Tratamiento vesical a largo plazo con sondaje intermitente en adultos y niños
Referencias
Referencias de los estudios incluidos en esta revisión
Referencias de los estudios excluidos de esta revisión
Referencias de los estudios en espera de evaluación
Referencias de los estudios en curso
Referencias adicionales
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | DESIGN: Randomised controlled trial | |
| Participants | N=11 | |
| Interventions | COATED VS UNCOATED: integrated catheter and bag system (all‐in‐one) or sterile technique with open catheter tray | |
| Outcomes | 3 urines for culture over a 24 hour period + meatal swabs | |
| Notes | no difference between groups but sample too small and time frame too short to make any inferences | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | DESIGN: Randomised controlled trial; ALLOCATION: done by investigator using sealed opaque envelopes. | |
| Participants | N=123 | |
| Interventions | COATED VS UNCOATED: one catheter over another; assessed at Day 15 then monthly x 12 m. | |
| Outcomes | Primary: UTI | |
| Notes | UTI described as "clinical infection with Sx of UTI and for which treatment was prescribed", however, lab analyses did not differ between groups. significant challenges in retaining subjects illustrating the difficulty of conducting trials in this group. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | DESIGN: Randomised controlled trial ALLOCATION: not described but did stratify subjects according to presence/ absence of UTI and study site. | |
| Participants | N=80 | |
| Interventions | STERILE TECHNIQUE VS CLEAN TECHNIQUE (ALSO SINGLE VS MULTIPLE USE): sterile equipment and procedure, cleaning with betadine; Clean techique: catheter washed with soap and water and reused x 1 week. | |
| Outcomes | Number of treatment episodes for UTI + urinalysis, and cost up to 90 days. | |
| Notes | Some subjects had indwelling catheters prior to enrolment in the study (unstated how many); weeks to onset of symptomatic UTI was 3.11 (3.12) for Treatment and 3.5 (3.02) for control. Dropout rate high after Day 15 with only 39 completing data collection to Day 90. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | DESIGN: Randomised controlled trial ALLOCATION: not described | |
| Participants | N=20 | |
| Interventions | OTHER STRATEGIES DESIGNED TO REDUCE INFECTION: Gentamycin cream (0.1%) versus lidocaine jelly used as separate lubricant for IC | |
| Outcomes | Number of episodes of asymptomatic bacteriuria (>= 100,000 CFU/ml) , number of patients with symptomatic UTI | |
| Notes | Repeated measures of asymptomatic bacteriuria reported for each participant. Final measure used in table of results. Asymptomatic bacteriuria similar in both groups 8/10 in gentamycin group 6/10 in lidocaine group. 1/10 developed symptomatic UTI in gentamycin group, 2/10 in Lidocaine group. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | D ‐ Not used |
| Methods | DESIGN: Randomised controlled cross over trial | |
| Participants | N=18 | |
| Interventions | COATED VS UNCOATED: Single use PVC (Nelaton) catheter vs pre lubricated non‐hydrophilic catheter; one catheter x 7 wks then crossover to other group. | |
| Outcomes | UTI measured by C&S at 2, 4 & 7 weeks; | |
| Notes | UTI defined as cloudy, odourous urine, onset of UI, increase autonomic dysreflexia, pyuria, bacteriuria; SS too small to draw conclusions. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | C ‐ Inadequate |
| Methods | DESIGN: Randomised controlled trial | |
| Participants | N=46 | |
| Interventions | STERILE VS CLEAN TECHNIQUE (also Single Use vs Multiple Use) catheterisation kit and sterile single use catheter, meatus cleansed with povidone iodine. | |
| Outcomes | daily urine dipslides + symptomatic UTI | |
| Notes | No statistically significant differences between urine cultures or Sx UTI; weeks to onset of UTI was 1.1 (0.87) for treatment and 1.2 (1.0) for control. Number of days in study varied from 1 to 28 with only | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | DESIGN: Randomised controlled cross‐over trial with each arm 6 months | |
| Participants | N=30 | |
| Interventions | SINGLE VS MULTI USE:sterile single use PVC or reused PVC | |
| Outcomes | Bacteriuria > 10x3 CFU/ml obtained monthly; no difference between groups. | |
| Notes | Symptomatic UTI defined as + symptoms; catheters washed with liquid soap and water, air dried and reused (does not indicate length of reuse); several subjects took prophylactic antibiotics. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | DESIGN: Randomised controlled trial | |
| Participants | N=36 | |
| Interventions | STERILE TECHNIQUE VS CLEAN TECHNIQUE Sterile single use PVC catheter with sterile technique or sterile single use PVC catheter with clean technique (clean gloves, clean container, non‐sterile wipes for cleansing pre catheterisation) | |
| Outcomes | Days to onset of symptomatic UTI | |
| Notes | UTI defined as >= 10x5 CFU/ml, pyuria + accompanying symptoms; no difference between groups. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
