Ejercicios para pacientes de edad avanzada hospitalizados por enfermedades agudas
Referencias
Referencias de los estudios incluidos en esta revisión
Referencias de los estudios excluidos de esta revisión
Referencias de los estudios en espera de evaluación
Referencias de los estudios en curso
Referencias adicionales
Referencias de otras versiones publicadas de esta revisión
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Study characteristics | ||
| Methods | Design: RCT Baseline time point (T1): first 48 hours of admission Outcome time point (T2): day of discharge from hospital | |
| Participants | Inclusion criteria: aged ≥ 65 years; admitted for an acute medical illness or exacerbation of a previous chronic condition; either participant or legal representative provided informed consent Exclusion criteria: none Exercise arm
Control arm
| |
| Interventions | Exercise arm
Control arm
| |
| Outcomes | Barthel Index (score of 0–100) at T2 Incidence of delirium during hospitalisation Falls during hospitalisation Mortality during hospitalisation Musculoskeletal injuries during hospitalisation Length of hospital stay | |
| Notes | Participants randomised to intervention group were admitted for stroke more often than the control group (19.7% with intervention vs 7.9% with control; P < 0.01) and presented greater ambulation‐dependence on admission (57.9% with intervention vs 41.8% with control; P < 0.01). | |
| Study characteristics | ||
| Methods | Design: RCT Baseline time point (T1): admission to hospital Outcome time point (T2): discharge from hospital Follow‐up time point (T3): 3 months after discharge | |
| Participants | Inclusion criteria: aged > 70 years; acutely admitted to University Hospital of Umeå for medical ailments Exclusion criteria: required treatment in specialised units, such as the intensive care unit, coronary care unit or acute stroke unit; or required treatment in 1 of the designated subspecialities such as in a renal care unit Exercise arm
Control arm
| |
| Interventions | Exercise arm
Control arm
| |
| Outcomes | Barthel Index (categorised scores only) at T2 and T3 Mini‐Mental State Examination at T3 Incidence of delirium during hospitalisation Length of hospital stay Adverse events (mortality during hospitalisation) New institutionalisation at discharge from hospital Readmission to an acute hospital during first 3 months after discharge | |
| Notes | ||
| Study characteristics | ||
| Methods | Design: RCT Baseline time point (T1): within first 24 hours of admission Outcome time point (T2): when deemed clinically stable Follow‐up time point (T3): 1 month after T2 | |
| Participants | Inclusion criteria: aged > 70 years; confined to bed or walking from bed to chair with human help, but independent for locomotion within 3 months; written consent from participants and surrogates Exclusion criteria: any neuromuscular diseases affecting lower limbs, chronic respiratory impairment, severe heart failure (New York Heart Association class IV), peripheral vascular disease, palliative care, use of drugs known to impair muscle function. Owing to PT availability, admitted patients in a period that was incompatible to PT intervention the following day after admission were excluded. Thus, no more than 5/20 patients admitted from Sunday to Thursday were included per week Exercise arm
Control arm
| |
| Interventions | Exercise arm
Control arm
| |
| Outcomes | Katz ADL score (0–12) at T2 and T3 Adverse events (mortality) | |
| Notes | The intervention group had a higher mean BMI approaching significance (P < 0.06) and a higher mean weight approaching significance (P < 0.07). | |
| Study characteristics | ||
| Methods | Design: RCT Baseline time point (T1): admission to hospital Outcome time point (T2): discharge from hospital Follow‐up time point (T3): 4 weeks after discharge | |
| Participants | Inclusion criteria: aged ≥ 65 years; with medical reason for admission; not being imminently terminal (death not expected in the next 30 days) Exclusion criteria: delirium (CAM score > 0); cognitive impairment (Mini‐Cognitive Assessment score < 3); self‐report of not being ambulatory with or without an assistive device in the 2 weeks before admission; significant language barrier that required a translator; being previously enrolled in the study Exercise arm
