拉莫三嗪治疗成人慢性神经病理性疼痛和纤维肌痛
摘要
研究背景
这是对发表于2007年第2期并更新于2011年第2期的题为拉莫三嗪治疗急性和慢性疼痛的原始Cochrane系统综述的更新。一些抗癫痫药物在治疗神经病理性疼痛(神经损伤引起的疼痛)中占有一席之地。此更新的系统综述没有增加新的、旨在寻找拉莫三嗪作为慢性神经病理性疼痛或纤维肌痛的有效治疗方法的证据的研究。此次更新使用了比以前更高的证据标准。
研究目的
评价拉莫三嗪治疗慢性神经病理性疼痛和纤维肌痛的镇痛效果,并评价研究中报告的不良反应。
检索策略
我们从MEDLINE、EMBASE和Cochrane对照试验中心注册库(Cochrane Central Register of Controlled Trials, CENTRAL)中确定了关于拉莫三嗪治疗慢性神经病理性疼痛和纤维肌痛(包括癌痛)的随机对照试验(randomised controlled trials, RCT)。我们在2006年检索了原始系统综述,在2011年进行了第一次更新检索,随后在2013年8月为此次更新进行了检索。我们从检索到的论文的参考文献列表中寻找更多的研究。原始系统综述和第一次的更新纳入了急性疼痛,但没有发现关于急性疼痛的研究。
纳入排除标准
研究使用拉莫三嗪(任何剂量、任何途径和任何研究持续时间)治疗慢性神经病理性疼痛或纤维肌痛的RCT。使用经过验证的量表评价疼痛强度或疼痛缓解情况,或两者兼而有之。受试者是18岁及以上的成年人。我们只纳入了完整的期刊发表文章。
资料收集与分析
两名系统综述作者独立提取有关有效性和不良事件的资料,并评价研究质量。我们使用三级证据进行分析。第一级使用的数据的研究报告的结局是疼痛与初始相比减轻至少50%,持续至少8周,采用平行组设计,涉及200名或更多的受试者进行比较,并报告了治疗意向分析。一级研究没有使用末次观测值结转法(ast observation carried forward, LOCF)或其他插补方法来计算脱落率。第二级使用了不符合此标准的数据,因此第二级研究结果可能存在偏差。
主要结果
在11篇出版物中共纳入12项研究(涉及1511名受试者),这些受试者均伴有慢性神经病理性疼痛:中枢性卒中后疼痛(1)、化疗引起的神经病理性疼痛(1)、糖尿病性神经病变(4)、HIV相关神经病变(2)、混合神经病理性疼痛(2)、脊髓损伤相关疼痛(1)和三叉神经痛(1)。我们没有发现任何额外的研究。受试者年龄在26至77岁之间。一项研究的研究持续时间为两周,其余研究至少六周;八项的持续时间为八周或更长时间。
没有研究提供关于疗效结局的一级证据。没有能够令人信服的证据表明每天服用200至400毫克剂量的拉莫三嗪可有效治疗神经病理性疼痛和纤维肌痛。几乎10%服用了拉莫三嗪的受试者报告发生了皮疹。
作者结论
报告个体受试者疼痛缓解临床有用水平的大型、高质量长期研究没有提供能够令人信服的证据,以表明每天服用约200至400毫克剂量的拉莫三嗪可有效治疗神经病理性疼痛和纤维肌痛。考虑到包括抗癫痫药物和抗抑郁药物在内的更有效的治疗方法的可用性,根据现有的证据,拉莫三嗪在治疗中不具有重要地位。拉莫三嗪的不良反应也值得关注。
PICO
简语概要
拉莫三嗪(一种抗癫痫药)用于治疗慢性神经病理性疼痛或纤维肌痛
神经病理性疼痛是由神经受损导致的疼痛。它不同于健康神经从受损组织(跌倒、割伤或关节炎膝盖)传递的疼痛信息。治疗神经病理性疼痛的药物与治疗受损组织疼痛的药物不同。扑热息痛或布洛芬等药物对神经病理性疼痛无效,而有时用于治疗抑郁症或癫痫的药物对某些神经病理性疼痛的患者来说可能非常有效。我们对纤维肌痛(持续,广泛的疼痛和压痛,睡眠问题和疲劳的病症)的认识尚不充分,但是纤维肌痛可以与神经病理性疼痛一样对同类药物有反应。
拉莫三嗪是一种用于治疗癫痫的药物,因此可能是治疗神经病理性疼痛或纤维肌痛的有用药物。
我们在2013年11月26日进行了检索,以寻找拉莫三嗪治疗神经病理性疼痛或纤维肌痛的临床试验。我们发现了12项质量合理的研究,这些研究在数周内测试了拉莫三嗪与安慰剂的对比。在1511名参与研究的人中,几乎有一半的人因糖尿病引起的神经损伤而四肢疼痛,研究人员还对七种不同的疼痛性神经病变进行了评价。没有研究关注纤维肌痛。
拉莫三嗪对疼痛没有帮助,除了引起更多副作用外,与安慰剂没有什么不同。拉莫三嗪组的不良事件比安慰剂组更频繁,27人中有1人发生皮疹。
Authors' conclusions
Summary of findings
| Lamotrigine compared with placebo for painful diabetic neuropathy | ||||||
| Patient or population: neuropathic pain (three studies in painful diabetic neuropathy) Settings: Community Intervention: oral lamotrigine 200 to 400 mg daily Comparison: placebo | ||||||
| Outcomes | Probable outcome with | Risk ratio NNTor NNH (95% CI) | No of studies, attacks, events | Quality of the evidence | Comments | |
| comparator | intervention | |||||
| At least 50% of maximum pain relief | 240 in 1000 | 260 in 1000 | RR 1.1 (0.82 to 1.4) NNT not calculated | 3 studies, 773 participants, 195 events | High | Unlikely that further research would reveal significant benefit, especially as potential high positive bias exists in the calculations we have because of LOCF imputation or completer analyses |
| Participants with at least 1 adverse event (all conditions) | 622 in 1000 | 717 in 1000 | RR 1.1 (1.01 to 1.2) NNH 10 (6.5 to 27) | 7 studies, 1121 participants, 768 events | High | Large numbers of events |
| Participants with a serious adverse event (all conditions) | No data | Very low | No data | |||
| Participants with rash (all conditions) | 56 in 1000 | 95 in 1000 | RR 1.4 (1.01 to 2.0) NNH 27 (16 to 89) | 12 studies, 1715 participants, 138 events | Moderate | Modest number of events |
| Deaths (all conditions) | No data | Very low | No data | |||
| GRADE Working Group grades of evidence | ||||||
| LOCF: last observation carried forward; NNT: number needed to treat for an additional beneficial outcome: NNH: number needed to treat for an additional harmful outcome; RR: risk ratio | ||||||
Background
This is an update of a review first published in 2007 (Wiffen 2007), which looked at lamotrigine to treat acute and chronic pain of any type. In 2011 we updated the review with the addition of five new studies (1111 participants, of whom 767 received lamotrigine), using stricter methodological criteria to reduce potential bias (Wiffen 2011a). We made the decision to update again, and to concentrate on chronic neuropathic pain and fibromyalgia. We changed the title from Lamotrigine for acute and chronic pain, because there have been further recent advances in the rigour with which we assess studies and report data. We also made this decision in order to conform with other reviews in the series on neuropathic pain and fibromyalgia, and because there is little or no use or intended use of lamotrigine and similar drugs in acute pain, or other forms of chronic pain.
