Heparin‐bonded catheters for prolonging the patency of central venous catheters in children
Abstract
Background
Central venous catheters (CVCs) are a mainstay in the management of critically ill children. However, these catheters are associated with mechanical and infectious complications which reduce their life span. Heparin bonding of catheters has shown promise in animal studies and in adults. This is the first update of a review published in 2007.
Objectives
The primary objective was to determine the effect of heparin‐bonded CVCs on the duration of catheter patency in children. Secondary objectives were to determine the effects of heparin‐bonded catheters on catheter‐related thrombosis, occlusion, blood stream infection and side effects.
Search methods
For this update the Cochrane Peripheral Vascular Diseases Group Trials Search Co‐ordinator searched the Specialised Register (last searched August 2013) and CENTRAL (2013, Issue 7). The authors searched MEDLINE (1946 to week 3 August 2013).
Selection criteria
We included randomized and quasi‐randomized controlled trials of heparin‐bonded catheters versus non‐heparin bonded catheters or antibiotic‐impregnated catheters that reported on any of the prespecified outcomes, without language restriction.
Data collection and analysis
We assessed the methodological quality of the trials using the information provided in the studies and by contacting authors. We extracted data and estimated the effect size reported as risk ratio (RR), risk difference (RD) or number needed to treat (NNT), as appropriate.
Main results
We included two eligible studies with a total of 287 participants; both had good methodological quality. There was no difference in the duration of catheter patency between heparin‐bonded and non‐heparin bonded catheters (median duration seven days versus six days) reported in one study. There was no difference in the risk of catheter‐related thrombosis (two studies, RR 0.34, 95% CI 0.01 to 7.68; I2 = 80%; RD ‐0.06, 95% CI ‐0.17 to 0.06). Data from one study revealed a statistically significant reduction in the risk of catheter occlusion (RR 0.06, 95% CI 0.00 to 1.07; RD ‐0.08, 95% CI ‐0.13 to ‐0.02; NNT 13, 95% CI 8 to 50), catheter‐related blood stream infections (RR 0.06, 95% CI 0.01 to 0.41; RD ‐0.17, 95% CI ‐0.25 to ‐0.10; NNT 6, 95% CI 4 to 10) and catheter colonization (RR 0.21, 95% CI 0.06 to 0.71; RD ‐0.11, 95% CI ‐0.19 to ‐0.04; NNT 9, 95% CI 5 to 25) in the heparin‐bonded catheter group. The second study did not report on these outcomes. There was no significant difference in risk of thrombocytopenia after catheter placement (RR 0.73, 95% CI 0.38 to 1.39; RD ‐0.02, 95% CI ‐0.10 to 0.07).
Authors' conclusions
Two eligible studies on the use of heparin‐bonded catheters versus placebo in children were identified. Meta‐analysis of the two studies revealed no reduction in catheter‐related thrombosis with heparin‐bonded catheters. One study reported a reduction in catheter‐related blood stream infection and colonization following the use of heparin‐bonded catheters. The strength of evidence is low and further well‐designed multicenter randomized controlled trials are warranted.
PICO
Plain language summary
Heparin‐bonded catheters for prolonging the patency of central venous catheters in children
Central venous catheters are used for prolonged intravenous therapy in the management of critically ill children, for parenteral nutrition, medication and monitoring. Having these catheters in place can cause blood clots in or around the end of the catheter as well as infection, either local or a blood stream infection. As a result, the catheter becomes blocked, eventually to the point that it is occluded and can no longer be used to give fluids. Anticoagulant drugs such as heparin can be given to prolong the usefulness of the catheter or the catheters can be coated with heparin (heparin‐bonded catheters). Heparin can cause side effects such as bleeding, allergic reactions, induced thrombocytopenia (an abnormal drop in the number of platelets in the blood) and osteoporosis with long‐term use.
The review authors identified two good quality controlled trials that randomized 287 children aged one day to 16 years to either a heparin‐bonded catheter or a standard catheter. The median duration of time that the catheter could be used to give fluids (its patency) was not clearly different with the two types of catheter. This was seven days in the heparin‐bonded catheter group and six days in the standard catheter group. There was a no difference between the two groups for risk of catheter‐related thrombosis over the time the catheter was in. There was a trend towards a reduction in the risk of catheter occlusion in the first week after catheter placement, reported in one study only.
The risks of catheter‐related blood stream infections and bacterial colonization of the catheter were significantly reduced using the heparin‐bonded catheter, with a longer time to develop infection (delayed in the heparin‐bonded catheter group); however, it was reported in one study only and the strength of evidence was low. There was no significant difference in risk of thrombocytopenia after catheter placement.
