拉莫三嗪治疗成人慢性神经病理性疼痛和纤维肌痛
Referencias
References to studies included in this review
References to studies excluded from this review
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | Randomised DB placebo controlled, parallel group study for 11 weeks. One‐week screening phase, 8‐week treatment phase, 2‐week post‐treatment phase | |
| Participants | 59 participants with painful diabetic neuropathy. Age 50 to 60 years Excluded: participants who had received antiepileptics or antidepressants for reasons other than pain and those who had received opioids | |
| Interventions | Lamotrigine 25 mg dispersible tablets or matching placebo Rescue analgesia as paracetamol, dipyrone or NSAIDs | |
| Outcomes | Daily pain intensity, McGill, Beck depression, Pain disability index, Global assessment. Responder: 50% reduction in pain measured in final 3 weeks of treatment | |
| Notes | Oxford Quality Score: R2, DB2, W1 = 5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomisation was done in blocks of four according to a computer generated random code" |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) | Low risk | "Patients in the placebo group received equal numbers of identical looking placebo tablets" |
| Selective reporting (reporting bias) | Unclear risk | Not stated |
| Missing data | High risk | Completer analysis ‐ data from withdrawals not carried forward |
| Duration | Low risk | "Eight weeks treatment phase" |
| Outcome | Unclear risk | Looked for reduction in pain intensity but reports numbers with 50% reduction. No mention of imputation method |
| Treatment arm size | High risk | 59 participants: 29 active, 30 placebo |
| Methods | Randomised DB placebo controlled, cross‐over study. One‐week baseline assessment, two 9‐week treatment periods separated by 2‐week washout | |
| Participants | 30 participants with neuropathic pain after traumatic spinal cord injury (SCI). Age 27 to 63 years | |
| Interventions | Lamotrigine tablets or identical placebo. Dose escalation to 400 mg a day. Weeks 1 and 2 at 25 mg daily, weeks 3 and 4 at 50 mg, 1 week each at 100 mg, 200 mg, and 300 mg then 2 weeks at 400 mg. Concomitant treatment with spasmolytics, sedatives for insomnia, and simple analgesics for other pain allowed in constant unchanged dose Rescue analgesia: paracetamol up to 3 g daily | |
| Outcomes | Average daily pain on 11‐point numeric scale. Change in median weekly pain score from baseline to final week. Participant preference, other measures included details of types of pain, impact on sleep, and use of rescue medication | |
| Notes | Oxford Quality Score: R2, DB2, W1 = 5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "assignment to treatment was random via a computer generated randomisation list with blocks of four" |
| Allocation concealment (selection bias) | Low risk | "The primary investigator was provided with sealed code envelopes‐ one for each patient‐ containing information on the treatment given . . . and envelopes were returned unopened to the monitor after the study termination." |
| Blinding (performance bias and detection bias) | Low risk | "lamotrigine and identical placebo" |
| Selective reporting (reporting bias) | Unclear risk | Not stated |
| Missing data | High risk | completer |
| Duration | Low risk | Nine week per arm treatment period |
| Outcome | Unclear risk | Looked for reduction in pain intensity but reports numbers with 50% reduction |
| Treatment arm size | High risk | 30 participants total, 22 completers |
| Methods | Randomised DB active controlled, cross‐over study. Two 6‐week treatment periods separated by 2‐week washout | |
| Participants | 53 participants, of whom 46 received both treatments, with Type 2 Diabetes and painful diabetic neuropathy for at least 1 month Excluded: participants taking antidepressants, antiepileptics, opioids and local anaesthetic agents | |
| Interventions | Lamotrigine dose escalation to 100 mg twice daily over 6 weeks or amitriptyline to 50 mg at night with matching placebo in the morning. Two‐week washout using placebo between treatment periods Rescue analgesia: paracetamol up to 3 g daily | |