Control arm
| |
| Interventions | Exercise arm
Control arm
| |
| Outcomes | Katz ADL score (7–21) at T2 and T3 Incidence of delirium during hospitalisation Falls during hospitalisation Mortality during hospitalisation Medical deterioration during hospitalisation Length of hospital stay | |
| Notes | ||
| Study characteristics | ||
| Methods | Design: RCT Baseline time point (T1): admission to hospital Outcome time point (T2): discharge from hospital Follow‐up time point (T3): 1, 3, 6 and 12 months after discharge | |
| Participants | Inclusion criteria: ≥ aged 70 years, community‐dwelling and admitted to a medicine or family practice service Exclusion criteria: transferred from a nursing facility or another hospital; required speciality unit admission (e.g. intensive care, coronary care, telemetry or oncology); admitted electively; had a length of stay < 2 days; had been previously enrolled in the study Exercise arm
Control arm
| |
| Interventions | Exercise arm
Control arm
| |
| Outcomes | Katz ADL score (0–5) at T1 and T2 Physical Performance and Mobility Examination at T2 Mortality during hospitalisation Length of hospital stay Readmissions at 1 month after discharge from hospital New institutionalisation at discharge from hospital | |
| Notes | ||
| Study characteristics | ||
| Methods | Design: RCT Baseline time point (T1): within 72 hours of admission Outcome time point (T2): 4 weeks after discharge from hospital Follow‐up time point (T3): 12 and 24 weeks after discharge | |
| Participants | Inclusion criteria: aged ≥ 65 years, admitted with a medical diagnosis, with ≥ 1 risk factor for readmission (i.e. aged ≥ 75, multiple hospital admissions in previous 6 months, multiple comorbidities, living alone, lack of social support, poor self‐rating of health, functional impairment, history of depression, or a combination of these) Exclusion criteria: requiring home oxygen; dependent on a wheelchair or unable to walk independently for 3 m, living in a nursing home; cognitive deficit; progressive neurological disease Exercise arm
Control arm
| |
| Interventions | Exercise arm
Control arm
| |
| Outcomes | Katz ADL score (0–6) at T1 and T3 only Lawton Instrumental Activities of Daily Living Scale score (0–7) at T1 and T3 only Walking Impairment Scale at T1 and T3 only Quality of life at T1 and T3 only Mortality Adverse events (undefined composite score) Length of hospital stay Readmissions at 6 months after discharge from hospital | |
| Notes | Poor self‐rating of health was higher in the intervention group compared to the control group (65% with intervention vs 47% with control; P = 0.038). | |
| Study characteristics | ||
| Methods | Design: RCT Baseline time point (T1): within 48 hours of admission Outcome time point (T2): within 48 hours of discharge from hospital Follow‐up time point (T3): 1 month after discharge from hospital | |
| Participants | Inclusion criteria: aged ≥ 65 years, diagnosed with a general medical condition, admitted to either of the 2 medical wards, and assessed within 48 hours of admission Exclusion criteria: admitted to hospital from a nursing home, assessed to need nursing home level of care or palliative care; had a stroke or a condition for which mobilisation was contraindicated (e.g. deep vein thrombosis or fracture); too medically unwell to ambulate or exercise; readmitted having previously participated in the study Exercise arm
Control arm
| |
| Interventions | Exercise arm
Control arm
| |
| Outcomes | Barthel Index (score of 0–100) at T2 Functional Ambulatory Category at T2 Falls during hospitalisation Mortality during hospitalisation Medical deterioration during hospitalisation Length of hospital stay Readmissions within the first 28 days after discharge New institutionalisation at discharge from hospital Timed Up and Go at T2 | |
| Notes | Participants in intervention group were a mean 2 years older. | |
| Study characteristics | ||
