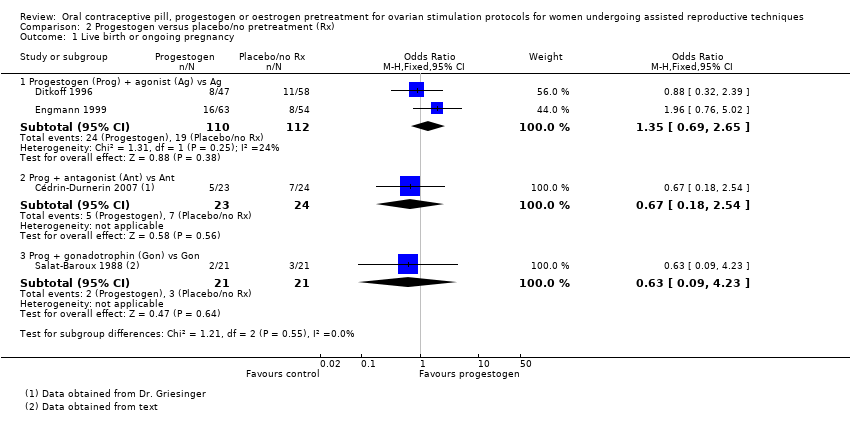
Comparison 2 Progestogen versus placebo/no pretreatment (Rx), Outcome 1 Live birth or ongoing pregnancy.

Comparison 2 Progestogen versus placebo/no pretreatment (Rx), Outcome 2 Pregnancy loss.

Comparison 2 Progestogen versus placebo/no pretreatment (Rx), Outcome 3 Clinical pregnancy rate.
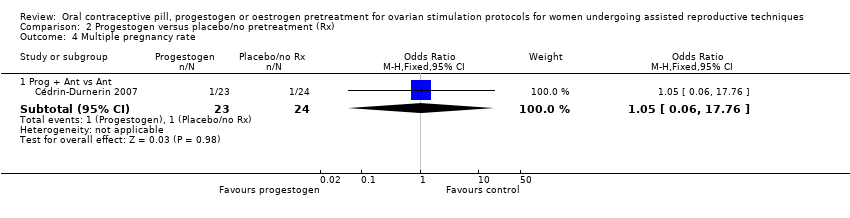
Comparison 2 Progestogen versus placebo/no pretreatment (Rx), Outcome 4 Multiple pregnancy rate.

Comparison 2 Progestogen versus placebo/no pretreatment (Rx), Outcome 5 Number of oocytes retrieved.

Comparison 2 Progestogen versus placebo/no pretreatment (Rx), Outcome 6 Days of gonadotrophin treatment.
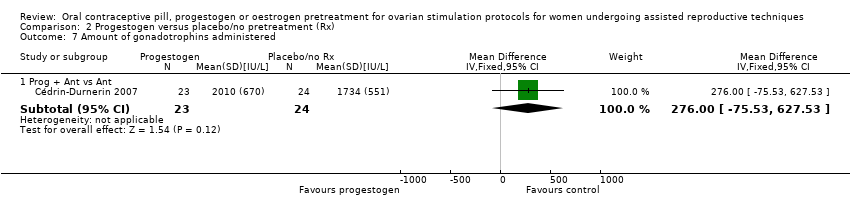
Comparison 2 Progestogen versus placebo/no pretreatment (Rx), Outcome 7 Amount of gonadotrophins administered.

Comparison 2 Progestogen versus placebo/no pretreatment (Rx), Outcome 8 Ovarian cyst formation rate.