| Methods | DESIGN: Randomised controlled crossover trial | |
| Participants | N=32 | |
| Interventions | COATED VS UNCOATED (ALSO SINGLE USE STERILE VS MULTIUSE CLEAN) hydrophilic (Lofric) single use) or PVC (multiple use) x 3 weeks each | |
| Outcomes | Urine for C&S at baseline and each 3 week point; haematuria; responses to catheter use questionnaire | |
| Notes | UTI defined as > 10 x 4 CFU/ml. No differences between groups in questionnaire response, bacteriuria or haematuria but short follow up and small sample size. Unable to use data in Table of Comparisons because of cross‐over design and no mid‐point data. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | DESIGN: Randomised controlled trial | |
| Participants | N=29 | |
| Interventions | SINGLE USE (STERILE) VS MULTIUSE (CLEAN) (also COATED VS UNCOATED) clean reused red rubber catheter (x 1 week) or integrated catheter + bag system | |
| Outcomes | UTI Urine for C&S collected weekly x X wks ‐‐ unclear on study time frame or endpoint. | |
| Notes | UTI as defined by NIDRR (1992); higher % of UTI in closed system (42% vs 29%) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | DESIGN: Randomised controlled trial | |
| Participants | N=30 | |
| Interventions | COATED VS UNCOATED: Integrated catheter + bag system or open sterile system | |
| Outcomes | UTI >10x5 CFU/ml + symptoms (fever, CV or SP tenderness) | |
| Notes | data only collected for 4 days | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | DESIGN: Randomised controlled cross over. | |
| Participants | N=10 | |
| Interventions | SINGLE VS MULTIUSE sterile 1 x use PVC or reused | |
| Outcomes | UTI weekly urine for C&S x 4 months | |
| Notes | UTI defined as + or > than 10x4 CFU/ml plus symptoms (fever, pain, change in continence, change in colour or odour of urine); | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | DESIGN: Randomised controlled trial | |
| Participants | N=33 | |
| Interventions | SINGLE VS MULTIUSE: hydrophilic (Lofric) (single use) or PVC reused catheter. | |
| Outcomes | UTI | |
| Notes | UTI defined as 10x5 CFU/ml + Sx (not defined); subjects with positive cultures were treated and reentered into the trial; no diff in bacteriuria b/w groups; haematuria lower in Lofric group but SS too small to draw conclusions and groups included gastric augmentation as well. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | DESIGN: Randomised controlled trial | |
| Participants | N=62 | |
| Interventions | COATED VS UNCOATED; ALSO SINGLE VS MULTIUSE: hydrophilic coated catheter (Lofric) (single use) vs PVC clean reused times 24 hours. | |
| Outcomes | UTI; | |
| Notes | UTI defined as 10x5 CFU/ml + at least one clinical symptom (fever, chills, malodorous urine, increased spasticity, malaise). Catheter cleaning not described; used 1 reused catheter per day. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Retrospective chart review of incidence of UTI in patients using standard practice IC in rehabilitation from 1985‐1988 then evaluation of UTI in 18 subjects of a closed catheter/bag system. Not an RCT. | |
| Patient satisfaction evaluated only; not randomized; does not evaluate UTI. | |
| Report on study published in British Journal of Urology 2001 comparing user impressions of different hydrophilic coated catheters available on the market at the time; does not compare PVC to hydrophilic or incidence of UTI ADRIAN: ? NOT RANDOMISED? | |
| Article in German; appears to be a review of catheterisation methods (based on short English Abstract) | |
| Review of the literature | |
| Laboratory study evaluating the likelihood of catheter contamination based on catheter design; did not compare incidence of UTI. Not an RCT | |
| Survey of catheter users who reused a silicone catheter ‐ does not compare different products or provide quantitative measure of UTI. Not an RCT | |
| Review article discussing various catheterisation methods. | |
| Indwelling catheterisation vs no indwelling catheter but IC in patients undergoing spina fusion. Not a suitable comparison. | |
| Not an RCT | |
| Hand washing comparison (30 s + double gloving) or 3 minutes hand to elbow + sterile gown on incidence of UTI in patients receiving indwelling catheterisation. IC not used. | |
| Survey of individuals using intermittent catheterisation and reusing the catheter. Not an RCT. | |
| Chart review of two difference cleaning methods and comparison of UTI (wash with soap and water then soak in povidone iodine or allow to air dry) . Not an RCT. | |
| Laboratory evaluation of friction force of 2 hydrophilic catheters and one non‐hydrophilic catheter. Not an RCT. | |
| Observational study of the incidence and time to onset of UTI in elderly in a Veterans Administration Centre (USA). Not an RCT. | |
| Abstract in ICS 2004 unpublished proceedings. Unable to reach author for further information on the study. No usable data. | |
| Case report of one type of catheter proposed by the authors "Wu Reusable Catheter". Not an RCT. |
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Multicentre trial comparing occurrence of UTI in patients with SCI using either coated or uncoated intermittent catheterisation |
