Exercise for acutely hospitalised older medical patients
Información
- DOI:
- https://doi.org/10.1002/14651858.CD005955.pub3Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 10 noviembre 2022see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Salud musculoesquelética
- Copyright:
-
- Copyright © 2022 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
PH performed the literature search; reviewed the search results for eligibility; identified all included trials; performed data extraction; assessed risk of bias of the included trials; conducted data analysis; drafted the updated protocol and the final review.
JK reviewed the search results for eligibility; identified all included trials; performed data extraction; assessed risk of bias of the included trials; provided judgements on the interpretation of the results and conclusions drawn; was involved in the writing and approval of the protocol and the final review.
KJ reviewed the search results for eligibility; identified all included trials; performed data extraction; assessed risk of bias of the included trials; provided judgements on the interpretation of the results and conclusions drawn; was involved in the writing and approval of the protocol and the final review.
MR reviewed the search results for eligibility; identified all included trials; performed data extraction; assessed risk of bias of the included trials; provided judgements on the interpretation of the results and conclusions drawn; was involved in the writing and approval of the protocol and the final review.
TS reviewed the search results for eligibility; identified all included trials; performed data extraction; assessed risk of bias of the included trials; provided judgements on the interpretation of the results and conclusions drawn; was involved in the writing and approval of the protocol and the final review.
Sources of support
Internal sources
-
No sources of support provided
External sources
-
Dunhill Medical Trust Research Training Fellowship, UK
Peter Hartley was funded by a research training fellowship from The Dunhill Medical Trust [grant number RTF115/0117] from October 2017 to March 2021.
-
Cambridge Biomedical Research Centre and The Addenbrooke’s Charitable Trust research grant, UK
Peter Hartley is funded by a fellowship from the Cambridge Biomedical Research Centre and The Addenbrooke’s Charitable Trust [grant reference: 03/20 A] (March 2020 to Feb 2021)
Declarations of interest
PH: none.
JK: none.
KJ: none.
MR: none.
TS: none.
Two review authors (JK, KJ) conducted included studies. They were not involved in the screening, data extraction or risk of bias assessments of their studies.
Acknowledgements
The review authors would like to thank the Cochrane Musculoskeletal Editorial Team and the Cochrane Central Executive Methods Team for their input and help with this review. We would also like to thank Dr Jason Wallis, Monash‐Cabrini Department of Musculoskeletal Health and Clinical Epidemiology, Cabrini Health, Melbourne, Australia, and Anne Asher, Cochrane Consumer Referee, for their reviews and feedback.
Version history
| Published | Title | Stage | Authors | Version |
| 2022 Nov 10 | Exercise for acutely hospitalised older medical patients | Review | Peter Hartley, Jennifer L Keating, Kimberley J Jeffs, Melissa JM Raymond, Toby O Smith | |
| 2007 Jan 24 | Exercise for acutely hospitalised older medical patients | Review | Natalie de Morton, Jennifer L Keating, Kim Jeffs | |
| 2006 Apr 19 | Exercise for acutely hospitalised older medical patients | Protocol | Natalie A de Morton, Jennifer L Keating, Kim Jeffs | |
Differences between protocol and review
We planned to report adverse events during hospitalisation as a combined outcome. As adverse events were expected to be defined differently by different studies, the plan was to include any and all of participant mortality, falls, medical deterioration and musculoskeletal injury as a combined adverse‐event outcome. However, we changed this for three reasons.
-
Combining the outcomes might have led to double counting of participants who experienced an adverse event (e.g. if the same participant experienced both a fall and medical deterioration).
-
The estimate of baseline risk of experiencing an adverse event would have been very different depending on the number and type of adverse events reported by the different studies.
-
Interpretation of the analysis for the combined outcome would be very challenging due to the very different natures of the individual outcomes.
Therefore, we decided not to combine the outcomes, but to analyse each separately.
We did not plan separate analyses for studies that compared exercise interventions to a sham‐control intervention and those that did not, as per the reasons outlined in Types of interventions section. However, after discussions with the editors, we made a post‐hoc decision to conduct subgroup analyses to examine the effect of sham interventions in addition to usual care for the outcomes: independence with activities of daily living at hospital discharge and functional mobility at hospital discharge. We compared exercise interventions to usual care (excluding studies using sham‐control interventions) and separately compared exercise interventions to sham control interventions.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
Medical Subject Headings Check Words
Aged; Aged, 80 and over; Female; Humans; Male;
PICO
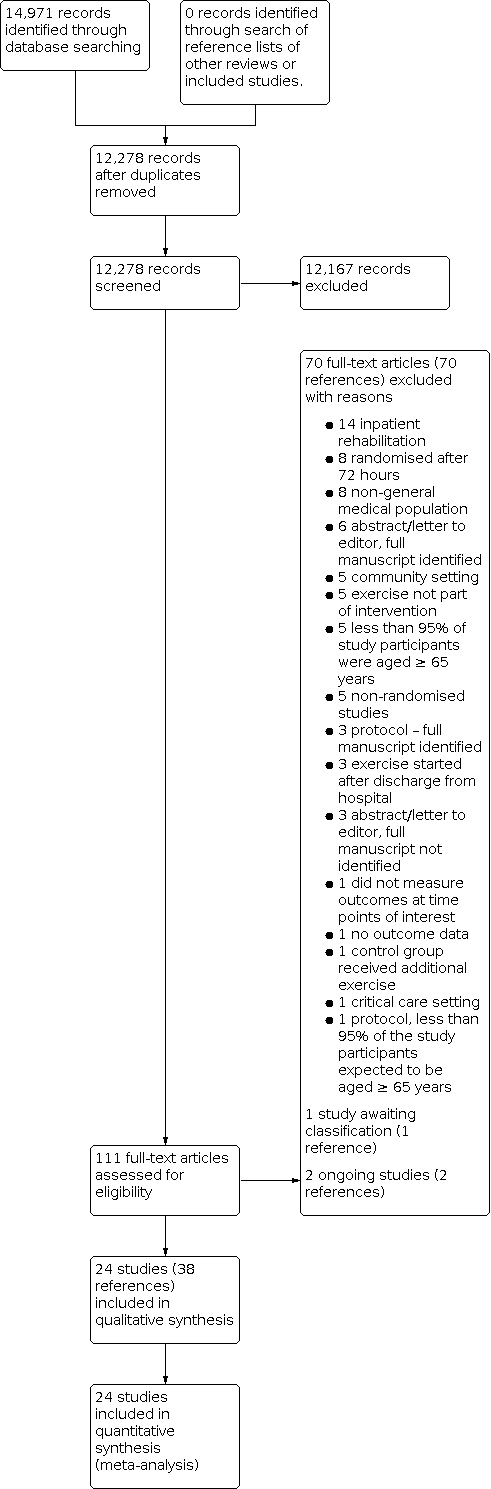
Study flow diagram

Funnel plot: independence with activities of daily living at discharge from hospital.
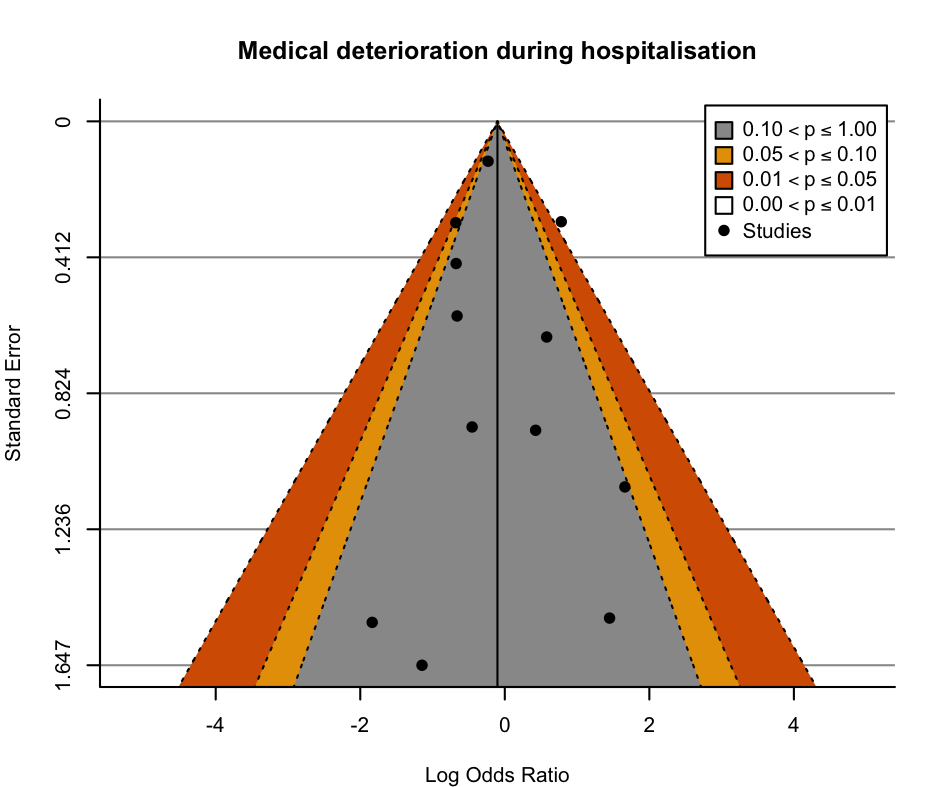
Funnel plot: medical deterioration during hospitalisation.

Funnel plot: mortality during hospitalisation.
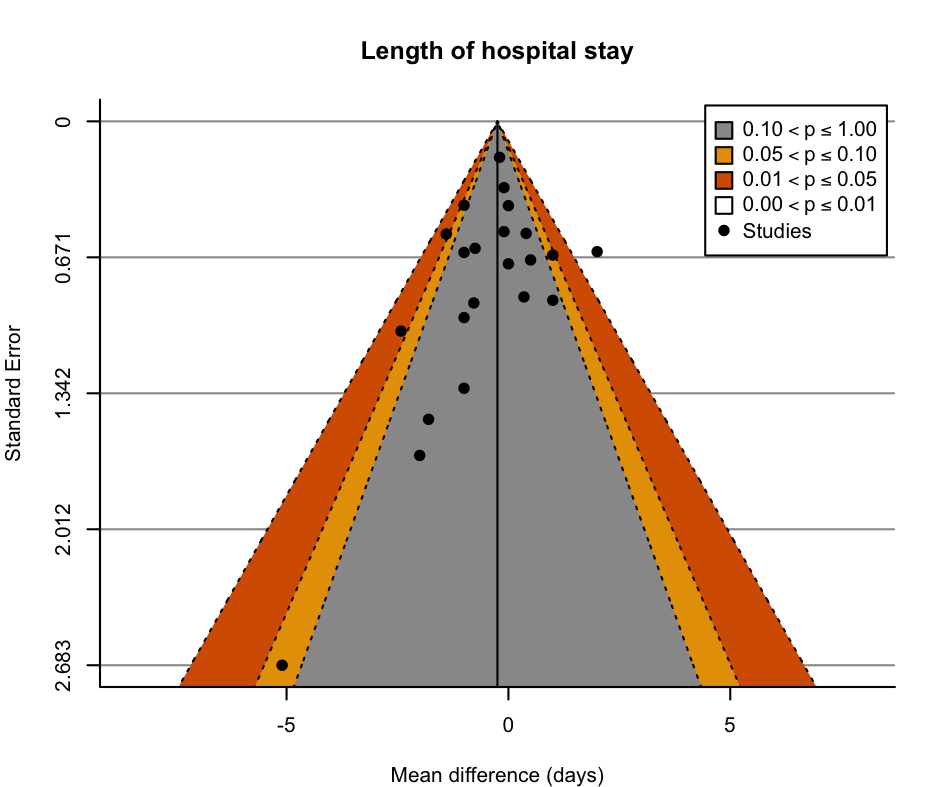
Funnel plot: length of hospital stay.

Funnel plot: readmissions to hospital.

Comparison 1: Major outcomes, Outcome 1: Functional ability: independence with activities of daily living at discharge from hospital

Comparison 1: Major outcomes, Outcome 2: Functional ability: functional mobility at discharge from hospital

Comparison 1: Major outcomes, Outcome 3: Functional ability: new incidence of delirium during hospitalisation

Comparison 1: Major outcomes, Outcome 4: Quality of life at discharge from hospital

Comparison 1: Major outcomes, Outcome 5: Falls during hospitalisation

Comparison 1: Major outcomes, Outcome 6: Medical deterioration during hospitalisation

Comparison 2: Minor outcomes, Outcome 1: Death during hospitalisation

Comparison 2: Minor outcomes, Outcome 2: Hospital length of stay (days)

Comparison 2: Minor outcomes, Outcome 3: New institutionalisation at hospital discharge

Comparison 2: Minor outcomes, Outcome 4: Hospital readmission

Comparison 2: Minor outcomes, Outcome 5: Walking performance at discharge from hospital
| Exercise interventions compared to usual care with or without sham interventions for acutely hospitalised older medical patients | ||||||
| Patient or population: acutely hospitalised medical patients | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Risk with usual care ± sham interventions | Risk with exercise interventions | |||||
| Functional ability: independence with activities of daily living at discharge from hospital | The mean functional ability: independence with activities of daily living at discharge from hospital ranged from 42 to 96 points on the Barthel Indexa | MD 1.8 points on the Barthel Index higher | ‐ | 5174 | ⊕⊕⊝⊝ | Exercise interventions may result in little to no difference in independence with activities of daily living at discharge from hospital (SMD 0.09, 95% CI −0.02 to 0.19). A change of 11 points on the Barthel Index is thought to represent a minimally clinically important difference (MCID). |
| Functional ability: functional mobility at discharge from hospital | The mean functional ability: functional mobility at discharge from hospital ranged from 3.7 to 4.9 points on the Short Physical Performance Battery e | MD 0.78 points on the Short Physical Performance Battery higher | ‐ | 2369 | ⊕⊝⊝⊝ | The evidence is very uncertain about the effect of exercise on functional mobility at discharge from hospital (SMD 0.28, 95% CI −0.01 to 0.56). A change of 1.0 points on the Short Physical Performance Battery is thought to represent an MCID. |
| Functional ability: new incidence of delirium during hospitalisation | 81 per 1000 | 73 per 1000 | RR 0.90 | 2088 | ⊕⊝⊝⊝ | The evidence suggests that exercise results in little to no difference in incidence of delirium during hospitalisation. |
| Quality of life at discharge from hospital | The mean quality of life at discharge from hospital ranged from 48.7 to 64.7 points on the EQ‐5D VAS | MD 6.04 points on the EQ‐5D VAS higher | ‐ | 875 | ⊕⊕⊝⊝ | Exercise interventions may result in a small clinically unimportant improvement in quality of life at discharge from hospital. A change of 10 points on the EQ‐5D VAS is thought to represent a MCID. |
| Falls during hospitalisation | 34 per 1000 | 34 per 1000 | RR 0.99 | 1787 | ⊕⊕⊕⊝ | Exercise interventions probably result in little to no difference in falls during hospitalisation. |
| Medical deterioration during hospitalisation | 71 per 1000 | 73 per 1000 | RR 1.02 | 2730 | ⊕⊝⊝⊝ | Exercise interventions may have no effect on medical deterioration during hospitalisation. |
| Participant global assessment of success | Not pooled | Not pooled | Not pooled | (0 studies) | ‐ | No studies reported participant global assessment of success. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| See interactive version of this table: https://gdt.gradepro.org/presentations/#/isof/isof_question_revman_web_423027971375700878. | ||||||
| a Range based on the seven studies that measured activities of daily living using a Barthel Index (range 0–100). | ||||||
| Rehabilitation‐related activity interventions compared to usual care with or without sham interventions for acutely hospitalised older medical patients | ||||||
| Patient or population: acutely hospitalised older medical patients | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Risk with usual care ± sham interventions | Risk with rehabilitation‐related activities | |||||
| Functional ability: independence with activities of daily living at discharge from hospital | The mean functional ability: independence with activities of daily living at discharge from hospital was 42 points on the Barthel Indexa | MD 0 points on the Barthel Index | ‐ | 2838 | ⊕⊕⊝⊝ | Rehabilitation‐related activities may result in little to no difference in independence with activities of daily living at discharge from hospital (standardised mean difference (SMD) 0.00, 95% CI −0.12 to 0.13). A change of 11 points on the Barthel Index is thought to represent a minimally clinically important difference (MCID). |
| Functional ability: functional mobility at discharge from hospital | The mean functional ability: functional mobility at discharge from hospital was 5 points on the Physical Performance and Mobility Examination | MD 0.14 points on the Physical Performance and Mobility Examination higher | ‐ | 975 | ‐ | Included only 1 study categorised as delivering a rehabilitation‐related activity intervention. The effect of rehabilitation‐related activities on functional mobility at discharge from hospital was very uncertain. |
| Incidence of new delirium during hospitalisation | 107 per 1000 | 92 per 1000 | RR 0.86 | 732 | ⊕⊝⊝⊝ | The evidence was very uncertain with regard to the effect of rehabilitation‐related activity interventions on incidence of delirium during hospitalisation. |
| Falls during hospitalisation | 24 per 1000 | 32 per 1000 | RR 1.33 | 250 | ‐ | Only 1 study categorised as delivering a rehabilitation‐related activity intervention was included. The effect of rehabilitation‐related activities on falls during hospitalisation was very uncertain. |
| Quality of life at discharge from hospital | The mean quality of life at discharge from hospital was 48.9 points on the EQ‐5D VAS | MD 2.2 points on the EQ‐5D VAS higher | ‐ | 350 | ‐ | Only 1 study reported a quality‐of‐life outcome at hospital discharge. The effect of rehabilitation‐related activities on the incidence of delirium during hospitalisation was very uncertain. |
| Medical deterioration during hospitalisation | 107 per 1000 | 92 per 1000 | RR 0.86 | 732 | ⊕⊝⊝⊝ | The evidence was very uncertain with regard to the effect of rehabilitation‐related activity interventions on incidence of medical deterioration during hospitalisation. |
| Participant global assessment of success | Not pooled | Not pooled | Not pooled | (0 studies) | ‐ | No studies reported participant global assessment of success. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| See interactive version of this table: https://gdt.gradepro.org/presentations/#/isof/isof_question_revman_web_423062461138566957. | ||||||
| a Based on the one study that measured activities of daily living using a Barthel Index (range of possible scores 0–100). | ||||||
| Structured exercise interventions compared to usual care with or without sham interventions for acutely hospitalised older medical patients | ||||||
| Patient or population: acutely hospitalised older medical patients | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Risk with usual care ± sham interventions | Risk with structured exercise interventions | |||||
| Functional ability: independence with activities of daily living at discharge from hospital | The mean functional ability: independence with activities of daily living at discharge from hospital ranged from 55 to 56 points on the Barthel Indexa | MD 2.6 points on the Barthel Index higher | ‐ | 648 | ⊕⊕⊝⊝ | Structured exercise may result in little to no difference in independence with activities of daily living at discharge from hospital (standardised mean difference (SMD) 0.12, 95% CI −0.21 to 0.45). A change of 11 points on the Barthel Index is thought to represent a minimally clinically important difference (MCID). |