In particular we considered study size and duration, outcomes reported, and method of imputation for withdrawals, and we now report results in two tiers according to outcome and freedom from known sources of bias. We wanted to bring this review in line with a template protocol so that it could easily be included in the overview of antiepileptics for chronic neuropathic pain and fibromyalgia in adults (Wiffen 2013a). Reviews of carbamazepine (Wiffen 2011b), clonazepam (Corrigan 2012), gabapentin (Moore 2011a), lacosamide (Hearn 2012), phenytoin (Birse 2012), pregabalin (Moore 2009a), topiramate (Wiffen 2013b), and valproic acid (Gill 2011) have been completed.
The aim is for all reviews to use the same methods, based on new criteria for what constitutes reliable evidence in chronic pain (Moore 2010a; Moore 2012b; Appendix 1). A Cochrane review of pregabalin in neuropathic pain and fibromyalgia demonstrated different response rates for different types of chronic pain (higher in diabetic neuropathy and postherpetic neuralgia and lower in central pain and fibromyalgia) (Moore 2009a). This indicated that different neuropathic pain conditions should be treated separately from one another, and that pooling should not be done unless there are good grounds for doing so. While fibromyalgia is considered to have a different aetiology from chronic neuropathic pain, it is a condition that responds to the same therapies. Because of limitations in the number of available clinical trials, it is convenient to consider fibromyalgia together with neuropathic pain. We make no presumption to pool data across individual neuropathic pain conditions or fibromyalgia, but will consider each condition separately.
Description of the condition
The 2011 International Association for the Study of Pain definition of neuropathic pain is "pain caused by a lesion or disease of the somatosensory system" (Jensen 2011) based on an earlier consensus meeting (Treede 2008). Neuropathic pain may be caused by nerve damage, but is often followed by changes in the central nervous system (CNS) (Moisset 2007). Neuropathic pain tends to be chronic and may be present for months or years. It is complex (Apkarian 2011; Tracey 2011), and neuropathic pain features can be found in patients with joint pain (Soni 2013).
Fibromyalgia is defined as widespread pain for longer than three months with pain on palpation at 11 or more of 18 specified tender points (Wolfe 1990), and is frequently associated with other symptoms such as poor sleep, fatigue, and depression. More recently, a definition of fibromyalgia has been proposed based on symptom severity and the presence of widespread pain (Wolfe 2010). The cause, or causes, are not well understood, but it has features in common with neuropathic pain, including changes in the CNS. Moreover, patients with neuropathic pain and those with fibromyalgia experience similar sensory phenomena (Koroschetz 2011). Many people with these conditions are significantly disabled with moderate or severe pain for many years.
In primary care in the United Kingdom (UK), the incidences per 100,000 person‐years' observation have been reported as 28 (95% confidence interval (CI) 27 to 30) for postherpetic neuralgia, 27 (26 to 29) for trigeminal neuralgia, 0.8 (0.6 to 1.1) for phantom limb pain, and 21 (20 to 22) for painful diabetic neuropathy (Hall 2008). Estimates varied between studies, often because of small numbers of cases. The incidence of trigeminal neuralgia has been estimated at 4 in 100,000 per year (Katusic 1991; Rappaport 1994), while more recently, a study of facial pain in The Netherlands found incidences per 100,000 person‐years of 12.6 for trigeminal neuralgia and 3.9 for postherpetic neuralgia (Koopman 2009). A systematic review of chronic pain demonstrated that some neuropathic pain conditions, such as painful diabetic neuropathy, can be more common, with prevalence rates up to 400 per 100,000 person‐years (McQuay 2007). The prevalence of neuropathic pain was reported as being 3.3% in Austria (Gustorff 2008), 6.9% in France (Bouhassira 2008) and as high as 8% in the UK (Torrance 2006), and about 7% in a systematic review of studies published since 2000 (Moore 2013a). Some forms of neuropathic pain, such as diabetic neuropathy and post surgical chronic pain (which is often neuropathic in origin) are increasingly common (Hall 2008). Fibromyalgia is common, especially in women, with an all‐age prevalence of 12%, and a female to male ratio of 6:1 (McNally 2006).