| Methods | |
| Participants | newly SCI adults requiring self or health care provider intermittent cahteterisation |
| Interventions | randomized to sterile single use PVC or sterile single use hydrophilic catheters (Speedicath) |
| Outcomes | incidence of symptomatic UTI, haematuria, satisfaction with products by subject and staff; antibiotic use; appointments missed |
| Starting date | July 2006 |
| Contact information | Darin Hurninan |
| Notes |
| Trial name or title | Incidence of UTI in children with spina bifida using clean reused PVC or sterile single use hydrophilic catheters |
| Methods | |
| Participants | children with spina bifida requiring intermittent catheterisation |
| Interventions | sterile single use hydrophilic or standard care (clean reused PVC catheter) |
| Outcomes | incidence of symptomatic UTI; haematuria; antibiotic use, days missed from school, physician appointments, subject satisfaction |
| Starting date | January 2007 |
| Contact information | Katherine Moore University of Alberta, Canada |
| Notes |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number with asymptomatic bacteriuria Show forest plot | 1 | 36 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.47, 2.03] |
| Analysis 1.1  Comparison 1 Sterile technique versus clean technique, Outcome 1 Number with asymptomatic bacteriuria. | ||||
| 1.1 uncoated (sterile catheter) both arms | 1 | 36 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.47, 2.03] |
| 1.2 single use (uncoated, sterile catheter) versus multiple use (uncoated, clean catheter) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Number with symptomatic UTI Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.2  Comparison 1 Sterile technique versus clean technique, Outcome 2 Number with symptomatic UTI. | ||||
| 2.1 uncoated (sterile catheter) both arms | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 single use (uncoated, sterile catheter) versus multiple use (uncoated, clean catheter) | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Number with urethral trauma/bleeding | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.1 uncoated (sterile catheter) both arms | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Number with stricture formation | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.1 uncoated (sterile catheter) both arms | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Number with microscopic haematuria | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.1 uncoated (sterile catheter) both arms | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Number with urethritis, epididymitis, or orchitis | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.1 uncoated (sterile catheter) both arms | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Weeks to onset of symptomatic UTI Show forest plot | 3 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.7  Comparison 1 Sterile technique versus clean technique, Outcome 7 Weeks to onset of symptomatic UTI. | ||||
| 7.1 uncoated (sterile catheter) both arms | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.2 single use (uncoated) versus multiple use (uncoated) | 2 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 Number reporting satisfaction with catheter product | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.1 uncoated (sterile catheter) both arms | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Number reporting comfort and ease of insertion | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9.1 uncoated (sterile catheter) both arms | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Number reporting preference | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10.1 uncoated (sterile catheter) both arms | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number with asymptomatic bacteriuria Show forest plot | 1 | 11 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.17 [0.01, 2.92] |
| Analysis 2.1  Comparison 2 Coated versus uncoated catheter, Outcome 1 Number with asymptomatic bacteriuria. | ||||
| 1.1 sterile technique and catheter (both arms) | 1 | 11 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.17 [0.01, 2.92] |
| 1.2 clean technique (sterile catheter) both arms | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.3 single use (sterile catheter) clean technique versus multiple use (clean catheter) clean technique | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Number with symptomatic UTI Show forest plot | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.2  Comparison 2 Coated versus uncoated catheter, Outcome 2 Number with symptomatic UTI. | ||||