| Functional ability: functional mobility at discharge from hospital | The mean functional ability: functional mobility at discharge from hospital was 14.13 units on the Elderly Mobility Scalee | MD 1.79 units on the Elderly Mobility Scale higher | ‐ | 416 | ⊕⊝⊝⊝ | The evidence was very uncertain with regard to the effect of structured exercise programmes on functional mobility at discharge from hospital (SMD 0.30 95% CI, ‐0.96, 1.57). A change of 2 points on the Elderly Mobility Scale is thought to represent an MCID. |
| Functional ability: new incidence of delirium during hospitalisation | Only 1 study reported the outcome. The study found only 1 incidence of delirium in the intervention group and 0 in the control group. | 100 | ‐ | Included only 1 study categorised as delivering a structured exercise intervention. The effect of structured exercise on the incidence of new delirium during hospitalisation was very uncertain. | ||
| Quality of life at discharge from hospital | The mean quality of life at discharge from hospital was 64.74 points on the EQ‐5D VAS | MD 3.74 points on the EQ‐5D VAS higher | ‐ | 76 | ‐ | Only 1 study reported a quality‐of‐life outcome at hospital discharge. The effect of structured exercise interventions on quality of life at discharge from hospital was very uncertain. |
| Falls during hospitalisation | 40 per 1000 | 31 per 1000 | RR 0.76 | 542 | ⊕⊕⊝⊝ | Structured exercise interventions may result in little to no difference in falls during hospitalisation. |
| Medical deterioration during hospitalisation | 20 per 1000 | 51 per 1000 | RR 2.56 | 200 | ⊕⊝⊝⊝ | The evidence was very uncertain with regard to the effect of structured exercise programmes on medical deterioration during hospitalisation. |
| Participant global assessment of success | Not pooled | Not pooled | Not pooled | (0 studies) | ‐ | No studies reported participant global assessment of success. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| See interactive version of this table: https://gdt.gradepro.org/presentations/#/isof/isof_question_revman_web_423064120727928815. | ||||||
| a Range based on the two studies that measured activities of daily living using a Barthel Index (range of possible scores 0–100). | ||||||
| Progressive resistance exercise interventions compared to usual care with or without sham interventions for acutely hospitalised older medical patients | ||||||
| Patient or population: acutely hospitalised older medical patients | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Risk with usual care ± sham interventions | Risk with progressive resistance exercise | |||||
| Functional ability: independence with activities of daily living at discharge from hospital | The mean functional ability: independence with activities of daily living at discharge from hospital ranged from 75 to 96 points on the Barthel Indexa | MD 0.14 points on the Barthel Index higher | ‐ | 1688 | ⊕⊕⊝⊝ | The evidence is classified as very uncertain with regard to the effect of progressive resistance exercise on independence with activities of daily living at discharge from hospital (SMD 0.14, 95% CI −0.05 to 0.32). A change of 11 points on the Barthel Index is thought to represent a minimally clinically important difference (MCID). |
| Functional ability: functional mobility at discharge from hospital | The mean functional ability: functional mobility at discharge from hospital ranged from 3.7 to 4.9 points on the Short Physical Performance Battery e | MD 0.24 points on the Short Physical Performance Battery higher | ‐ | 978 | ⊕⊝⊝⊝ | The evidence is classified as very uncertain with regard to the effect of progressive resistance exercise on functional mobility at discharge from hospital. (SMD 0.63, 95% CI‐0.28, 1.55). A change of 1.0 points on the Short Physical Performance Battery is thought to represent a MCID. |
| Functional ability: new incidence of delirium during hospitalisation | 71 per 1000 | 68 per 1000 | RR 0.96 | 1256 | ⊕⊕⊝⊝ | The evidence is classified as very uncertain with regard to the effect of progressive resistance exercise on incidence of delirium during hospitalisation. |
| Quality of life at discharge from hospital | The mean quality of life at discharge from hospital ranged from 57.5 to 62.4 points on the EQ‐5D VAS | MD 8.9 points on the EQ‐5D VAS higher | ‐ | 449 | ⊕⊕⊕⊝ | Progressive resistance exercise probably increases quality of life at discharge from hospital slightly. A change of 10 points on the EQ‐5D VAS is thought to represent a MCID. |
| Falls during hospitalisation | 34 per 1000 | 33 per 1000 | RR 0.96 | 995 | ⊕⊕⊝⊝ | Progressive resistance exercise may result in little to no difference in falls during hospitalisation. |
| Medical deterioration during hospitalisation | 62 per 1000 | 61 per 1000 | RR 0.99 | 1798 | ⊕⊝⊝⊝ | The evidence is classified as very uncertain with regard to the effect of progressive resistance exercise on medical deterioration during hospitalisation. |
| Participant global assessment of success | Not pooled | Not pooled | Not pooled | (0 studies) | ‐ | This outcome was not measured by any of the included studies. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| See interactive version of this table: https://gdt.gradepro.org/presentations/#/isof/isof_question_revman_web_423057317911555615. | ||||||
| a Range based on the four studies that measured activities of daily living using a Barthel Index (range of possible scores 0–100). | ||||||
| Study ID | Usual care setting and description | Control/sham intervention | Intervention group setting and description | Intervention subgroup category | Exercise component of intervention | Exercise dose prescription | Exercise intervention adherence |
|---|---|---|---|---|---|---|---|
| Acute geriatric unit. Geriatrician‐led care, physiotherapy requested by the geriatrician as required. | None. | Usual care conditions with additional occupational therapy interventions. | Rehabilitation‐related activities. | Occupational therapy including practice of activities of daily living. | 45 minutes, 5 times per week (Monday–Friday), for the duration of hospital admission. | Mean 5 sessions per participant. | |
| Medical ward. Internist‐led care, physiotherapy and occupational therapy not routinely available. No geriatrician. | None. | Acute geriatric ward. Care provided by both geriatricians and internists. Multidisciplinary team included physiotherapists, occupational therapists and dietitians. Emphasis on interdisciplinary care. | Rehabilitation‐related activities. | Exercise component not specifically described, intervention included early start of rehabilitation and routine physiotherapy and occupational therapy assessments. | No information. | No information. | |
| Acute care geriatric medicine unit. Physiotherapy provided from day 3 of admission, for 3 sessions per week until discharge. | None. | Usual care conditions with additional physiotherapy. | Structured exercise. | Early physiotherapy starting from day 1–2 of admission consisting of bed and standing exercises. | 30 minutes, 2 times per day, 5 times per week, until deemed clinically stable. | No information. | |
| Medical ward. Physicians could order physiotherapy services. | Usual care with daily 15‐ to 20‐minute visits from research assistants, up to twice daily, 7 days per week. Participants requested to keep a diary of their visitors. | Usual care conditions + mobility programme, and encouragement to increase time out of bed. | Structured exercise | Assisted/ supervised mobility programme with behavioural intervention to encourage additional physical activity outside the supervised intervention. | 15–20 minutes, up to twice per day, 7 days per week, for the duration of hospital admission. | 122/238 (51.3%) potential walks were completed. | |
| Usual care units. Not described.
| None. | Acute Care for Elders Unit Renovated ward with a physiotherapy room. Daily interdisciplinary team rounds provided by geriatrician medical director and geriatric clinical nurse specialist who created care plans. Care processes designed to promote functional independence. | Rehabilitation related activities. | Exercise component not specifically described, intervention included a mobility protocol and physiotherapy. | No information. | No information. | |
| Medical ward. Routine care, discharge planning and rehabilitation advice normally provided. | None. | Usual care with additional exercise. | Progressive resistance exercise. | With 72 hours of admission a care plan was produced by a nurse and physiotherapist which included: facilitated stretching, balance training, walking and strengthening exercises. | Walking for up to 15 minutes (duration of other exercise not specified), up to 2–4 times per week, for the duration of the hospital admission. | No information regarding in‐hospital adherence. | |
| Medical wards. Daily medical assessment, and allied health service on referral. | None. | Usual care with additional exercise. | Progressive resistance exercise. | Supervised strengthening and mobility exercise. | 20–30 minutes, twice per day, 5 days per week, for the duration of the hospital admission. | No information. | |
| Acute medical care unit. Care led by physicians specialising in internal medicine. Physiotherapy/ occupational therapy available for counselling only. | None. | Comprehensive geriatric assessment unit. Structured comprehensive geriatric assessment and care led by physicians specialising in internal medicine, family medicine, geriatrics or a combination. Unit staff included physiotherapists and occupational therapists. | Rehabilitation‐related activities. | Exercise component not specifically described, intervention included routine physiotherapy and occupational therapy. | No information. | No information. | |
| Medical or surgical floors. Description not provided other than 'standard medical care'. | None. | Senior Care Unit Functional assessment on admission, 3 clinical team meetings and 1 administration meeting weekly. Geriatric assessment team included nurse co‐ordinators and a physiotherapist. Emphasise interdisciplinary comprehensive geriatric assessment and intervention. | Rehabilitation‐related activities. | Exercise component not specifically described, intervention included routine functional assessment and physiotherapy. | No information. | No information. | |
| Geriatric unit. Care led by a geriatrician and provided by multidisciplinary team. | None. | Usual care with a walking intervention guided by geriatrician, delivered by nurses. | Structured exercise. | Assisted walking programme. | 20–30 minutes, daily, 5 days per week, for the duration of hospitalisation. | A mean time of 32 minutes per session (range 10–67), with a mean distance of 89 m (range 0–260). Mean number of intervention days for each participant was 5.8. | |
| Medical wards. Not described. | None. | Usual care conditions with mobility programme. | Structured exercise. | Assisted or supervised exercise, including balance, pedalling and mobility activities. | Up to 30 minutes per day, for the duration of hospital admission. | No information. | |
| Medical unit. Daily medical assessment and allied health professionals available via referral. | None. | Usual care conditions with additional exercise and orientation. | Progressive resistance exercise. | Progressive resistance exercise and mobility training. | 20–30 minutes per day (Monday–Friday), twice per day, for the duration of hospitalisation. | Median of 1.4 therapy sessions per day or 38 minutes per day (including weekends and routine therapy). This was equivalent to approximately 1.4 sessions or 42 minutes of additional therapy per weekday compared to the control group. | |
| General medical wards. Allied health interventions including physiotherapy available. | None. | Usual care conditions with additional exercise. | Progressive resistance exercise. | Individualised assisted or supervised strength, balance and functional exercises. | 30 minutes, twice per day for the duration of hospitalisation. | Median of 160 minutes (IQR 120–360) participating in the exercise intervention. | |
| Medical units. Physiotherapy available. | None. | Usual care conditions with additional assisted/ supervised walking. | Structured exercise. | Assisted or supervised walking programme. | Twice per day, 7 days per week, for 7 days. The distance walked was the maximum distance able to be comfortably walked as decided by that individual at that time. | No information. | |
| General medical unit. Care led by attending physician, nursing:participant ratio approximately 1:2. Access to hospital wide support services including physiotherapy. | None. | Acute Care for Elders Unit Care led by medical and nursing directors. Increased funded multidisciplinary team hours compared to usual care (including physiotherapy) with care protocols and ward environment designed to promote independence and early discharge. | Rehabilitation‐related activities | Exercise component not specifically described, intervention included a mobility protocol and physiotherapy. | No information. | No information. | |
| Acute Care for the Elderly Unit. Care led by a geriatrician with routine physiotherapy available when needed. | None | Usual care conditions with additional exercise. | Progressive resistance exercise | Supervised morning sessions included progressive resistance, balance and walking exercises. Unsupervised functional exercises in evenings. | 20 minutes, twice per day for 5–7 consecutive days (including weekends). | The mean number of completed morning sessions per participant was 5 (SD 1) and evening sessions was 4 (SD 1). Adherence to the intervention was 95.8% for the morning sessions (i.e. 806 successfully completed sessions of 841 total possible sessions) and 83.4% in the evening sessions (574 of 688 successfully completed sessions). | |
| All wards admitting older medical patients. Physiotherapy available to all participants (mean 3 sessions per week).