Neuropathic pain and fibromyalgia are known to be difficult to treat effectively, with only a minority of individuals experiencing a clinically relevant benefit from any one intervention. A multidisciplinary approach is now advocated, with pharmacological interventions being combined with physical or cognitive interventions or both. Conventional analgesics are usually not effective. Some patients with neuropathic pain may derive some benefit from topical lidocaine patch or low concentration topical capsaicin, although evidence of benefit is uncertain (Derry 2012; Khaliq 2013). High concentration topical capsaicin may benefit some patients with postherpetic neuralgia (Derry 2013). Treatment is more usually by so‐called unconventional analgesics such as antidepressants like duloxetine and amitriptyline (Lunn 2009; Moore 2012a; Sultan 2008) or antiepileptics like gabapentin or pregabalin (Moore 2009a; Moore 2011a). The proportion of patients who achieve worthwhile pain relief (typically defined as at least 50% pain intensity reduction (Moore 2013b)) is small, typically 10% to 25% more than with placebo, with numbers needed to treat for an additional beneficial outcome (NNTs) usually between 4 and 10.
Description of the intervention
Lamotrigine, a phenyltriazine, is chemically unrelated to other antiepileptic drugs. The drug is available as standard oral tablets (25 mg to 200 mg) and chewable, dispersible tablets (2 mg to 100 mg), and a new extended release tablet is available in some parts of the world.
How the intervention might work
Lamotrigine is an antiepileptic drug exerting its antiepileptic effect via sodium channels. There is some evidence that agents that block sodium channels are useful in the treatment of neuropathic pain (McCleane 2000). There is evidence from animal models supporting the use of lamotrigine in neuropathic pain, and for an effect in experimental pain models such as cold‐induced pain in humans (McCleane 2000). Lamotrigine is chemically unrelated to existing antiepileptic agents. There has also been discussion of the role of lamotrigine as a pre‐emptive analgesic to reduce postsurgical pain (Bonicalzi 1997). More recently it has been shown that neuronal alpha4 beta2 nicotinic acetylcholine receptors may be a target for lamotrigine, and this may mediate its antiepileptic effects (Zheng 2010).
Why it is important to do this review
The standards used to assess evidence in chronic pain trials have changed substantially in recent years, with particular attention being paid to trial duration, withdrawals, and statistical imputation following withdrawal, all of which can substantially alter estimates of efficacy. The most important change is the move from using average pain scores, or average change in pain scores, to using the number of participants who have a large decrease in pain (by at least 50%); this level of pain relief has been shown to correlate with improvements in comorbid symptoms, function, and quality of life. These standards are set out in the reference guide for pain studies (AUREF 2012) and reflect what patients with chronic pain want from treatment (Moore 2013a).
This Cochrane review assesses evidence in ways that make both statistical and clinical sense, and uses developing criteria for what constitutes reliable evidence in chronic pain (Moore 2010a). Trials included and analysed need to meet a minimum of reporting quality (blinding, randomisation), validity (duration, dose and timing, diagnosis, outcomes, etc), and size (ideally at least 500 participants in a comparison in which the NNT is four or more (Moore 1998)).
Lamotrigine is not widely prescribed for neuropathic pain, though it is prescribed for some cases of painful HIV‐related neuropathy. It is important to know its place in the range of drugs used to treat the various types of neuropathic pain. This updated review brings the evidence for lamotrigine into line with that for other medicines used in these conditions, and will form part of an overview of antiepileptic drugs for chronic neuropathic pain and fibromyalgia.
Objectives
To assess the analgesic efficacy of lamotrigine in the treatment of chronic neuropathic pain and fibromyalgia, and to evaluate adverse effects reported in the studies.
Methods
Criteria for considering studies for this review
Types of studies
We included studies if they were randomised controlled trials (RCTs) with at least 10 participants per treatment group and double‐blind (participant and observers) assessment of participant‐reported outcomes, following two weeks of treatment or longer, although the emphasis of the review is on studies of six weeks or longer. We required full journal publication, with the exception of extended abstracts of otherwise unpublished clinical trials (for example, detailed information from PDFs of posters that typically included all important details of methodology used and results obtained). We did not include short abstracts (usually meeting reports with inadequate or no reporting of data). We excluded studies of experimental pain, case reports, and clinical observations.
In the earlier review, we excluded studies of lamotrigine used to treat pain produced by other drugs; in this version we have included one study for chemotherapy‐induced pain, but have not combined results from this study in the analysis (Rao 2008).
Types of participants
Studies included adult participants aged 18 years and above. Participants could have one or more of a wide range of chronic neuropathic pain conditions including (but not limited to):
-
cancer‐related neuropathy;
-
central neuropathic pain;
-
complex regional pain syndrome (CRPS) Type II;
-
human immunodeficiency virus (HIV) neuropathy;
-
painful diabetic neuropathy;
-
phantom limb pain;
-
postherpetic neuralgia;
-
postoperative or traumatic neuropathic pain;
-
spinal cord injury;
-
trigeminal neuralgia;
and
-
fibromyalgia;
-
CRPS Type I.
Types of interventions
Lamotrigine in any dose, by any route, administered for the relief of neuropathic pain or fibromyalgia, and compared to placebo, no intervention or any other active comparator.
Types of outcome measures
We anticipated that studies would use a variety of outcome measures, with the majority of studies using standard subjective scales (numerical rating scale (NRS) or visual analogue scale (VAS)) for pain intensity or pain relief, or both. We were particularly interested in Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials (IMMPACT) definitions for moderate and substantial benefit in chronic pain studies (Dworkin 2008). These are defined as at least 30% pain relief over baseline (moderate), at least 50% pain relief over baseline (substantial), much or very much improved on patient global impression of change (PGIC) (moderate), and very much improved on PGIC (substantial). These outcomes are different from those set out in the earlier review (Wiffen 2007), concentrating as they do on dichotomous outcomes where pain responses do not follow a normal (Gaussian) distribution. People with chronic pain desire high levels of pain relief, ideally more than 50%, and with pain not worse than mild (O'Brien 2010).
