Medicamentos de venta libre para reducir la tos como complemento de los antibióticos para la neumonía aguda en niños y adultos
Referencias
Referencias de los estudios incluidos en esta revisión
Referencias de los estudios excluidos de esta revisión
Referencias adicionales
Referencias de otras versiones publicadas de esta revisión
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | Single‐centre double blind parallel placebo controlled RCT. Method of recruitment was not specified. Compliance not mentioned. Inclusion and exclusion criteria described in next column. Description of withdrawals or dropouts not mentioned. Assessment of quality | |
| Participants | 14 subjects allocated to neltenexine, 14 to placebo. Three within group had pneumonia but data specific to pneumonia was unavailable. Mean age of total group was 57.5 years (SD 3.04). | |
| Interventions | Neltenexine (a mucolytic), 37.4 mg tds or placebo (one tablet tds) for 10 to 12 days. | |
| Outcomes | Overall physicians' assessment of efficacy scored; excellent, good, moderate, not satisfactory. Exact quantification unspecified. | |
| Notes | Wrote to authors with no response. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Single‐centre double blind placebo controlled RCT. Subjects recruited from inpatients in the geriatric unit of Barnet General Hospital, England. The method of randomisation and allocation was not described. When the medication (active or placebo) was considered ineffective, the pharmacist was asked to change to alternate treatment. Data card and observation record prepared for each subject, other medications recorded and authors indicated that these factors were taken into account when assessing response to trial drugs (but did not specify how). Inclusion and exclusion criteria not described. Description of withdrawals or dropouts not mentioned. Assessment of quality | |
| Participants | Total randomised unknown. Total described in group unclear as some subjects could have been counted twice given potential crossover methodology. If assumed crossover was undertaken for all, total randomised would be 34. Age of subjects not given. Subjects had variety of aetiological factors for cough (pneumonia, acute and chronic bronchitis, bronchiectasis, carcinoma, cardiac failure, cor pulmonale, nervous cough, coryza, influenza). Inclusion and exclusion criteria not described. | |
| Interventions | Dimyril (active ingredient = isoaminile citrate, a codeine derivative) or placebo in identical bottles. Dose used varied. Initially 3 to 4 times/day followed by 'as necessary' dosing of up to 5 times a day (1 to 2 g). | |
| Outcomes | Outcomes not clearly specified. Paper stated: | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Multi‐centre double blind parallel placebo controlled RCT. Children recruited from 3 hospitals in Italy. Potential subjects admitted into hospital for symptoms of pneumonia screened for inclusion criteria (next column). Double blinded study and all subjects were treated as inpatients and re‐evaluated daily for heart rate, respiratory rate, maximal rectal body temperature. Cough, dyspnoea and chest pathological scores also recorded daily. CXR on admission and end of treatment. Compliance not mentioned but presumed excellent given inpatient study. All children given antibiotics (see column on intervention). Other co‐treatment (e.g. anti‐pyretic agents) not mentioned. Inclusion and exclusion criteria described in next column. Description of withdrawals or dropouts not mentioned. As children were inpatients, assumed most followed up. CXR follow‐up rate 115/120 = 95.8%. Assessment of quality | |
| Participants | Total of 120 children randomised ‐ 60 in each arm. Outcome measure available for 115 children (57 active arm, 58 controls), 95.8%. Antibiotic with ambroxol group: mean age not given, 11 aged < 1 year, 9 aged 1 to 2 years, 19 aged 2 to 5 years, 21 aged 5 to 12 years. Gender ‐ M: 28; F: 32. Mean body weight 17.1 kg (SD 1.08). Inclusion criteria: Children admitted into hospital for pneumonia. Have had blood culture performed before commencement of antibiotics and positive for well defined bacterium or a CXR showing lobar and sub lobar involvement, with ESR ≥ 30 mm/h and C‐reactive protein ≥ 25 μg/mL. Exclusion criteria: Taken antibiotics, mucolytics or mucoregulatory drugs in the preceding week. | |