Authors' conclusions
Summary of findings
| Heparin‐bonded catheter compared with non‐heparin bonded catheter for central venous catheter in children | ||||||
| Participant or population: Children with central venous catheter Settings: Hospital Intervention: Heparin‐bonded central venous catheter Comparison: Non‐heparin bonded central venous catheter | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Non heparin‐bonded catheter | Heparin‐bonded catheter | |||||
| Days of catheter patency | median of 6 days | median of 7 days | 200 (1 study) | ⊕⊕⊝⊝ | Only one study reported this outcome | |
| Catheter‐related thrombosis at any time during catheter stay | 175 per 1000 | 59 per 1000 (2 to 1344) | RR 0.34 (0.01 to 7.68) | 287 (2 studies) | ⊕⊕⊕⊝ | |
| Occlusion of catheter within one week of catheter insertion | 78 per 1000 | 5 per 1000 (0 to 83) | RR 0.06 (0.00 to 1.07) | 200 (1 study) | ⊕⊕⊝⊝ | Only one study reported this outcome |
| Catheter‐related blood stream infection and CVC‐related colonization | 184 per 1000 | 11 per 1000 (2 to 75) | RR 0.06 (0.01 to 0.41) | 200 (1 study) | ⊕⊕⊝⊝ | Only one study reported this outcome |
| Side effects: Thrombocytopenia after catheter insertion | 133 per 1000 | 97 per 1000 (50 to 85) | RR 0.73 (0.38 to 1.39) | 287 (2 studies) | ⊕⊕⊝⊝ | No systematic evaluation of thrombocytopenia was conducted in either study |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
Background
Description of the condition
Central venous catheters (CVCs) are important for the management of prolonged intravenous therapy in children who need parenteral nutrition, medication or hemodynamic monitoring (Klerk 2003). However, these catheters are associated with mechanical and infectious complications. Mechanical complications include thrombosis (clotting) (Andrew 1994; Massicotte 1998), extravasation (leakage of blood or lymph, intravenous drugs, or intravenous nutrition from a vein into the surrounding tissue), occlusion (blockage) and dislodgement of the catheter. The incidence of CVC‐related thrombosis is reported to be 10% to 26% (Beck 1998) or 3.5 per 10,000 pediatric admissions (Massicotte 1998) and is higher in patients with malignancy. Several factors have been implicated in the causation of thrombosis. These include patient characteristics, for example younger age, and infusion‐related characteristics such as low flow states, rate of infusion (Beck 1998), site of catheter placement (Massicotte 1998) and duration of catheter placement. Infections can be caused by bacteria or fungi. Additionally, thromboses can also act as a nidus (breeding ground) for infection (Timsit 1998).
Description of the intervention
Several measures have been employed to prevent CVC‐related thrombosis. These include anticoagulants such as unfractionated heparin at prophylactic or therapeutic dosages (Abdelkefi 2004; Bailey 1979; Brismar 1982); low molecular weight heparins (for example dalteparin); warfarin; heparin‐bonded catheters; infusion of nitroglycerin (Jacobs 2001); and vitamin C (Rabe 2002). In a systematic review of six studies on the prophylactic or therapeutic use of heparin flushes or antithrombotic agents in adults, Klerk (Klerk 2003) concluded that the addition of heparin to parenteral fluids did not reduce the incidence of CVC‐related thrombosis. In a randomized controlled trial of 152 children, heparinization was found to reduce the risk for non‐patency of arterial catheters but not for CVCs (de Neef 2002). However, another meta‐analysis of 11 studies of heparin infusion or heparin bonding for CVCs reported a reduction of CVC‐related venous thrombosis (risk ratio 0.43, 95% confidence interval 0.23 to 0.78) with prophylactic heparinization (Randolph 1998). A review of the effectiveness of antibiotic‐impregnated and heparin‐bonded catheters in preventing infections reported a significant reduction in the incidence of infections. However, only one study of heparin‐bonded catheters was included in this review (Marin 2000). Carrasco 2004 compared heparin‐coated catheters with chlorhexidine and silver sulfadiazine‐coated CVCs in adult intensive care units and reported reduced colonization by gram‐positive cocci and Candida species in the latter group.
How the intervention might work
Thrombosis has been observed within 24 hours of placement of intravenous catheters and by 72 hours thrombi started to detach (Hofbauer 2000). Bonding of the catheter surface with heparin benzalkonium has been shown to reduce thrombogenicity and thrombophlebitis in rabbits by reducing damage to the intima (the inner layer of a blood vessel) and by reducing platelet aggregation (Di Costanzo 1984). Heparin bonding of catheters, in which heparin is impregnated and thus slowly released on a continuous basis, should be differentiated from heparin flushes which involve intermittent infusion of heparin alongside other infusates. Heparinization has also been shown to reduce adherence by coagulase negative Staphylococcus aureus when compared with catheters without heparinization (Appelgren 1996). Heparin coating was believed to reduce fibronectin adhesion (a substance that interacts with extracellular substances such as fibrin) on catheters and thereby reduce bacterial attachment (Appelgren 1996).
Why it is important to do this review
Bennegard 1982 demonstrated an increased incidence of thrombophlebitis in the first four days after catheterization in heparin‐coated polyethylene catheters with no reduction in the incidence of thrombotic complications. Mollenholt 1987 cautioned against overestimation of the protective effects of heparin bonding of pulmonary arterial catheters because effectiveness was found to decrease with time. Additionally, heparin is associated with side effects such as bleeding, allergic reactions and heparin‐induced thrombocytopenia (HIT) (Warkentin 1990). These side effects could be aggravated in critically ill children if they have other intravascular devices in place or are receiving additional heparin. It is important to do this review in order to assess the effectiveness and safety of heparin‐bonded CVCs in children.
Objectives
The primary objective was to determine the effect of heparin‐bonded CVCs on the duration of catheter patency in children.