| Methods | Design: quasi‐RCT Baseline time point (T1): admission to hospital Outcome time point (T2): (quote) "intention was to perform the initial physical tests during the latter part of the hospital stay, before discharge." Follow‐up time point (T3): 3 months after discharge | |
| Participants | Inclusion criteria: aged ≥ 75 years, in need of in‐hospital treatment, and with ≥ 2 of the following FRail Elderly Support researcH group (FRESH) criteria: general fatigue, tiredness from a short walk, dependence in shopping, frequent falls/anticipation of falls, or ≥ 3 more visits to the emergency ward during the last 12 months Exclusion criteria: person clearly suited for care in a conventional acute medical care unit due to the severity and type of condition: acute stroke, acute myocardial infarction, sepsis, or other acute life‐threatening conditions; patient declined participation in study; informed consent could not be obtained from the patient (and it was not possible to obtain informed consent from a relative); or the patient was a previously defined MÄVA (acute elderly care CGA units) patient Exercise arm
Control arm
| |
| Interventions | Exercise arm
Control arm
| |
| Outcomes | ADL staircase (0–9) assessed at T2 and T3 6‐minute walk test assessed at T2 and T3 Timed Up and Go assessed at T2 and T3 Quality of life (EQ‐5D VAS) assessed at T2 and T3 Mortality during hospitalisation Length of hospital stay Readmissions in first 3 months following discharge | |
| Notes | Higher proportion of control group (38%) compared to the intervention group (29%) were from home living without help. Intervention group had a higher mean Charlson Co‐morbidity Index score than the control group (7.4 with intervention vs 6.2 with control). | |
| Study characteristics | ||
| Methods | Design: RCT Baseline time point (T1): first 24 hours of admission to hospital Outcome time point (T2): discharge from hospital Follow‐up time point (T3): 6 weeks, 3 and 6 months after randomisation | |
| Participants | Inclusion criteria: aged ≥ 75 years, not on protocol treatment or require admission to coronary or intensive care; if their physician provided consent Exclusion criteria: none Exercise arm
Control arm
| |
| Interventions | Exercise arm
Control arm
| |
| Outcomes | Katz ADL (score of 0–5) at T3 only Modified Mini‐Mental State Examination at T3 only Mortality during hospitalisation Length of hospital stay New institutionalisation at discharge from hospital | |
| Notes | ||
| Study characteristics | ||
| Methods | Design: RCT Baseline time point (T1): within 24 hours of hospital admission Outcome time point (T2): hospital discharge Follow‐up time point (T3): 1 and 3 months posthospital discharge | |
| Participants | Inclusion criteria: all participants consecutively admitted to the geriatric unit between October 2018 and January 2020, if they were aged ≥ 65 years and if they were potentially able to walk, as assessed through geriatrician's clinical judgement based on participant's current and preadmission status Exclusion criteria: independent walking ability at admission; diagnosis of femoral fractures or stroke (due to the presence of specific rehabilitation pathways for these patients), coma and severe dementia; unable to provide informed consent or refused to participate in the study Exercise arm
Control arm
| |
| Interventions | Exercise arm
Control arm
| |
| Outcomes | Barthel Index at T2 Mobility Barden Activity Subscale at T2 Falls during hospitalisation Mortality during hospitalisation Length of hospital stay Hospital readmissions at T3 | |
| Notes | Unpublished data from email correspondence Barthel Index: the mean scores at discharge (T2) were: 65.20 (SD 24.18) for the intervention group and 56.07 (SD 23.74) for the control group. Mobility Barden Activity Subscale: the mean scores at discharge (T2) were: 3.43 (SD 0.64) for the intervention group and 2.80 (SD 0.69) for the control group. Hospital readmissions: at 30‐day follow‐up (T3) 33/174 of intervention group vs 40/165 of control group had been readmitted. | |
| Study characteristics | ||
| Methods | Design: RCT (3 arms) Baseline time point (T1): within 48 hours of hospital admission Outcome time point (T2): at hospital discharge Follow‐up time point (T3): 1 and 3 months after hospital discharge | |