| Interventions | Trial medications consisted of ambroxol (1.5 to 2 mg/kg/day in two divided doses) or placebo for 10 days. All children also given antibiotics, chosen on basis of microbiological data or in accordance with literature on most probable aetiology for each age, for 7 to 10 days. Children aged < 5 years given oral amoxil or intramuscular ampicillin (50 mg/kg in 3 to 4 divided doses). Older children had oral erythromycin ethylsuccinate (50 mg/kg/day in 4 doses). | |
| Outcomes | Cough, dyspnoea and chest pathological signs scored, ranging from 0 (absent) to 3 (very severe). CXR findings at the end of treatment was compared to pre‐treatment CXR and expressed as normalised, improved or unchanged. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Multi‐centre double blind parallel RCT comparing amoxicillin plus bromhexine versus amoxycillin alone. Subjects recruited from 22 centres involving internalists or pulmonologists in the Philippines. Potential subjects evaluated for inclusion criteria by history, examination, CXR, laboratory tests (blood counts, sputum). The method of randomisation and allocation was not described. Double blinded study and all subjects were treated as outpatients and re‐evaluated on days 3, 5, 7 and 10. Compliance monitored by pill counting. Subjects allowed to receive medications for fever and constitutional symptoms but not any other cough expectorants or antimicrobials. Inclusion and exclusion criteria described in next column. Description of withdrawals or dropouts mentioned for entire group. Maximum follow‐up rate 375/407 = 92% but less for other aspects. Assessment of quality | |
| Participants | Total of 407 subjects randomised ‐ 201 in active Rx and 206 in control group. 392 completed study (192 active, 200 controls). Compliance of 80% in active group and 85% in control group. Amoxil with bromhexine group: Mean age 32 (SD 13) years, gender ‐ 117 M: 75 F; 51 with pneumonia, 141 with bronchitis. Inclusion criteria: Adolescents and adults aged 15 to 60 years with uncomplicated community acquired lower respiratory tract infection (pneumonia or bronchitis), clinically assessed to be bacterial in aetiology. Pneumonia defined as presence of cough < 2 weeks, purulent phlegm, fever and/or leucocytosis (> 10,000 mm3), and pulmonary infiltrates on CXR. Acute bronchitis defined as presence of cough < 2 weeks, purulent phlegm, fever and/or leucocytosis (> 10,000 mm3). Sputum culture had to be sensitive to amoxil or if organism resistant, subject included if clinical response at Day 3 occurred on amoxil. Exclusion criteria: Frank respiratory failure, coexistent chronic disease (diabetes, renal failure, liver or renal impairment, terminal illness such as cancer, active tuberculosis, healed tuberculosis with bronchiectasis, chronic bronchitis or emphysema, heavy smokers (undefined)), pregnant or lactating, hypersensitivity to study drugs, or recent (< 2 weeks) treatment with antibiotics. | |
| Interventions | Active Rx = amoxil 240 mg and bromhexine 8 mg, both 4 times/day for 7 days. Control group: amoxil alone, 250 mg 4 times/day for 7 days. | |
| Outcomes | Days 3, 5, 7 and 10. Subjects evaluated for clinical response, bacteriological response, subjective symptom scores, adverse events, compliance, complete blood count. Clinical response: Clinical symptoms: Bacteriologic response: | |
| Notes | Wrote to authors with no response. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
tds: three times a day
CXR: chest X‐ray
Rx: treatment
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| Non‐placebo trial. Study involves comparing levodropropizine, codeine and cloperastine to levocloperastine | |
| Erdosteine is not legally available as an over‐the‐counter medication in countries such as Australia, UK and USA. Study compared amoxil plus erdosteine to amoxil‐placebo in children with acute lower respiratory tract infections | |
| Non‐placebo study comparing nimesulide to lysine‐aspirin in children | |
| Non‐controlled study in 40 adults using anti‐phlogistic‐balsamic compound (in Italian) | |
| Non‐placebo study comparing neltenexine against N‐acetylcysteine | |
| Randomised controlled study but subjects did not have pneumonia (in Italian) | |
| A double blind study in adults with acute and chronic bronchitis (not pneumonia) | |
| Non‐placebo study comparing drops to syrup formulation of an antitussive in infants and young children (in German) | |
| Study examined role of bromhexine in prevention of post‐operative pneumonia | |
| A review article describing three studies on dimemorfan, a dextromethorphan analogue. Of the three cited studies, one was a placebo‐controlled trial. Insufficient details were included in the text and further data were not available from the author, who was unable to be contacted | |