Secondary objectives were to determine the effect of heparin‐bonded catheters on:
-
CVC‐related thrombosis (determined by color‐coded Doppler ultrasonography or venography);
-
occlusion of CVCs (defined as inability to infuse fluids through the catheter due to blockage);
-
episodes of CVC‐related blood stream infection and CVC‐related colonization (CVC‐related blood stream infection was defined as the presence of symptoms and signs suggestive of blood stream infection, accompanied by positive blood cultures obtained from a normally sterile site different to the CVC and distant from the CVC or CVC tip, each growing the same micro‐organism; CVC‐related colonization was defined as the presence of micro‐organisms in the CVC only and not from another sterile site);
-
number of additional CVC insertions;
-
Side effects of heparin (allergic reactions, hemorrhage (any site in the body), heparin‐induced thrombocytopenia (HIT) (development of thrombocytopenia after insertion of a heparin‐bonded CVC in an infant with a previously normal platelet count after exclusion of all other causes of thrombocytopenia, and a positive antibody test), abnormal coagulation profile, osteoporosis);
-
mortality;
-
any other reported adverse outcome (not prespecified).
Methods
Criteria for considering studies for this review
Types of studies
We included randomized controlled trials (RCTs) of heparin‐bonded CVCs in children with CVCs and also studies that used alternative methods of randomization such as alternate days of the week, odd or even dates of birth or hospital numbers (quasi‐randomized). We did not include studies that used historical controls. For any study in which the unit of randomization was the catheter, we contacted the primary authors to obtain data for the first catheter after randomization and planned to include only those data. If authors were unable to provide the data or could not be contacted, we described the study and included only data on side effects and long‐term outcomes such as hemorrhage or mortality.
Types of participants
Children (from birth to 18 years) who required CVCs. Studies of mixed populations (children and adults were only included if the study authors provided data for pediatric participants separately.
Types of interventions
Heparin‐bonded CVCs versus control (CVCs without heparin bonding or CVCs impregnated with antibiotics) without any restriction to the amount of heparin released.
Types of outcome measures
Studies that reported on one or more of the following outcomes amongst all those participants randomized.
Primary outcomes
Days of catheter patency (duration of patency of first catheter, in days).
Secondary outcomes
1. Catheter‐related thrombosis (along the length or at the tip of the catheter, or at adjacent sites) as determined clinically and confirmed by Doppler ultrasonography or contrast venography. This was determined as dichotomous data (yes or no) and the timescales of catheter‐related thrombosis were:
(a) within one week of catheter placement;
(b) within two weeks of catheter placement;
(c) within four weeks of catheter placement;
(d) after four weeks of catheter placement.
2. Occlusion of the catheter (identified by inability to infuse fluids). This was determined as dichotomous data (yes or no) and the timescales of occlusion of the catheter were:
(a) within one week of catheter placement;
(b) within two weeks of catheter placement;
(c) within four weeks of catheter placement;
(d) after four weeks of catheter placement.
3. Episodes of CVC‐related blood stream infection and CVC‐related colonization (children with one or more episodes).
4. Number of additional CVCs needed.
5. Side effects of heparin (allergic reactions, hemorrhage, heparin‐induced thrombocytopenia (HIT), abnormal coagulation profile, osteoporosis).
6. Mortality.
7. Any other reported adverse outcome (not prespecified).
Search methods for identification of studies
There was no restriction on language of publication.
Electronic searches
For this update the Cochrane Peripheral Vascular Diseases (PVD) Group Trials Search Co‐ordinator (TSC) searched the Specialised Register (last searched August 2013) and the Cochrane Central Register of Controlled Trials (CENTRAL) (2013, Issue 7), part of The Cochrane Library, (www.thecochranelibrary.com). See Appendix 1 for details of the search strategy used to search CENTRAL. The Specialised Register is maintained by the TSC and is constructed from weekly electronic searches of MEDLINE, EMBASE, CINAHL, AMED, and through handsearching relevant journals. The full list of the databases, journals and conference proceedings which have been searched, as well as the search strategies used, are described in the Specialised Register section of the Cochrane Peripheral Vascular Diseases Group module in The Cochrane Library (www.thecochranelibrary.com).
For this update, the review authors also searched Ovid MEDLINE® (1946 to week 3 August 2013) using the search strategy in Appendix 2.
Searching other resources
In addition, we searched the reference lists of identified trials.
Data collection and analysis
Selection of studies
Both review authors (PS, NS) independently assessed all published articles identified by the literature search as potentially relevant for inclusion in the review. Disagreements were resolved by discussion. In order to be included the trial had to meet the following criteria:
-
the study population was children;
-
the intervention was heparin‐bonded CVCs compared with standard catheters or antibiotic‐impregnated catheters;
-
the study was a randomized or quasi‐randomized controlled trial;
-
one or more of the primary or secondary outcome measures were reported.
Data extraction and management
Both review authors (PS, NS) independently extracted data from the retrieved articles. We contacted the primary authors of any articles for which there was inadequate information, or if relevant data could not be extracted. Any discrepancies were resolved by consensus.
Assessment of risk of bias in included studies
For this updated review (2013), risk of bias was assessed independently by the review authors (PS, NS) using the 'Risk of bias' tool from The Cochrane Collaboration and the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Measures of treatment effect
Statistical methods included risk ratio (RR), risk difference (RD), number needed to treat (NNT) and mean difference (MD), where appropriate, using the methods recommended by the Cochrane PVD Review Group.