| Participants | Inclusion criteria: aged ≥ 65 years, if their hospital admission with a medical diagnosis had been unplanned, and if they had been able to walk independently 2 weeks before admission (participants independently using walking aids were included) Exclusion criteria: admission due to severe acute illness (immediately requiring intensive care); needing hospice care or surgery; having severe cognitive impairment; admitted for < 72 hours; or being diagnosed with an illness requiring activity restraint Exercise arm
'Reminder' arm (not included in meta‐analysis)
Control arm
| |
| Interventions | Exercise arm
'Reminder' arm (not included in meta‐analysis)
Control arm
| |
| Outcomes | Katz ADL score (7–21) EQ‐5D VAS at T2 Medical deterioration during hospitalisation Mortality during hospitalisation Length of hospital stay Timed Up and Go at T2 | |
| Notes | ||
| Study characteristics | ||
| Methods | Design: RCT Baseline time point (T1): within 48 hours of admission Outcome time point (T2): within 24 hours of discharge from hospital | |
| Participants | Inclusion criteria: aged ≥ 65 years, admitted to a medical unit in the study area and in hospital for < 48 hours at the point of recruitment Exclusion criteria: severe dysphasia rendering communication impossible; death expected within 24 hours; isolation for infection control; documented contraindication to mobilisation; admission to the stroke unit or to critical care (intensive or coronary care); planned admission of < 48 hours; major psychiatric diagnosis (e.g. schizophrenia); previous inclusion in the study; delirium documented in the admission notes; transfer from another hospital Exercise arm
Control arm
| |
| Interventions | Exercise arm
Control arm
| |
| Outcomes | Barthel Index at hospital discharge Mini‐Mental State Examination at hospital discharge Incidence of delirium during hospitalisation Mortality during hospitalisation Adverse events (composite score only) during hospitalisation Length of hospital stay New institutionalisation at hospital discharge | |
| Notes | Unpublished data from email correspondence and author's PhD thesis: Barthel Index: the median and IQR of scores at discharge (T2) were: 95 (IQR 78 to 100; 305 participants) in the intervention group, and 96 (IQR 77 to 100; 343 participants) in the control group. | |
| Study characteristics | ||
| Methods | Design: RCT Baseline time point (T1): within 48 hours of admission Outcome time point (T2): within 24 hours of discharge from hospital | |
| Participants | Inclusion criteria: aged ≥ 65 years, general medical admission to the general medical wards; provided informed consent Exclusion criteria: admitted from a nursing home or who were receiving nursing home level of care at home; medically unstable or where mobilisation was contraindicated by the treating medical team; admitted to the delirium management unit; non‐weight‐bearing; not assessed within 48 hours of admission; assessed as requiring palliative care; admitted to hospital with a diagnosis known to cause functional impairment; or who had an expected length of stay < 24 hours Exercise arm
Control arm
| |
| Interventions | Exercise arm
Control arm
| |
| Outcomes | Barthel Index (score 0–100) at T2 Falls during hospitalisation Medical deterioration during hospitalisation Mortality during hospitalisation Length of hospital stay Timed Up and Go at T2 | |
| Notes | The control group had lower proportion of people from their own home compared to the intervention group (88.8% with intervention vs 76.3% with control). Mean modified Barthel Index was higher in the intervention group compared to the control (71 with intervention vs 61 with control). | |
| Study characteristics | ||
| Methods | Design: RCT Baseline time point (T1): hospital admission Outcome time point (T2): 7 days after T1 | |
| Participants | Inclusion criteria: aged ≥ 70 years; admitted to the medical wards; unable to walk by themselves, or displayed inhibition going for a walk by themselves; a provisional diagnoses including heart‐, lung‐ and diabetes‐related morbidities Exclusion criteria: unable to understand plain English statement and consent form; people with a stroke and undergoing rehabilitation with the physiotherapist; significant dementia precluding the possibility of gathering reliable exercise self‐efficacy data Exercise arm