| Non‐placebo study comparing two cough formulations | |
| The paper summarises 3 studies which were not referenced. The first of the 3 studies described a RCT in children with "acute lower respiratory affections (e.g. acute bronchitis, bronchoalveolitis)". Unknown if children with pneumonia included and results stated reduction in cough scores with no specific data given. We wrote to authors and no response was received The other two studies described were in adults with '"superinfected chronic bronchitis" and "hypersecretory chronic obstructive bronchopneumopathies". | |
| Non‐randomised, non‐placebo study in 26 adults (in Italian) | |
| Erdosteine is not legally available as an over‐the‐counter medication in countries such as Australia, UK and USA. Multi‐centre RCT compared ampicillin plus erdosteine to ampicillin‐placebo in children with acute lower respiratory tract infections | |
| Non‐randomised, non‐placebo study using fenspiride in 20 adults (in Italian) | |
| Study used Fuxiong plaster (i.e. not an OTC). Randomised controlled study in children with pneumonia | |
| Placebo but non‐randomised study comparing placebo to prenodiazine in 84 adults (in German) | |
| Study used Toubiao Qingfei (an externally applied therapy, i.e. not an OTC). Randomised controlled study in children with fever from pneumonia | |
| Non‐placebo, double blind study comparing Sinecod‐Hommel to a codeine based antitussive in 95 adults (in German) |
OTC: Over‐the‐counter
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Not cured or not improved Show forest plot | 1 | 120 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.40 [0.10, 1.62] |
| Analysis 1.1  Comparison 1 Children ‐ global assessment, Outcome 1 Not cured or not improved. | ||||
| 2 Not improved Show forest plot | 1 | 120 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.40 [0.10, 1.62] |
| Analysis 1.2  Comparison 1 Children ‐ global assessment, Outcome 2 Not improved. | ||||
| 3 Not cured Show forest plot | 1 | 120 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.16, 0.77] |
| Analysis 1.3  Comparison 1 Children ‐ global assessment, Outcome 3 Not cured. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mean cough score at day 3 Show forest plot | 1 | 120 | Mean Difference (IV, Fixed, 95% CI) | ‐0.25 [‐0.33, ‐0.17] |
| Analysis 2.1  Comparison 2 Children ‐ cough score, Outcome 1 Mean cough score at day 3. | ||||
| 2 Mean score at day 10 Show forest plot | 1 | 120 | Mean Difference (IV, Fixed, 95% CI) | ‐0.15 [‐0.17, ‐0.13] |
| Analysis 2.2  Comparison 2 Children ‐ cough score, Outcome 2 Mean score at day 10. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Not cured or not improved Show forest plot | 1 | 101 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.48, 3.04] |
| Analysis 3.1  Comparison 3 Adults ‐ global assessment, Outcome 1 Not cured or not improved. | ||||
| 2 Not improved Show forest plot | 1 | 101 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.48, 3.04] |
| Analysis 3.2  Comparison 3 Adults ‐ global assessment, Outcome 2 Not improved. | ||||
| 3 Not cured Show forest plot | 1 | 101 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.13, 0.75] |
| Analysis 3.3  Comparison 3 Adults ‐ global assessment, Outcome 3 Not cured. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Not cured or not improved Show forest plot | 2 | 221 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.40, 1.80] |
| Analysis 4.1  Comparison 4 Combined children and adults, Outcome 1 Not cured or not improved. | ||||
| 2 Not improved Show forest plot | 2 | 221 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.38, 1.67] |
| Analysis 4.2  Comparison 4 Combined children and adults, Outcome 2 Not improved. | ||||
| 3 Not cured Show forest plot | 2 | 221 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.19, 0.60] |
| Analysis 4.3  Comparison 4 Combined children and adults, Outcome 3 Not cured. | ||||

Comparison 1 Children ‐ global assessment, Outcome 1 Not cured or not improved.

Comparison 1 Children ‐ global assessment, Outcome 2 Not improved.

Comparison 1 Children ‐ global assessment, Outcome 3 Not cured.
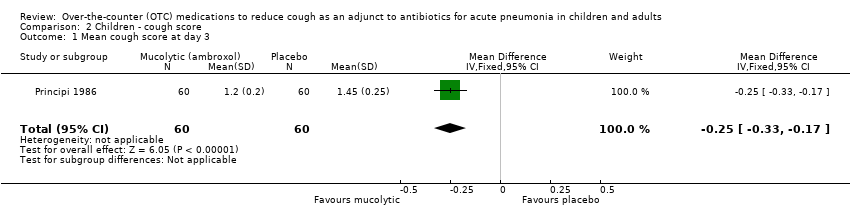
Comparison 2 Children ‐ cough score, Outcome 1 Mean cough score at day 3.
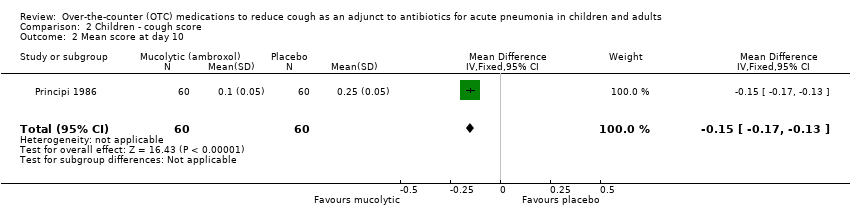
Comparison 2 Children ‐ cough score, Outcome 2 Mean score at day 10.

Comparison 3 Adults ‐ global assessment, Outcome 1 Not cured or not improved.

Comparison 3 Adults ‐ global assessment, Outcome 2 Not improved.

Comparison 3 Adults ‐ global assessment, Outcome 3 Not cured.