| Outcomes | Patient global assessment (> 50% pain relief = good, > 25% pain relief), VAS PI, short form McGill, 5‐point categorical scale for pain and Hamilton depression scale | |
| Notes | CONSORT flow chart indicated 23 patients randomised to lamotrigine and 30 to amitriptyline on first cross‐over arm, 23 each on second. 46 participants included in ITT analysis. Outcomes reported for both arms of cross‐over, with 46 as denominator for efficacy Oxford Quality Score: R2, DB2, W1 = 5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "numbers generated using random number tables by block randomisation" |
| Allocation concealment (selection bias) | Low risk | "blinding and randomisation carried out by independent person unrelated to the study", "drug codes were maintained under lock and key" |
| Blinding (performance bias and detection bias) | Low risk | "drugs were blinded, packed and numbered serially" |
| Selective reporting (reporting bias) | Unclear risk | Not stated |
| Missing data | Unclear risk | LOCF used |
| Duration | High risk | 6 weeks dose escalation then cross over |
| Outcome | Low risk | "VAS score showing improvement of > 50%, > 25% and < 25%" used |
| Treatment arm size | High risk | 53 participants; 23 per treatment arm, with 46 completers |
| Methods | Randomised DB placebo controlled, parallel group study. Eight‐week treatment period | |
| Participants | 100 participants with intractable neuropathic pain. Mean age placebo group 44.7 years, lamotrigine group 47.1 years. All had failed response to codeine or NSAID‐based analgesics Excluded: participants taking antiepileptics | |
| Interventions | Lamotrigine 25 mg dispersible tablets or matching placebo Rescue analgesia not reported | |
| Outcomes | Daily participant‐recorded VAS for PI, shooting pain, burning pain, paraesthesia, numbness, QOL, mobility, sleep and mood. Daily analgesic consumption | |
| Notes | 18 withdrew: 8 nausea (5 placebo, 3 lamotrigine); 2 skin rash (1 lamotrigine); 2 bad taste of tablets (1 lamotrigine); 6 due to lack of analgesia (2 placebo, 4 lamotrigine). Eight failed to attend final assessment Oxford Quality Score: R2, DB2, W1 = 5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients randomly assigned in equal numbers to one of two groups using computer generated random number lists" |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) | Low risk | "patients received either lamotrigine.. . . . or identical looking dispersible placebo tablets" |
| Selective reporting (reporting bias) | Unclear risk | Not stated |
| Missing data | Unclear risk | Not stated |
| Duration | Low risk | 8‐week study |
| Outcome | Unclear risk | VAS recorded |
| Treatment arm size | High risk | 74 participants; placebo 38, lamotrigine 36 |
| Methods | Randomised DB placebo controlled, parallel group study. Ten‐week treatment period, followed by 4‐week tapered withdrawal | |
| Participants | 125 participants (63 received lamotrigine) with diagnosis of symptomatic chemotherapy‐induced peripheral neuropathy > 1 month due to neurotoxic agents. Age 29 to 84 years. Average pain > 4 on NRS Excluded: participants taking drugs for treating neuropathic pain, including antiepileptics, opioids or topical analgesics at study entry; NSAIDs were permitted. | |
| Interventions | Lamotrigine or matching placebo. 25 mg once daily for 2 weeks, then 25 mg, 50 mg, 100 mg, 150 mg twice daily for 2 weeks at each dose, then 4 weeks taper down | |
| Outcomes | Average daily pain score using NRS and ENS (Eastern Cooperative Oncology neuropathy scale). | |
| Notes | Oxford Quality Score: R1, DB2, W1 = 4 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | stated to be randomised |
| Allocation concealment (selection bias) | Unclear risk | Not statement |
| Blinding (performance bias and detection bias) | Low risk | "an identical appearing placebo" |
| Selective reporting (reporting bias) | Unclear risk | Not stated |
| Missing data | Unclear risk | Not stated |
| Duration | Low risk | 10 weeks |
| Outcome | Unclear risk | Average daily pain scores |