Control arm
| |
| Interventions | Exercise arm
Control arm
| |
| Outcomes | Barthel Index at T2 Falls during hospitalisation Mortality during hospitalisation Maximum distance able to walk at T2 | |
| Notes | Excluded participants discharged before day 7 of hospitalisation (9 participants in intervention group vs 7 participants in control group), and those who completed < 70% of the intervention (2 participants). | |
| Study characteristics | ||
| Methods | Design: RCT Baseline time point (T1): admission Outcome time point (T2): discharge Follow‐up time point (T3): 3 month after discharge from hospital | |
| Participants | Inclusion criteria: aged ≥ 70 years, admitted for general medical care Exclusion criteria: people who were admitted to a speciality unit (e.g. intensive care, cardiology–telemetry, or oncology). At the time of admission beds were not available in both the intervention and usual care units Exercise arm
Control arm
| |
| Interventions | Exercise arm
Control arm
| |
| Outcomes | ADL (score of 0–5) at T2 and T3 IADL (score of 0–7) at T3 Mini‐Mental State Examination at T2 Mortality during hospitalisation Length of hospital stay Readmissions in the first 3 months after discharge | |
| Notes | Participants assigned to the intervention group reported better overall health status at admission (P = 0.04) and were less likely to have a clinical diagnosis of cerebrovascular disease (P = 0.05). | |
| Study characteristics | ||
| Methods | Design: RCT Baseline time point (T1): within 48 hours of admission Outcome time point (T2): discharge from hospital Follow‐up time point (T3): 3 months after discharge | |
| Participants | Inclusion criteria: aged ≥ 75 years; Barthel Index ≥ 60; able to ambulate with/without assistance; able to communicate and collaborate with the research team Exclusion criteria: expected length of stay < 6 days; very severe cognitive decline (i.e. Global Deterioration Scale score 7); terminal illness; uncontrolled arrhythmias; acute pulmonary embolism; recent myocardial infarction; recent major surgery; extremity bone fracture in past 3 months Exercise arm
Control arm
| |
| Interventions | Exercise arm
Control arm
| |
| Outcomes | Barthel Index (score 0–100) at T2 Short Physical Performance Battery at T2 Mini‐Mental State Examination at T2 Incidence of delirium/confusion during hospitalisation EQ‐5D at T2 Falls during hospitalisation Mortality during hospitalisation Medical deterioration during hospitalisation Length of hospital stay Readmissions within the first 3 months of discharge | |
| Notes | Unpublished data from email correspondence Barthel Index: mean scores at discharge (T2): 84.5 (SD 14.5; 146 participants) for the intervention group and 78.2 (SD 19.5; 143 participant) for the control group. Short Physical Performance Battery: mean scores at discharge (T2): 6.79 (SD 3.21; 150 participants) for the intervention group and 4.91 (SD 2.89; 153 participants) for the control group. Delirium: 152 participants were assessed for delirium in the intervention group, 157 participants in the control group. EQ‐5D: mean scores at discharge (T2): 69.5 (SD 18.8; 139 participants) in the intervention group and 57.5 (SD 20.6; 135 participants) for the control group. Falls: 0 falls/139 participants were recorded in the intervention group, 4 falls/146 participants were recorded in the control group. Readmissions: 29 participants were readmitted in the intervention group, 31 participants were readmitted in the control group. | |
| Study characteristics | ||
| Methods | Design: RCT Baseline time point (T1): within the first 48 hours of admission to hospital Outcome time point (T2): within 24 hours of planned discharge from hospital Follow‐up time point (T3): 2–3 months following discharge from hospital | |