Comparison 3 Oestrogen versus no pretreatment (Rx), Outcome 1 Live birth or ongoing pregnancy.
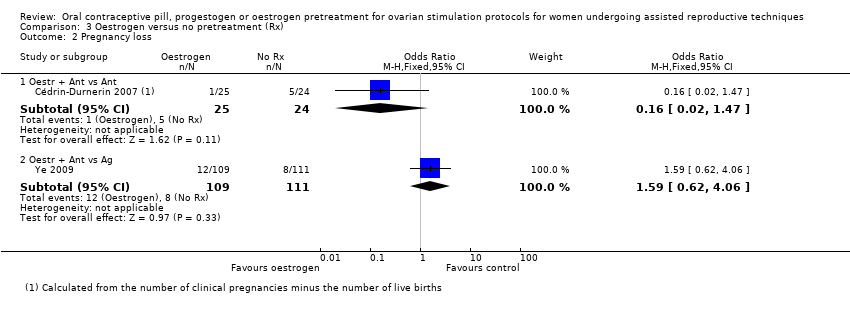
Comparison 3 Oestrogen versus no pretreatment (Rx), Outcome 2 Pregnancy loss.
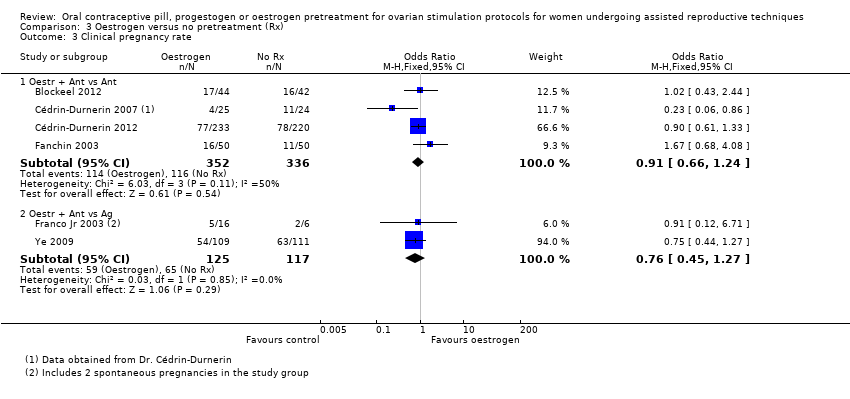
Comparison 3 Oestrogen versus no pretreatment (Rx), Outcome 3 Clinical pregnancy rate.
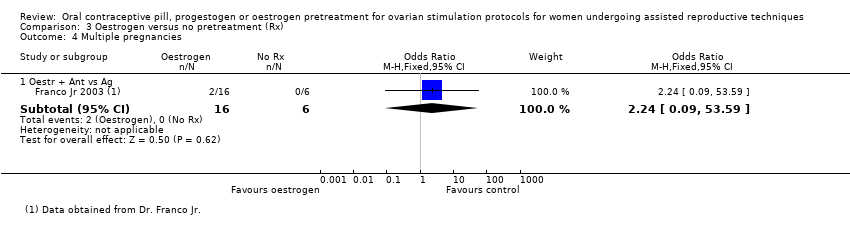
Comparison 3 Oestrogen versus no pretreatment (Rx), Outcome 4 Multiple pregnancies.

Comparison 3 Oestrogen versus no pretreatment (Rx), Outcome 5 Ovarian hyperstimulation syndrome rate.

Comparison 3 Oestrogen versus no pretreatment (Rx), Outcome 6 Number of oocytes retrieved.

Comparison 3 Oestrogen versus no pretreatment (Rx), Outcome 7 Days of gonadotrophin treatment.

Comparison 3 Oestrogen versus no pretreatment (Rx), Outcome 8 Amount of gonadotrophins administered.

Comparison 4 Combined oral contraceptive pill (COCP) versus progestogen, Outcome 1 Live birth or ongoing pregnancy.