We include a 'Summary of findings' table as set out in the Cochrane Pain, Palliative and Supportive Care Group author guide (AUREF 2012). The 'Summary of findings' table includes outcomes of at least 30% and at least 50% pain intensity reduction, PGIC, adverse event withdrawals, serious adverse events and death (summary of findings Table for the main comparison).
Primary outcomes
-
Participant‐reported pain intensity reduction of 30% or greater.
-
Participant‐reported pain intensity reduction of 50% or greater.
-
Participant‐reported global impression of clinical change (Patient Global Impression of Change, PGIC) much or very much improved.
-
Participant‐reported global impression of clinical change (PGIC) very much improved.
Secondary outcomes
-
Any pain‐related outcome indicating some improvement.
-
Withdrawals due to lack of efficacy.
-
Participants experiencing any adverse event.
-
Participants experiencing any serious adverse event.
-
Withdrawals due to adverse events.
-
Specific adverse events, particularly somnolence and dizziness.
These outcomes are not eligibility criteria for this review, but are outcomes of interest within whichever studies are included.
Search methods for identification of studies
Electronic searches
We identified studies by several methods. For the original review we identified RCTs of lamotrigine (and key brand names Lamictal, Lamictin, Neurium) in acute and chronic pain in the Cochrane Central Register of Controlled Trials (CENTRAL, 2010, Issue 12), MEDLINE (via Ovid) from 1966 to January 2011, and EMBASE (via Ovid) from 1994 to January 2011.
For this update we searched for new studies in chronic neuropathic pain and fibromyalgia in:
-
Cochrane Central Register of Controlled Trials (CENTRAL, 2013, Issue 11 in The Cochrane Library);
-
MEDLINE (via Ovid) (January 2010 to 26 November 2013);
-
EMBASE (via Ovid) (January 2010 to 26 November 2013);
-
PhRMA clinical study results database (clinicaltrials.gov) to 26 November 2013;
-
WHO International Clinical Trials Registry Platform (apps.who.int/trialsearch) to 26 November 2013.
Given the limited literature in this area, we undertook a sensitive search strategy. See Appendix 2 for the MEDLINE search strategy, Appendix 3 for the EMBASE search strategy, and Appendix 4 for the CENTRAL search strategy.
Searching other resources
For the original review, we identified additional studies from the reference lists of the retrieved papers and by contacting study authors. We applied no language restrictions.
Data collection and analysis
Selection of studies
Two review authors independently read the titles and abstracts of all studies identified by the search, and the full text of all potentially relevant studies. We reached agreement on eligibility by discussion. We did not anonymise the studies in any way before assessment.
Data extraction and management
Two review authors extracted data using a standard form, and agreed data before entry into Review Manager 5 (RevMan 2012) or any other analysis method. Data extracted included information about the pain condition and number of participants treated, drug and dosing regimen, study design, study duration and follow‐up, analgesic outcome measures and results, withdrawals and adverse events (participants experiencing any adverse event or a serious adverse event).
Assessment of risk of bias in included studies
We independently scored each study for quality using a three‐item scale (Jadad 1996) and agreed a 'consensus' score for each study. Scores of two and below have been associated with greater estimates of efficacy than studies of higher quality (Khan 1996). Quality scores were not used to weight the results in any way.
We used the 'Risk of bias' tool to assess the likely impact on the strength of the evidence of various study characteristics relating to methodological quality (randomisation, allocation concealment, blinding, freedom from selective reporting), study validity (duration, outcome reporting, and handling of missing data), and size (Moore 2010a).
Two review authors independently assessed risks of bias for each study, using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) and adapted from those used by the Cochrane Pregnancy and Childbirth Group, with any disagreements resolved by discussion. We assessed the following for each study.
-
Random sequence generation (checking for possible selection bias). We assessed the method used to generate the allocation sequence as: low risk of bias (any truly random process, eg random number table; computer random number generator); unclear risk of bias (method used to generate sequence not clearly stated). We excluded studies using a non‐random process (eg odd or even date of birth; hospital or clinic record number).
-
Allocation concealment (checking for possible selection bias). The method used to conceal allocation to interventions before assignment determines whether intervention allocation could have been foreseen in advance of or during recruitment, or changed after assignment. We assessed the methods as: low risk of bias (eg telephone or central randomisation; consecutively numbered sealed opaque envelopes); unclear risk of bias (method not clearly stated). We excluded studies that did not conceal allocation (eg open list).
-
Blinding of outcome assessment (checking for possible detection bias). We assessed the methods used to blind study participants and outcome assessors from knowledge of which intervention a participant received. We assessed the methods as: low risk of bias (study states that it was blinded and describes the method used to achieve blinding, eg identical tablets; matched in appearance and smell); unclear risk of bias (study states that it was blinded but does not provide an adequate description of how this was achieved). We excluded studies that were not double‐blind.
-
Incomplete outcome data (checking for possible attrition bias due to the amount, nature, and handling of incomplete outcome data). We assessed the methods used to deal with incomplete data as: low risk of bias (< 10% of participants did not complete the study, or used ‘baseline observation carried forward’ analysis, or both); unclear risk of bias (used 'last observation carried forward' (LOCF) analysis); high risk of bias (used 'completer' analysis).
-
Size of study (checking for possible biases confounded by small size). We assessed studies as being at low risk of bias (≥ 200 participants per treatment arm); unclear risk of bias (50 to 199 participants per treatment arm); high risk of bias (< 50 participants per treatment arm).