| Participants | Inclusion criteria: irrespective of ward allocation, medical inpatients aged ≥ 65 years, needing an aid or assistance to walk (or both) on admission, and admitted from and planned for discharge home (rather than for institutional care), with an anticipated hospital stay ≥ 3 days were recruited Exclusion criteria: inpatients admitted for > 48 hours prior to screening; unable to follow simple commands in the English language; admitted with an acute psychiatric condition, or requiring end‐of‐life or critical care; ordered bedrest, or contraindications to walking (e.g. hip fracture or high ventricular rate atrial fibrillation); baseline Short Physical Performance Battery score 0/1; participated in the trial within the previous 12 months Exercise arm
Control arm
| |
| Interventions | Exercise arm
Control arm
| |
| Outcomes | Short physical performance battery at T2 and T3 6‐Item Cognitive Impairment Test at T2 EQ‐5D‐5L VAS at T2 and T3 Falls during hospitalisation Length of hospital stay Mortality during hospitalisation Hospital readmissions in the 3 months following discharge | |
| Notes | There was a higher proportion of women in the intervention group (64%) compared to the control group (41%). The mean of the intervention group was 79.9 (SD 7.5) and the control group was 81.6 (SD 7.33) (P = 0.07). All multivariate analyses adjusted for age. Unpublished data from email correspondence Delirium: the number of participants with delirium at discharge that was not present at admission were 2 in the intervention group and 3 in the control group. Length of hospital stay: mean 9.88 (SD 7.12) days in the intervention group and 11.42 (SD 9.46) days in the control group. | |
| Study characteristics | ||
| Methods | Design: RCT Baseline time point (T1): within 48 hours of admission Outcome time point (T2): day of discharge from hospital or day 7 of hospital admission if earlier | |
| Participants | Inclusion criteria: aged > 65 years; admitted to hospital within the preceding 48 hours; able to sit in a chair independently and follow 1‐stage commands; expected length of stay at least a further 48 hours Exclusion criteria: terminally ill or moribund; needing isolation precautions; bed bound prior to admission; who had a condition that made them unable to use the pedal exerciser (e.g. lower limb fracture, lower limb pain, leg amputation or foot deformity) Exercise arm
Control arm
| |
| Interventions | Exercise arm
Control arm
| |
| Outcomes | Elderly Mobility Scale at hospital discharge Length of hospital stay | |
| Notes | Intervention group was older (87 in intervention group vs 83 in control group) with a greater proportion of women (67% in intervention group vs 54% in control group). | |
| Study characteristics | ||
| Methods | Design: quasi‐RCT Baseline time point (T1): within 48 hours of admission to hospital Outcome time point (T2): within 48 hours of discharge from hospital | |
| Participants | Inclusion criteria: aged ≥ 65 years, admitted to an internal medicine unit for ≥ 3 days and received at least some of their care on the designated intervention or control ward Exclusion criteria: already fully dependent before their admission; came from a high‐level residential care facility or were medically too unstable for early assessment or terminally ill; discharged or transferred within 72 hours; died in hospital; or did not gain admission to study wards during their admission Exercise arm
Control arm
| |
| Interventions | Exercise arm
Control arm
| |
| Outcomes | Barthel Index (score of 0–100) at T2 Incidence of delirium during hospitalisation Falls during hospitalisation Mortality during hospitalisation Length of hospital stay Readmissions within 30 days of hospital discharge New institutionalisation at hospital discharge Timed Up and Go at T2 (categorised scores only) | |
| Notes | ||
| Study characteristics | ||
| Methods | Design: quasi‐RCT Baseline time point (T1): admission to hospital Outcome time point (T2): discharge from hospital Follow‐up time point (T3): 3 months after discharge | |
| Participants | Inclusion criteria: aged > 75 years, admitted to an acute care of the elderly unit Exclusion criteria: non‐ambulatory or dependent in all basic ADLs at baseline (i.e. 2 weeks before admission, as assessed by retrospective interview), had unstable cardiovascular disease or any other major medical condition contraindicating exercise, terminal illness, severe dementia (i.e. ≥ 8 errors in the Spanish version of the Short Portable Mental Status Questionnaire), an expected length of hospitalisation < 3 days, were transferred from another hospital unit or had a scheduled admission (which was usually associated with a length of hospitalisation < 3 days) Exercise arm