| 2.1 sterile technique and catheter (both arms) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 sterile catheter (clean technique) both arms | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 single use (sterile catheter) clean technique versus multiple use (clean catheter) clean technique | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Number with urethral trauma/bleeding | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.1 sterile technique and catheter (both arms) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 sterile catheter (clean technique) both arms | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.3 single use (sterile catheter) clean technique versus multiple use (clean catheter) clean technique | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Number with stricture formation Show forest plot | 1 | 58 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.92 [0.17, 92.43] |
| Analysis 2.4  Comparison 2 Coated versus uncoated catheter, Outcome 4 Number with stricture formation. | ||||
| 4.1 sterile technique and catheter (both arms) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 sterile catheter (clean technique) both arms | 1 | 58 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.92 [0.17, 92.43] |
| 4.3 single use (sterile catheter) clean technique versus multiple use (clean catheter) clean technique | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Number with microscopic haematuria Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.5  Comparison 2 Coated versus uncoated catheter, Outcome 5 Number with microscopic haematuria. | ||||
| 5.1 sterile technique and catheter (both arms) | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 sterile catheter (clean technique) both arms | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.3 single use (sterile catheter) clean technique versus multiple use (clean catheter) clean technique | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Number with urethritis, epididymitis, or orchitis | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.1 sterile technique and catheter (both arms) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.2 sterile catheter (clean technique) both arms | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.3 single use (sterile catheter) clean technique versus multiple use (clean catheter) clean technique | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Weeks to onset of symptomatic UTI | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7.1 sterile technique and catheter (both arms) | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7.2 sterile catheter (clean technique) both arms | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7.3 single use (sterile catheter) clean technique versus multiple use (clean catheter) clean technique | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Number reporting satisfaction with catheter product | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.1 sterile technique and catheter (both arms) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.2 sterile catheter (clean technique) both arms | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.3 single use (sterile catheter) clean technique versus multiple use (clean catheter) clean technique | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Number reporting comfort and ease of insertion | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9.1 sterile technique and catheter (both arms) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9.2 sterile catheter (clean technique) both arms | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9.3 single use (sterile catheter) clean technique versus multiple use (clean catheter) clean technique | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Number reporting preference Show forest plot | 1 | 58 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.70 [0.73, 3.93] |
| Analysis 2.10  Comparison 2 Coated versus uncoated catheter, Outcome 10 Number reporting preference. | ||||
| 10.1 sterile technique and catheter (both arms) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10.2 sterile catheter (clean technique) both arms | 1 | 58 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.70 [0.73, 3.93] |
| 10.3 single use (sterile catheter) clean technique versus multiple use (clean catheter) clean technique | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number with asymptomatic bacteriuria Show forest plot | 1 | 66 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.66, 1.72] |
| Analysis 3.1  Comparison 3 Single use (sterile) versus multiple use (clean) catheter, Outcome 1 Number with asymptomatic bacteriuria. | ||||
| 1.1 uncoated catheter, clean technique (both arms) | 1 | 66 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.66, 1.72] |
| 1.2 coated versus uncoated (clean technique both arms) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.3 sterile technique versus clean technique (uncoated catheter both arms) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Number with symptomatic UTI Show forest plot | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 3.2  Comparison 3 Single use (sterile) versus multiple use (clean) catheter, Outcome 2 Number with symptomatic UTI. | ||||