Comparison 4 Combined oral contraceptive pill (COCP) versus progestogen, Outcome 2 Pregnancy loss.
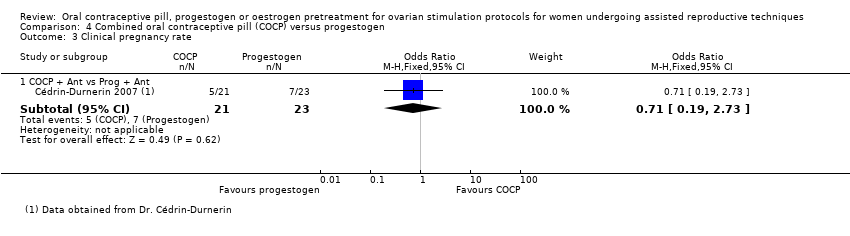
Comparison 4 Combined oral contraceptive pill (COCP) versus progestogen, Outcome 3 Clinical pregnancy rate.
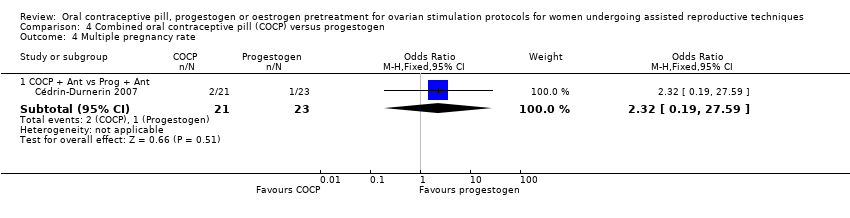
Comparison 4 Combined oral contraceptive pill (COCP) versus progestogen, Outcome 4 Multiple pregnancy rate.
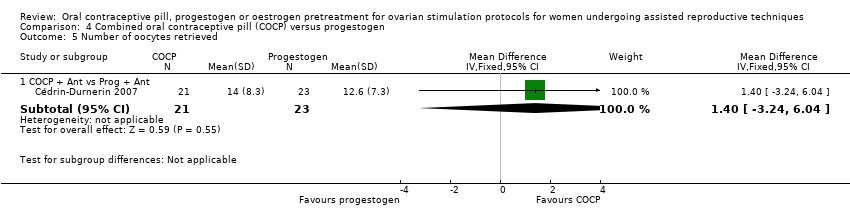
Comparison 4 Combined oral contraceptive pill (COCP) versus progestogen, Outcome 5 Number of oocytes retrieved.
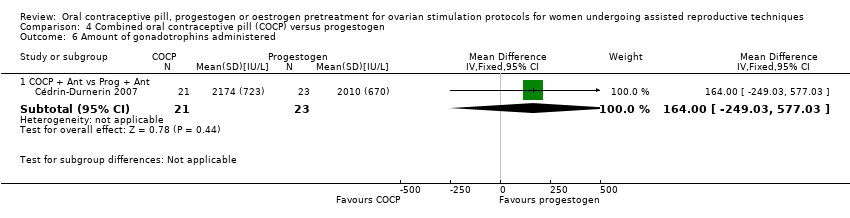
Comparison 4 Combined oral contraceptive pill (COCP) versus progestogen, Outcome 6 Amount of gonadotrophins administered.
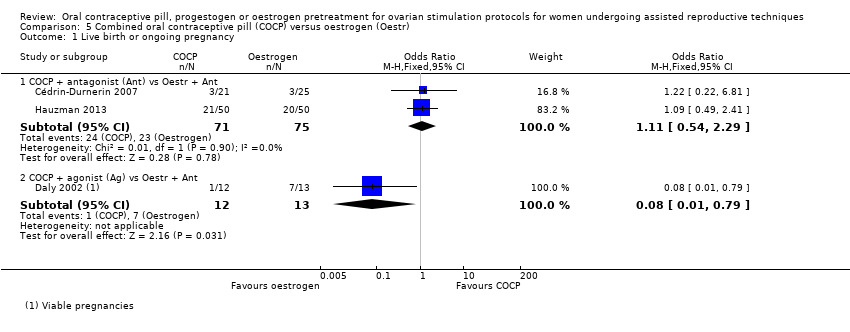
Comparison 5 Combined oral contraceptive pill (COCP) versus oestrogen (Oestr), Outcome 1 Live birth or ongoing pregnancy.
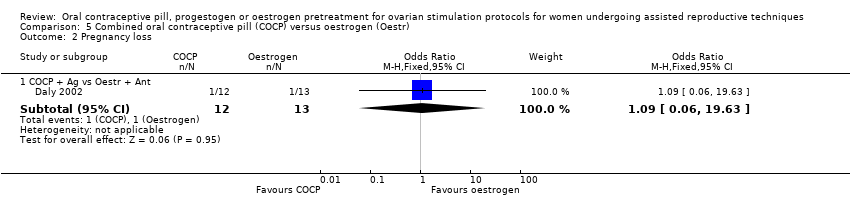
Comparison 5 Combined oral contraceptive pill (COCP) versus oestrogen (Oestr), Outcome 2 Pregnancy loss.
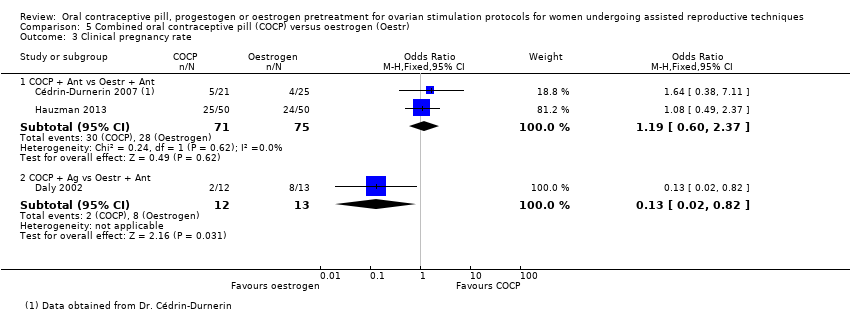
Comparison 5 Combined oral contraceptive pill (COCP) versus oestrogen (Oestr), Outcome 3 Clinical pregnancy rate.