Unit of analysis issues
The unit of analysis was the individual participant.
Dealing with missing data
We only included the data reported in the study publications or those provided by study authors. We did not impute any missing data.
Assessment of heterogeneity
We assessed heterogeneity clinically from the description of studies, visually from forest plots, and by using the I2 statistic. An I2 > 50% indicates moderate to substantial heterogeneity (Higgins 2011).
Assessment of reporting biases
We were unable to assess publication bias using a funnel plot because only two studies were included in the review. Higgins 2011 recommends a minimum of 10 studies to assess publication bias using funnel plots.
Data synthesis
We used a fixed‐effect model for the meta‐analyses unless heterogeneity was high (I2 > 50%), when a random‐effects model was used.
Subgroup analysis and investigation of heterogeneity
The following subgroup analyses were planned according to the control intervention:
1 heparin‐bonded CVCs versus standard CVCs (without any coating);
2 heparin‐bonded CVCs versus antibiotic‐impregnated CVCs.
Sensitivity analysis
No sensitivity analyses were conducted as only two studies were available for this review.
Results
Description of studies
Results of the search
See Figure 1.
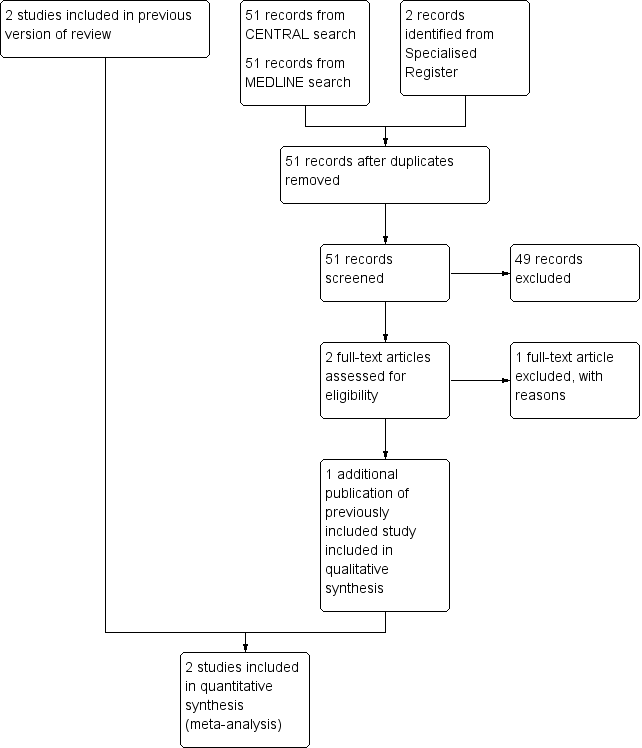
Study flow diagram for updated review.
Included studies
See Characteristics of included studies.
For this review update an additional publication was identified for the Anton 2009 study which had previously been included as an abstract only. In this update the full publication was included.
In total, two studies (Anton 2009; Pierce 2000) were included in this review.
Anton 2009 was a single center study. A total of 97 participants were randomized but 87 participants completed the study. A total of 10 participants (five in each arm) were lost to the final analysis due to either not receiving the study intervention or being unable to obtain final imaging for diagnosis. Children less than one year of age with congenital heart disease who required a short‐term central venous line for standard clinical care were randomized to receive either a heparin‐bonded catheter or non‐heparin bonded catheter. The catheters were identical in appearance. Randomization was held at the company, which manufactured identical catheters, to preserve blinding. The primary outcome of the study was the incidence of symptomatic and silent CVL‐associated venous thromboembolism, assessed by prespecified criteria. Clinical thrombosis was defined as swelling of the affected limb, discoloration or tenderness, or an inability to draw back or flush the CVL after the use of alteplase to flush the catheter. An ultrasound was performed in all participants if they were symptomatic or within 48 hours of removal of the catheter in asymptomatic infants. If the ultrasound was positive, the participant was confirmed positive for the thrombosis outcome. If the ultrasound was negative, the participant underwent venography. All ultrasounds were reviewed by a blinded central adjudication committee. Of 97 randomized participants, 53 were allocated to the non‐heparin bonded catheter group (of whom 52 received the intervention as one participant did not receive a catheter) and 44 were randomized to the heparin‐bonded catheter group (of whom 42 received the intervention as two participants did not receive a catheter). Four participants in the non‐heparin bonded catheter and three participants in the heparin‐bonded catheter group were lost to follow up as they did not have ultrasound assessment as planned. Thus, 47 participants in the non‐heparin bonded catheter group and 40 in the heparin‐bonded catheter group were analysed. After enrollment of these children, the first batch of blinded catheters reached their expiration date. Before producing another batch, the steering committee evaluated (blinded) the outcomes of 87 participants. Based on the results of the analyses, the steering committee stopped the trial based on futility as there was strong evidence against the presumed 50% reduction in thrombosis.