| Usual care with twice‐daily sessions (Monday–Friday) each 20–30 minutes of stretching and relaxation exercises in lying or sitting. Participants encouraged to talk about their condition and exercise, none given education, encouragement or assisted to exercise or walk more. | Usual care with additional exercise. | Progressive resistance exercise. | Assisted or supervised tailored strengthening, balance and gait exercises. | Up to 30 minutes, 2 times per day (Monday–Friday) for the duration of hospital admission. | 63/95 participants completed ≥ 75% of possible exercise sessions; 16/95 participants completed 50–74% of possible exercise sessions. 13/95 participants completed 25–49% of possible exercise sessions. 3/95 participants completed < 25% of possible exercise sessions. | |
| Acute medical wards for older people. Not described. | None. | Usual care with additional pedalling exercise. | Structured exercise. | Unsupervised pedalling exercise. | 5 minutes, 3 times per day. | The median number of revolutions cycled throughout the entire study period with the pedal exerciser was 152 (IQR 43.5–464.5) revolutions. The median time spent on the pedal exerciser was 5.08 (IQR 2.03–20.05) minutes across the whole study period. | |
| Medical ward. Multidisciplinary care included daily discussion of participant progress and discharge plan. Referrals made to physiotherapy or occupational therapy when needed. | None. | Medical ward Usual care with additional exercise and cognitive group therapy to encourage mobility. Intervention ward staff, participants and carers educated to encourage mobility and functional independence. | Progressive resistance exercise. | Graduated and tailored supervised exercise programme. | Twice per day for the duration of hospital admission. | 92% of participants in the intervention group received an exercise diary and made some record of exercise; 1/3 completed their diary every day. | |
| Acute care of older patient units Not described. | None. | Usual care with additional exercise. | Progressive resistance exercise. | Supervised walking and sit to stand exercises. | 1–3 sessions per day, with a total duration of approximately 20 minutes per day (Monday–Friday). | Participants performed a median of 3 training days (IQR 2) and 2 training sessions per day (IQR 2), with a mean total exercise time per day of 20 minutes (for each session, the median duration of the walking part was 5 minutes (IQR 4, range 0–10), and participants performed a mean of 9 (SD 6, range 0 to 30) sit‐to‐stands). | |
| Acute medical ward and internal medicine ward. National targets to assess function and nutrition and make an appropriate plan within 24–48 hours of admission. Rehabilitation often started during hospitalisation. | None. | Usual care with additional exercise and protein supplements. | Progressive resistance exercise. | Supervised progressive strength training based on sit to stand exercises. | 20 minutes daily (Monday–Friday) for the duration of hospital admission. | 78.8% of participants started the intervention 0–2 days after admission. Overall (during and after hospitalisation), 43% (18/42) of the participants randomised to the intervention group were very compliant with the intervention (80% of sessions performed with 2 sets of 8 repetitions). | |
| General medical elderly care wards. Therapy provided by ward occupational therapist and physiotherapist on weekdays only. | None. | General medical elderly care wards. Therapy provided by community therapy team including occupational therapist and physiotherapist 7 days per week if appropriate. | Rehabilitation related activities. | Exercise component not specifically described, intervention included daily rehabilitation with a physiotherapist or occupational therapist. | Daily, duration dependent on needs. | No information. | |
| General medical unit. Description not provided. | None. | General Medical Unit. In addition to usual care, a geriatric team consisting of a geriatrician, physiotherapist and liaison nurse provided care including daily physiotherapy. The aim of the team was to optimise function and mobility. | Rehabilitation related activities | Exercise component not specifically described, intervention included daily physiotherapy. | No information. | No information. | |
| Internal medical care unit. Care led by internist physician and had access to physical and occupational therapy by referral. | None. | Geriatric care unit. Care led by geriatrician and ward team included physiotherapist and occupational therapist. | Rehabilitation‐related activities | Exercise component not specifically described, intervention included a mobility protocol and physiotherapy. | No information. | No information. | |
| IQR: interquartile range; SD: standard deviation. | |||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1.1 Functional ability: independence with activities of daily living at discharge from hospital Show forest plot | 16 | 5174 | Std. Mean Difference (IV, Random, 95% CI) | 0.09 [‐0.02, 0.19] |
| 1.1.1 Rehabilitation‐related activities | 4 | 2838 | Std. Mean Difference (IV, Random, 95% CI) | 0.00 [‐0.12, 0.13] |
| 1.1.2 Structured exercise | 5 | 648 | Std. Mean Difference (IV, Random, 95% CI) | 0.12 [‐0.21, 0.45] |
| 1.1.3 Progressive resistance exercise | 7 | 1688 | Std. Mean Difference (IV, Random, 95% CI) | 0.14 [‐0.05, 0.32] |
| 1.2 Functional ability: functional mobility at discharge from hospital Show forest plot | 8 | 2369 | Mean Difference (IV, Random, 95% CI) | 0.54 [0.09, 0.99] |
| 1.2.1 Rehabilitation‐related activities | 1 | 975 | Mean Difference (IV, Random, 95% CI) | 0.60 [0.06, 1.14] |
| 1.2.2 Structured exercise | 2 | 416 | Mean Difference (IV, Random, 95% CI) | 0.30 [‐0.96, 1.57] |
| 1.2.3 Progressive resistance exercise | 5 | 978 | Mean Difference (IV, Random, 95% CI) | 0.63 [‐0.28, 1.55] |
| 1.3 Functional ability: new incidence of delirium during hospitalisation Show forest plot | 7 | 2088 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.58, 1.41] |
| 1.3.1 Rehabilitation‐related activities | 2 | 732 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.30, 2.50] |
| 1.3.2 Structured exercise | 1 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 3.00 [0.13, 71.92] |
| 1.3.3 Progressive resistance exercise | 4 | 1256 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.55, 1.68] |
| 1.4 Quality of life at discharge from hospital Show forest plot | 4 | 875 | Mean Difference (IV, Random, 95% CI) | 6.04 [0.90, 11.18] |
| 1.4.1 Rehabilitation‐related activities | 1 | 350 | Mean Difference (IV, Random, 95% CI) | 2.20 [‐1.90, 6.30] |
| 1.4.2 Structured exercise | 1 | 76 | Mean Difference (IV, Random, 95% CI) | 3.74 [‐6.32, 13.80] |
| 1.4.3 Progressive resistance exercise | 2 | 449 | Mean Difference (IV, Random, 95% CI) | 8.90 [2.35, 15.45] |
| 1.5 Falls during hospitalisation Show forest plot | 9 | 1787 | Risk Ratio (IV, Random, 95% CI) | 0.99 [0.59, 1.65] |
| 1.5.1 Rehabilitation‐related activities | 1 | 250 | Risk Ratio (IV, Random, 95% CI) | 1.33 [0.30, 5.84] |
| 1.5.2 Structured exercise | 3 | 542 | Risk Ratio (IV, Random, 95% CI) | 0.76 [0.23, 2.53] |
| 1.5.3 Progressive resistance exercise | 5 | 995 | Risk Ratio (IV, Random, 95% CI) | 0.96 [0.48, 1.91] |
| 1.6 Medical deterioration during hospitalisation Show forest plot | 11 | 2730 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.62, 1.68] |
| 1.6.1 Rehabilitation‐related activities | 2 | 732 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.30, 2.50] |
| 1.6.2 Structured exercise | 2 | 200 | Risk Ratio (M‐H, Random, 95% CI) | 2.56 [0.48, 13.54] |
| 1.6.3 Progressive resistance exercise | 7 | 1798 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.52, 1.87] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 2.1 Death during hospitalisation Show forest plot | 20 | 6822 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.79, 1.22] |
| 2.1.1 Rehabilitation‐related activities | 7 | 3926 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.78, 1.34] |
| 2.1.2 Structured exercise | 5 | 740 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.54, 1.56] |
| 2.1.3 Progressive resistance exercise | 8 | 2156 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.54, 1.48] |
| 2.2 Hospital length of stay (days) Show forest plot | 22 | 7182 | Mean Difference (IV, Random, 95% CI) | ‐0.25 [‐0.62, 0.12] |
| 2.2.1 Rehabilitation‐related activities | 9 | 4388 | Mean Difference (IV, Random, 95% CI) | ‐0.55 [‐1.42, 0.32] |
| 2.2.2 Structured exercise | 4 | 635 | Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.93, 0.89] |
| 2.2.3 Progressive resistance exercise | 9 | 2159 | Mean Difference (IV, Random, 95% CI) | ‐0.24 [‐0.63, 0.16] |
| 2.3 New institutionalisation at hospital discharge Show forest plot | 5 | 2364 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.74, 1.12] |
| 2.3.1 Rehabilitation‐related activities | 3 | 2004 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.74, 1.13] |
| 2.3.2 Progressive resistance exercise | 2 | 360 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.37, 2.01] |
| 2.4 Hospital readmission Show forest plot | 14 | 4689 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.81, 1.11] |
| 2.4.1 Rehabilitation‐related activities | 6 | 2960 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.78, 1.16] |
| 2.4.2 Structured exercise | 1 | 339 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.52, 1.18] |
| 2.4.3 Progressive resistance exercise | 7 | 1390 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.72, 1.36] |
| 2.5 Walking performance at discharge from hospital Show forest plot | 6 | 682 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.13 [‐0.35, 0.09] |
| 2.5.1 Rehabilitation‐related activities | 1 | 273 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.29 [‐0.53, ‐0.05] |
| 2.5.2 Structured exercise | 2 | 131 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.32 [‐0.80, 0.16] |
| 2.5.3 Progressive resistance exercise | 3 | 278 | Std. Mean Difference (IV, Random, 95% CI) | 0.09 [‐0.15, 0.33] |
| Bias | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study | Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | ||||||
| Subgroup 1.1.1 Rehabilitation‐related activities | ||||||||||||
| Abizanda 2011 |  |  |  |  |  |  | ||||||
| Allocation sequence was random (computer generated random numbers). Allocation occurred after enrolment by an investigator not involved in the participants' clinical management. Higher number of participants admitted with a stroke in the intervention group (39 vs. 16), in addition, the 'Others' subgroup the Barthel index is higher in the control group than the intervention group (29.1 vs. 23.1). This is thought to be compatible with chance. | ||||||||||||
| Its assumed participants and the Occupational Therapist (OT) delivering the intervention were aware of the assigned intervention allocation. All participants received their allocated intervention. Therefore, even though intention to treat analysis not specified, analysis deemed appropriate. | ||||||||||||
| Given the nature of this population (older adults during and after acute hospitalisation) we considered a threshold of 90% of participants with data as sufficient. We felt this would be consistent with measurements outside of a trial context. When accounting for mortality, 95% and 94% assessed at discharge in intervention and control arms respectively. | ||||||||||||
| The use of the Barthel Index to measure independence with ADLs is considered appropriate, and there were no differences in measurement or ascertainment of the outcomes between groups. The OT assessor was blinded. | ||||||||||||
| The data analyst was blinded, and followed a pre‐specified statistical plan. It is not thought that results were from multiple outcome measurements or multiple analyses. | ||||||||||||
| The study is judged to be at low risk of bias in all domains for this outcome. | ||||||||||||
| Counsell 2000 |  |  |  |  |  |  | ||||||
| Allocation sequence was random (computer generated random numbers) and sequence concealed (opaque sealed envelope). Participant characteristics were balanced. | ||||||||||||
| Participants and those delivering the interventions were aware of intervention assignments. Seventy‐nine participants were not admitted to the unit to which they were assigned. Deviations may have affected the effect estimate, however they were well‐balanced between groups. Intention to treat analysis was used. | ||||||||||||
| Functional data was obtained in 1476 of 1483 of surviving patients (99.5%) at discharge. | ||||||||||||
| The Katz ADL score is considered appropriate, and there were no differences in the measurement or ascertainment between groups. The assessors were not blinded, and it was therefore considered likely that knowledge of the intervention could influence the outcome, given the likely strong belief in the benefits of the intervention ward. | ||||||||||||
| A pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to be at high risk of bias due to measurement of the outcome. | ||||||||||||
| Ekerstad 2017 |  |  |  |  |  |  | ||||||
| Randomisation was based on the availability of beds, and there was no allocation concealment as participants were allocated a ward prior to enrolment. Participant characteristics appeared balanced. | ||||||||||||
| Participants and clinicians delivering care were aware of treatment assignments. There was no evidence of deviations from intended interventions and intention to treat analysis was used. | ||||||||||||
| No missing data at hospital discharge. | ||||||||||||
| The ADL Staircase is considered appropriate, and there were no differences in the measurement or ascertainment between groups. The assessors were not blinded, and it was therefore considered likely that knowledge of the intervention could influence the outcome, given the likely strong belief in the benefits of the intervention ward. | ||||||||||||
| A detailed pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to be at high risk of bias due to bias in the measurement of the outcome and bias arising from the randomisation process. | ||||||||||||
| Landefeld 1995 |  |  |  |  |  |  | ||||||
| Allocation sequence was random (computer generated random numbers) and balanced participant characteristics, but no information was provided regarding allocation concealment. | ||||||||||||
| It is assumed participants and clinicians were aware of the treatment assignments. There was no evidence of deviations from the assigned interventions and evidence of an intention to treat approach being used for analysis. | ||||||||||||
| Accounting for mortality (24 died in each arm) all participants had outcome data at discharge. | ||||||||||||
| The Katz ADL score is considered appropriate, and there were no differences in the measurement or ascertainment between groups. The assessors were not blinded, and it was therefore considered likely that knowledge of the intervention could influence the outcome, given the likely strong belief in the benefits of the intervention ward. | ||||||||||||
| A pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to be at high risk of bias in measurement of the outcome for this outcome. | ||||||||||||
| Subgroup 1.1.2 Structured exercise | ||||||||||||
| Blanc‐Bisson 2008 |  |  |  |  |  |  | ||||||
| No information provided on randomisation methods or sequence concealment other than to say participants were randomised. Differences in participant characteristics appear compatible with chance. | ||||||||||||
| It is assumed that participants and those delivering the interventions were aware of intervention assignments. However, all participants received their intended intervention and intention to treat was specified in the analysis. | ||||||||||||
| When accounting for mortality, data was available for only 72% and 81% of intervention and control group respectively at T1. Some missing data is likely to be dependent on its true value, as the main reason for missing data was adverse events, and we consider participants who experience adverse events to be more likely to have a lower ADL score. | ||||||||||||
| The Katz ADL score is considered appropriate, and there were no differences in the measurement or ascertainment between groups. The assessors were not blinded, and it was therefore considered likely that knowledge of the intervention could influence the outcome, given the likely strong belief in the benefits of the intervention ward. | ||||||||||||
| A pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to be at high risk of bias due to missing outcome data and measurement of the outcome. | ||||||||||||
| Brown 2016 |  |  |  |  |  |  | ||||||
| No description of randomisation sequence generation, but the paper describes a block randomisation strategy and sequence concealed (sealed envelopes). The patient characteristics appear well‐balanced between groups. | ||||||||||||
| We assume both participants and those delivering the interventions were aware of intervention assignments. 6 participants did not receive allocated intervention, this is considered to be consistent with what could occur outside the trial context, and intention to treat analysis was used. | ||||||||||||
| No missing data. | ||||||||||||
| The Katz ADL score is considered appropriate, and there were no differences in the measurement or ascertainment between groups. The assessors were blinded. | ||||||||||||
| The study protocol details statistical analysis plan which is in accordance with results presented, and it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to be at low risk of bias in all domains for this outcome. | ||||||||||||
| Gazineo 2021 |  |  |  |  |  |  | ||||||
| Allocation sequence was random (generated using an online system) and the allocation sequence was concealed until participants were enrolled (opaque concealed and sealed envelopes). Participant characteristics were well‐balanced. | ||||||||||||
| Participants and those delivering the interventions were aware of intervention allocations. There was no evidence of deviations from the intended interventions and intention to treat analysis used. | ||||||||||||
| No missing data after accounting for mortality. | ||||||||||||
| The Barthel Index is considered appropriate, and there were no differences in the measurement or ascertainment between groups. The assessors were not blinded, and it was therefore considered likely that knowledge of the intervention could influence the outcome, given the likely strong belief in the benefits of the intervention ward. | ||||||||||||
| A pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to be at high risk of bias due to measurement of the outcome. | ||||||||||||
| Hu 2020 |  |  |  |  |  |  | ||||||
| Allocation sequence was random (computer generated random numbers) and sequence concealed (blinded project coordinator allocated participants). Participant characteristics were balanced. | ||||||||||||
| Participants and those delivering the interventions were aware of intervention allocations. There was no evidence of deviations from the intended interventions and as such it is presumed intention to treat analysis was used. | ||||||||||||
| At discharge data was available for 76% of reablement group, (78% of reminder group) and 80% of the control group. Only participants who had complete data (i.e. data collected at baseline, discharge and follow‐up) had outcomes reported. Although missing data is well‐balanced between all three groups, it is considered likely that reason for missing data is related to its true value. | ||||||||||||
| The Katz ADL score is considered appropriate, and there were no differences in the measurement or ascertainment between groups. The assessors were blinded. | ||||||||||||
| A pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to be at high risk of bias due to missing outcome data. | ||||||||||||
| Killey 2006 |  |  |  |  |  |  | ||||||
| Allocation not random and sequence predictable as based on alternation. There is limited data on participant characteristics, but available data suggests balanced characteristics between groups. | ||||||||||||
| Participants and those delivering the interventions were aware of intervention allocations. There was no evidence of deviations from the intended interventions. Per‐protocol analysis appears to have been used. 2 participants in the intervention group were excluded from analysis as they completed less than 70% of their walks. The 2 participants that were excluded accounted for 7% of the remaining sample, it is therefore considered that there was potential for a significant impact on the results. | ||||||||||||
| Only 71% of intervention and 74% of control group had outcome data. A significant proportion of missing data was related to early discharge from hospital. These participants could be expected to have a higher functional level than those who remained in hospital. However, the missing data was well‐balanced between the two groups. | ||||||||||||
| The Barthel Index is considered appropriate, and there were no differences in the measurement or ascertainment between groups. The assessors were not blinded, and it was therefore considered likely that knowledge of the intervention could influence the outcome, given the likely strong belief in the benefits of the intervention ward. | ||||||||||||
| A pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to be at high risk of bias in three domains for this outcome. | ||||||||||||
| Subgroup 1.1.3 Progressive resistance exercise | ||||||||||||
| de Morton 2007 |  |  |  |  |  |  | ||||||
| Allocation of wards was random (coin toss) and the allocating officer unaware of study. Baseline differences between groups were thought to be compatible with chance. | ||||||||||||
| Participants and clinicians delivering care were believed to be aware of treatment assignments. There was no evidence of deviations from intended interventions and intention to treat analysis was used. | ||||||||||||
| After accounting for mortality, discharge Barthel Index (BI) scores were available for 75% in intervention group and 70% in control group. Although it is feasible that following discharge patients with lower levels of functional ability are more likely to be missing, we do not think this applies in this situation as assessment prior to discharge. We do not see a situation where it would be more likely to miss an assessment due to high/low level of disability at discharge. | ||||||||||||
| The use of the BI to measure independence with ADLs is considered appropriate, and there were no differences in the measurement or ascertainment between groups. The assessors were not blinded, and it is considered likely that knowledge of the intervention could influence the outcome, given the likely strong belief in the benefits of the intervention. | ||||||||||||