Measures of treatment effect
We used the risk ratio (relative risk, RR) to establish statistical difference. We used numbers needed to treat for an additional beneficial outcome (NNT) or for an additional harmful outcome (NNH) and pooled percentages as absolute measures of benefit or harm.
The following terms are used to describe adverse outcomes in terms of harm or prevention of harm:
-
When significantly fewer adverse outcomes occurred with lamotrigine than with control (placebo or active) we used the term the number needed to treat to prevent one event (NNTp);
-
When significantly more adverse outcomes occurred with lamotrigine compared with control (placebo or active) we used the term the number needed to harm or cause one event (NNH).
Unit of analysis issues
The control treatment arm would be split between active treatment arms in a single study if the active treatment arms were not combined for analysis.
Dealing with missing data
We used intention‐to‐treat (ITT) analysis. The ITT population consisted of participants who were randomised, took the assigned study medication and provided at least one post‐baseline assessment. Missing participants were assigned zero improvement where this could be done. We were aware that imputation methods might be problematical and examined trial reports for information about them.
Assessment of heterogeneity
We dealt with clinical heterogeneity by combining studies that examined similar painful conditions, and not combining results from dissimilar painful conditions. We assessed statistical heterogeneity visually (L'Abbe 1987) and with the use of the I² statistic (Higgins 2003).
Assessment of reporting biases
The aim of this review was to use dichotomous data of known utility (Moore 2010a). The review did not depend on what authors of the original studies chose to report or not report, though clearly there were difficulties with studies failing to report any dichotomous results. We extracted continuous data, which probably poorly reflected efficacy and utility, and used them only when useful for illustrative purposes.
We undertook no statistical assessment of publication bias.
Data synthesis
We considered individual painful conditions separately because placebo response rates with the same outcome can vary between conditions, as can the drug‐specific effects (Moore 2009a).
We analysed data for each painful condition in three tiers, according to outcome and freedom from known sources of bias.
-
The first tier uses data meeting current best standards, where studies report the outcome of at least 50% pain intensity reduction over baseline (or its equivalent), without the use of last observation carried forward (LOCF) or other imputation method for dropouts, report an intention‐to‐treat (ITT) analysis, last eight or more weeks, have a parallel‐group design, and have at least 200 participants (preferably at least 400) in the comparison (Moore 2010a; Moore 2012b). These top‐tier results are reported first.
-
The second tier uses data from at least 200 participants but where one or more of the above conditions is not met (for example reporting at least 30% pain intensity reduction, using LOCF or a completer analysis, or lasting four to eight weeks).
-
The third tier of evidence relates to data from fewer than 200 participants, or where there are expected to be significant problems because, for example, of very short duration studies of less than four weeks, where there is major heterogeneity between studies, or where there are shortcomings in allocation concealment, attrition, or incomplete outcome data. For this third tier of evidence, no data synthesis is reasonable, and may be misleading, but an indication of beneficial effects might be possible.
We used dichotomous data to calculate risk ratio for benefit with 95% confidence intervals (CIs), using a fixed‐effect model, together with numbers needed to treat for an additional beneficial outcome (NNTs) (Cook 1995). This was done for effectiveness, for adverse effects, and for drug‐related study withdrawal. We also undertook meta‐analysis when appropriate data were available. We calculated NNTs as the reciprocal of the absolute risk reduction (McQuay 1998). For unwanted effects, the NNH (number needed to treat for an additional harmful outcome) is calculated in the same way. In the absence of dichotomous data, we have reported summary continuous data where available and appropriate, but did not carry out any analysis. We undertook meta‐analysis using a fixed‐effect model.
Subgroup analysis and investigation of heterogeneity
We planned no subgroup analyses, beyond separate analysis of different conditions, as we expected that there would be insufficient study data.
Sensitivity analysis
We planned no sensitivity analyses because we knew the evidence base to be too small to allow reliable analysis.
Results
Description of studies
Results of the search
The previous review identified 23 studies, in 23 publications. New searches for this update identified one potentially relevant study.
Included studies
There were seven studies (400 participants) in the original review (Eisenberg 2001; Finnerup 2002a; McCleane 1999; Simpson 2000; Simpson 2003; Vestergaard 2001; Zakrzewska 1997). In the first update five studies were added (Jose 2007; Rao 2008; Silver 2007; Vinik 2007a; Vinik 2007b), with 1111 participants, almost trebling the number of participants since the previous review. These studies were generally larger in size and of longer duration. We found no new studies for this update that satisfied the inclusion criteria.
Twelve previously identified studies (12 publications), involving 1511 participants are therefore included (Eisenberg 2001; Finnerup 2002a; Jose 2007; McCleane 1999; Rao 2008; Silver 2007; Simpson 2000; Simpson 2003; Vestergaard 2001; Vinik 2007a; Vinik 2007b; Zakrzewska 1997). Two studies were reported in one publication (Vinik 2007a; Vinik 2007b), and an incomplete report of Eisenberg 2001 (Lurie 2000) provided no additional data, but is included and linked to the primary study. Additional data for Silver 2007 (NPP30010) were identified during the latest searches in a results summary posted on the GlaxoSmithKline Clinical Trials Register (Figure 1).
Study flow diagram.
Included studies covered the following conditions: central post‐stroke pain (Vestergaard 2001), chemotherapy‐induced peripheral neuropathic pain (Rao 2008), diabetic neuropathy (Eisenberg 2001; Jose 2007; Vinik 2007a; Vinik 2007b) HIV‐related neuropathy (Simpson 2000; Simpson 2003), mixed neuropathic pain (McCleane 1999; Silver 2007), spinal cord injury‐related pain (Finnerup 2002a), and trigeminal neuralgia (Zakrzewska 1997). There were no studies using lamotrigine to treat fibromyalgia.