| Treatment arm size | Unclear risk | 125 participants; lamotrigine 63, placebo 62 |
| Methods | Randomised DB placebo controlled, parallel group, 'add on study'. Fourteen‐week treatment period consisting of 8 weeks dose escalation and 6 weeks at fixed dose, followed by 1 week tapered withdrawal | |
| Participants | Neuropathic pain defined as DN, PHN, nerve injury, spinal cord injury, MS or HIV neuropathy. Mean age 60 years (SD 12). Mean weekly pain score > 4 on 11‐point scale. Participants on stable (≥ 4 weeks) treatment with gabapentin, tricyclics or non‐opioid analgesics Excluded: back and neck pain | |
| Interventions | Lamotrigine 200 to 400 mg daily or placebo in addition to other (inadequate) treatments as above Rescue analgesia: paracetamol up to 3 g daily | |
| Outcomes | Numerical PR (11‐point), sleep interference, short form McGill, neuropathic pain scale, Patient Global Impression of Change | |
| Notes | Oxford Quality Score: R1, DB2, W1 = 4 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomised in a 1:1 ratio" |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) | Unclear risk | Placebo tablets were "identical in appearance" |
| Selective reporting (reporting bias) | Unclear risk | Not stated |
| Missing data | Unclear risk | LOCF |
| Duration | Low risk | 14 week treatment |
| Outcome | Unclear risk | Change in daily pain intensity |
| Treatment arm size | Unclear risk | 111 participants lamotrigine, 109 placebo |
| Methods | Multicentre randomised DB placebo controlled, parallel study. Fourteen‐week treatment period | |
| Participants | 42 participants with painful HIV associated polyneuropathy. Mean age 44 years Excluded: participants taking valproate | |
| Interventions | Lamotrigine or placebo. Week 1 and 2 at 25 mg daily, weeks 3 and 4 at 50 mg daily, week 5 at 100 mg daily, week 6 at 100 mg twice daily, then weeks 7 to 14 at 150 mg twice daily | |
| Outcomes | Average and peak neuropathic pain using Gracely Pain Scale. Difference in weekly mean pain scores. Pain assessed in weeks 1 and 14, also slope of change in pain scores | |
| Notes | Oxford Quality Score: R2, DB2, W1 = 5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The biostatistician generated a list of treatment assignments in random order using a program written in SAS" |
| Allocation concealment (selection bias) | Low risk | "The biostatistician had no contact with patients nor did he communicate these to anyone other than the pharmacists' (to supply the medicines)" |
| Blinding (performance bias and detection bias) | Low risk | "Lamotrigine and matching placebo" |
| Selective reporting (reporting bias) | Unclear risk | LOCF used for part of the analysis |
| Missing data | High risk | Combination of LOCF and completer |
| Duration | Low risk | 14 weeks including dose escalation |
| Outcome | Unclear risk | Difference in weekly mean pain scores between baseline and final week |
| Treatment arm size | High risk | 42 participants in total at start |
| Methods | Randomised DB placebo controlled parallel multicentre trial. One‐week screening phase, then 11‐week treatment period. Randomisation stratified according to use of neurotoxic antiretroviral therapy (ART) | |
| Participants | 227 participants with HIV‐associated sensory neuropathy. Age 32 to 67 years Excluded: participants with previous or current use of lamotrigine | |
| Interventions | Lamotrigine or placebo. Weeks 1 and 2 at 25 mg on alternate days (daily if taking enzyme‐inducing drugs), then dose escalation over 5 weeks to a target dose of 400 mg daily (up to 600 mg daily allowed if taking enzyme‐inducing drugs), followed by 4‐week maintenance phase. Concomitant medication allowed if stable (≥ 4 weeks) and unchanged Rescue analgesia: opioid and non‐opioid analgesics as needed | |
| Outcomes | Daily pain rating of average pain and worst pain on Gracely Pain Scale. VAS PI and short form McGill at end of baseline and beginning and end of maintenance phase, PGIC | |