| A pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to be at high risk of bias in measurement of the outcome. | ||||||||||||
| Jeffs 2013 |  |  |  |  |  |  | ||||||
| Sequence concealment was achieved via a member of the research team not involved in recruitment and using sealed envelopes. There was no information regarding method of generating random sequence, other than to say that there was randomisation, given details of allocation concealment, assumption made that appropriate method used. Patient characteristics between groups appear well‐balanced. | ||||||||||||
| Participants and those delivering the interventions were likely to be aware of intervention allocations. One participant was not allocated to a group due to an administrative error and 35 participants did not receive the intervention as planned (17 in the intervention group). This is thought to be consistent with what could occur outside of the trial context. Intention to treat analysis was used. | ||||||||||||
| No missing data. | ||||||||||||
| The Barthel Index is considered appropriate, and there were no differences in the measurement or ascertainment between groups. The assessors were blinded. | ||||||||||||
| A pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to raise some bias concerns in selection of the reported result. | ||||||||||||
| Jones 2006 |  |  |  |  |  |  | ||||||
| Allocation sequence was random (computer generated random numbers). Sequence allocation was not concealed, but performed by a member of staff independent of the enrolment procedures. ). Participant characteristics were balanced between groups and differences compatible with chance. | ||||||||||||
| It is assumed that both participants and clinicians delivering care were aware of assigned interventions. There is no evidence of deviations from intended interventions and intention to treat analysis used. | ||||||||||||
| Admission and discharge modified Barthel Index (mBI) scores were available for 78.8% of patients (126/160). Although we felt that patients with lower levels of functional ability were more likely to be missing at follow‐up, we do not think this applies in this situation as assessment was prior to discharge. We do not see a situation where it would be more likely to miss an assessment due to high/low level of disability at discharge | ||||||||||||
| The use of the mBI to measure independence with ADLs is considered appropriate, and there were no differences in the measurement or ascertainment between groups. The assessors were blinded. | ||||||||||||
| A pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to raise some concerns due to missing outcome data and in selection of the reported results. | ||||||||||||
| Martinez‐Velilla 2019 |  |  |  |  |  |  | ||||||
| Allocation sequence was random (www.randomizer.org) and personal communication with author confirms allocation concealment. Baseline differences between groups are thought to be compatible with chance. | ||||||||||||
| Both participants and clinicians delivering care were aware of assigned interventions. There is no evidence of deviations from intended interventions and intention to treat analysis used. | ||||||||||||
| Assuming that those that discontinued the study did not provide Barthel Index (BI) outcome data, and accounting for mortality, 80% of intervention group and 78% of control group had outcome data. Although there were participants who discontinued the study due to medical deterioration, which could have and is considered likely to have biased the results (i.e. medical deterioration being associated with poor functional outcomes), the numbers were relatively low (only 6% of the sample) and were well‐balanced between groups. We therefore feel that this was unlikely to bias the results. | ||||||||||||
| The use of the BI to measure independence with ADLs is considered appropriate, and there were no differences in the measurement or ascertainment between groups. The assessors were blinded. | ||||||||||||
| The trial protocol reflects the analyses as that were conducted, and it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to raise some concerns due to missing outcome data. | ||||||||||||
| Mudge 2008 |  |  |  |  |  |  | ||||||
| Pseudo‐randomisation, allocation was based on admitting unit and bed availability, and admitting unit was determined by a rotating roster. No evidence of baseline differences in patient characteristics other than those thought to be compatible with chance. | ||||||||||||
| Both participants and clinicians delivering care were aware of assigned interventions. There is no evidence of deviations from intended interventions and intention to treat analysis used. | ||||||||||||
| No missing data. | ||||||||||||
| The use of the modified Barthel Index to measure independence with ADLs is considered appropriate, and there were no differences in the measurement or ascertainment between groups. The assessors were blinded. | ||||||||||||
| A pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to be at high risk of bias due to the randomisation process. | ||||||||||||
| Ortiz‐Alonso 2020 |  |  |  |  |  |  | ||||||
| The allocation sequence was based on recruitment blocks of 4‐8 weeks. Activity of daily living (ADL) scores at baseline and admission were significantly lower in the intervention group than the control group, this is thought to be an important prognostic factor and therefore a concern regarding the randomisation process. | ||||||||||||
| Methods were designed to blind participants from group assignments however due to the nature of the intervention and lack of sham intervention the success of this blinding is felt unlikely. Ward staff and research staff were not blinded to the participant's assigned intervention. There were no deviations from the intended interventions due to trial context described and although intention to treat analysis not specified given the lack of deviations it is assumed. | ||||||||||||
| After adjusting for mortality 97% in intervention group and 97% in control group had outcome data. | ||||||||||||
| The Katz ADL score is considered appropriate, and there were no differences in the measurement or ascertainment between groups. The assessors were not blinded, and it was therefore considered likely that knowledge of the intervention could influence the outcome, given the likely strong belief in the benefits of the intervention ward. | ||||||||||||
| Analysis reflects plan in trial registration and study protocol, and results are not thought to be from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to be at high risk of bias due to the randomisation process and measurement of the outcome. | ||||||||||||
| Pedersen 2019 |  |  |  |  |  |  | ||||||
| Allocation sequence was random (computer‐generated block randomisation), and allocation sequence concealed from the investigators. Participant characteristics were well‐balanced between groups. | ||||||||||||
| Participants and clinicians were aware of the treatment assignments. There was no evidence of deviations from the assigned interventions and an intention to treat approach was used for analysis. | ||||||||||||
| When accounting for mortality only 93% of participants in intervention group and 67% in control group completed measures at discharge. The difference in the proportion of missing data, and that the assessments were carried out home after discharge, it was judged likely that the reasons for missing data may have depended on its true value. | ||||||||||||
| The Barthel Index is considered appropriate, and there were no differences in the measurement or ascertainment between groups. The assessors were blinded. | ||||||||||||
| The analysis is in accordance with a pre‐specified analysis in a published protocol, and results are not thought to be from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to be at high risk of bias due to missing outcome data. | ||||||||||||
| Bias | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study | Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | ||||||
| Subgroup 1.2.1 Rehabilitation‐related activities | ||||||||||||
| Counsell 2000 |  |  |  |  |  |  | ||||||
| Allocation sequence was random (computer generated random numbers) and sequence concealed (opaque sealed envelope). Participant characteristics were balanced. | ||||||||||||
| Participants and those delivering the interventions were aware of intervention assignments. Seventy‐nine participants were not admitted to the unit to which they were assigned. Deviations may have affected the effect estimate, however the were well‐balanced between groups. Intention to treat analysis was used. | ||||||||||||
| Functional data was obtained in 1476 of 1483 of surviving patients (99.5%) at discharge. | ||||||||||||
| The Physical Performance and Mobility Examination (PPME) is considered appropriate, and there were no differences in the measurement or ascertainment between groups. The assessors were not blinded, and it was therefore considered likely that knowledge of the intervention could influence the outcome, given the likely strong belief in the benefits of the intervention ward. | ||||||||||||
| A pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to be at high risk of bias due to measurement of the outcome. | ||||||||||||
| Subgroup 1.2.2 Structured exercise | ||||||||||||
| Gazineo 2021 |  |  |  |  |  |  | ||||||
| Allocation sequence was random (generated using an online system) and the allocation sequence was concealed until participants were enrolled (opaque concealed and sealed envelopes). Participant characteristics were well‐balanced. | ||||||||||||
| Participants and those delivering the interventions were aware of intervention allocations. There was no evidence of deviations from the intended interventions and intention to treat analysis used. | ||||||||||||
| No missing data after accounting for mortality. | ||||||||||||
| The Barden Activity Subscale to measure functional mobility is considered appropriate, and there were no differences in the measurement or ascertainment between groups. The assessors were not blinded, and it was therefore considered likely that knowledge of the intervention could influence the outcome, given the likely strong belief in the benefits of the intervention ward. | ||||||||||||
| A pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to be at high risk of bias due to measurement of the outcome. | ||||||||||||
| McGowan 2018a |  |  |  |  |  |  | ||||||
| Allocation sequence was random (www.randomization.com) and the allocation sequence was only known by the chief investigator who was not involved with screening patients. Baseline differences between groups are thought to be compatible with chance. | ||||||||||||
| Both participants and clinicians delivering care were aware of assigned interventions. There is no evidence of deviations from intended interventions and intention to treat analysis not explicitly stated, but all participants appear to have been analysed in the group to which they were randomised. | ||||||||||||
| 96% of participants had complete data sets. | ||||||||||||
| The use of the Elderly Mobility Scale (EMS) to measure functional mobility is considered appropriate, and there were no differences in the measurement or ascertainment between groups. The assessors were not blinded, and it is considered likely that knowledge of the intervention could influence the outcome, given the likely strong belief in the benefits of the intervention. | ||||||||||||
| The trial protocol and registration reflects the analyses as that were conducted, and it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to raise some concerns due to the measurement of the outcome. | ||||||||||||
| Subgroup 1.2.3 Progressive resistance exercise | ||||||||||||
| de Morton 2007 |  |  |  |  |  |  | ||||||
| Allocation of wards was random (coin toss) and the allocating officer unaware of study. Baseline differences between groups were thought to be compatible with chance. | ||||||||||||
| Participants and clinicians delivering care were believed to be aware of treatment assignments. There was no evidence of deviations from intended interventions and intention to treat analysis was used. | ||||||||||||
| After accounting for mortality, discharge Functional Ambulation Classification (FAC) scores were available for 75% in intervention group and 70% in control group. Although it is feasible that following discharge patients with lower levels of functional ability are more likely to be missing, we do not think this applies in this situation as assessment prior to discharge. We do not see a situation where it would be more likely to miss an assessment due to high/low level of disability at discharge. | ||||||||||||
| The use of the FAC to measure functional mobility is considered appropriate, and there were no differences in the measurement or ascertainment between groups. The assessors were not blinded, and it is considered likely that knowledge of the intervention could influence the outcome, given the likely strong belief in the benefits of the intervention. | ||||||||||||
| A pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to be at high risk of bias in measurement of the outcome. | ||||||||||||
| Martinez‐Velilla 2019 |  |  |  |  |  |  | ||||||
| Allocation sequence was random (www.randomizer.org) and personal communication with author confirms allocation concealment. Baseline differences between groups are thought to be compatible with chance. | ||||||||||||
| Both participants and clinicians delivering care were aware of assigned interventions. There is no evidence of deviations from intended interventions and intention to treat analysis used. | ||||||||||||
| Assuming that those that discontinued the study did not provide Short Physical Performance Battery (SPPB) outcome data, and accounting for mortality, 83% of intervention group and 86% of control group had outcome data. Although there were participants who discontinued the study due to medical deterioration, which could have and is considered likely to have biased the results (i.e. medical deterioration being associated with poor functional outcomes), the numbers were relatively low (only 6% of the sample) and were well‐balanced between groups. We therefore feel that this was unlikely to bias the results. | ||||||||||||
| The use of the SPPB to measure functional mobility is considered appropriate, and there were no differences in the measurement or ascertainment between groups. The assessors were blinded. | ||||||||||||
| The trial protocol reflects the analyses as that were conducted, and it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to raise some concerns due to missing outcome data. | ||||||||||||
| McCullagh 2020 |  |  |  |  |  |  | ||||||
| Allocation sequence was random (computer‐generated) and allocation sequence was concealed. Baseline differences are thought to be compatible with chance. | ||||||||||||
| Both participants and clinicians delivering care were aware of assigned interventions. There is no evidence of deviations from intended interventions and intention to treat analysis used. | ||||||||||||
| 90% of intervention group and 94% of the control group had Short Physical Performance Battery (SPPB) data. Given the nature of this population (older adults during and after acute hospitalisation) we considered a threshold of 90% of participants with data as sufficient. | ||||||||||||
| The use of SPPB to measure functional mobility is considered appropriate, and there were no differences in the measurement or ascertainment between groups. The assessors were blinded. | ||||||||||||
| The trial protocol reflects the analyses as that were conducted, and it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to be at low risk of bias for all domains for this outcome. | ||||||||||||
| Ortiz‐Alonso 2020 |  |  |  |  |  |  | ||||||
| The allocation sequence was based on recruitment blocks of 4‐8 weeks. Activity of daily living (ADL) scores at baseline and admission were significantly lower in the intervention group than the control group, this is thought to be an important prognostic factor and therefore a concern regarding the randomisation process. | ||||||||||||
| Methods were designed to blind participants from group assignments however due to the nature of the intervention and lack of sham intervention the success of this blinding is felt unlikely. Ward staff and research staff were not blinded to the participant's assigned intervention. There were no deviations from the intended interventions due to trial context described and although intention to treat analysis not specified given the lack of deviations it is assumed. | ||||||||||||
| After adjusting for mortality 97% in intervention group and 97% in control group had outcome data. | ||||||||||||
| The use of the Short Physical Performance Battery (SPPB) for measuring functional mobility is considered appropriate, and there were no differences in the measurement or ascertainment between groups. The assessors were not blinded, and it was therefore considered likely that knowledge of the intervention could influence the outcome, given the likely strong belief in the benefits of the intervention ward. | ||||||||||||
| Analysis reflects plan in trial registration and study protocol, and results are not thought to be from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to be at high risk of bias due to the randomisation process and measurement of the outcome. | ||||||||||||
| Pedersen 2019 |  |  |  |  |  |  | ||||||
| Allocation sequence was random (computer‐generated block randomisation), and allocation sequence concealed from the investigators. Participant characteristics were well‐balanced between groups. | ||||||||||||
| Participants and clinicians were aware of the treatment assignments. There was no evidence of deviations from the assigned interventions and an intention to treat approach was used for analysis. | ||||||||||||
| When accounting for mortality only 93% of participants in intervention group and 67% in control group completed measures at discharge. The difference in the proportion of missing data, and that the assessments were carried out home after discharge, it was judged likely that the reasons for missing data may have depended on its true value. | ||||||||||||
| The use of the de Morton Mobility Index (DEMMI) is considered appropriate, and there were no differences in the measurement or ascertainment between groups. The assessors were blinded. | ||||||||||||
| The analysis is in accordance with a pre‐specified analysis in a published protocol, and results are not thought to be from multiple outcome measures or multiple analyses. | ||||||||||||
| As per RoB2 algorithm, the study is judged to be at high risk of bias due to missing outcome data. | ||||||||||||
| Bias | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study | Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | ||||||
| Subgroup 1.3.1 Rehabilitation‐related activities | ||||||||||||
| Abizanda 2011 |  |  |  |  |  |  | ||||||
| Allocation sequence was random (computer generated random numbers). Allocation occurred after enrolment by an investigator not involved in the participants' clinical management. Higher number of participants admitted with a stroke in the intervention group (39 vs. 16), in addition, the 'Others' subgroup the Barthel index is higher in the control group than the intervention group (29.1 vs. 23.1). This is thought to be compatible with chance. | ||||||||||||
| Its assumed participants and the Occupational Therapist (OT) delivering the intervention were aware of the assigned intervention allocation. All participants received their allocated intervention. Therefore, even though intention to treat analysis not specified, analysis deemed appropriate. | ||||||||||||
| Given the nature of this population (older adults during and after acute hospitalisation) we considered a threshold of 90% of participants with data as sufficient. We felt this would be consistent with measurements outside of a trial context. When accounting for mortality, 93% and 91% assessed at discharge in intervention and control arms respectively. | ||||||||||||
| The use of the Confusion Assessment Method (CAM) to assess for delirium is considered appropriate, and there were no differences in measurement or ascertainment of the outcomes between groups. The OT assessor was blinded. | ||||||||||||
| The data analyst was blinded, and followed a pre‐specified statistical plan. It is not thought that results were from multiple outcome measurements or multiple analyses. | ||||||||||||
| The study is judged to be at low risk of bias in all domains for this outcome. | ||||||||||||
| Asplund 2000 |  |  |  |  |  |  | ||||||
| The method of allocation sequence is not described, but the use of sealed envelopes suggests a random component was used. Participant characteristics were balanced. | ||||||||||||
| Participants and those delivering the interventions were aware of intervention assignments. Twenty‐five patients were excluded due to them not meeting the set eligibility criteria, however, these: protocol violations are not expected to influence the effect estimate of the outcome as per protocol analyses used. The per protocol analyses was not thought to have a substantial impact on the result given the main reason for exclusion was inappropriate recruitment. Excluding this 25, the other 6 exclusions represent approximately 1% of the total sample size. | ||||||||||||
| Data at discharge from hospital was available for 98% of participants. | ||||||||||||
| The confusion assessment method (CAM) instrument was considered appropriate, and there were no differences in the measurement or ascertainment between groups. The assessors were not blinded, and it was therefore considered likely that knowledge of the intervention could influence the outcome, given the likely strong belief in the benefits of the intervention ward. | ||||||||||||
| A pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to be at high risk of bias due to measurement of the outcome. | ||||||||||||
| Subgroup 1.3.2 Structured exercise | ||||||||||||
| Brown 2016 |  |  |  |  |  |  | ||||||
| No description of randomisation sequence generation, but the paper describes a block randomisation strategy and sequence concealed (sealed envelopes). The patient characteristics appear well‐balanced between groups. | ||||||||||||