Pierce 2000 was a single center study. A total of 209 participants between the ages of one day and 16 years were randomized to either heparin‐bonded catheters or non‐heparin bonded catheters. The authors reported that at the end of the study data were available for 97 participants randomized to heparin‐bonded catheters and 103 participants randomized to non‐heparin bonded catheters for the analyses. Both types of catheter were made from polyurethane. The catheters were either size 4F in diameter; double or triple lumen; and 5 cm, 8 cm or 15 cm in length. The catheters had similar appearance and construction. The decision to remove a catheter was made by the attending team. All participants had color Doppler ultrasound examination within three days of insertion of the catheter and then every three days until the removal of the catheter. Thrombosis was defined as the presence of an echogenic mass within the lumen disrupting the blood flow. The radiologist reviewing the ultrasonography was unaware of the treatment allocation. All participants had blood cultures taken from the CVC and another site on insertion of the catheter and then every three days until the removal of the catheter. Additional blood cultures were obtained when clinically indicated. Infections were considered significant when the same organism was grown from two sets of cultures from two different sites and the organism was different from the one isolated at the start of catheter placement. Catheter‐related blood stream infections were considered when the same organism was isolated from two sets of cultures from two separate sites. The microbiologist reporting infection was unaware of treatment allocation.
Excluded studies
See Characteristics of excluded studies.
For this review update one additional study was excluded (Hitz 2012). In total seven studies were excluded. Six studies were excluded because the studies included adult participants only (Appelgren 1996; Bennegard 1982; Carrasco 2004; Hitz 2012; Hoar 1981; Mangano 1982). One study was excluded because it was not randomized (Krafte‐Jacobs 1995).
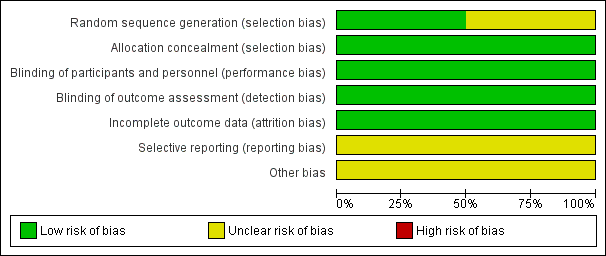
Risk of bias in included studies

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
In Pierce 2000 randomization was performed by selecting consecutively numbered opaque envelopes. It is not clear who prepared these envelopes. For the Anton 2009 study the pharmaceutical company personnel prepared the list and the group assignment was kept off‐site, the study was described as a triple‐blind study.
Blinding
Both studies were blinded studies.
Anton 2009 was described as a triple‐blind study. The ultrasound examinations were reviewed by a masked radiology adjudication committee.
In Pierce 2000 the investigators were unaware of treatment allocation. The radiologist reporting the results of the ultrasound Doppler and the microbiologist reporting results of infection were unaware of allocation. The attending physician deciding about the removal of a catheter was also unaware of treatment allocation.
Incomplete outcome data
Pierce 2000 had nine participants and Anton 2009 had 10 participants for whom data were not available after randomization.
In Pierce 2000 nine participants (five participants in the heparin‐bonded catheter group and four participants in the non‐heparin bonded catheter group) were removed from the study after randomization. One participant needed to be changed to a different type of catheter for hemofiltration and in the other eight participants the catheters were removed before microbiological data could be collected.
In Anton 2009, in Group 1 four participants did not have an ultrasound measurement and one participant did not have the study catheter inserted; and in Group 2 three participants did not have an ultrasound measurement and two participants did not have the study catheters inserted.
Selective reporting
We did not have access to either protocol, so selective reporting could not be ruled out.
Other potential sources of bias
The role of the pharmaceutical manufacturer in Anton 2009 was not completely clear.
Effects of interventions
See: Summary of findings for the main comparison
Heparin‐bonded CVCs versus standard CVCs
Both Anton 2009 and Pierce 2000 included neonates (< 28 days of age) and children (> 28 days of age). Data on neonates only could not be separated. The results reported below included all participants and contains both published and unpublished information obtained by contacting the study authors.
Primary outcome
Days of catheter patency (duration of patency of first catheter, in days)
Pierce 2000 reported these data in survival curve format for development of infection. The study authors provided data that the catheter remained patent for more than seven days in 47/97 participants in the heparin‐bonded catheter group versus 45/103 participants in the non‐heparin bonded catheter group. There was no significant difference in the median duration of catheter patency (seven days in the heparin‐bonded catheter group versus six days in the non‐heparin bonded catheter group). Anton 2009 were contacted but the study authors confirmed they did not collect this information.
Secondary outcomes
The time points reported for catheter‐related thrombosis and occlusion of the catheter were different from those predefined (Secondary outcomes); however, we analyzed these data as reported in the included studies in order to show all available data.
1. Catheter‐related thrombosis (along the length or at the tip of the catheter, or at adjacent sites) as determined clinically and confirmed by Doppler ultrasonography or contrast venography
Pierce 2000 reported data on any thrombosis during the time the catheter was in position in the published report and provided additional data on the number of infants with thrombosis within the first week of therapy and after the first week of therapy. Anton 2009 reported that 17/40 participants developed thrombosis in the non‐heparin bonded catheter group whereas 21/47 participants had thrombosis in the heparin‐bonded catheter group, at any time during the study.
(a) At any time during catheter stay (Analysis 1.1)
Both studies reported these data. There were no statistically significant beneficial effects of heparin‐bonded catheters in reducing the risk of catheter‐related thrombosis (RR 0.34, 95% CI 0.01 to 7.68; I2 = 80%; RD ‐0.06, 95% CI ‐0.17 to 0.06) (Figure 4).