Eleven studies used a placebo comparator, and one (Jose 2007) used amitriptyline as the comparator. Two studies added lamotrigine or placebo to existing treatments for neuropathic pain (Silver 2007; Zakrzewska 1997). The studies included participants in the age range of 26 to 77 years. One study was for two weeks (Zakrzewska 1997); the remainder were at least six weeks, and eight were of eight‐week duration or longer. Four were cross‐over studies (Finnerup 2002a; Jose 2007; Vestergaard 2001; Zakrzewska 1997). Details of all eligible studies are given in the Characteristics of included studies table and results for individual studies are in a separate table (Appendix 5).
Excluded studies
Eleven studies were excluded from the earlier review (Bonicalzi 1997; Breuer 2007; Carrieri 1998; Devulder 2000; Di Vadi 1998; Eisenberg 1998; Eisenberg 2003; Eisenberg 2005; Lunardi 1997; Petersen 2003; Sandner‐Kiesling 2002), and one more for this update (Shaikh 2011). Details of the reasons for exclusion are in the Characteristics of excluded studies table.
Risk of bias in included studies
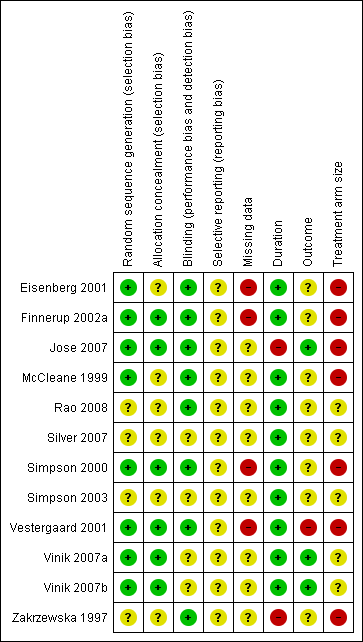
Each study was scored for quality using the three‐item Oxford Quality Score scale (Jadad 1996) and agreed by the review authors. All scored 3/5 or greater, with one scoring 3/5, five scoring 4/5, and six scoring 5/5.
In this update we have used the 'Risk of bias' tool. The comments on individual studies are reported in the Risk of bias section of the Characteristics of included studies table. The findings are displayed in Figure 2; no sensitivity analysis was undertaken. The greatest risks of bias came from imputation after study withdrawal, outcomes used of little relevance, and small study size.
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Effects of interventions
For measures of efficacy we considered each condition separately, but for adverse outcomes we combined data across conditions. There were no data for fibromyalgia.
Efficacy
No study provided first‐tier evidence for an efficacy outcome. We judged results as second‐ or third‐tier because of use of LOCF imputation or completer analysis, and small size. Details of efficacy outcomes in individual studies are in Appendix 5.
Painful diabetic neuropathy (PDN)
Second tier evidence
Four studies (Eisenberg 2001; Jose 2007; Vinik 2007a; Vinik 2007b) looked at the role of lamotrigine for PDN (758 participants). None of these demonstrated any major benefits.
In one study (Eisenberg 2001), a 50% reduction in pain measured in the last three weeks of treatment was reported by 12/27 on lamotrigine titrated up to 400 mg daily and 5/26 on placebo. In two large randomised studies of lamotrigine 200 mg to 400 mg daily, with a 12‐week maintenance phase, there was no difference between lamotrigine and placebo for the outcome of at least 50% pain relief (Vinik 2007a; Vinik 2007b). Combining these studies, 145/567 (26%) of participants experienced at least 50% pain relief with lamotrigine 200 mg to 400 mg daily, and 50/206 (24%) with placebo. There was no overall significant difference between lamotrigine and placebo (RR 1.1 (95% CI 0.8 to 1.4)) (Figure 3). A similar non‐significant difference was found for participants reporting "marked improvement".
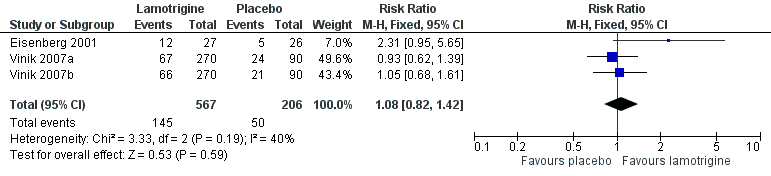
Forest plot of comparison: 1 Painful diabetic neuropathy, outcome: 1.1 50% pain relief.
In the fourth study, a 20% reduction in pain after six weeks of treatment was reported by 19/46 taking lamotrigine 200 mg daily and 13/46 taking 50 mg amitriptyline at night (Jose 2007). There were insufficient data for analysis.
Mixed neuropathic pain
Third tier evidence
One study of 100 participants examined the use of lamotrigine 200 mg daily in participants with intractable neuropathic pain diagnosed by symptoms of shooting/lancinating pain, burning, numbness, allodynia and paraesthesia/dysaesthesia (McCleane 1999). At least three of these symptoms were required for participation. Participants already taking an antiepileptic were excluded. No useful analgesic benefit was demonstrated. There was a reduction in the overall pain score of 1/100 mm.
A second study used an 'add‐on' design for lamotrigine titrated up to 400 mg daily on top of gabapentin, tricyclic antidepressant, or non‐opioid analgesic where pain was inadequately controlled (Silver 2007). No additional analgesic benefit could be demonstrated over 14 weeks using responder definitions of ≥ 50% and ≥ 30% pain reduction or PGIC outcomes of much or very much improved.
Central post‐stroke pain
Third tier evidence
Thirty participants took part in a single cross‐over study, and only 20 completed both arms (Vestergaard 2001). The difference between lamotrigine 200 mg and placebo for clinical response was significant when assessed at eight weeks. Lower pain scores (reduction of 2/10 or more) were reported by 12 participants with lamotrigine and three with placebo.