| Notes | Oxford Quality Score: R1, DB1, W1 = 3 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomised" |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) | Unclear risk | "double blind placebo controlled" |
| Selective reporting (reporting bias) | Unclear risk | Not stated |
| Missing data | Unclear risk | observed scores used ‐ meaning unclear |
| Duration | Low risk | 13 weeks including dose escalation |
| Outcome | Unclear risk | "average pain and worse pain" recorded |
| Treatment arm size | Unclear risk | 227 participants; 150 lamotrigine, 77 placebo |
| Methods | Randomised DB placebo controlled, cross‐over study. Two 8‐week treatment periods, separated by 2‐week washout | |
| Participants | 30 participants with central post‐stroke pain with score of > 4 on an 11‐point scale. Age 37 to 77 years | |
| Interventions | Lamotrigine soluble tablets or matching placebo. Initial dose of 25 mg daily increased every 2nd week to 200 mg daily. No concomitant use of antidepressants, antiepileptics or analgesics allowed Rescue analgesia: paracetamol 500 mg as needed | |
| Outcomes | Average daily pain score during last week of treatment (11‐point Likert scale). Clinical responders defined as 2/10 reduction on lamotrigine compared with placebo period. CAT PR and CAT PI. Use of rescue medication | |
| Notes | Oxford Quality Score: R2, DB2, W1 = 5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "patients were randomised to treatment according to a computer generated randomisation list with a cluster size of six" |
| Allocation concealment (selection bias) | Low risk | "code envelopes were kept by the investigator during the trial and returned unopened to the monitor at the termination of the study. The blinding was maintained throughout" |
| Blinding (performance bias and detection bias) | Low risk | "soluble lamotrigine and identical placebo" |
| Selective reporting (reporting bias) | Unclear risk | Not stated |
| Missing data | High risk | Completer analysis |
| Duration | Low risk | Two 8‐week cross‐over periods |
| Outcome | High risk | Clinical response stated to be 2 or more points lower for lamotrigine compared to placebo |
| Treatment arm size | High risk | 30 participants; 16 lamotrigine, 13 placebo at initial randomisation, with 20 completers |
| Methods | Randomised DB placebo controlled parallel group. Nineteen‐week treatment period comprising 7‐week dose escalation and 12‐week fixed dose maintenance phase. Study no NPP30004 | |
| Participants | 360 participants with diabetic neuropathy (type1 or type 2 diabetics). Pain > 6 months and pain score > 4 on 11‐point Likert scale. Mean age 59 years (SD 11). | |
| Interventions | Lamotrigine at daily dose of 200 mg, 300 mg, 400 mg, or placebo. Dose doubled initially every 2nd week, then weekly to target dose. Concomitant gabapentin and TCAs allowed Rescue analgesia: paracetamol up to 4 g daily 91/360 received gabapentin, 17/360 received TCAs | |
| Outcomes | Average pain intensity (11 point pain NRS). Sleep disturbance. Short form McGill | |
| Notes | Oxford Qulaity Score: R2, DB1, W1 = 4 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 'in accordance with a computer generated randomisation schedule. A central randomisation procedure was used' |
| Allocation concealment (selection bias) | Low risk | 'the study center called into a central system' |
| Blinding (performance bias and detection bias) | Unclear risk | stated to be double blind |
| Selective reporting (reporting bias) | Unclear risk | Not stated |
| Missing data | Unclear risk | LOCF |
| Duration | Low risk | Seven week dose escalation and 12 weeks fixed dose |
| Outcome | Low risk | 50% reduction in pain intensity |
| Treatment arm size | Unclear risk | 360 participants; 90 patients randomised per group |
| Methods | Randomised DB placebo controlled parallel group. Nineteen week treatment period comprising 7 week dose escalation and 12 week fixed dose maintenance phase. Study no NPP30005 | |
| Participants | 360 participants with diabetic neuropathy (type 1 or type 2 diabetics). Pain > 6 months and pain score > 4 on 11 point Likert scale. Mean age 60 years (SD 11). Gabapentin and TCAs allowed. Paracetamol as rescue. | |