Comparison 5 Combined oral contraceptive pill (COCP) versus oestrogen (Oestr), Outcome 4 Number of oocytes retrieved.

Comparison 5 Combined oral contraceptive pill (COCP) versus oestrogen (Oestr), Outcome 5 Days of gonadotropin treatment.
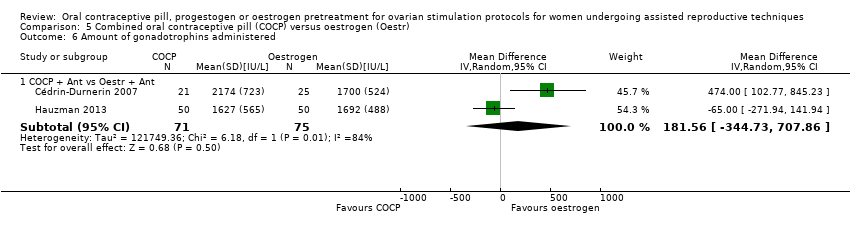
Comparison 5 Combined oral contraceptive pill (COCP) versus oestrogen (Oestr), Outcome 6 Amount of gonadotrophins administered.

Comparison 6 Progestogen (Prog) versus oestrogen (Oestr), Outcome 1 Live birth or ongoing pregnancy.

Comparison 6 Progestogen (Prog) versus oestrogen (Oestr), Outcome 2 Clinical pregnancy rate.