| 2.1 uncoated catheter, clean technique (both arms) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 coated versus uncoated (clean technique both arms) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 sterile technique versus clean technique (uncoated catheter both arms) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.4 coated (sterile technique) versus uncoated (clean technique) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Number with urethral trauma/bleeding | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.1 uncoated catheter, clean technique (both arms) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 coated versus uncoated (clean technique both arms) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.3 sterile technique versus clean technique (uncoated catheter both arms) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Number with stricture formation | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.1 uncoated catheter, clean technique (both arms) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 coated versus uncoated (clean technique both arms) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.3 sterile technique versus clean technique (uncoated catheter both arms) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Number with microscopic haematuria Show forest plot | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.24, 0.95] |
| Analysis 3.5  Comparison 3 Single use (sterile) versus multiple use (clean) catheter, Outcome 5 Number with microscopic haematuria. | ||||
| 5.1 uncoated catheter, clean technique (both arms) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 coated versus uncoated (clean technique both arms) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.3 sterile technique versus clean technique (uncoated catheter both arms) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.4 coated versus uncoated | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.24, 0.95] |
| 6 Number with urethritis, epididymitis, or orchitis | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.1 uncoated catheter, clean technique (both arms) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.2 coated versus uncoated (clean technique both arms) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.3 sterile technique versus clean technique (uncoated catheter both arms) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Weeks to onset of symptomatic UTI Show forest plot | 2 | 85 | Mean Difference (IV, Fixed, 95% CI) | ‐0.11 [‐0.64, 0.43] |
| Analysis 3.7  Comparison 3 Single use (sterile) versus multiple use (clean) catheter, Outcome 7 Weeks to onset of symptomatic UTI. | ||||
| 7.1 uncoated catheter, clean technique (both arms) | 1 | 39 | Mean Difference (IV, Fixed, 95% CI) | 0.38 [‐1.55, 2.31] |
| 7.2 coated versus uncoated (clean technique both arms) | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7.3 sterile technique versus clean technique (uncoated catheter both arms) | 1 | 46 | Mean Difference (IV, Fixed, 95% CI) | ‐0.15 [‐0.71, 0.41] |
| 8 Number reporting satisfaction with catheter product | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.1 uncoated catheter, clean technique (both arms) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.2 coated versus uncoated (clean technique both arms) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.3 sterile technique versus clean technique (uncoated catheter both arms) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Number reporting comfort and ease of insertion | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9.1 uncoated catheter, clean technique (both arms) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9.2 coated versus uncoated (clean technique both arms) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9.3 sterile technique versus clean technique (uncoated catheter both arms) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Number reporting preference | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10.1 uncoated catheter, clean technique (both arms) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10.2 coated versus uncoated (clean technique both arms) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10.3 sterile technique versus clean technique (uncoated catheter both arms) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10.4 coated versus uncoated | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Preference score Show forest plot | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | ‐1.60 [‐3.57, 0.37] |
| Analysis 3.11  Comparison 3 Single use (sterile) versus multiple use (clean) catheter, Outcome 11 Preference score. | ||||
| 11.1 uncoated catheter, clean technique (both arms) | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11.2 coated versus uncoated (clean technique both arms) | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | ‐1.60 [‐3.57, 0.37] |
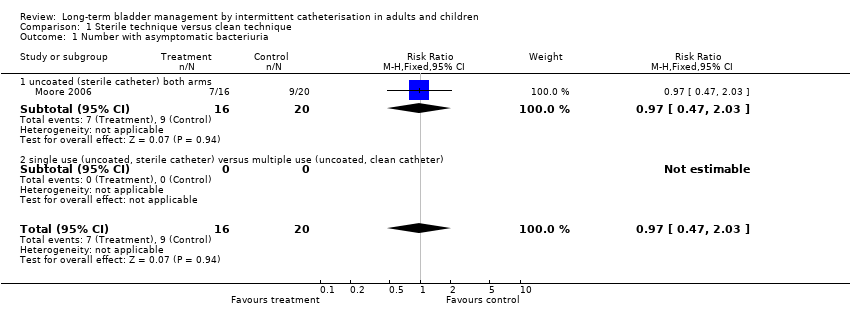
Comparison 1 Sterile technique versus clean technique, Outcome 1 Number with asymptomatic bacteriuria.

Comparison 1 Sterile technique versus clean technique, Outcome 2 Number with symptomatic UTI.
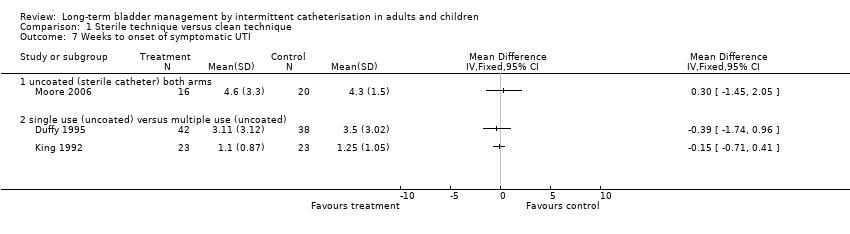
Comparison 1 Sterile technique versus clean technique, Outcome 7 Weeks to onset of symptomatic UTI.
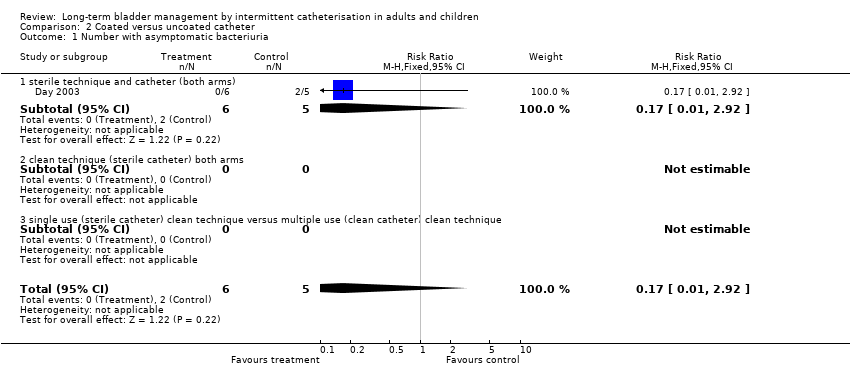
Comparison 2 Coated versus uncoated catheter, Outcome 1 Number with asymptomatic bacteriuria.