| We assume both participants and those delivering the interventions were aware of intervention assignments. 6 participants did not receive allocated intervention, this is considered to be consistent with what could occur outside the trial context, and intention to treat analysis was used. | ||||||||||||
| No missing data. | ||||||||||||
| The Confusion Assessment Method (CAM) score is considered appropriate, and there were no differences in the measurement or ascertainment between groups. The assessors were blinded at admission, discharge and follow up, but not the research assistants as they were delivering the intervention. “The research assistants also used the CAM at each patient visit throughout the hospital stay to ensure that patients in either group did not develop incident delirium.” Therefore, assessors not considered to be blinded, and it is likely given that there is judgement involved in scoring of the CAM, that knowledge of the intervention could influence the scoring of the CAM given the likely strong beliefs in the benefits of the intervention ward. | ||||||||||||
| The study protocol details statistical analysis plan which did not refer to assessment of delirium after baseline assessments, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to be at high risk of bias in measurement of the outcome. | ||||||||||||
| Subgroup 1.3.3 Progressive resistance exercise | ||||||||||||
| Jeffs 2013 |  |  |  |  |  |  | ||||||
| Sequence concealment was achieved via a member of the research team not involved in recruitment and using sealed envelopes. There was no information regarding method of generating random sequence, other than to say that there was randomisation, given details of allocation concealment, assumption made that appropriate method used. Patient characteristics between groups appear well‐balanced. | ||||||||||||
| Participants and those delivering the interventions were likely to be aware of intervention allocations. One participant was not allocated to a group due to an administrative error and 35 participants did not receive the intervention as planned (17 in the intervention group). This is thought to be consistent with what could occur outside of the trial context. Intention to treat analysis was used. | ||||||||||||
| No missing data. | ||||||||||||
| The Confusion Assessment Method (CAM) is considered appropriate, and there were no differences in the measurement or ascertainment between groups. The assessors were blinded. | ||||||||||||
| A pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to raise some concerns in the selection of the reported results. | ||||||||||||
| Martinez‐Velilla 2019 |  |  |  |  |  |  | ||||||
| Allocation sequence was random (www.randomizer.org) and personal communication with author confirms allocation concealment. Baseline differences between groups are thought to be compatible with chance. | ||||||||||||
| Both participants and clinicians delivering care were aware of assigned interventions. There is no evidence of deviations from intended interventions and intention to treat analysis used. | ||||||||||||
| Assuming that those that discontinued the study did not provide Barthel Index (BI) outcome data, and accounting for mortality, 84% of intervention group and 86% of control group had outcome data. Although there were participants who discontinued the study due to medical deterioration, which could have and is considered likely to have biased the results (i.e. medical deterioration being associated with poor functional outcomes), the numbers were relatively low (only 6% of the sample) and were well‐balanced between groups. We therefore feel that this was unlikely to bias the results. | ||||||||||||
| The use of the Confusion Assessment Method (CAM) to measure delirium is considered appropriate, and there were no differences in the measurement or ascertainment between groups. The assessors were blinded. | ||||||||||||
| The trial protocol reflects the analyses as that were conducted, and it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to raise some concerns due to missing outcome data. | ||||||||||||
| McCullagh 2020 |  |  |  |  |  |  | ||||||
| Allocation sequence was random (computer‐generated) and allocation sequence was concealed. Baseline differences are thought to be compatible with chance. | ||||||||||||
| Both participants and clinicians delivering care were aware of assigned interventions. There is no evidence of deviations from intended interventions and intention to treat analysis used. | ||||||||||||
| 90% of intervention group and 94% of the control group had outcome data. Given the nature of this population (older adults during and after acute hospitalisation) we considered a threshold of 90% of participants with data as sufficient. | ||||||||||||
| The use of the Six‐item Cognitive Impairment Test (6CIT) to assess for delirium is considered appropriate, and there were no differences in the measurement or ascertainment between groups. The assessors were blinded. | ||||||||||||
| The trial protocol reflects the analyses as that were conducted, and it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to be at low risk of bias in all domains for this outcome. | ||||||||||||
| Mudge 2008 |  |  |  |  |  |  | ||||||
| Pseudo‐randomisation, allocation was based on admitting unit and bed availability, and admitting unit was determined by a rotating roster. No evidence of baseline differences in patient characteristics other than those thought to be compatible with chance. | ||||||||||||
| Both participants and clinicians delivering care were aware of assigned interventions. There is no evidence of deviations from intended interventions and intention to treat analysis used. | ||||||||||||
| No missing data. | ||||||||||||
| Identifying delirium according to chart review using validated methodology is considered appropriate, and there were no differences in the measurement or ascertainment between groups. The assessors were blinded. | ||||||||||||
| A pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to be at high risk of bias due to the randomisation process. | ||||||||||||
| Bias | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study | Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | ||||||
| Subgroup 1.4.1 Rehabilitation‐related activities | ||||||||||||
| Ekerstad 2017 |  |  |  |  |  |  | ||||||
| Randomisation was based on the availability of beds, and there was no allocation concealment as participants were allocated a ward prior to enrolment. Participant characteristics appeared balanced. | ||||||||||||
| Participants and clinicians delivering care were aware of treatment assignments. There was no evidence of deviations from intended interventions and intention to treat analysis was used. | ||||||||||||
| No missing data. | ||||||||||||
| The EQ‐VAS is considered appropriate, and there were no differences in the measurement or ascertainment between groups. The assessors were not blinded, and it was therefore considered likely that knowledge of the intervention could influence the outcome, given the likely strong belief in the benefits of the intervention ward. | ||||||||||||
| A detailed pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to be at high risk of bias due to bias in the measurement of the outcome and bias arising from the randomisation process. | ||||||||||||
| Subgroup 1.4.2 Structured exercise | ||||||||||||
| Hu 2020 |  |  |  |  |  |  | ||||||
| Allocation sequence was random (computer generated random numbers) and sequence concealed (blinded project coordinator allocated participants). Participant characteristics were balanced. | ||||||||||||
| Participants and those delivering the interventions were aware of intervention allocations. There was no evidence of deviations from the intended interventions and as such it is presumed intention to treat analysis was used. | ||||||||||||
| At discharge data was available for 76% of reablement group, (78% of reminder group) and 80% of the control group. Only participants who had complete data (i.e. data collected at baseline, discharge and follow‐up) had outcomes reported. Although missing data is well‐balanced between all three groups, it is considered likely that reason for missing data is related to its true value. | ||||||||||||
| The EQ5D is considered appropriate, and there were no differences in the measurement or ascertainment between groups. The assessors were blinded. | ||||||||||||
| A pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to be at high risk of bias due to missing outcome data. | ||||||||||||
| Subgroup 1.4.3 Progressive resistance exercise | ||||||||||||
| Martinez‐Velilla 2019 |  |  |  |  |  |  | ||||||
| Allocation sequence was random (www.randomizer.org) and personal communication with author confirms allocation concealment. Baseline differences between groups are thought to be compatible with chance. | ||||||||||||
| Both participants and clinicians delivering care were aware of assigned interventions. There is no evidence of deviations from intended interventions and intention to treat analysis used. | ||||||||||||
| Assuming that those that discontinued the study did not provide e EQ‐5D outcome data, and accounting for mortality, 83% of intervention group and 86% of control group had outcome data. Although there were participants who discontinued the study due to medical deterioration, which could have and is considered likely to have biased the results (i.e. medical deterioration being associated with poor functional outcomes), the numbers were relatively low (only 6% of the sample) and were well‐balanced between groups. We therefore feel that this was unlikely to bias the results. | ||||||||||||
| The EQ‐5D is considered appropriate, and there were no differences in the measurement or ascertainment between groups. The assessors were blinded. | ||||||||||||
| The trial protocol reflects the analyses as that were conducted, and it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to raise some concerns due to missing outcome data. | ||||||||||||
| McCullagh 2020 |  |  |  |  |  |  | ||||||
| Allocation sequence was random (computer‐generated) and allocation sequence was concealed. Baseline differences are thought to be compatible with chance. | ||||||||||||
| Both participants and clinicians delivering care were aware of assigned interventions. There is no evidence of deviations from intended interventions and intention to treat analysis used. | ||||||||||||
| 90% of intervention group and 94% of the control group had outcome data. Given the nature of this population (older adults during and after acute hospitalisation) we considered a threshold of 90% of participants with data as sufficient. | ||||||||||||
| The use of EQ‐5D5L to measure quality of life is considered appropriate, and there were no differences in the measurement or ascertainment between groups. The assessors were blinded. | ||||||||||||
| The trial protocol reflects the analyses as that were conducted, and it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to be at low risk of bias for all domains for this outcome. | ||||||||||||
| Bias | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study | Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | ||||||
| Subgroup 1.5.1 Rehabilitation‐related activities | ||||||||||||
| Sahota 2017 |  |  |  |  |  |  | ||||||
| No information on randomisation methods other than to say participants were randomised, however, patient characteristics appear well‐balanced between groups and differences compatible with chance. | ||||||||||||
| It is assumed that participants and those delivering the interventions were aware of intervention assignments. There were 15 protocol deviations in the CIRACT group and 8 in the THB‐Rehab group. The deviations were thought to be in keeping with what would be expected outside of a trial context. Intention to treat not explicitly stated though it appears that the 1 participant who did not receive the intended intervention was analysed within the group to which they were assigned. | ||||||||||||
| No missing data | ||||||||||||
| Methods of measuring the number of falls was considered appropriate and there were no differences in measurement or ascertainment of the outcomes between groups. The assessor was not blinded, but it is thought that due to the lack of judgement in the measurement, that the knowledge of the intervention did not influence the outcomes. | ||||||||||||
| A pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to raise some concerns in the selection of the reported results and the randomisation process. | ||||||||||||
| Subgroup 1.5.2 Structured exercise | ||||||||||||
| Brown 2016 |  |  |  |  |  |  | ||||||
| No description of randomisation sequence generation, but the paper describes a block randomisation strategy and sequence concealed (sealed envelopes). The patient characteristics appear well‐balanced between groups. | ||||||||||||
| We assume both participants and those delivering the interventions were aware of intervention assignments. 6 participants did not receive allocated intervention, this is considered to be consistent with what could occur outside the trial context, and intention to treat analysis was used. | ||||||||||||
| No missing data. | ||||||||||||
| Methods of measuring the number of falls was considered appropriate and there were no differences in measurement or ascertainment of the outcomes between groups. The assessor was blinded. | ||||||||||||
| The study protocol details statistical analysis plan which is in accordance with results presented, and it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to be at low risk of bias in all domains for this outcome. | ||||||||||||
| Gazineo 2021 |  |  |  |  |  |  | ||||||
| Allocation sequence was random (generated using an online system) and the allocation sequence was concealed until participants were enrolled (opaque concealed and sealed envelopes). Participant characteristics were well‐balanced. | ||||||||||||
| Participants and those delivering the interventions were aware of intervention allocations. There was no evidence of deviations from the intended interventions and intention to treat analysis used. | ||||||||||||
| No missing data after accounting for mortality. | ||||||||||||
| Methods of measuring the number of falls was considered appropriate and there were no differences in measurement or ascertainment of the outcomes between groups. The assessor was not blinded, but it is thought that due to the lack of judgement in the measurement, that the knowledge of the intervention did not influence the outcomes. | ||||||||||||
| A pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to raise some concerns in the selection of the reported results . | ||||||||||||
| Killey 2006 |  |  |  |  |  |  | ||||||
| Allocation not random and sequence predictable as based on alternation. There is limited data on participant characteristics, but available data suggests balanced characteristics between groups. | ||||||||||||
| Participants and those delivering the interventions were aware of intervention allocations. There was no evidence of deviations from the intended interventions. Per‐protocol analysis appears to have been used. 2 participants in the intervention group were excluded from analysis as they completed less than 70% of their walks. The 2 participants that were excluded accounted for 7% of the remaining sample, it is therefore considered that there was potential for a significant impact on the results. | ||||||||||||
| Only 71% of intervention and 74% of control group had outcome data. A significant proportion of missing data was related to early discharge from hospital. These participants could be expected to have a higher functional level than those who remained in hospital. However, the missing data was well‐balanced between the two groups. | ||||||||||||
| Methods of measuring the number of falls was considered appropriate and there were no differences in measurement or ascertainment of the outcomes between groups. The assessor was not blinded, but it is thought that due to the lack of judgement in the measurement, that the knowledge of the intervention did not influence the outcomes. | ||||||||||||
| A pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to be at high risk of bias arising from the randomisation process and due to deviations from the intended interventions. | ||||||||||||
| Subgroup 1.5.3 Progressive resistance exercise | ||||||||||||
| de Morton 2007 |  |  |  |  |  |  | ||||||
| Allocation of wards was random (coin toss) and the allocating officer unaware of study. Baseline differences between groups were thought to be compatible with chance. | ||||||||||||
| Participants and clinicians delivering care were believed to be aware of treatment assignments. There was no evidence of deviations from intended interventions and intention to treat analysis was used. | ||||||||||||
| No missing data. | ||||||||||||
| Methods of measuring the number of falls was considered appropriate and there were no differences in measurement or ascertainment of the outcomes between groups. The assessor was not blinded, but it is thought that due to the lack of judgement in the measurement, that the knowledge of the intervention did not influence the outcomes. | ||||||||||||
| A pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to raise some concerns in the selection of the reported result. | ||||||||||||
| Jones 2006 |  |  |  |  |  |  | ||||||
| Allocation sequence was random (computer generated random numbers). Sequence allocation was not concealed, but performed by a member of staff independent of the enrolment procedures. ). Participant characteristics were balanced between groups and differences compatible with chance. | ||||||||||||
| It is assumed that both participants and clinicians delivering care were aware of assigned interventions. There is no evidence of deviations from intended interventions and intention to treat analysis used. | ||||||||||||
| After accounting for mortality, falls data available for 99% of control group and 93% of intervention group. Given the nature of this population (older adults during and after acute hospitalisation) we considered a threshold of 90% of participants with data as sufficient. | ||||||||||||
| Methods of measuring the number of falls was considered appropriate and there were no differences in measurement or ascertainment of the outcomes between groups. The assessor was blinded. | ||||||||||||
| A pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to raise some concerns due to the selection of the reported results. | ||||||||||||
| Martinez‐Velilla 2019 |  |  |  |  |  |  | ||||||
| Allocation sequence was random (www.randomizer.org) and personal communication with author confirms allocation concealment. Baseline differences between groups are thought to be compatible with chance. | ||||||||||||
| Both participants and clinicians delivering care were aware of assigned interventions. There is no evidence of deviations from intended interventions and intention to treat analysis used. | ||||||||||||
| No missing data | ||||||||||||
| Methods of measuring the number of falls was considered appropriate and there were no differences in measurement or ascertainment of the outcomes between groups. The assessor was blinded. | ||||||||||||
| The trial protocol reflects the analyses as that were conducted, and it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to be at low risk of bias in all domains for this outcome. | ||||||||||||
| McCullagh 2020 |  |  |  |  |  |  | ||||||
| Allocation sequence was random (computer‐generated) and allocation sequence was concealed. Baseline differences are thought to be compatible with chance. | ||||||||||||
| Both participants and clinicians delivering care were aware of assigned interventions. There is no evidence of deviations from intended interventions and intention to treat analysis used. | ||||||||||||
| No missing data. | ||||||||||||
| Methods of measuring the number of falls was considered appropriate and there were no differences in measurement or ascertainment of the outcomes between groups. The assessor was blinded. | ||||||||||||
| The trial protocol reflects the analyses as that were conducted, and it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| As per RoB2 algorithm, the study is judged to be at low risk of bias for all domains for this outcome. | ||||||||||||
| Mudge 2008 |  |  |  |  |  |  | ||||||
| Pseudo‐randomisation, allocation was based on admitting unit and bed availability, and admitting unit was determined by a rotating roster. No evidence of baseline differences in patient characteristics other than those thought to be compatible with chance. | ||||||||||||
| Both participants and clinicians delivering care were aware of assigned interventions. There is no evidence of deviations from intended interventions and intention to treat analysis used. | ||||||||||||
| No missing data. | ||||||||||||
| Methods of measuring the number of falls was considered appropriate and there were no differences in measurement or ascertainment of the outcomes between groups. The assessor was blinded. | ||||||||||||
| A pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to be at high risk of bias due to the randomisation process. | ||||||||||||
| Bias | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study | Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | ||||||
| Subgroup 1.6.1 Rehabilitation‐related activities | ||||||||||||
| Abizanda 2011 |  |  |  |  |  |  | ||||||
| Allocation sequence was random (computer generated random numbers). Allocation occurred after enrolment by an investigator not involved in the participants' clinical management. Higher number of participants admitted with a stroke in the intervention group (39 vs. 16), in addition, the 'Others' subgroup the Barthel index is higher in the control group than the intervention group (29.1 vs. 23.1). This is thought to be compatible with chance. | ||||||||||||
| Its assumed participants and the Occupational Therapist (OT) delivering the intervention were aware of the assigned intervention allocation. All participants received their allocated intervention. Therefore, even though intention to treat analysis not specified, analysis deemed appropriate. | ||||||||||||
| Given the nature of this population (older adults during and after acute hospitalisation) we considered a threshold of 90% of participants with data as sufficient. We felt this would be consistent with measurements outside of a trial context. When accounting for mortality, 93% and 91% assessed at discharge for confusion in intervention and control arms respectively. | ||||||||||||