Comparison 6 Progestogen (Prog) versus oestrogen (Oestr), Outcome 3 Number of oocytes retrieved.
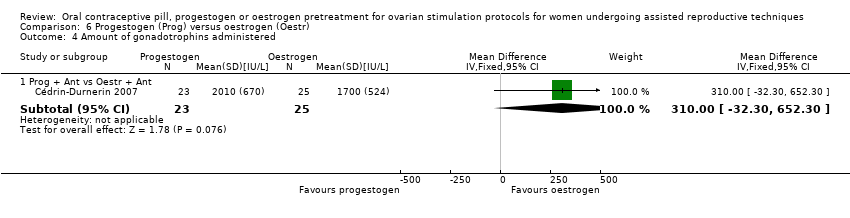
Comparison 6 Progestogen (Prog) versus oestrogen (Oestr), Outcome 4 Amount of gonadotrophins administered.
| Combined oral contraceptive pill compared to no pretreatment for ovarian stimulation protocols for women undergoing assisted reproductive techniques | ||||||
| Population: women undergoing ART Settings: ART clinic Intervention: COCP Comparison: no pretreatment | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk1 | Corresponding risk | |||||
| No pretreatment | COCP | |||||
| Live birth or ongoing pregnancy (COCP + Ant vs Ant) | 270 per 1000 | 215 per 1000 | OR 0.74 | 1335 | ⊕⊕⊕⊝ | ‐ |
| Live birth or ongoing pregnancy (COCP + Ant vs Ag) | 296 per 1000 | 273 per 1000 | OR 0.89 | 724 | ⊕⊕⊕⊝ | ‐ |
| Pregnancy loss (COCP + Ant vs Ant) | 64 per 1000 | 85 per 1000 | OR 1.36 | 868 | ⊕⊕⊕⊝ | ‐ |
| Pregnancy loss (COCP + Ant vs Ag) | 103 per 1000 | 44 per 1000 | OR 0.40 | 780 | ⊕⊕⊕⊝ | ‐ |
| Multiple pregnancy rate (COCP + Ant vs Ant) | 47 per 1000 | 98 per 1000 | OR 2.21 | 125 | ⊕⊕⊝⊝ | ‐ |
| Multiple pregnancy rate (COCP + Ant vs Ag) | 147 per 1000 | 189 per 1000 | OR 1.36 | 546 | ⊕⊕⊕⊝ | ‐ |
| OHSS rate (COCP + Ant vs Ant) | 16 per 1000 | 16 per 1000 (4 to 52) | OR 0.98 (0.28 to 3.40) | 642 (2 studies) | ⊕⊕⊝⊝ | ‐ |
| OHSS rate (COCP + Ant vs Ag) | 55 per 1000 | 35 per 1000 (11 to 102) | OR 0.63 (0.20 to 1.96) | 290 (2 studies) | ⊕⊕⊝⊝ | ‐ |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Mean baseline risk of control group. | ||||||
| Progestogen compared to placebo or no pretreatment for ovarian stimulation protocols for women undergoing assisted reproductive techniques | ||||||
| Patient or population: ovarian stimulation protocols for women undergoing ART Settings: Intervention: progestogen Comparison: placebo or no pretreatment | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk1 | Corresponding risk | |||||
| Placebo or no pretreatment | Prog | |||||
| Live birth or ongoing pregnancy (Prog + Ag vs Ag) | 170 per 1000 | 217 per 1000 | OR 1.35 | 222 | ⊕⊕⊝⊝ | ‐ |
| Live birth or ongoing pregnancy (Prog + Ant vs Ant) | 292 per 1000 | 217 per 1000 | OR 0.67 | 47 | ⊕⊕⊝⊝ | ‐ |
| Pregnancy loss (Prog + Ag vs Ag) | 36 per 1000 | 78 per 1000 | OR 2.26 | 222 | ⊕⊕⊝⊝ | ‐ |
| Pregnancy loss (Prog + Ant vs Ant) | 208 per 1000 | 86 per 1000 | OR 0.36 | 47 | ⊕⊕⊝⊝ | ‐ |
| Multiple pregnancy rate (Prog + Ag vs Ag) | No data available | ‐ | ‐ | |||
| Multiple pregnancy rate (Prog + Ant vs Ant) | 42 per 1000 | 44 per 1000 | OR 1.05 | 47 | ⊕⊕⊝⊝ | ‐ |
| OHSS rate (Prog + Ag vs Ag) | No data available | ‐ | ‐ | |||
| OHSS rate (Prog + Ant vs Ant) | No data available | ‐ | ‐ | |||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Mean baseline risk of control group. | ||||||
| Oestrogencompared to no pretreatment for ovarian stimulation protocols for women undergoing assisted reproductive techniques | ||||||
| Patient or population: ovarian stimulation protocols for women undergoing ART Settings: Intervention: oestrogen Comparison: no pretreatment | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk1 | Corresponding risk | |||||
| No pretreatment | Oestr | |||||
| Live birth or ongoing pregnancy (Oestr + Ant vs Ant) | 299 per 1000 | 252 per 1000 | OR 0.79 | 502 | ⊕⊕⊕⊝ | ‐ |
| Live birth or ongoing pregnancy (Oestr + Ant vs Ag) | 350 per 1000 | 322 per 1000 | OR 0.88 | 242 | ⊕⊝⊝⊝ | ‐ |
| Pregnancy loss (Oestr + Ant vs Ant) | 208 per 1000 | 40 per 1000 | OR 0.16 | 49 | ⊕⊝⊝⊝ | ‐ |
| Pregnancy loss (Oestr + Ant vs Ag) | 72 per 1000 | 110 per 1000 | OR 1.59 | 220 | ⊕⊝⊝⊝ | ‐ |
| Multiple pregnancy rate (Oestr + Ant vs Ant) | No data available | ‐ | ‐ | |||
| Multiple pregnancy rate (Oestr + Ant vs Ag) | Not calculable ‐ see comment | OR 2.24 | 22 | ⊕⊝⊝⊝ | Only 2 events (both in oestrogen group) | |
| OHSS rate (Oestr + Ant vs Ant) | No data available | ‐ | ‐ | |||