| Interventions | Lamotrigine at daily dose of 200 mg, 300 mg, 400 mg, or placebo. Dose doubled initially every 2nd week, then weekly to target dose. Concomitant gabapentin and TCAs allowed. Rescue analgesia: paracetamol up to 4 g daily 76/360 received gabapentin, 23/360 received TCAs | |
| Outcomes | Average pain intensity (11‐point pain NRS). Sleep disturbance. Short form McGill Greater than 50% reduction in average pain intensity | |
| Notes | Oxford Quality Score: R2, DB1, W1 = 4 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "in accordance with a computer generated randomisation schedule. A central randomisation procedure was used" |
| Allocation concealment (selection bias) | Low risk | "the study center called into a central system" |
| Blinding (performance bias and detection bias) | Unclear risk | stated to be double blind |
| Selective reporting (reporting bias) | Unclear risk | Not stated |
| Missing data | Unclear risk | LOCF |
| Duration | Low risk | 7‐week dose escalation and 12 weeks fixed dose |
| Outcome | Low risk | 50% reduction in pain intensity |
| Treatment arm size | Unclear risk | 360 participants; 90 randomised per group |
| Methods | Randomised DB placebo controlled, cross‐over, 'add on study'. Two 2‐week treatment periods separated by 3‐day washout. Lamotrigine added to existing antiepileptic treatment | |
| Participants | 14 participants with refractory trigeminal neuralgia. Age 44 to 75 (mean 60 years) | |
| Interventions | Lamotrigine or placebo added to existing stable regimen of carbamazepine or phenytoin, or both. Day 1 at 50 mg, day 2 at 100 mg, day 3 at 200 mg, then days 4 to 14 at 400 mg. Rescue analgesia: increased dose of carbamazepine or phenytoin used for uncontrollable pain | |
| Outcomes | No of pain paroxysms. CAT PI, CAT PR and global assessment at the end of each treatment period | |
| Notes | Oxford Quality Score: R1, DB2, W1 = 4 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomised" |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) | Low risk | "dispersible lamotrigine and identical placebo" |
| Selective reporting (reporting bias) | Unclear risk | Not stated |
| Missing data | Unclear risk | unclear |
| Duration | High risk | 2 weeks per arm |
| Outcome | Unclear risk | composite efficacy index |
| Treatment arm size | High risk | 14 participants |
AEs = adverse effects, DB = double blind, CAT PI = categorical scale of pain intensity, CAT PR = categorical scale of pain relief, LOCF = last observation carried forward, NNTB = number needed to treat for an additional beneficial outcome, NRS = numerical rating scale, NSAID = non‐steroidal anti‐inflammatory drug, PI = pain intensity, QOL = quality of life, R = randomisation, TCA = tricyclic antidepressant, VAS = visual analogue scale, W = withdrawals
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| Pre‐emptive study but all participants also received a known analgesic‐buprenorphine | |
| RCT of multiple sclerosis pain. Not neuropathic pain | |
| Case study | |
| Survey not RCT | |
| Case report only | |
| Not randomised | |
| Not randomised | |
| Review article | |
| Case series | |
| RCT but healthy volunteers | |
| Case report | |
| Not randomised or double blind |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 50% pain relief Show forest plot | 3 | 773 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.82, 1.42] |
| Analysis 1.1 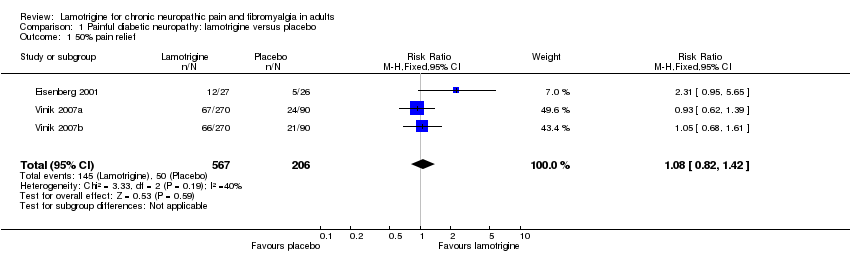 Comparison 1 Painful diabetic neuropathy: lamotrigine versus placebo, Outcome 1 50% pain relief. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 At least one adverse event Show forest plot | 7 | 1121 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [1.01, 1.22] |
| Analysis 2.1  Comparison 2 All conditions: lamotrigine versus placebo, Outcome 1 At least one adverse event. | ||||
| 2 Rash Show forest plot | 12 | 1715 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.43 [1.01, 2.03] |
| Analysis 2.2  Comparison 2 All conditions: lamotrigine versus placebo, Outcome 2 Rash. | ||||

Study flow diagram.