| The use of the Confusion Assessment Method (CAM) to assess for delirium is considered appropriate, and there were no differences in measurement or ascertainment of the outcomes between groups. The OT assessor was blinded. | ||||||||||||
| The data analyst was blinded, and followed a pre‐specified statistical plan. It is not thought that results were from multiple outcome measurements or multiple analyses. | ||||||||||||
| The study is judged to be at low risk of bias in all domains for this outcome. | ||||||||||||
| Asplund 2000 |  |  |  |  |  |  | ||||||
| The method of allocation sequence is not described, but the use of sealed envelopes suggests a random component was used. Participant characteristics were balanced. | ||||||||||||
| Participants and those delivering the interventions were aware of intervention assignments. Twenty‐five patients were excluded due to them not meeting the set eligibility criteria, however, these: protocol violations are not expected to influence the effect estimate of the outcome as per protocol analyses used. The per protocol analyses was not thought to have a substantial impact on the result given the main reason for exclusion was inappropriate recruitment. Excluding this 25, the other 6 exclusions represent approximately 1% of the total sample size. | ||||||||||||
| Data at discharge from hospital was available for 98% of participants. | ||||||||||||
| The confusion assessment method (CAM) instrument was considered appropriate, and there were no differences in the measurement or ascertainment between groups. The assessors were not blinded, and it was therefore considered likely that knowledge of the intervention could influence the outcome, given the likely strong belief in the benefits of the intervention ward. | ||||||||||||
| A pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to be at high risk of bias due to measurement of the outcome. | ||||||||||||
| Subgroup 1.6.2 Structured exercise | ||||||||||||
| Brown 2016 |  |  |  |  |  |  | ||||||
| No description of randomisation sequence generation, but the paper describes a block randomisation strategy and sequence concealed (sealed envelopes). The patient characteristics appear well‐balanced between groups. | ||||||||||||
| We assume both participants and those delivering the interventions were aware of intervention assignments. Six participants did not receive allocated intervention, this is considered to be consistent with what could occur outside the trial context, and intention to treat analysis was used. | ||||||||||||
| No missing data. | ||||||||||||
| Measures used to measure medical deterioration are considered appropriate and there and there were no differences in the measurement or ascertainment between groups. The assessors were blinded. | ||||||||||||
| The study protocol details statistical analysis plan which is in line with the analyses conducted, and it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to be at low risk of bias in all domains for this outcome. | ||||||||||||
| Hu 2020 |  |  |  |  |  |  | ||||||
| Allocation sequence was random (computer generated random numbers) and sequence concealed (blinded project coordinator allocated participants). Participant characteristics were balanced. | ||||||||||||
| Participants and those delivering the interventions were aware of intervention allocations. There was no evidence of deviations from the intended interventions and as such it is presumed intention to treat analysis was used. | ||||||||||||
| No missing data for medical deterioration during hospitalisation. | ||||||||||||
| The measures are considered appropriate, and there were no differences in the measurement or ascertainment between groups. The assessors were blinded. | ||||||||||||
| A pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to have some concerns in the selection of the reported result. | ||||||||||||
| Subgroup 1.6.3 Progressive resistance exercise | ||||||||||||
| de Morton 2007 |  |  |  |  |  |  | ||||||
| Allocation of wards was random (coin toss) and the allocating officer unaware of study. Baseline differences between groups were thought to be compatible with chance. | ||||||||||||
| Participants and clinicians delivering care were believed to be aware of treatment assignments. There was no evidence of deviations from intended interventions and intention to treat analysis was used. | ||||||||||||
| No missing data. | ||||||||||||
| The measures are considered appropriate, and there were no differences in the measurement or ascertainment between groups. The assessors were not blinded, but it is thought that due to the lack of judgement in the outcome, that the knowledge of the intervention did not influence the outcomes. | ||||||||||||
| A pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to raise some concerns in the selection of the reported result. | ||||||||||||
| Jeffs 2013 |  |  |  |  |  |  | ||||||
| Sequence concealment was achieved via a member of the research team not involved in recruitment and using sealed envelopes. There was no information regarding method of generating random sequence, other than to say that there was randomisation, given details of allocation concealment, assumption made that appropriate method used. Patient characteristics between groups appear well‐balanced. | ||||||||||||
| Participants and those delivering the interventions were likely to be aware of intervention allocations. One participant was not allocated to a group due to an administrative error and 35 participants did not receive the intervention as planned (17 in the intervention group). This is thought to be consistent with what could occur outside of the trial context. Intention to treat analysis was used. | ||||||||||||
| No missing data. | ||||||||||||
| The Confusion Assessment Method (CAM) is considered appropriate, and there were no differences in the measurement or ascertainment between groups. The assessors were blinded. | ||||||||||||
| A pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to raise some concerns in the selection of the reported results. | ||||||||||||
| Jones 2006 |  |  |  |  |  |  | ||||||
| Allocation sequence was random (computer generated random numbers). Sequence allocation was not concealed, but performed by a member of staff independent of the enrolment procedures. ). Participant characteristics were balanced between groups and differences compatible with chance. | ||||||||||||
| It is assumed that both participants and clinicians delivering care were aware of assigned interventions. There is no evidence of deviations from intended interventions and intention to treat analysis used. | ||||||||||||
| No missing data. | ||||||||||||
| Measurement methods are considered appropriate, and there were no differences in the measurement or ascertainment between groups. The assessors were blinded. | ||||||||||||
| A pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to raise some concerns in the selection of the reported results. | ||||||||||||
| Martinez‐Velilla 2019 |  |  |  |  |  |  | ||||||
| Allocation sequence was random (www.randomizer.org) and personal communication with author confirms allocation concealment. Baseline differences between groups are thought to be compatible with chance. | ||||||||||||
| Both participants and clinicians delivering care were aware of assigned interventions. There is no evidence of deviations from intended interventions and intention to treat analysis used. | ||||||||||||
| The methods of measurement are considered appropriate, and there were no differences in the measurement or ascertainment between groups. The assessors were blinded. | ||||||||||||
| The trial protocol reflects the analyses as that were conducted, and it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to be at low risk of bias in all domains for this outcome. | ||||||||||||
| The study is judged to be at low risk of bias in all domains for this outcome. | ||||||||||||
| McCullagh 2020 |  |  |  |  |  |  | ||||||
| Allocation sequence was random (computer‐generated) and allocation sequence was concealed. Baseline differences are thought to be compatible with chance. | ||||||||||||
| Both participants and clinicians delivering care were aware of assigned interventions. There is no evidence of deviations from intended interventions and intention to treat analysis used. | ||||||||||||
| 90% of intervention group and 94% of the control group had outcome data. Given the nature of this population (older adults during and after acute hospitalisation) we considered a threshold of 90% of participants with data as sufficient. | ||||||||||||
| The use of the Six‐item Cognitive Impairment Test (6CIT) to assess for delirium is considered appropriate, and there were no differences in the measurement or ascertainment between groups. The assessors were blinded. | ||||||||||||
| The trial protocol reflects the analyses as that were conducted, and it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to be at low risk of bias in all domains for this outcome. | ||||||||||||
| Mudge 2008 |  |  |  |  |  |  | ||||||
| Pseudo‐randomisation, allocation was based on admitting unit and bed availability, and admitting unit was determined by a rotating roster. No evidence of baseline differences in patient characteristics other than those thought to be compatible with chance. | ||||||||||||
| Both participants and clinicians delivering care were aware of assigned interventions. There is no evidence of deviations from intended interventions and intention to treat analysis used. | ||||||||||||
| No missing data. | ||||||||||||
| Identifying delirium according to chart review using validated methodology is considered appropriate, and there were no differences in the measurement or ascertainment between groups. The assessors were blinded. | ||||||||||||
| A pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to be at high risk of bias due to the randomisation process. | ||||||||||||
| Pedersen 2019 |  |  |  |  |  |  | ||||||
| Allocation sequence was random (computer‐generated block randomisation), and allocation sequence concealed from the investigators. Participant characteristics were well‐balanced between groups. | ||||||||||||
| Participants and clinicians were aware of the treatment assignments. There was no evidence of deviations from the assigned interventions and an intention to treat approach was used for analysis. | ||||||||||||
| No missing data. | ||||||||||||
| The measure is considered appropriate, and there were no differences in the measurement or ascertainment between groups. The assessors were blinded. | ||||||||||||
| The analysis is in accordance with a pre‐specified analysis in a published protocol, and results are not thought to be from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to be at low risk of bias in all domains for this outcome. | ||||||||||||
| Bias | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study | Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | ||||||
| Subgroup 2.2.1 Rehabilitation‐related activities | ||||||||||||
| Abizanda 2011 |  |  |  |  |  |  | ||||||
| Allocation sequence was random (computer generated random numbers). Allocation occurred after enrolment by an investigator not involved in the participants' clinical management. Higher number of participants admitted with a stroke in the intervention group (39 vs. 16), in addition, the 'Others' subgroup the Barthel index is higher in the control group than the intervention group (29.1 vs. 23.1). This is thought to be compatible with chance. | ||||||||||||
| Its assumed participants and the Occupational Therapist (OT) delivering the intervention were aware of the assigned intervention allocation. All participants received their allocated intervention. Therefore, even though intention to treat analysis not specified, analysis deemed appropriate. | ||||||||||||
| No missing data. | ||||||||||||
| The method is considered appropriates, and there were no differences in measurement or ascertainment of the outcomes between groups. The OT assessor was blinded. | ||||||||||||
| The data analyst was blinded, and followed a pre‐specified statistical plan. It is not thought that results were from multiple outcome measurements or multiple analyses. | ||||||||||||
| The study is judged to be at low risk of bias in all domains for this outcome. | ||||||||||||
| Asplund 2000 |  |  |  |  |  |  | ||||||
| The method of allocation sequence is not described, but the use of sealed envelopes suggests a random component was used. Participant characteristics were balanced. | ||||||||||||
| Participants and those delivering the interventions were aware of intervention assignments. Twenty‐five patients were excluded due to them not meeting the set eligibility criteria, however, these: protocol violations are not expected to influence the effect estimate of the outcome as per protocol analyses used. The per protocol analyses was not thought to have a substantial impact on the result given the main reason for exclusion was inappropriate recruitment. Excluding this 25, the other 6 exclusions represent approximately 1% of the total sample size. | ||||||||||||
| Data at discharge from hospital complete for 98% of participants. | ||||||||||||
| The measurement of length of stay is considered appropriate, and there were no differences in measurement or ascertainment between groups. Assessors were not blinded, but is thought that due to the lack of judgement in scoring a 'hard outcome' like length of stay, that it is unlikely that the knowledge of the intervention could influence the outcome. | ||||||||||||
| A pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to raise some concerns due to deviations from intended interventions and the selection of the reported results. | ||||||||||||
| Counsell 2000 |  |  |  |  |  |  | ||||||
| Allocation sequence was random (computer generated random numbers) and sequence concealed (opaque sealed envelope). Participant characteristics were balanced. | ||||||||||||
| Participants and those delivering the interventions were aware of intervention assignments. Seventy‐nine participants were not admitted to the unit to which they were assigned. Deviations may have affected the effect estimate, however they were well‐balanced between groups. Intention to treat analysis was used. | ||||||||||||
| No missing data. | ||||||||||||
| The methods of measuring length of stay are considered appropriate, and there were no differences in measurement or ascertainment between groups. Assessors were not blinded, but is thought that due to the lack of judgement in scoring a 'hard outcome' like length of hospital stay, that it is unlikely that the knowledge of the intervention could influence the outcome. | ||||||||||||
| A pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to raise some concerns in the selection of the reported results and deviations from the intended interventions. | ||||||||||||
| Ekerstad 2017 |  |  |  |  |  |  | ||||||
| Randomisation was based on the availability of beds, and there was no allocation concealment as participants were allocated a ward prior to enrolment. Participant characteristics appeared balanced. | ||||||||||||
| Participants and clinicians delivering care were aware of treatment assignments. There was no evidence of deviations from intended interventions and intention to treat analysis was used. | ||||||||||||
| No missing data. | ||||||||||||
| The methods of measuring length of hospital stay are considered appropriate, and there were no differences in measurement or ascertainment between groups. Assessors were not blinded, but is thought that due to the lack of judgement in scoring a 'hard outcome' like length of hospital stay, that it is unlikely that the knowledge of the intervention could influence the outcome. | ||||||||||||
| A detailed pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to be at high risk of bias arising from the randomisation process. | ||||||||||||
| Fretwell 1990 |  |  |  |  |  |  | ||||||
| “Patients were randomised only when both a treatment and control bed were available”. Although not stated, we believe the necessity for available beds on both wards indicate that a random component was likely used. Sequence allocation concealment is not discussed, but no significant differences in patient characteristics other than those compatible with chance were observed. | ||||||||||||
| Participants and those delivering the interventions were aware of intervention assignments. There were 30 randomisation errors which has been interpreted to mean 'allocated to the ward that they were not randomised to' however there is no explanation of this term. These deviations were thought likely to have affected the outcome, though were relative well‐balanced. It appears a per protocol analyses was used, though given the low number (7%) and relative balance between groups, the omission of the participants randomised incorrectly probably did not have substantial impact on the results. | ||||||||||||
| All participants accounted for at discharge. | ||||||||||||
| The methods of measuring length of stay are considered appropriate, and there were no differences in measurement or ascertainment between groups. Assessors were not blinded, but is thought that due to the lack of judgement in scoring a 'hard outcome' like length of stay, that it is unlikely that the knowledge of the intervention could influence the outcome. | ||||||||||||
| A detailed pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to raise some concerns in deviations from the intended interventions, methods of randomisation and selection of the reported result. | ||||||||||||
| Landefeld 1995 |  |  |  |  |  |  | ||||||
| Allocation sequence was random (computer generated random numbers) and balanced participant characteristics, but no information was provided regarding allocation concealment. | ||||||||||||
| It is assumed participants and clinicians were aware of the treatment assignments. There was no evidence of deviations from the assigned interventions and evidence of an intention to treat approach being used for analysis. | ||||||||||||
| Accounting for mortality (24 died in each arm) all participants had outcome data at discharge. | ||||||||||||
| The methods of measuring length of hospital stay are considered appropriate, and there were no differences in measurement or ascertainment between groups. Assessors were not blinded, but is thought that due to the lack of judgement in scoring a 'hard outcome' like length of hospital stay, that it is unlikely that the knowledge of the intervention could influence the outcome. | ||||||||||||
| A pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to have some concerns due to the methods of randomisation and in the selection of the reported result. | ||||||||||||
| Sahota 2017 |  |  |  |  |  |  | ||||||
| No information on randomisation methods other than to say participants were randomised, however, patient characteristics appear well‐balanced between groups and differences compatible with chance. | ||||||||||||
| It is assumed that participants and those delivering the interventions were aware of intervention assignments. There were 15 protocol deviations in the CIRACT group and 8 in the THB‐Rehab group. The deviations were thought to be in keeping with what would be expected outside of a trial context. Intention to treat not explicitly stated though it appears that the 1 participant who did not receive the intended intervention was analysed within the group to which they were assigned. | ||||||||||||
| No missing data | ||||||||||||
| The methods of measuring length of hospital stay are considered appropriate, and there were no differences in measurement or ascertainment between groups. Assessors were not blinded, but is thought that due to the lack of judgement in scoring a 'hard outcome' like length of hospital stay, that it is unlikely that the knowledge of the intervention could influence the outcome. | ||||||||||||
| A pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to raise some concerns in the selection of the reported results and the randomisation process. | ||||||||||||
| Slaets 1997 |  |  |  |  |  |  | ||||||
| Randomisation methods are described only as: “an alternating randomisation procedure”. However, there was a centrally administered method to allocate interventions, and baseline differences did not appear to show a significant problem with randomisation process other than those thought to be compatible with chance. | ||||||||||||
| Participants and clinicians delivering care were aware of allocated interventions. There was no evidence of deviations from intended intervention, and although intention to treat analysis was not specified, there was no information to suggest participants were not assessed in the group to which they were randomised. | ||||||||||||
| After accounting for mortality (6 patients died), data was available for 91% of participants. | ||||||||||||
| The methods of measuring length of hospital stay in hospital are considered appropriate, and there were no differences in measurement or ascertainment between groups. Assessors were not blinded, but is thought that due to the lack of judgement in scoring a 'hard outcome' like length of stay, that it is unlikely that the knowledge of the intervention could influence the outcome. | ||||||||||||
| A pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to raise some concerns in the randomisation process and in the selection of the reported results. | ||||||||||||
| Zelada 2009 |  |  |  |  |  |  | ||||||
| The allocation sequence was based on bed availability, and therefore not random, and sequence is believed to be predictable. However, baseline differences did not appear to show a significant problem with randomisation process other than those thought to be compatible with chance. | ||||||||||||
| Participants and clinicians delivering care were aware of treatment allocations. There is no evidence of deviations from intended intervention, or that participants moved between the intervention and usual care units during the study period. Intention to treat analysis was not specified, however there was no information to suggest participants were not assessed in the group to which they were randomised. | ||||||||||||
| No missing data. | ||||||||||||
| The methods of measuring length of stay are considered appropriate, and there were no differences in measurement or ascertainment between groups. Assessors were not blinded, but is thought that due to the lack of judgement in scoring a 'hard outcome' like length of stay, that it is unlikely that the knowledge of the intervention could influence the outcome. | ||||||||||||
