Lamotrigina para el dolor neuropático crónico y la fibromialgia en adultos
Información
- DOI:
- https://doi.org/10.1002/14651858.CD006044.pub4Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 03 diciembre 2013see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Dolor y cuidados paliativos
- Copyright:
-
- Copyright © 2019 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
All authors contributed equally to updating this review.
Sources of support
Internal sources
-
Oxford Pain Relief Trust, UK.
General institutional support for the original review and updates
External sources
-
No sources of support supplied
Declarations of interest
SD and PW have received research support from charities, government and industry sources at various times, but none relate to this review.
RAM has consulted for various pharmaceutical companies and received lecture fees from pharmaceutical companies related to analgesics and other healthcare interventions, including (in the past five years) AstraZeneca, Eli Lilly, Flynn Pharma, Furtura Medical, Grünenthal, GSK, Horizon Pharma, Lundbeck, Menarini, MSD, Pfizer, Reckitt Benckiser, Sanofi Aventis, Urgo, Astellas, and Vifor Pharma.
Acknowledgements
Jayne Edwards (Rees) contributed as an author to the original review.
Version history
| Published | Title | Stage | Authors | Version |
| 2013 Dec 03 | Lamotrigine for chronic neuropathic pain and fibromyalgia in adults | Review | Philip J Wiffen, Sheena Derry, R Andrew Moore | |
| 2011 Feb 16 | Lamotrigine for acute and chronic pain | Review | Philip J Wiffen, Sheena Derry, R Andrew Moore | |
| 2007 Apr 18 | Lamotrigine for acute and chronic pain | Review | Philip J Wiffen, Jayne Rees | |
| 2006 Apr 19 | Lamotrigine for acute and chronic pain | Protocol | Philip Wiffen, Henry HJ McQuay, R A Moore, Andrew Moore | |
Differences between protocol and review
The updated review conforms to more stringent evidence standards than those pertaining at the time of the original protocol.
Notes
A restricted search in May 2016 did not identify any potentially relevant studies likely to change the conclusions. Therefore, this review has now been stabilised following discussion with the authors and editors. If appropriate, we will update the review if new evidence likely to change the conclusions is published, or if standards change substantially which necessitate major revisions.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
Medical Subject Headings Check Words
Adult; Humans;
PICO

Study flow diagram.
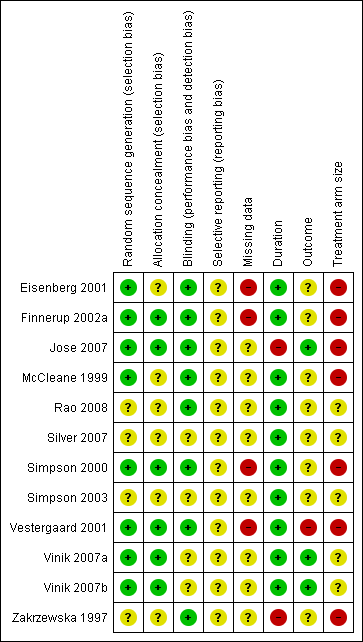
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Forest plot of comparison: 1 Painful diabetic neuropathy, outcome: 1.1 50% pain relief.

Forest plot of comparison: 2 All conditions: lamotrigine versus placebo, outcome: 2.2 Rash.

Comparison 1 Painful diabetic neuropathy: lamotrigine versus placebo, Outcome 1 50% pain relief.

Comparison 2 All conditions: lamotrigine versus placebo, Outcome 1 At least one adverse event.

Comparison 2 All conditions: lamotrigine versus placebo, Outcome 2 Rash.
| Lamotrigine compared with placebo for painful diabetic neuropathy | ||||||
| Patient or population: neuropathic pain (three studies in painful diabetic neuropathy) Settings: Community Intervention: oral lamotrigine 200 to 400 mg daily Comparison: placebo | ||||||
| Outcomes | Probable outcome with | Risk ratio NNTor NNH (95% CI) | No of studies, attacks, events | Quality of the evidence | Comments | |
| comparator | intervention | |||||
| At least 50% of maximum pain relief | 240 in 1000 | 260 in 1000 | RR 1.1 (0.82 to 1.4) NNT not calculated | 3 studies, 773 participants, 195 events | High | Unlikely that further research would reveal significant benefit, especially as potential high positive bias exists in the calculations we have because of LOCF imputation or completer analyses |
| Participants with at least 1 adverse event (all conditions) | 622 in 1000 | 717 in 1000 | RR 1.1 (1.01 to 1.2) NNH 10 (6.5 to 27) | 7 studies, 1121 participants, 768 events | High | Large numbers of events |
| Participants with a serious adverse event (all conditions) | No data | Very low | No data | |||
| Participants with rash (all conditions) | 56 in 1000 | 95 in 1000 | RR 1.4 (1.01 to 2.0) NNH 27 (16 to 89) | 12 studies, 1715 participants, 138 events | Moderate | Modest number of events |
| Deaths (all conditions) | No data | Very low | No data | |||
| GRADE Working Group grades of evidence | ||||||
| LOCF: last observation carried forward; NNT: number needed to treat for an additional beneficial outcome: NNH: number needed to treat for an additional harmful outcome; RR: risk ratio | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 50% pain relief Show forest plot | 3 | 773 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.82, 1.42] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 At least one adverse event Show forest plot | 7 | 1121 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [1.01, 1.22] |
| 2 Rash Show forest plot | 12 | 1715 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.43 [1.01, 2.03] |