Comparison 2 Coated versus uncoated catheter, Outcome 2 Number with symptomatic UTI.
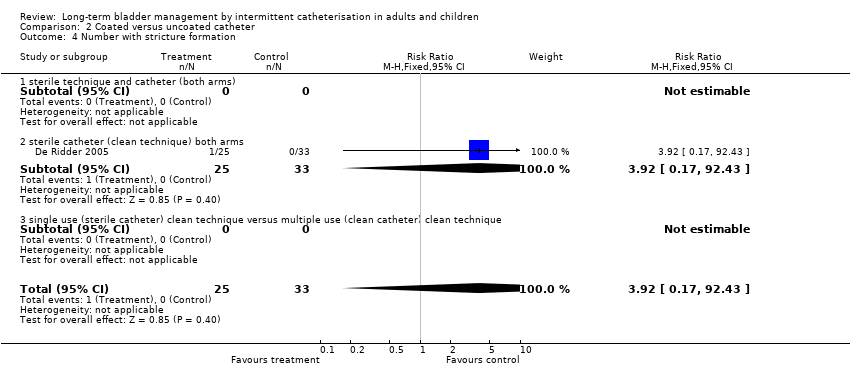
Comparison 2 Coated versus uncoated catheter, Outcome 4 Number with stricture formation.

Comparison 2 Coated versus uncoated catheter, Outcome 5 Number with microscopic haematuria.
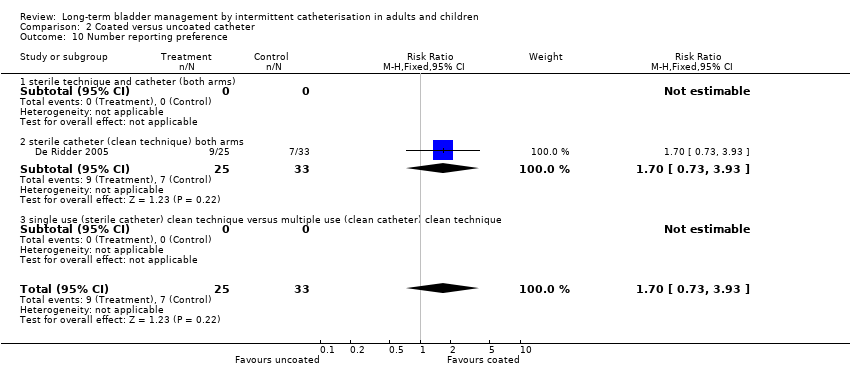
Comparison 2 Coated versus uncoated catheter, Outcome 10 Number reporting preference.

Comparison 3 Single use (sterile) versus multiple use (clean) catheter, Outcome 1 Number with asymptomatic bacteriuria.
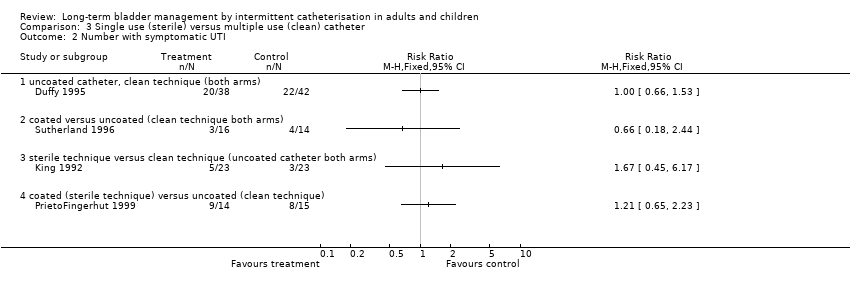
Comparison 3 Single use (sterile) versus multiple use (clean) catheter, Outcome 2 Number with symptomatic UTI.
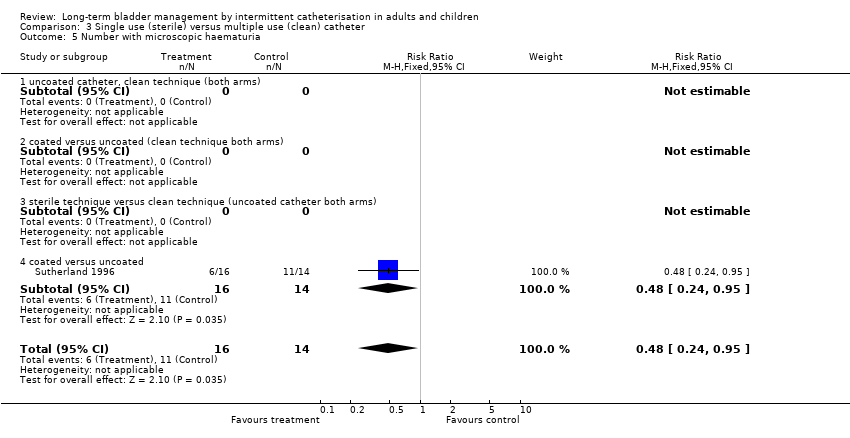
Comparison 3 Single use (sterile) versus multiple use (clean) catheter, Outcome 5 Number with microscopic haematuria.