| OHSS rate (Oestr + Ant vs Ag) | 18 per 1000 | 27 per 1000 (5 to 147) | OR 1.54 (0.25 to 9.42) | 220 (1 study) | ⊕⊝⊝⊝ | ‐ |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Mean baseline risk of control group. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Live birth or ongoing pregnancy Show forest plot | 8 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 COCP + antagonist (Ant) vs Ant | 6 | 1335 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.58, 0.95] |
| 1.2 COCP + Ant vs agonist (Ag) | 4 | 724 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.64, 1.25] |
| 1.3 COCP + Ant vs Ant, low response | 1 | 80 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.71 [0.61, 4.79] |
| 1.4 COCP + Ant vs Ag, low response | 1 | 80 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.43, 2.98] |
| 2 Pregnancy loss Show forest plot | 8 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 COCP + Ant vs Ant | 5 | 868 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.36 [0.82, 2.26] |
| 2.2 COCP + Ant vs Ag | 5 | 780 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.40 [0.22, 0.72] |
| 2.3 COCP + Ant vs Ant, low response | 1 | 80 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.05 [0.18, 23.59] |
| 2.4 COCP + Ant vs Ag, low response | 1 | 80 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.13, 7.47] |
| 3 Clinical pregnancy rate Show forest plot | 8 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 COCP + Ant vs Ant | 5 | 740 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.63, 1.15] |
| 3.2 COCP + Ant vs Ag | 4 | 546 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.59, 1.20] |
| 3.3 COCP + Ant vs Ant, low response | 1 | 80 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.85 [0.69, 4.97] |
| 3.4 COCP + Ant vs Ag, low response | 1 | 80 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.44, 2.83] |
| 4 Multiple pregnancy rate Show forest plot | 5 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 COCP + Ant vs Ant | 2 | 125 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.21 [0.53, 9.26] |
| 4.2 COCP + Ant vs Ag | 4 | 546 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.36 [0.85, 2.19] |
| 4.3 COCP + Ant vs Ant, low response | 1 | 80 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.11 [0.36, 12.24] |
| 4.4 COCP + Ant vs Ag, low response | 1 | 80 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.37 [0.29, 6.56] |
| 5 Ovarian hyperstimulation syndrome rate Show forest plot | 3 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 COCP + Ant vs Ant | 2 | 642 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.28, 3.40] |
| 5.2 COCP + Ant vs Ag | 2 | 290 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.20, 1.96] |
| 6 Number of oocytes retrieved Show forest plot | 8 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 7 Days of gonadotrophin treatment Show forest plot | 8 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7.1 COCP + Ant vs Ant | 6 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.2 COCP + Ant vs Ag | 4 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.3 COCP + Ant vs Ant, low response | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.4 COCP + Ant vs Ag, low response | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 Amount of gonadotrophins administered Show forest plot | 8 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 8.1 COCP + Ant vs Ant | 7 | 1275 | Mean Difference (IV, Fixed, 95% CI) | 190.10 [134.91, 245.28] |
| 8.2 COCP + Ant vs Ag | 3 | 496 | Mean Difference (IV, Fixed, 95% CI) | 9.96 [‐104.09, 124.02] |
| 8.3 COCP + Ant vs Ant, low response | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | 20.0 [‐165.39, 205.39] |
| 8.4 COCP + Ant vs Ag, low response | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | ‐349.0 [‐537.92, ‐160.08] |
| 9 Ovarian cyst formation rate Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 9.1 COCP + Ant vs Ant | 1 | 64 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.08, 2.75] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Live birth or ongoing pregnancy Show forest plot | 4 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Progestogen (Prog) + agonist (Ag) vs Ag | 2 | 222 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.35 [0.69, 2.65] |