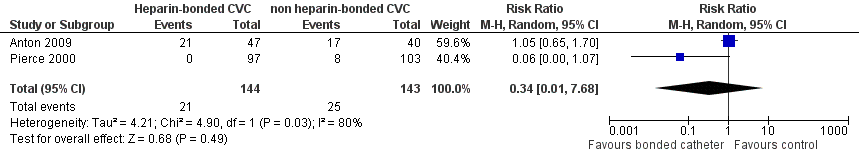
Forest plot of comparison: 1 Heparin‐bonded catheter versus non‐heparin bonded catheters, outcome: 1.1 Catheter‐related thrombosis at any time during catheter stay.
(b) Within one week of catheter placement (Analysis 1.2)
Only Pierce 2000 provided data on this outcome. There was no statistically significant reduction in catheter‐related thrombosis in the first week after catheter placement (RR 0.10, 95% CI 0.01 to 1.72; RD ‐0.05, 95% CI ‐0.09 to 0.00).
(c) After the first week of catheter placement (Analysis 1.3)
Only Pierce 2000 provided data on this outcome. There was no statistically significant reduction in catheter‐related thrombosis after the first week of catheter placement (RR 0.14, 95% CI 0.01 to 2.58; RD ‐0.07, 95% CI ‐0.15 to 0.02).
2. Occlusion of the catheter (identified by inability to infuse fluids)
These data were provided by Pierce 2000 in two categories, occlusion within the first week and occlusion after the first week of catheter placement.
(a) Within one week of catheter placement (Analysis 1.4)
Pierce 2000 provided data for this outcome. There was a trend towards a reduction in the risk of catheter occlusion in the first week after catheter placement in the heparin‐bonded catheter group compared to the non‐heparin bonded group (RR 0.06, 95% CI 0.00 to 1.07; RD ‐0.08, 95% CI ‐0.13 to ‐0.02). The number of children needed to be treated to prevent one catheter occlusion within the first week with the use of a heparin‐bonded catheter was 13 (95% CI 8 to 50).
(b) After the first week of catheter placement (Analysis 1.5)
Pierce 2000 reported a statistically significant reduction in the risk of catheter occlusion after the first week of catheter placement in the heparin‐bonded catheter group compared to the non‐heparin bonded catheter group (RR 0.22, 95% CI 0.07 to 0.72; RD ‐0.23, 95% CI ‐0.37 to ‐0.08). The number of children needed to be treated to prevent one catheter occlusion after the first week with the use of heparin‐bonded catheters was 5 (95% CI 3 to 13).
3. Episodes of CVC‐related blood stream infection and CVC‐related colonization (children with one or more episodes)
Pierce 2000 reported a statistically significant reduction in the risk of catheter‐related infection in the heparin‐bonded catheter group compared to the non‐heparin bonded catheter group (RR 0.06, 95% CI 0.01 to 0.41; RD ‐0.17, 95% CI ‐0.25 to ‐0.10). The number of children that needed to be treated with a heparin‐bonded catheter to prevent one catheter‐related infection was 6 (95% CI 4 to 10). There was a statistically significant reduction in the risk of catheter colonization in the heparin‐bonded catheter group compared to the non‐heparin bonded catheter group (RR 0.21, 95% CI 0.06 to 0.71; RD ‐0.11, 95% CI ‐0.19 to ‐0.04). The number of children needed to be treated with heparin‐bonded catheters to prevent one catheter colonization with microbes was 9 (95% CI 5 to 25). The authors reported the survival function for time to development of infection. There was a statistically significant difference in the time to develop infection (delayed in the heparin‐bonded catheters group) with an adjusted hazard ratio of 0.14 and P < 0.001.
4. Number of additional CVCs needed
This outcome was not reported in any of the included studies.
5. Side effects of heparin (allergic reactions, hemorrhage, heparin‐induced thrombocytopenia (HIT), abnormal coagulation profile, osteoporosis)
Data on incidence of thrombocytopenia were available from both studies (Anton 2009; Pierce 2000). None of the participants in Anton 2009 developed thrombocytopenia; however, platelet counts were not assessed systematically. There was no significant difference in risk of thrombocytopenia after catheter placement in the Pierce 2000 study (RR 0.73, 95% CI 0.38 to 1.39; RD ‐0.02, 95% CI ‐0.10 to 0.07) (Figure 5). Participants were not investigated for heparin‐induced thrombocytopenia. No other side effects were reported.
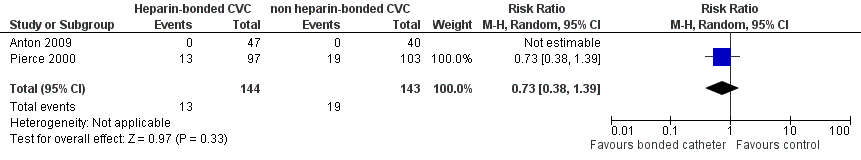
Forest plot of comparison: 1 Heparin‐bonded catheter versus non‐heparin bonded catheters, outcome: 1.8 Thrombocytopenia after catheter insertion.
6. Mortality
This outcome was not reported in the included studies.
7. Any other reported adverse outcome (not prespecified)
No other outcomes were reported in any of the included studies.