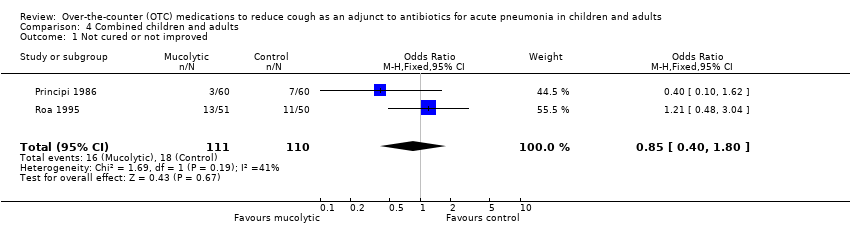
Comparison 4 Combined children and adults, Outcome 1 Not cured or not improved.
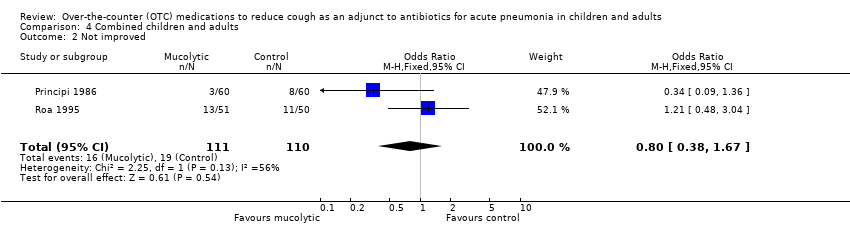
Comparison 4 Combined children and adults, Outcome 2 Not improved.
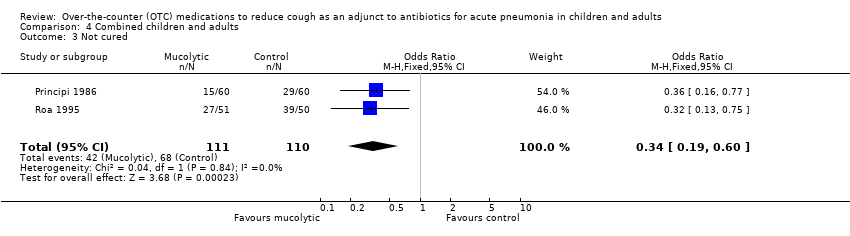
Comparison 4 Combined children and adults, Outcome 3 Not cured.
| Mucolytics as an adjunct to antibiotics to reduce cough in acute pneumonia in children and adults | ||||||
| Patient or population: children and adults with acute pneumonia Settings: any Intervention: mucolytics (and antibiotics)1 Comparision: antibiotics only | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| antibiotics only | mucolytics (and antibiotics) | |||||
| Cough score | The mean cough score in the control groups was | The mean Cough score in the intervention groups was | 120 | ⊕⊕⊝⊝ | Data for children only. | |
| Number of people who had not improved or had not been cured | 16 per 100 | 14 per 100 | OR 0.85 | 221 | ⊕⊕⊝⊝ | Fewer people represents a benefit |
| Adverse events | See comment | See comment | Not estimable | 120 | See comment | 1 study in children provided data specific to participants with pneumonia ‐ there were no adverse events. |
| Complications (e.g. medication change) | See comment | See comment | Not estimable | 0 | See comment | Complications were not measured in the trials. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidance | ||||||
| 1 In addition to antibiotics, people with pneumonia often use over‐the‐counter (OTC) cough medications when at home or request OTC cough medicaitons when in hospital to suppress an annoying cough. There is a question as to whether suppressing cough may prolong pneumonia. Over‐the‐counter cough medications can include anti‐tussives, expectorants, anti‐histamine‐decongestants, anti‐histamines and mucolytics (such as bromhexine, ambroxol and neltenexine). 2 Allocation concealment unclear. 3 Scale not validated. 4 Sparse data. 5 Sparse data; confidence interval does not rule out the potential for "more people" not improved or cured with mucolytics. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Not cured or not improved Show forest plot | 1 | 120 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.40 [0.10, 1.62] |
| 2 Not improved Show forest plot | 1 | 120 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.40 [0.10, 1.62] |
| 3 Not cured Show forest plot | 1 | 120 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.16, 0.77] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mean cough score at day 3 Show forest plot | 1 | 120 | Mean Difference (IV, Fixed, 95% CI) | ‐0.25 [‐0.33, ‐0.17] |
| 2 Mean score at day 10 Show forest plot | 1 | 120 | Mean Difference (IV, Fixed, 95% CI) | ‐0.15 [‐0.17, ‐0.13] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Not cured or not improved Show forest plot | 1 | 101 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.48, 3.04] |
| 2 Not improved Show forest plot | 1 | 101 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.48, 3.04] |
| 3 Not cured Show forest plot | 1 | 101 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.13, 0.75] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Not cured or not improved Show forest plot | 2 | 221 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.40, 1.80] |
| 2 Not improved Show forest plot | 2 | 221 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.38, 1.67] |
| 3 Not cured Show forest plot | 2 | 221 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.19, 0.60] |