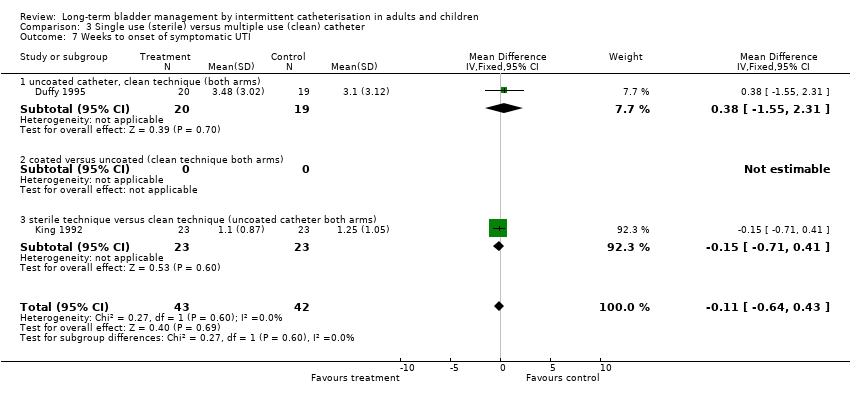
Comparison 3 Single use (sterile) versus multiple use (clean) catheter, Outcome 7 Weeks to onset of symptomatic UTI.

Comparison 3 Single use (sterile) versus multiple use (clean) catheter, Outcome 11 Preference score.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number with asymptomatic bacteriuria Show forest plot | 1 | 36 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.47, 2.03] |
| 1.1 uncoated (sterile catheter) both arms | 1 | 36 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.47, 2.03] |
| 1.2 single use (uncoated, sterile catheter) versus multiple use (uncoated, clean catheter) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Number with symptomatic UTI Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 uncoated (sterile catheter) both arms | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 single use (uncoated, sterile catheter) versus multiple use (uncoated, clean catheter) | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Number with urethral trauma/bleeding | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.1 uncoated (sterile catheter) both arms | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Number with stricture formation | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.1 uncoated (sterile catheter) both arms | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Number with microscopic haematuria | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.1 uncoated (sterile catheter) both arms | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Number with urethritis, epididymitis, or orchitis | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.1 uncoated (sterile catheter) both arms | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Weeks to onset of symptomatic UTI Show forest plot | 3 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7.1 uncoated (sterile catheter) both arms | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.2 single use (uncoated) versus multiple use (uncoated) | 2 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 Number reporting satisfaction with catheter product | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.1 uncoated (sterile catheter) both arms | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Number reporting comfort and ease of insertion | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9.1 uncoated (sterile catheter) both arms | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Number reporting preference | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10.1 uncoated (sterile catheter) both arms | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number with asymptomatic bacteriuria Show forest plot | 1 | 11 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.17 [0.01, 2.92] |
| 1.1 sterile technique and catheter (both arms) | 1 | 11 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.17 [0.01, 2.92] |
| 1.2 clean technique (sterile catheter) both arms | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.3 single use (sterile catheter) clean technique versus multiple use (clean catheter) clean technique | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Number with symptomatic UTI Show forest plot | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 sterile technique and catheter (both arms) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 sterile catheter (clean technique) both arms | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 single use (sterile catheter) clean technique versus multiple use (clean catheter) clean technique | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Number with urethral trauma/bleeding | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.1 sterile technique and catheter (both arms) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 sterile catheter (clean technique) both arms | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.3 single use (sterile catheter) clean technique versus multiple use (clean catheter) clean technique | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Number with stricture formation Show forest plot | 1 | 58 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.92 [0.17, 92.43] |
| 4.1 sterile technique and catheter (both arms) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 sterile catheter (clean technique) both arms | 1 | 58 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.92 [0.17, 92.43] |
| 4.3 single use (sterile catheter) clean technique versus multiple use (clean catheter) clean technique | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Number with microscopic haematuria Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5.1 sterile technique and catheter (both arms) | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 sterile catheter (clean technique) both arms | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.3 single use (sterile catheter) clean technique versus multiple use (clean catheter) clean technique | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Number with urethritis, epididymitis, or orchitis | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.1 sterile technique and catheter (both arms) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.2 sterile catheter (clean technique) both arms | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.3 single use (sterile catheter) clean technique versus multiple use (clean catheter) clean technique | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Weeks to onset of symptomatic UTI | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7.1 sterile technique and catheter (both arms) | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7.2 sterile catheter (clean technique) both arms | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7.3 single use (sterile catheter) clean technique versus multiple use (clean catheter) clean technique | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Number reporting satisfaction with catheter product | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.1 sterile technique and catheter (both arms) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.2 sterile catheter (clean technique) both arms | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.3 single use (sterile catheter) clean technique versus multiple use (clean catheter) clean technique | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Number reporting comfort and ease of insertion | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9.1 sterile technique and catheter (both arms) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9.2 sterile catheter (clean technique) both arms | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9.3 single use (sterile catheter) clean technique versus multiple use (clean catheter) clean technique | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Number reporting preference Show forest plot | 1 | 58 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.70 [0.73, 3.93] |