Discussion
Summary of main results
After an extensive literature review only two studies which have evaluated this intervention were identified (Anton 2009; Pierce 2000). Both studies were well‐conducted single center studies with adequate methodological quality. The primary outcome of this review, duration of catheter patency, was only evaluated in a single study that showed no difference in median duration of catheter patency. Pierce 2000 reported a trend towards a reduction in the risk of catheter‐related thrombosis and risk of catheter occlusion in the heparin‐bonded catheter group whereas Anton 2009 reported no significant difference in the risk of catheter‐related thrombosis. There was a significant reduction in the risks of catheter‐related infection and catheter colonization in the study by Pierce 2000. The incidence of catheter occlusion was reduced in the heparin‐bonded catheter group within the first week of catheter placement according to Pierce 2000. There was no increase in the risk of thrombocytopenia associated with the use of heparin‐bonded catheters; however, no systematic attempts were made for evaluation of side effects in either study. This leads to inconclusive information regarding toxic effects or lack thereof.
Overall completeness and applicability of evidence
We have searched extensively to identify, select and appraise studies for inclusion in this review. Overall completeness of the results in this review is quite satisfactory based on the information available to date. Contact with both study authors led to additional information from Pierce 2000 but not from Anton 2009.
A major difference in the clinical characteristics of the two included studies is the study population. Pierce 2000 enrolled newborn infants irrespective of diagnosis while Anton 2009 only enrolled a high‐risk group of children with congenital heart disease. Presence of congenital heart disease is in itself a predisposing factor for vascular thrombosis, second to polycythemia associated with cyanotic heart disease. A slower, altered circulatory state and increased likelihood of undergoing surgery are observed in this group of infants, increasing the probability of thrombosis. Anton 2009 appears to be a negative result study whereas Pierce 2000 reports positive results as far as the efficacy of heparin‐bonded catheters in preventing vascular thrombosis and its associated complications are concerned. These studies reflect a fair amount of clinical and statistical heterogeneity.
Quality of the evidence
Both studies were well‐conducted with good rigor and low risks of bias.
The quality of the evidence according to GRADE was graded as moderate to low for individual outcomes. The evidence was graded as low when only one study provided an estimate, in this case with wide confidence limits due to the lack of adequate power.
Potential biases in the review process
A comprehensive literature review was conducted, searching for published and unpublished abstracts and manuscripts. This search was not limited to a particular language. Authors were contacted to supplement any data missing in the original publications or abstracts. However, a minor limitation of this review is the fact that it was not possible to collect all relevant information from the included trials, particularly regarding data on several outcomes planned for this review.
Agreements and disagreements with other studies or reviews
Heparin‐bonded catheters have been well studied in the adult population, with different types of catheters. Appelgren 1996 reported reduced colonization by staphylococcal organisms in heparin‐bonded catheters. Bennegard 1982 did not identify any advantage of heparin‐bonded catheters in preventing thrombotic complications. Carrasco 2004 reported reduced colonization with antibiotic‐impregnated catheters compared with heparin‐bonded catheters.
Hoar 1981 reported reduced thrombogenicity with heparin‐bonded pulmonary catheters when examined at 24 hours of age during bypass surgery. Mangano 1982 reported that heparin‐bonded catheters provided protection from thrombosis when examined at 24 hours of age during atriotomy. Krafte‐Jacobs 1995 prospectively evaluated 50 consecutive but non‐randomized pediatric patients who had heparin‐bonded (n = 25) and non‐heparin bonded (n = 25) catheters. They reported thrombotic complications in 44% of patients in the non‐heparin bonded catheter group versus 8% of patients in the heparin‐bonded catheter group (P = 0.004). The incidence of positive blood cultures was 24% in the non‐heparin bonded catheter group versus none in the heparin‐bonded catheter group (P = 0.009). None of these studies reported a higher incidence of complications associated with the use of heparin‐bonded catheters.
Thrombophlebitis, hemorrhage and heparin‐induced thrombocytopenia are major concerns with the use of heparin. An animal study (Di Costanzo 1984) showed that heparin‐benzalkonium bonded silicone catheters provided protection against thrombophlebitis due to less damage to the intima and slowed aggregation of platelets. Nichols 1984 revealed that heparin‐bonded catheters prevented catheter‐induced platelet alpha granule release and fibrin formation on the catheter surfaces, which thus may prevent thrombus formation.
Gilbert 2003 reported that, despite the study by Pierce 2000, the use of heparin‐bonded catheters is not prevalent in the UK due to licensing issues. Only the center where the study was conducted is using these types of catheters with full legal responsibility and on a named‐patient basis.
Due to variability in the results, the findings of the studies by Anton 2009 and Pierce 2000 need to be confirmed in other studies before specific recommendations can be made. Exposure to inadvertent use of heparin for pediatric patients needs to be studied carefully, especially with emerging reports of heparin‐induced thrombocytopenia and associated thrombosis in adult and pediatric patients (Schmugge 2002). Exposure at this early age may lead to sensitization and could render an individual prone to further complications in later life. There was no difference in the duration of catheter patency in these studies, which is usually the effect seen with heparin. The authors have not documented the clinically important and significant outcome of mortality. Further multicenter evaluation of heparin‐bonded catheters is warranted.

Study flow diagram for updated review.