| 1.2 Prog + antagonist (Ant) vs Ant | 1 | 47 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.18, 2.54] |
| 1.3 Prog + gonadotrophin (Gon) vs Gon | 1 | 42 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.09, 4.23] |
| 2 Pregnancy loss Show forest plot | 4 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Prog + Ag vs Ag | 2 | 222 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.26 [0.67, 7.55] |
| 2.2 Prog + Ant vs Ant | 1 | 47 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.06, 2.09] |
| 2.3 Prog + Gon vs Gon | 1 | 42 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.06, 17.12] |
| 3 Clinical pregnancy rate Show forest plot | 5 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Prog + Ag vs Ag | 3 | 374 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.99 [1.20, 3.28] |
| 3.2 Prog + Ant vs Ant | 1 | 47 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.16, 1.71] |
| 3.3 Prog + Gon vs Gon | 1 | 42 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.14, 3.64] |
| 4 Multiple pregnancy rate Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Prog + Ant vs Ant | 1 | 47 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.06, 17.76] |
| 5 Number of oocytes retrieved Show forest plot | 4 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5.1 Prog + Ag vs Ag | 2 | 222 | Mean Difference (IV, Fixed, 95% CI) | ‐0.52 [‐2.07, 1.02] |
| 5.2 Prog + Ant vs Ant | 1 | 47 | Mean Difference (IV, Fixed, 95% CI) | 2.70 [‐0.98, 6.38] |
| 5.3 Prog + Gon vs Gon | 1 | 29 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.57, 0.57] |
| 6 Days of gonadotrophin treatment Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6.1 Prog + Ag vs Ag | 2 | 222 | Mean Difference (IV, Fixed, 95% CI) | 0.11 [‐0.30, 0.52] |
| 7 Amount of gonadotrophins administered Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 7.1 Prog + Ant vs Ant | 1 | 47 | Mean Difference (IV, Fixed, 95% CI) | 276.0 [‐75.53, 627.53] |
| 8 Ovarian cyst formation rate Show forest plot | 3 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.1 Prog + Ag vs Ag | 3 | 374 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.16 [0.08, 0.32] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Live birth or ongoing pregnancy Show forest plot | 4 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Oestrogen (Oestr) + antagonist (Ant) vs Ant | 2 | 502 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.53, 1.17] |
| 1.2 Oestr + Ant vs agonist (Ag) | 2 | 242 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.51, 1.50] |
| 2 Pregnancy loss Show forest plot | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Oestr + Ant vs Ant | 1 | 49 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.16 [0.02, 1.47] |
| 2.2 Oestr + Ant vs Ag | 1 | 220 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.59 [0.62, 4.06] |
| 3 Clinical pregnancy rate Show forest plot | 6 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Oestr + Ant vs Ant | 4 | 688 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.66, 1.24] |
| 3.2 Oestr + Ant vs Ag | 2 | 242 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.45, 1.27] |
| 4 Multiple pregnancies Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Oestr + Ant vs Ag | 1 | 22 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.24 [0.09, 53.59] |
| 5 Ovarian hyperstimulation syndrome rate Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Oestr + Ant vs Ag | 1 | 220 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.54 [0.25, 9.42] |
| 6 Number of oocytes retrieved Show forest plot | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6.1 Oestr + Ant vs Ant | 2 | 139 | Mean Difference (IV, Fixed, 95% CI) | 2.23 [0.71, 3.75] |
| 6.2 Oestr + Ant vs Ag | 1 | 22 | Mean Difference (IV, Fixed, 95% CI) | 0.40 [‐4.47, 5.27] |