| A pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to be at high risk of bias due to bias arising from the randomisation process. | ||||||||||||
| Subgroup 2.2.2 Structured exercise | ||||||||||||
| Brown 2016 |  |  |  |  |  |  | ||||||
| No description of randomisation sequence generation, but the paper describes a block randomisation strategy and sequence concealed (sealed envelopes). The patient characteristics appear well‐balanced between groups. | ||||||||||||
| We assume both participants and those delivering the interventions were aware of intervention assignments. 6 participants did not receive allocated intervention, this is considered to be consistent with what could occur outside the trial context, and intention to treat analysis was used. | ||||||||||||
| No missing data. | ||||||||||||
| The methods of measuring length of hospital stay are considered appropriate, and there were no differences in measurement or ascertainment between groups. The assessor was blinded. | ||||||||||||
| The study protocol details statistical analysis plan which is in accordance with results presented, and it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to be at low risk of bias in all domains for this outcome. | ||||||||||||
| Gazineo 2021 |  |  |  |  |  |  | ||||||
| Allocation sequence was random (generated using an online system) and the allocation sequence was concealed until participants were enrolled (opaque concealed and sealed envelopes). Participant characteristics were well‐balanced. | ||||||||||||
| Participants and those delivering the interventions were aware of intervention allocations. There was no evidence of deviations from the intended interventions and intention to treat analysis used. | ||||||||||||
| No missing data after accounting for mortality. | ||||||||||||
| Methods of measuring length of hospital stay are considered appropriate and there were no differences in measurement or ascertainment of the outcomes between groups. The assessor was not blinded, but it is thought that due to the lack of judgement in the measurement, that the knowledge of the intervention did not influence the outcomes. | ||||||||||||
| A pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to raise some concerns in the selection of the reported results . | ||||||||||||
| Hu 2020 |  |  |  |  |  |  | ||||||
| Allocation sequence was random (computer generated random numbers) and sequence concealed (blinded project coordinator allocated participants). Participant characteristics were balanced. | ||||||||||||
| Participants and those delivering the interventions were aware of intervention allocations. There was no evidence of deviations from the intended interventions and as such it is presumed intention to treat analysis was used. | ||||||||||||
| No missing data for length of hospital stay. | ||||||||||||
| The methods of measuring legnth of hospital stay are considered appropriate, and there were no differences in measurement or ascertainment between groups. The assessors were blinded. | ||||||||||||
| A pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to have some concerns in the selection of the reported result. | ||||||||||||
| McGowan 2018a |  |  |  |  |  |  | ||||||
| Allocation sequence was random (www.randomization.com) and the allocation sequence was only known by the chief investigator who was not involved with screening patients. Baseline differences between groups are thought to be compatible with chance. | ||||||||||||
| Both participants and clinicians delivering care were aware of assigned interventions. There is no evidence of deviations from intended interventions and intention to treat analysis not explicitly stated, but all participants appear to have been analysed in the group to which they were randomised. | ||||||||||||
| 96% of participants had complete data sets. | ||||||||||||
| The measurement of length of hospital stay is considered appropriate, and there were no differences in the measurement or ascertainment between groups. The assessors were not blinded, but is thought that due to the lack of judgement in scoring a 'hard outcome' like length of hospital stay, that it is unlikely that the knowledge of the intervention could influence the outcome. | ||||||||||||
| The trial protocol and registration reflects the analyses as that were conducted, and it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to raise some concerns due to the measurement of the outcome. | ||||||||||||
| Subgroup 2.2.3 Progressive resistance exercise | ||||||||||||
| Courtney 2009 |  |  |  |  |  |  | ||||||
| Allocation sequence was random (computer generated random numbers) and the allocation sequence concealed until participants were enrolled (via project coordinator blinded to baseline data). Participant characteristics appear well‐balanced. | ||||||||||||
| Participants and clinicians delivering care were believed to be aware of treatment assignments. 6/64 participants in the intervention group did not receive allocated treatment, the reasons are considered consistent with what could occur outside the trial context, and intention to treat analysis was used. | ||||||||||||
| No missing length of stay data. | ||||||||||||
| The measurement of hospital length of stay is considered appropriate, and there were no differences in measurement or ascertainment between groups. The assessors are believed to be blinded to treatment allocation. | ||||||||||||
| A pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to raise some concerns in the selection of the reported result. | ||||||||||||
| de Morton 2007 |  |  |  |  |  |  | ||||||
| Allocation of wards was random (coin toss) and the allocating officer unaware of study. Baseline differences between groups were thought to be compatible with chance. | ||||||||||||
| Participants and clinicians delivering care were believed to be aware of treatment assignments. There was no evidence of deviations from intended interventions and intention to treat analysis was used. | ||||||||||||
| No missing data. | ||||||||||||
| The measure of hospital length of stay considered appropriate, and there were no differences in measurement or ascertainment between groups. Assessors were not blinded, but is thought that due to the lack of judgement in scoring a 'hard outcome' like length of stay, that it is unlikely that the knowledge of the intervention could influence the outcome. | ||||||||||||
| A pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to raise some concerns in the selection of the reported result. | ||||||||||||
| Jeffs 2013 |  |  |  |  |  |  | ||||||
| Sequence concealment was achieved via a member of the research team not involved in recruitment and using sealed envelopes. There was no information regarding method of generating random sequence, other than to say that there was randomisation, given details of allocation concealment, assumption made that appropriate method used. Patient characteristics between groups appear well‐balanced. | ||||||||||||
| Participants and those delivering the interventions were likely to be aware of intervention allocations. One participant was not allocated to a group due to an administrative error and 35 participants did not receive the intervention as planned (17 in the intervention group). This is thought to be consistent with what could occur outside the trial context. Intention to treat analysis was used. | ||||||||||||
| No missing data. | ||||||||||||
| Measurement of length of hospital stay is considered appropriate, and there were no differences in the measurement or ascertainment between groups. The assessors were blinded. | ||||||||||||
| A pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to raise some concerns in the selection of the reported results. | ||||||||||||
| Jones 2006 |  |  |  |  |  |  | ||||||
| Allocation sequence was random (computer generated random numbers). Sequence allocation was not concealed, but performed by a member of staff independent of the enrolment procedures. ). Participant characteristics were balanced between groups and differences compatible with chance. | ||||||||||||
| It is assumed that both participants and clinicians delivering care were aware of assigned interventions. There is no evidence of deviations from intended interventions and intention to treat analysis used. | ||||||||||||
| After accounting for mortality, length of stay data available for 99% of control group and 93% of intervention group. Given the nature of this population (older adults during and after acute hospitalisation) we considered a threshold of 90% of participants with data as sufficient. | ||||||||||||
| Measurement methods are considered appropriate, and there were no differences in the measurement or ascertainment between groups. The assessors were blinded. | ||||||||||||
| A pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to raise some concerns in the selection of the reported results. | ||||||||||||
| Martinez‐Velilla 2019 |  |  |  |  |  |  | ||||||
| Allocation sequence was random (www.randomizer.org) and personal communication with author confirms allocation concealment. Baseline differences between groups are thought to be compatible with chance. | ||||||||||||
| Both participants and clinicians delivering care were aware of assigned interventions. There is no evidence of deviations from intended interventions and intention to treat analysis used. | ||||||||||||
| No missing data. | ||||||||||||
| The methods of measurement are considered appropriate, and there were no differences in the measurement or ascertainment between groups. The assessors were blinded. | ||||||||||||
| The trial protocol reflects the analyses as that were conducted, and it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to be at low risk of bias in all domains for this outcome. | ||||||||||||
| McCullagh 2020 |  |  |  |  |  |  | ||||||
| Allocation sequence was random (computer‐generated) and allocation sequence was concealed. Baseline differences are thought to be compatible with chance. | ||||||||||||
| Both participants and clinicians delivering care were aware of assigned interventions. There is no evidence of deviations from intended interventions and intention to treat analysis used. | ||||||||||||
| No missing data. | ||||||||||||
| The measure is considered appropriate, and there were no differences in the measurement or ascertainment between groups. The assessors were blinded. | ||||||||||||
| The trial protocol reflects the analyses as that were conducted, and it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to be at low risk of bias in all domains for this outcome. | ||||||||||||
| Mudge 2008 |  |  |  |  |  |  | ||||||
| Pseudo‐randomisation, allocation was based on admitting unit and bed availability, and admitting unit was determined by a rotating roster. No evidence of baseline differences in patient characteristics other than those thought to be compatible with chance. | ||||||||||||
| Both participants and clinicians delivering care were aware of assigned interventions. There is no evidence of deviations from intended interventions and intention to treat analysis used. | ||||||||||||
| No missing data. | ||||||||||||
| The method is considered appropriate, and there were no differences in the measurement or ascertainment between groups. The assessors were blinded. | ||||||||||||
| A pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to be at high risk of bias due to the randomisation process. | ||||||||||||
| Ortiz‐Alonso 2020 |  |  |  |  |  |  | ||||||
| The allocation sequence was based on recruitment blocks of 4‐8 weeks. Activity of daily living (ADL) scores at baseline and admission were significantly lower in the intervention group than the control group, this is thought to be an important prognostic factor and therefore a concern regarding the randomisation process. | ||||||||||||
| Methods were designed to blind participants from group assignments however due to the nature of the intervention and lack of sham intervention the success of this blinding is felt unlikely. Ward staff and research staff were not blinded to the participant's assigned intervention. There were no deviations from the intended interventions due to trial context described and although intention to treat analysis not specified given the lack of deviations it is assumed. | ||||||||||||
| No missing outcome data. | ||||||||||||
| The measure is considered appropriate, and there were no differences in the measurement or ascertainment between groups. The assessors were not blinded, but it is thought that due to the lack of judgement in the outcome, that the knowledge of the intervention did not influence the outcome. | ||||||||||||
| Analysis reflects plan in trial registration and study protocol, and results are not thought to be from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to be at high risk of bias due to the randomisation process. | ||||||||||||
| Pedersen 2019 |  |  |  |  |  |  | ||||||
| Allocation sequence was random (computer‐generated block randomisation), and allocation sequence concealed from the investigators. Participant characteristics were well‐balanced between groups. | ||||||||||||
| Participants and clinicians were aware of the treatment assignments. There was no evidence of deviations from the assigned interventions and an intention to treat approach was used for analysis. | ||||||||||||
| No missing data | ||||||||||||
| The measure is considered appropriate, and there were no differences in the measurement or ascertainment between groups. The assessors were blinded. | ||||||||||||
| The analysis is in accordance with a pre‐specified analysis in a published protocol, and results are not thought to be from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to be at low risk of bias for all domains for this outcome. | ||||||||||||
| Bias | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study | Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | ||||||
| Subgroup 2.3.1 Rehabilitation‐related activities | ||||||||||||
| Asplund 2000 |  |  |  |  |  |  | ||||||
| The method of allocation sequence is not described, but the use of sealed envelopes suggests a random component was used. Participant characteristics were balanced. | ||||||||||||
| Participants and those delivering the interventions were aware of intervention assignments. Twenty‐five patients were excluded due to them not meeting the set eligibility criteria, however, these: protocol violations are not expected to influence the effect estimate of the outcome as per protocol analyses used. The per protocol analyses was not thought to have a substantial impact on the result given the main reason for exclusion was inappropriate recruitment. Excluding this 25, the other 6 exclusions represent approximately 1% of the total sample size. | ||||||||||||
| Data at discharge from hospital complete for 98% of participants. | ||||||||||||
| The measurement of new institutionalisation is considered appropriate, and there were no differences in measurement or ascertainment between groups. Assessors were not blinded, but is thought that due to the lack of judgement in scoring a 'hard outcome' like new institutionalisation, that it is unlikely that the knowledge of the intervention could influence the outcome. | ||||||||||||
| A pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to raise some concerns due to deviations from intended interventions and the selection of the reported results. | ||||||||||||
| Counsell 2000 |  |  |  |  |  |  | ||||||
| Allocation sequence was random (computer generated random numbers) and sequence concealed (opaque sealed envelope). Participant characteristics were balanced. | ||||||||||||
| Participants and those delivering the interventions were aware of intervention assignments. Seventy‐nine participants were not admitted to the unit to which they were assigned. Deviations may have affected the effect estimate, however they were well‐balanced between groups. Intention to treat analysis was used. | ||||||||||||
| No missing data. | ||||||||||||
| The methods of measuring length of stay are considered appropriate, and there were no differences in measurement or ascertainment between groups. Assessors were not blinded, but is thought that due to the lack of judgement in scoring a 'hard outcome' like length of hospital stay, that it is unlikely that the knowledge of the intervention could influence the outcome. | ||||||||||||
| A pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to raise some concerns in the selection of the reported results and deviations from the intended interventions. | ||||||||||||
| Fretwell 1990 |  |  |  |  |  |  | ||||||
| “Patients were randomised only when both a treatment and control bed were available”. Although not stated, we believe the necessity for available beds on both wards indicate that a random component was likely used. Sequence allocation concealment is not discussed, but no significant differences in patient characteristics other than those compatible with chance were observed. | ||||||||||||
| Participants and those delivering the interventions were aware of intervention assignments. There were 30 randomisation errors which has been interpreted to mean 'allocated to the ward that they were not randomised to' however there is no explanation of this term. These deviations were thought likely to have affected the outcome, though were relative well‐balanced. It appears a per protocol analyses was used, though given the low number (7%) and relative balance between groups, the omission of the participants randomised incorrectly probably did not have substantial impact on the results. | ||||||||||||
| All participants accounted for at discharge. | ||||||||||||
| The methods of measuring new institutionalisation are considered appropriate, and there were no differences in measurement or ascertainment between groups. Assessors were not blinded, but is thought that due to the lack of judgement in scoring a 'hard outcome' like new institutionalisation, that it is unlikely that the knowledge of the intervention could influence the outcome. | ||||||||||||
| A detailed pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to raise some concerns in deviations from the intended interventions, methods of randomisation and selection of the reported result. | ||||||||||||
| Subgroup 2.3.2 Progressive resistance exercise | ||||||||||||
| de Morton 2007 |  |  |  |  |  |  | ||||||
| Allocation of wards was random (coin toss) and the allocating officer unaware of study. Baseline differences between groups were thought to be compatible with chance. | ||||||||||||
| Participants and clinicians delivering care were believed to be aware of treatment assignments. There was no evidence of deviations from intended interventions and intention to treat analysis was used. | ||||||||||||
| No missing data. | ||||||||||||
| The measure of new institutionalisation considered appropriate, and there were no differences in measurement or ascertainment between groups. Assessors were not blinded, but is thought that due to the lack of judgement in scoring a 'hard outcome' like new institutionalisation, that it is unlikely that the knowledge of the intervention could influence the outcome. | ||||||||||||
| A pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to raise some concerns in the selection of the reported result. | ||||||||||||
| Mudge 2008 |  |  |  |  |  |  | ||||||
| Pseudo‐randomisation, allocation was based on admitting unit and bed availability, and admitting unit was determined by a rotating roster. No evidence of baseline differences in patient characteristics other than those thought to be compatible with chance. | ||||||||||||
| Both participants and clinicians delivering care were aware of assigned interventions. There is no evidence of deviations from intended interventions and intention to treat analysis used. | ||||||||||||
| No missing data. | ||||||||||||
| The method is considered appropriate, and there were no differences in the measurement or ascertainment between groups. The assessors were blinded. | ||||||||||||
| A pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to be at high risk of bias due to the randomisation process. | ||||||||||||
| Bias | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study | Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | ||||||
| Subgroup 2.4.1 Rehabilitation‐related activities | ||||||||||||
| Asplund 2000 |  |  |  |  |  |  | ||||||
| The method of allocation sequence is not described, but the use of sealed envelopes suggests a random component was used. Participant characteristics were balanced. | ||||||||||||
| Participants and those delivering the interventions were aware of intervention assignments. Twenty‐five patients were excluded due to them not meeting the set eligibility criteria, however, these: protocol violations are not expected to influence the effect estimate of the outcome as per protocol analyses used. The per protocol analyses was not thought to have a substantial impact on the result given the main reason for exclusion was inappropriate recruitment. Excluding this 25, the other 6 exclusions represent approximately 1% of the total sample size. | ||||||||||||
| Data for readmissions complete for 98% of participants. | ||||||||||||
| The measurement of hospital readmissions is considered appropriate, and there were no differences in measurement or ascertainment between groups. Assessors were not blinded, but is thought that due to the lack of judgement in scoring a 'hard outcome' like hospital readmissions, that it is unlikely that the knowledge of the intervention could influence the outcome. | ||||||||||||
| A pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to raise some concerns due to deviations from intended interventions and the selection of the reported results. | ||||||||||||
| Counsell 2000 |  |  |  |  |  |  | ||||||
| Allocation sequence was random (computer generated random numbers) and sequence concealed (opaque sealed envelope). Participant characteristics were balanced. | ||||||||||||
| Participants and those delivering the interventions were aware of intervention assignments. Seventy‐nine participants were not admitted to the unit to which they were assigned. Deviations may have affected the effect estimate, however they were well‐balanced between groups. Intention to treat analysis was used. | ||||||||||||