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
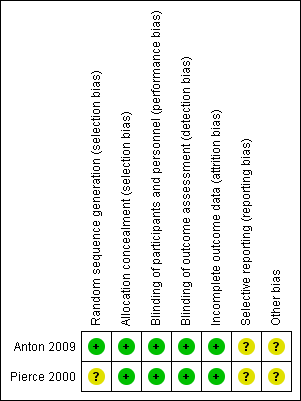
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Forest plot of comparison: 1 Heparin‐bonded catheter versus non‐heparin bonded catheters, outcome: 1.1 Catheter‐related thrombosis at any time during catheter stay.

Forest plot of comparison: 1 Heparin‐bonded catheter versus non‐heparin bonded catheters, outcome: 1.8 Thrombocytopenia after catheter insertion.

Comparison 1 Heparin‐bonded catheter versus non‐heparin bonded catheters, Outcome 1 Catheter‐related thrombosis at any time during catheter stay.

Comparison 1 Heparin‐bonded catheter versus non‐heparin bonded catheters, Outcome 2 Catheter‐related thrombosis within one week of catheter insertion.

Comparison 1 Heparin‐bonded catheter versus non‐heparin bonded catheters, Outcome 3 Catheter‐related thrombosis after one week of insertion.
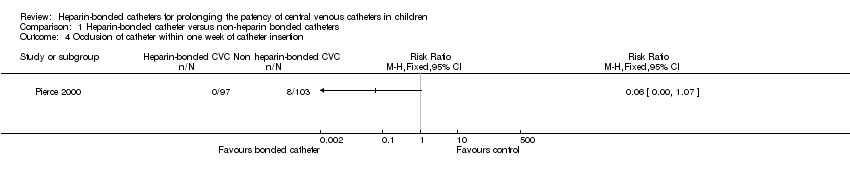
Comparison 1 Heparin‐bonded catheter versus non‐heparin bonded catheters, Outcome 4 Occlusion of catheter within one week of catheter insertion.

Comparison 1 Heparin‐bonded catheter versus non‐heparin bonded catheters, Outcome 5 Occlusion of catheter after one week of catheter insertion.
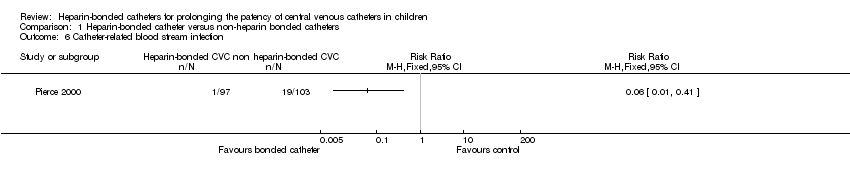
Comparison 1 Heparin‐bonded catheter versus non‐heparin bonded catheters, Outcome 6 Catheter‐related blood stream infection.

Comparison 1 Heparin‐bonded catheter versus non‐heparin bonded catheters, Outcome 7 Colonization of catheter with microbes.

Comparison 1 Heparin‐bonded catheter versus non‐heparin bonded catheters, Outcome 8 Thrombocytopenia after catheter insertion.
| Heparin‐bonded catheter compared with non‐heparin bonded catheter for central venous catheter in children | ||||||
| Participant or population: Children with central venous catheter Settings: Hospital Intervention: Heparin‐bonded central venous catheter Comparison: Non‐heparin bonded central venous catheter | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Non heparin‐bonded catheter | Heparin‐bonded catheter | |||||
| Days of catheter patency | median of 6 days | median of 7 days | 200 (1 study) | ⊕⊕⊝⊝ | Only one study reported this outcome | |
| Catheter‐related thrombosis at any time during catheter stay | 175 per 1000 | 59 per 1000 (2 to 1344) | RR 0.34 (0.01 to 7.68) | 287 (2 studies) | ⊕⊕⊕⊝ | |
| Occlusion of catheter within one week of catheter insertion | 78 per 1000 | 5 per 1000 (0 to 83) | RR 0.06 (0.00 to 1.07) | 200 (1 study) | ⊕⊕⊝⊝ | Only one study reported this outcome |
| Catheter‐related blood stream infection and CVC‐related colonization | 184 per 1000 | 11 per 1000 (2 to 75) | RR 0.06 (0.01 to 0.41) | 200 (1 study) | ⊕⊕⊝⊝ | Only one study reported this outcome |
| Side effects: Thrombocytopenia after catheter insertion | 133 per 1000 | 97 per 1000 (50 to 85) | RR 0.73 (0.38 to 1.39) | 287 (2 studies) | ⊕⊕⊝⊝ | No systematic evaluation of thrombocytopenia was conducted in either study |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Catheter‐related thrombosis at any time during catheter stay Show forest plot | 2 | 287 | Risk Ratio (M‐H, Random, 95% CI) | 0.34 [0.01, 7.68] |
| 2 Catheter‐related thrombosis within one week of catheter insertion Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Catheter‐related thrombosis after one week of insertion Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4 Occlusion of catheter within one week of catheter insertion Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5 Occlusion of catheter after one week of catheter insertion Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6 Catheter‐related blood stream infection Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 7 Colonization of catheter with microbes Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 8 Thrombocytopenia after catheter insertion Show forest plot | 2 | 287 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.38, 1.39] |