| 10.1 sterile technique and catheter (both arms) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10.2 sterile catheter (clean technique) both arms | 1 | 58 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.70 [0.73, 3.93] |
| 10.3 single use (sterile catheter) clean technique versus multiple use (clean catheter) clean technique | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number with asymptomatic bacteriuria Show forest plot | 1 | 66 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.66, 1.72] |
| 1.1 uncoated catheter, clean technique (both arms) | 1 | 66 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.66, 1.72] |
| 1.2 coated versus uncoated (clean technique both arms) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.3 sterile technique versus clean technique (uncoated catheter both arms) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Number with symptomatic UTI Show forest plot | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 uncoated catheter, clean technique (both arms) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 coated versus uncoated (clean technique both arms) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 sterile technique versus clean technique (uncoated catheter both arms) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.4 coated (sterile technique) versus uncoated (clean technique) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Number with urethral trauma/bleeding | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.1 uncoated catheter, clean technique (both arms) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 coated versus uncoated (clean technique both arms) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.3 sterile technique versus clean technique (uncoated catheter both arms) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Number with stricture formation | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.1 uncoated catheter, clean technique (both arms) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 coated versus uncoated (clean technique both arms) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.3 sterile technique versus clean technique (uncoated catheter both arms) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Number with microscopic haematuria Show forest plot | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.24, 0.95] |
| 5.1 uncoated catheter, clean technique (both arms) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 coated versus uncoated (clean technique both arms) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.3 sterile technique versus clean technique (uncoated catheter both arms) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.4 coated versus uncoated | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.24, 0.95] |
| 6 Number with urethritis, epididymitis, or orchitis | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.1 uncoated catheter, clean technique (both arms) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.2 coated versus uncoated (clean technique both arms) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.3 sterile technique versus clean technique (uncoated catheter both arms) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Weeks to onset of symptomatic UTI Show forest plot | 2 | 85 | Mean Difference (IV, Fixed, 95% CI) | ‐0.11 [‐0.64, 0.43] |
| 7.1 uncoated catheter, clean technique (both arms) | 1 | 39 | Mean Difference (IV, Fixed, 95% CI) | 0.38 [‐1.55, 2.31] |
| 7.2 coated versus uncoated (clean technique both arms) | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7.3 sterile technique versus clean technique (uncoated catheter both arms) | 1 | 46 | Mean Difference (IV, Fixed, 95% CI) | ‐0.15 [‐0.71, 0.41] |
| 8 Number reporting satisfaction with catheter product | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.1 uncoated catheter, clean technique (both arms) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.2 coated versus uncoated (clean technique both arms) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.3 sterile technique versus clean technique (uncoated catheter both arms) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Number reporting comfort and ease of insertion | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9.1 uncoated catheter, clean technique (both arms) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9.2 coated versus uncoated (clean technique both arms) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9.3 sterile technique versus clean technique (uncoated catheter both arms) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Number reporting preference | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10.1 uncoated catheter, clean technique (both arms) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10.2 coated versus uncoated (clean technique both arms) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10.3 sterile technique versus clean technique (uncoated catheter both arms) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10.4 coated versus uncoated | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Preference score Show forest plot | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | ‐1.60 [‐3.57, 0.37] |
| 11.1 uncoated catheter, clean technique (both arms) | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11.2 coated versus uncoated (clean technique both arms) | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | ‐1.60 [‐3.57, 0.37] |