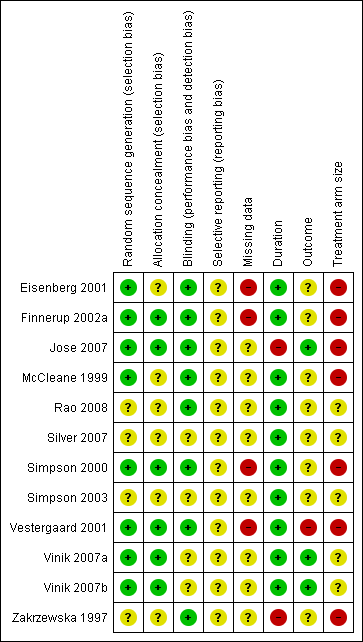
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Forest plot of comparison: 1 Painful diabetic neuropathy, outcome: 1.1 50% pain relief.

Forest plot of comparison: 2 All conditions: lamotrigine versus placebo, outcome: 2.2 Rash.

Comparison 1 Painful diabetic neuropathy: lamotrigine versus placebo, Outcome 1 50% pain relief.

Comparison 2 All conditions: lamotrigine versus placebo, Outcome 1 At least one adverse event.

Comparison 2 All conditions: lamotrigine versus placebo, Outcome 2 Rash.
| Lamotrigine compared with placebo for painful diabetic neuropathy | ||||||
| Patient or population: neuropathic pain (three studies in painful diabetic neuropathy) Settings: Community Intervention: oral lamotrigine 200 to 400 mg daily Comparison: placebo | ||||||
| Outcomes | Probable outcome with | Risk ratio NNTor NNH (95% CI) | No of studies, attacks, events | Quality of the evidence | Comments | |
| comparator | intervention | |||||
| At least 50% of maximum pain relief | 240 in 1000 | 260 in 1000 | RR 1.1 (0.82 to 1.4) NNT not calculated | 3 studies, 773 participants, 195 events | High | Unlikely that further research would reveal significant benefit, especially as potential high positive bias exists in the calculations we have because of LOCF imputation or completer analyses |
| Participants with at least 1 adverse event (all conditions) | 622 in 1000 | 717 in 1000 | RR 1.1 (1.01 to 1.2) NNH 10 (6.5 to 27) | 7 studies, 1121 participants, 768 events | High | Large numbers of events |
| Participants with a serious adverse event (all conditions) | No data | Very low | No data | |||
| Participants with rash (all conditions) | 56 in 1000 | 95 in 1000 | RR 1.4 (1.01 to 2.0) NNH 27 (16 to 89) | 12 studies, 1715 participants, 138 events | Moderate | Modest number of events |
| Deaths (all conditions) | No data | Very low | No data | |||
| GRADE Working Group grades of evidence | ||||||
| LOCF: last observation carried forward; NNT: number needed to treat for an additional beneficial outcome: NNH: number needed to treat for an additional harmful outcome; RR: risk ratio | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 50% pain relief Show forest plot | 3 | 773 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.82, 1.42] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 At least one adverse event Show forest plot | 7 | 1121 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [1.01, 1.22] |
| 2 Rash Show forest plot | 12 | 1715 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.43 [1.01, 2.03] |