Chemotherapy‐induced peripheral neuropathic pain
Third tier evidence
In a study of 125 participants (Rao 2008), average pain scores decreased in both the active and placebo groups with no significant difference between the groups. The study authors concluded that lamotrigine was not effective in this condition.
HIV‐related neuropathy
Third tier evidence
There were two studies involving participants with HIV‐related neuropathy. The first study of 42 participants (Simpson 2000) claimed effectiveness for lamotrigine 300 mg/day, but over 50% of the treatment group dropped out, making results difficult to interpret. The second study (Simpson 2003) analysed the results according to whether participants were receiving antiretroviral therapy (ART) or not. For those who were receiving antiretroviral therapy there did appear to be some benefits in terms of attainment of moderate or better pain relief with lamotrigine (35/62, 57%) than with placebo (7/30, 23%); for PGIC, marked improvement was recorded by 29/62 (47%) of participants with lamotrigine and 4/30 (13%) with placebo.
Spinal cord injury related pain
Third tier evidence
Thirty participants with neuropathic pain following traumatic spinal cord injury were included (Finnerup 2002a). Doses of up to 400 mg daily for lamotrigine were used but the study authors reported no significant difference from placebo for the outcomes of ≥ 50% or ≥ 30% pain relief.
Trigeminal neuralgia
Third tier evidence
Fourteen participants participated in a cross‐over 'add‐on' study comparing lamotrigine with placebo in a cross‐over study of two two‐week phases separated by a three‐day long washout (Zakrzewska 1997). All participants continued on carbamazepine or phenytoin throughout the study period. Lamotrigine was not significantly more effective than placebo in this small study; 10/13 participants stated that lamotrigine was better or much better, compared with 8/14 on placebo, using a global evaluation.
Adverse events
Adverse events were not consistently reported across studies. Seven studies, with 1121 participants (Eisenberg 2001; Finnerup 2002a; Silver 2007; Vestergaard 2001; Vinik 2007a; Vinik 2007b; Zakrzewska 1997) reported the number of participants who experienced at least one adverse event. Combining the studies across conditions, 531/740 (72%) of participants had an adverse event with lamotrigine and 237/381 (62%) with placebo. The RR just reached statistical significance at 1.1 (1.01 to 1.2) (Analysis 2.1) and the NNH was 10 (6.5 to 27).
Rash can be problematic with lamotrigine. It was mentioned as an adverse event or adverse event withdrawal in 11 studies, and omitted from a long list of adverse events in the other (Zakrzewska 1997). Combining studies, the overall incidence of rash was 9.5% with lamotrigine and 5.6% with placebo, barely achieving statistical significance (RR 1.4 (1.01 to 2.0)) (Figure 4). This would indicate that rash with lamotrigine would affect about one person in 27 who would not have been affected with placebo.
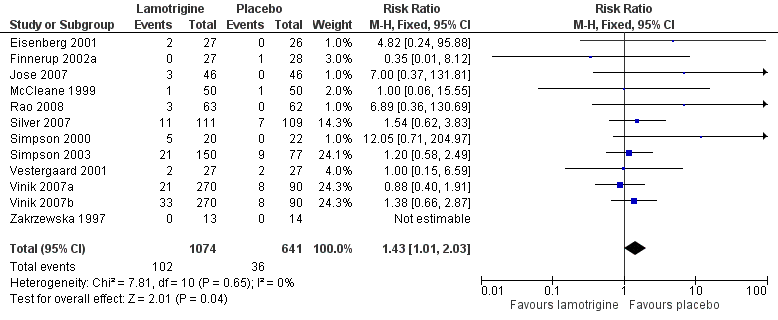
Forest plot of comparison: 2 All conditions: lamotrigine versus placebo, outcome: 2.2 Rash.
Discussion
Antiepileptic drugs have been used in the treatment of neuropathic pain since lamotrigine was first used for trigeminal neuralgia in the 1960s. Other antiepileptic drugs have been examined, with good evidence of efficacy for gabapentin (Moore 2011a) and pregabalin (Moore 2009a), and these two are now widely used. Although various drugs may be useful in controlling seizures, they have different mechanisms of action. There is no reason why a drug effective at seizure control should necessarily be effective in treating neuropathic pain. Lamotrigine, a relatively new antiepileptic drug, has therefore been investigated in neuropathic painful conditions.
Summary of main results
Large, high‐quality, long‐duration studies reporting clinically useful levels of pain relief for individual participants provide no convincing evidence that lamotrigine is effective in treating neuropathic pain and fibromyalgia at doses of about 200 mg to 400 mg daily. There is very limited evidence for a possible effect of lamotrigine in central post‐stroke pain and in a subgroup of patients with HIV‐related neuropathy receiving antiretroviral therapy. No benefit was demonstrated for diabetic neuropathy, in intractable neuropathic pain, spinal cord injury, or trigeminal neuralgia. We found no studies testing lamotrigine in fibromyalgia. The small number of studies and the small number of participants are insufficient to provide robust evidence for effect.
Safety is an important aspect of the choice of treatment even in difficult conditions. In this review, about 10% of participants developed a rash; this fits with wider epidemiological work (Hirsch 2006). The results are consistent with reports in the manufacturer's summary of product characteristics. Serious potentially life‐threatening rashes such as Stevens Johnson Syndrome are estimated to occur at an incidence of 1 in 1000 (SPC 2013).
Overall completeness and applicability of evidence
The difficulties of dose titration and adverse effects are likely to dissuade many clinicians from choosing lamotrigine to treat neuropathic pain, and it is possible that those conducting the studies have chosen to include the more difficult participants in terms of severity and duration of pain.