| 7 Days of gonadotrophin treatment Show forest plot | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 7.1 Oestr + Ant vs Ant | 2 | 529 | Mean Difference (IV, Fixed, 95% CI) | 0.83 [0.58, 1.08] |
| 7.2 Oestr + Ant vs Ag | 1 | 22 | Mean Difference (IV, Fixed, 95% CI) | ‐2.5 [‐4.07, ‐0.93] |
| 8 Amount of gonadotrophins administered Show forest plot | 5 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 8.1 Oestr + Ant vs Ant | 4 | 668 | Mean Difference (IV, Fixed, 95% CI) | 168.35 [111.53, 225.17] |
| 8.2 Oestr + Ant vs Ag | 1 | 22 | Mean Difference (IV, Fixed, 95% CI) | ‐16.0 [‐470.12, 438.12] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Live birth or ongoing pregnancy Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 COCP + antagonist (Ant) vs progestogen (Prog) + Ant | 1 | 44 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.6 [0.12, 2.89] |
| 2 Pregnancy loss Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 COCP + Ant vs Prog + Ant | 1 | 44 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.14, 8.64] |
| 3 Clinical pregnancy rate Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 COCP + Ant vs Prog + Ant | 1 | 44 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.19, 2.73] |
| 4 Multiple pregnancy rate Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 COCP + Ant vs Prog + Ant | 1 | 44 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.32 [0.19, 27.59] |
| 5 Number of oocytes retrieved Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5.1 COCP + Ant vs Prog + Ant | 1 | 44 | Mean Difference (IV, Fixed, 95% CI) | 1.40 [‐3.24, 6.04] |
| 6 Amount of gonadotrophins administered Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6.1 COCP + Ant vs Prog + Ant | 1 | 44 | Mean Difference (IV, Fixed, 95% CI) | 164.0 [‐249.03, 577.03] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Live birth or ongoing pregnancy Show forest plot | 3 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 COCP + antagonist (Ant) vs Oestr + Ant | 2 | 146 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.54, 2.29] |
| 1.2 COCP + agonist (Ag) vs Oestr + Ant | 1 | 25 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.08 [0.01, 0.79] |
| 2 Pregnancy loss Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 COCP + Ag vs Oestr + Ant | 1 | 25 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.06, 19.63] |
| 3 Clinical pregnancy rate Show forest plot | 3 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 COCP + Ant vs Oestr + Ant | 2 | 146 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.60, 2.37] |
| 3.2 COCP + Ag vs Oestr + Ant | 1 | 25 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.13 [0.02, 0.82] |
| 4 Number of oocytes retrieved Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.1 COCP + Ant vs Oestr + Ant | 1 | 46 | Mean Difference (IV, Fixed, 95% CI) | 0.90 [‐3.59, 5.39] |
| 5 Days of gonadotropin treatment Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5.1 COCP + Ant vs Oestr + Ant | 1 | 100 | Mean Difference (IV, Fixed, 95% CI) | ‐0.60 [‐1.23, 0.03] |
| 6 Amount of gonadotrophins administered Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 6.1 COCP + Ant vs Oestr + Ant | 2 | 146 | Mean Difference (IV, Random, 95% CI) | 181.56 [‐344.73, 707.86] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Live birth or ongoing pregnancy Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Prog + antagonist (Ant) vs Oestr + Ant | 1 | 48 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.04 [0.43, 9.70] |
| 2 Clinical pregnancy rate Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Prog + Ant vs Oestr + Ant | 1 | 48 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.30 [0.57, 9.22] |
| 3 Number of oocytes retrieved Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 Prog + Ant vs Oestr + Ant | 1 | 48 | Mean Difference (IV, Fixed, 95% CI) | ‐0.5 [‐4.55, 3.55] |
| 4 Amount of gonadotrophins administered Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.1 Prog + Ant vs Oestr + Ant | 1 | 48 | Mean Difference (IV, Fixed, 95% CI) | 310.0 [‐32.30, 652.30] |