| Given the nature of this population (older adults during and after acute hospitalisation) we considered a threshold of 90% of participants with data as sufficient, data was available for approximately 93% at 6 month follow‐up participants at discharge. | ||||||||||||
| The methods of measuring length of stay are considered appropriate, and there were no differences in measurement or ascertainment between groups. Assessors were not blinded, but is thought that due to the lack of judgement in scoring a 'hard outcome' like length of hospital stay, that it is unlikely that the knowledge of the intervention could influence the outcome. | ||||||||||||
| A pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to raise some concerns in the selection of the reported results and deviations from the intended interventions. | ||||||||||||
| Ekerstad 2017 |  |  |  |  |  |  | ||||||
| Randomisation was based on the availability of beds, and there was no allocation concealment as participants were allocated a ward prior to enrolment. Participant characteristics appeared balanced. | ||||||||||||
| Participants and clinicians delivering care were aware of treatment assignments. There was no evidence of deviations from intended interventions and intention to treat analysis was used. | ||||||||||||
| No missing data. | ||||||||||||
| The methods of measuring length of hospital stay are considered appropriate, and there were no differences in measurement or ascertainment between groups. Assessors were not blinded, but is thought that due to the lack of judgement in scoring a 'hard outcome' like length of hospital stay, that it is unlikely that the knowledge of the intervention could influence the outcome. | ||||||||||||
| A detailed pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to be at high risk of bias arising from the randomisation process. | ||||||||||||
| Landefeld 1995 |  |  |  |  |  |  | ||||||
| Allocation sequence was random (computer generated random numbers) and balanced participant characteristics, but no information was provided regarding allocation concealment. | ||||||||||||
| It is assumed participants and clinicians were aware of the treatment assignments. There was no evidence of deviations from the assigned interventions and evidence of an intention to treat approach being used for analysis. | ||||||||||||
| Accounting for mortality (24 died in each arm) all participants had outcome data at discharge. At follow up (a further 42, and 40 in intervention and control group had died), data was missing for just 6 participants (1%).at follow up (a further 42, and 40 in intervention and control group had died), data was missing for just 6 participants (1%). | ||||||||||||
| The measure is considered appropriate, and there were no differences in measurement or ascertainment between groups. Assessors were not blinded, but is thought that due to the lack of judgement in scoring a 'hard outcome' like hospital readmissions, that it is unlikely that the knowledge of the intervention could influence the outcome. | ||||||||||||
| A pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to have some concerns due to the methods of randomisation and in the selection of the reported result. | ||||||||||||
| Sahota 2017 |  |  |  |  |  |  | ||||||
| No information on randomisation methods other than to say participants were randomised, however, patient characteristics appear well‐balanced between groups and differences compatible with chance. | ||||||||||||
| It is assumed that participants and those delivering the interventions were aware of intervention assignments. There were 15 protocol deviations in the CIRACT group and 8 in the THB‐Rehab group. The deviations were thought to be in keeping with what would be expected outside of a trial context. Intention to treat not explicitly stated though it appears that the 1 participant who did not receive the intended intervention was analysed within the group to which they were assigned. | ||||||||||||
| No missing data. | ||||||||||||
| The measure is considered appropriate, and there were no differences in measurement or ascertainment between groups. Assessors were not blinded, but is thought that due to the lack of judgement in scoring a 'hard outcome' like hospital readmissions, that it is unlikely that the knowledge of the intervention could influence the outcome. | ||||||||||||
| A pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to raise some concerns in the selection of the reported results and the randomisation process. | ||||||||||||
| Slaets 1997 |  |  |  |  |  |  | ||||||
| Randomisation methods are described only as: “an alternating randomisation procedure”. However, there was a centrally administered method to allocate interventions, and baseline differences did not appear to show a significant problem with randomisation process other than those thought to be compatible with chance. | ||||||||||||
| Participants and clinicians delivering care were aware of allocated interventions. There was no evidence of deviations from intended intervention, and although intention to treat analysis was not specified, there was no information to suggest participants were not assessed in the group to which they were randomised. | ||||||||||||
| After accounting for mortality (6 patients died), data was available for 91% of participants. | ||||||||||||
| The methods of measuring hospital readmissions are considered appropriate, and there were no differences in measurement or ascertainment between groups. Assessors were not blinded, but is thought that due to the lack of judgement in scoring a 'hard outcome' like hospital readmissions, that it is unlikely that the knowledge of the intervention could influence the outcome. | ||||||||||||
| A pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to raise some concerns in the randomisation process and in the selection of the reported results. | ||||||||||||
| Subgroup 2.4.2 Structured exercise | ||||||||||||
| Gazineo 2021 |  |  |  |  |  |  | ||||||
| Allocation sequence was random (generated using an online system) and the allocation sequence was concealed until participants were enrolled (opaque concealed and sealed envelopes). Participant characteristics were well‐balanced. | ||||||||||||
| Participants and those delivering the interventions were aware of intervention allocations. There was no evidence of deviations from the intended interventions and intention to treat analysis used. | ||||||||||||
| No missing data after accounting for mortality. | ||||||||||||
| Methods of measuring hospital readmissions are considered appropriate and there were no differences in measurement or ascertainment of the outcomes between groups. The assessor was not blinded, but it is thought that due to the lack of judgement in the measurement, that the knowledge of the intervention did not influence the outcomes. | ||||||||||||
| A pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to raise some concerns in the selection of the reported results. | ||||||||||||
| Subgroup 2.4.3 Progressive resistance exercise | ||||||||||||
| Courtney 2009 |  |  |  |  |  |  | ||||||
| Allocation sequence was random (computer generated random numbers) and the allocation sequence concealed until participants were enrolled (via project coordinator blinded to baseline data). Participant characteristics appear well‐balanced. | ||||||||||||
| Participants and clinicians delivering care were believed to be aware of treatment assignments. 6/64 participants in the intervention group did not receive allocated treatment, the reasons are considered consistent with what could occur outside the trial context, and intention to treat analysis was used. | ||||||||||||
| Data regarding readmissions available for 92% of intervention and 98% of control group participants. Given the nature of this population (older adults during and after acute hospitalisation) we considered a threshold of 90% of participants with data as sufficient. | ||||||||||||
| The measurement of hospital readmissions is considered appropriate, and there were no differences in measurement or ascertainment between groups. The assessors are believed to be blinded to treatment allocation. | ||||||||||||
| A pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to raise some concerns in the selection of the reported result. | ||||||||||||
| de Morton 2007 |  |  |  |  |  |  | ||||||
| Allocation of wards was random (coin toss) and the allocating officer unaware of study. Baseline differences between groups were thought to be compatible with chance. | ||||||||||||
| Participants and clinicians delivering care were believed to be aware of treatment assignments. There was no evidence of deviations from intended interventions and intention to treat analysis was used. | ||||||||||||
| No missing data. | ||||||||||||
| The measure of hospital readmissions is considered appropriate, and there were no differences in measurement or ascertainment between groups. Assessors were not blinded, but is thought that due to the lack of judgement in scoring a 'hard outcome' like hospital readmissions, that it is unlikely that the knowledge of the intervention could influence the outcome. | ||||||||||||
| A pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to raise some concerns in the selection of the reported result. | ||||||||||||
| Martinez‐Velilla 2019 |  |  |  |  |  |  | ||||||
| Allocation sequence was random (www.randomizer.org) and personal communication with author confirms allocation concealment. Baseline differences between groups are thought to be compatible with chance. | ||||||||||||
| Both participants and clinicians delivering care were aware of assigned interventions. There is no evidence of deviations from intended interventions and intention to treat analysis used. | ||||||||||||
| No missing data. | ||||||||||||
| The methods of measurement are considered appropriate, and there were no differences in the measurement or ascertainment between groups. The assessors were blinded. | ||||||||||||
| The trial protocol reflects the analyses as that were conducted, and it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to be at low risk of bias in all domains for this outcome. | ||||||||||||
| McCullagh 2020 |  |  |  |  |  |  | ||||||
| Allocation sequence was random (computer‐generated) and allocation sequence was concealed. Baseline differences are thought to be compatible with chance. | ||||||||||||
| Both participants and clinicians delivering care were aware of assigned interventions. There is no evidence of deviations from intended interventions and intention to treat analysis used. | ||||||||||||
| No missing data. | ||||||||||||
| The measure is considered appropriate, and there were no differences in the measurement or ascertainment between groups. The assessors were blinded. | ||||||||||||
| The trial protocol reflects the analyses as that were conducted, and it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to be at low risk of bias in all domains for this outcome. | ||||||||||||
| Mudge 2008 |  |  |  |  |  |  | ||||||
| Pseudo‐randomisation, allocation was based on admitting unit and bed availability, and admitting unit was determined by a rotating roster. No evidence of baseline differences in patient characteristics other than those thought to be compatible with chance. | ||||||||||||
| Both participants and clinicians delivering care were aware of assigned interventions. There is no evidence of deviations from intended interventions and intention to treat analysis used. | ||||||||||||
| No missing data. | ||||||||||||
| The method is considered appropriate, and there were no differences in the measurement or ascertainment between groups. The assessors were blinded. | ||||||||||||
| A pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to be at high risk of bias due to the randomisation process. | ||||||||||||
| Ortiz‐Alonso 2020 |  |  |  |  |  |  | ||||||
| The allocation sequence was based on recruitment blocks of 4‐8 weeks. Activity of daily living (ADL) scores at baseline and admission were significantly lower in the intervention group than the control group, this is thought to be an important prognostic factor and therefore a concern regarding the randomisation process. | ||||||||||||
| Methods were designed to blind participants from group assignments however due to the nature of the intervention and lack of sham intervention the success of this blinding is felt unlikely. Ward staff and research staff were not blinded to the participant's assigned intervention. There were no deviations from the intended interventions due to trial context described and although intention to treat analysis not specified given the lack of deviations it is assumed. | ||||||||||||
| No missing outcome data. | ||||||||||||
| The measure is considered appropriate, and there were no differences in the measurement or ascertainment between groups. The assessors were not blinded, but it is thought that due to the lack of judgement in the outcome, that the knowledge of the intervention did not influence the outcome. | ||||||||||||
| Analysis reflects plan in trial registration and study protocol, and results are not thought to be from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to be at high risk of bias due to the randomisation process. | ||||||||||||
| Pedersen 2019 |  |  |  |  |  |  | ||||||
| Allocation sequence was random (computer‐generated block randomisation), and allocation sequence concealed from the investigators. Participant characteristics were well‐balanced between groups. | ||||||||||||
| Participants and clinicians were aware of the treatment assignments. There was no evidence of deviations from the assigned interventions and an intention to treat approach was used for analysis. | ||||||||||||
| No missing data | ||||||||||||
| The measure is considered appropriate, and there were no differences in the measurement or ascertainment between groups. The assessors were blinded. | ||||||||||||
| The analysis is in accordance with a pre‐specified analysis in a published protocol, and results are not thought to be from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to be at low risk of bias for all domains for this outcome. | ||||||||||||
| Bias | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study | Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | ||||||
| Subgroup 2.5.1 Rehabilitation‐related activities | ||||||||||||
| Ekerstad 2017 |  |  |  |  |  |  | ||||||
| Randomisation was based on the availability of beds, and there was no allocation concealment as participants were allocated a ward prior to enrolment. Participant characteristics appeared balanced. | ||||||||||||
| Participants and clinicians delivering care were aware of treatment assignments. There was no evidence of deviations from intended interventions and intention to treat analysis was used. | ||||||||||||
| The timed up and go (TUG) (when accounting for mortality) was available for 74% and 49% of participants in intervention and control groups at hospital discharge respectively. The difference in missing data for the TUG acompared to the activity of daily living data may be due to participants being unable to complete the walking tasks. Therefore a large proportion of missing data is thought to be due to the 'true' value. | ||||||||||||
| The use of the TUG to measure walking performance is considered appropriate and there was no differences in measurement between groups. The assessors were not blinded, and it was therefore considered likely that knowledge of the intervention could influence the outcome, given the likely strong belief in the benefits of the intervention ward. | ||||||||||||
| A detailed pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to be at high risk of bias due to bias in the measurement of the outcome and bias arising from the randomisation process. | ||||||||||||
| Subgroup 2.5.2 Structured exercise | ||||||||||||
| Hu 2020 |  |  |  |  |  |  | ||||||
| Allocation sequence was random (computer generated random numbers) and sequence concealed (blinded project coordinator allocated participants). Participant characteristics were balanced. | ||||||||||||
| Participants and those delivering the interventions were aware of intervention allocations. There was no evidence of deviations from the intended interventions and as such it is presumed intention to treat analysis was used. | ||||||||||||
| At discharge data was available for 76% of reablement group, (78% of reminder group) and 80% of the control group. Only participants who had complete data (i.e. data collected at baseline, discharge and follow‐up) had outcomes reported. Although missing data is well‐balanced between all three groups, it is considered likely that reason for missing data is related to its true value. | ||||||||||||
| The use of the timed up and go (TUG) is considered appropriate, and there were no differences in the measurement or ascertainment between groups. The assessors were blinded. | ||||||||||||
| A pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to be at high risk of bias due to missing outcome data. | ||||||||||||
| Killey 2006 |  |  |  |  |  |  | ||||||
| Allocation not random and sequence predictable as based on alternation. There is limited data on participant characteristics, but available data suggests balanced characteristics between groups. | ||||||||||||
| Participants and those delivering the interventions were aware of intervention allocations. There was no evidence of deviations from the intended interventions. Per‐protocol analysis appears to have been used. 2 participants in the intervention group were excluded from analysis as they completed less than 70% of their walks. The 2 participants that were excluded accounted for 7% of the remaining sample, it is therefore considered that there was potential for a significant impact on the results. | ||||||||||||
| Only 71% of intervention and 74% of control group had outcome data. A significant proportion of missing data was related to early discharge from hospital. These participants could be expected to have a higher functional level than those who remained in hospital. However, the missing data was well‐balanced between the two groups. | ||||||||||||
| The total distance able to walk is considered appropriate, and there were no differences in the measurement or ascertainment between groups. The assessors were not blinded, and it was therefore considered likely that knowledge of the intervention could influence the outcome, given the likely strong belief in the benefits of the intervention ward. | ||||||||||||
| A pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to be at high risk of bias in three domains for this outcome. | ||||||||||||
| Subgroup 2.5.3 Progressive resistance exercise | ||||||||||||
| de Morton 2007 |  |  |  |  |  |  | ||||||
| Allocation of wards was random (coin toss) and the allocating officer unaware of study. Baseline differences between groups were thought to be compatible with chance. | ||||||||||||
| Participants and clinicians delivering care were believed to be aware of treatment assignments. There was no evidence of deviations from intended interventions and intention to treat analysis was used. | ||||||||||||
| After accounting for mortality, at discharge, only 70% in intervention arm and 60% in control arm had timed up and go (TUG) assessed. There is a higher amount of missing data for the TUG than the Barthel Index which may indicate that some of the missing data is due to participants being unable to complete the TUG task. Therefore a proportion of missing data is believed to be due to the 'true' value. | ||||||||||||
| The use of the TUG to measure walking performance is considered appropriate, and there were no differences in the measurement or ascertainment between groups. The assessors were not blinded, and it is considered likely that knowledge of the intervention could influence the outcome, given the likely strong belief in the benefits of the intervention. | ||||||||||||
| A pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to be at high risk of bias in measurement of the outcome and missing data. | ||||||||||||
| Jones 2006 |  |  |  |  |  |  | ||||||
| Allocation sequence was random (computer generated random numbers). Sequence allocation was not concealed, but performed by a member of staff independent of the enrolment procedures. ). Participant characteristics were balanced between groups and differences compatible with chance. | ||||||||||||
| It is assumed that both participants and clinicians delivering care were aware of assigned interventions. There is no evidence of deviations from intended interventions and intention to treat analysis used. | ||||||||||||
| After accounting for mortality, timed up and go (TUG) data was available for 31% of control group and 51% of intervention group. "39.4% (63/160) were unable to complete the TUG either on admission or on discharge and therefore a change score could not be calculated." Therefore, a large proportion of missing data is due to the 'true' value. | ||||||||||||
| The use of the TUG to measure walking performance is considered appropriate, and there were no differences in the measurement or ascertainment between groups. The assessors were blinded. | ||||||||||||
| A pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to be at high risk of bias due to missing outcome data. | ||||||||||||
| Pedersen 2019 |  |  |  |  |  |  | ||||||
| Allocation sequence was random (computer‐generated block randomisation), and allocation sequence concealed from the investigators. Participant characteristics were well‐balanced between groups. | ||||||||||||
| Participants and clinicians were aware of the treatment assignments. There was no evidence of deviations from the assigned interventions and an intention to treat approach was used for analysis. | ||||||||||||
| When accounting for mortality only 93% of participants in intervention group and 67% in control group completed measures at discharge. The difference in the proportion of missing data, and that the assessments were carried out home after discharge, it was judged likely that the reasons for missing data may have depended on its true value. | ||||||||||||
| The use of the 4m timed walk for measuring gait speed/walking performance is considered appropriate, and there were no differences in the measurement or ascertainment between groups. The assessors were blinded. | ||||||||||||
| The analysis is in accordance with a pre‐specified analysis in a published protocol, and results are not thought to be from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to be at high risk of bias due to missing outcome data. | ||||||||||||