Efficacy and adverse event outcomes were not consistently reported across the studies, and this limited the analyses to some extent.
Quality of the evidence
The studies included in this review covered a number of different painful conditions. For some, like HIV neuropathy for instance, it is unclear whether antiepileptic drugs are effective in the condition, and any indication of benefit is welcome. The main quality issues involve reporting of outcomes of interest, particularly dichotomous outcomes equivalent to Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials (IMMPACT), as well as better reporting of adverse events. The earliest study was published in 1997, and there have been major changes in clinical trial reporting since then. The studies themselves appear to be largely well‐conducted, and individual patient analysis could overcome some of the shortcomings of reporting, though not the paucity of participants studied in each neuropathic pain condition.
Potential biases in the review process
The review was restricted to randomised double‐blind studies, thus limiting the potential for bias. Other possible sources of bias that could have affected the review included:
-
Duration ‐ NNT estimates of efficacy in chronic pain studies tend to increase (get worse) with increasing duration (Moore 2010d). However, all studies were six weeks or longer, and most longer than eight weeks.
-
Outcomes may affect estimates of efficacy, but the efficacy outcomes chosen were of participants achieving the equivalent of IMMPACT‐defined moderate or substantial improvement, and it is likely that lesser benefits, such as 'any benefit' or 'any improvement', are potentially related to inferior outcomes, though this remains to be clarified. Most authors attempted to report dichotomous outcomes of interest, especially in the larger, more recent studies.
-
The question of whether cross‐over trials exaggerate treatment effects in comparison with parallel‐group designs, as has been seen in some circumstances (Khan 1996), is unclear but unlikely to be the source of major bias (Elbourne 2002). Withdrawals meant that any results were more likely to be per protocol for completers than for a true ITT analysis. Parallel group studies were larger than cross‐over studies, and predominated analyses in terms of number of participants, with only about 100 participants in cross‐over studies.
-
The absence of publication bias (unpublished trials showing no benefit of lamotrigine over placebo) can never be proven. However, publication bias is irrelevant where the published studies show no effect of treatment.
-
Imputation methods used when participants withdrew were typically either last observation carried forward (LOCF) or were not stated; no study reported clearly that participants achieving acceptable levels of pain relief were unequivocally on treatment at the end of the study. Use of LOCF imputation can overestimate efficacy, particularly where adverse event withdrawals are high with active treatment (Moore 2012b).
Agreements and disagreements with other studies or reviews
This update does not substantially change the results of the 2011 update, which itself was broadly in agreement with the previous Cochrane review (Wiffen 2007).
A systematic review of pharmacological treatment of painful HIV‐associated sensory neuropathy (Phillips 2010) did not find lamotrigine 600 mg/day to be better than placebo. A non‐systematic review considered lamotrigine to be effective, but based on only a fraction of the results presented in this updated review (Jensen 2002), and Finnerup 2002b suggested that lamotrigine is as good as amitriptyline and is a first‐line agent in central neuropathic pain.
Guidelines from Europe (Attal 2010) and the International Association for the Study of Pain (Dworkin 2010) reported that there were few data for lamotrigine in any neuropathic pain condition, and recommended its use only as a third line medication, and probably only in a specialist setting. Guidelines from NICE in the UK do not recommend lamotrigine for neuropathic pain (NICE 2013).

Study flow diagram.
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Forest plot of comparison: 1 Painful diabetic neuropathy, outcome: 1.1 50% pain relief.

Forest plot of comparison: 2 All conditions: lamotrigine versus placebo, outcome: 2.2 Rash.

Comparison 1 Painful diabetic neuropathy: lamotrigine versus placebo, Outcome 1 50% pain relief.

Comparison 2 All conditions: lamotrigine versus placebo, Outcome 1 At least one adverse event.

Comparison 2 All conditions: lamotrigine versus placebo, Outcome 2 Rash.
| Lamotrigine compared with placebo for painful diabetic neuropathy | ||||||
| Patient or population: neuropathic pain (three studies in painful diabetic neuropathy) Settings: Community Intervention: oral lamotrigine 200 to 400 mg daily Comparison: placebo | ||||||
| Outcomes | Probable outcome with | Risk ratio NNTor NNH (95% CI) | No of studies, attacks, events | Quality of the evidence | Comments | |
| comparator | intervention | |||||
| At least 50% of maximum pain relief | 240 in 1000 | 260 in 1000 | RR 1.1 (0.82 to 1.4) NNT not calculated | 3 studies, 773 participants, 195 events | High | Unlikely that further research would reveal significant benefit, especially as potential high positive bias exists in the calculations we have because of LOCF imputation or completer analyses |
| Participants with at least 1 adverse event (all conditions) | 622 in 1000 | 717 in 1000 | RR 1.1 (1.01 to 1.2) NNH 10 (6.5 to 27) | 7 studies, 1121 participants, 768 events | High | Large numbers of events |
| Participants with a serious adverse event (all conditions) | No data | Very low | No data | |||
| Participants with rash (all conditions) | 56 in 1000 | 95 in 1000 | RR 1.4 (1.01 to 2.0) NNH 27 (16 to 89) | 12 studies, 1715 participants, 138 events | Moderate | Modest number of events |
| Deaths (all conditions) | No data | Very low | No data | |||
| GRADE Working Group grades of evidence | ||||||
| LOCF: last observation carried forward; NNT: number needed to treat for an additional beneficial outcome: NNH: number needed to treat for an additional harmful outcome; RR: risk ratio | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 50% pain relief Show forest plot | 3 | 773 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.82, 1.42] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 At least one adverse event Show forest plot | 7 | 1121 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [1.01, 1.22] |
| 2 Rash Show forest plot | 12 | 1715 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.43 [1.01, 2.03] |