Control arm
| |
| Interventions | Exercise arm
Control arm
| |
| Outcomes | ADL (score 0–6) at T2 and T3 Short Physical Performance Battery at T2 Functional Ambulatory Category at T2 and T3 Mortality during hospitalisation Falls Length of hospital stay Readmissions within 3 months of hospital discharge | |
| Notes | Intervention group had higher proportion of participants with a diagnosis of dementia (27% in intervention vs 12% in control), depression (32% in intervention vs 18% in control), history of falls (36% in intervention vs 16% in control), lower mean baseline ADL scores (4.0 in intervention vs 4.6 in control) and admission ADL scores (2.3 in intervention vs 3.1 in control). | |
| Study characteristics | ||
| Methods | Design: RCT Baseline time point (T1): admission to hospital Outcome time point (T2): within the first week after discharge from hospital Follow‐up time point (T3): 4 weeks after discharge and 6 months after discharge | |
| Participants | Inclusion criteria: aged ≥ 65 years, admitted with acute illness from their own home to the emergency department of hospital Exclusion criteria: terminal illness; in treatment for diagnosed cancer; diagnosis of COPD and participation in a COPD rehabilitation programme; living outside the 3 included municipalities; inability to speak or understand Danish; inability to cooperate in tests/exercises; transfer to the intensive care unit; isolation‐room stay; expected hospitalisation lasting < 24 hours; inability to stand Exercise arm
Control arm
| |
| Interventions | Exercise arm
Control arm
| |
| Outcomes | Barthel Index (score of 0–20) at T2 and T3 de Morton Mobility Index at T2 and T3 Mortality during hospitalisation Adverse events (composite score) during hospitalisation Length of hospital stay Readmissions within first 4 weeks and first 6 months after discharge (numbers not reported) Walking speed at T2 and T3 | |
| Notes | Intervention group had higher percentage of women (71.4% in intervention vs 60.5% in control). Unpublished data from email correspondence Hospital readmissions: at 4 weeks' follow‐up (T3), 8/43 participants of intervention group vs 6/42 participants of control group had been readmitted. | |
| Study characteristics | ||
| Methods | Design: RCT Baseline time point (T1): within 36 hours of admission to hospital Outcome time point (T2): within the first week after discharge from hospital Follow‐up time point (T3): 91 days after discharge | |
| Participants | Inclusion criteria: aged ≥ 70 years; general practitioner registered within the Nottingham City Clinical Commissioning Group's catchment area only (UK) Exclusion criteria: bed bound prior to admission or moribund on admission; receiving palliative care; previously included in the trial on an earlier admission; unable to be screened and recruited by the research team within 36 hours of admission to the study ward; nursing home residents Exercise arm
Control arm
| |
| Interventions | Exercise arm
Control arm
| |
| Outcomes | Barthel Index (score of 0–20) at T3 only EQ‐5D‐3L at T3 only Falls Mortality during hospitalisation Length of hospital stay Hospital readmissions in the first 28 after hospital discharge. | |
| Notes | ||
| Study characteristics | ||
| Methods | Design: quasi‐RCT Baseline time point (T1): admission to hospital Outcome time point (T2): discharge from hospital Follow‐up time point (T3): 6 months (readmissions) | |
| Participants | Inclusion criteria: aged ≥ 75 years, and referred to department of general medicine Exclusion criteria: admitted for day treatment Exercise arm
Control arm
| |
| Interventions | Exercise arm
Control arm
| |
| Outcomes | ADL (score of 0–7) at T2 (categorised only) Help index (score of 0–12) at T2 (categorised only) Mobility (score of 0–4) at T2 (categorised only) Length of hospital stay Readmissions within first 6 months of discharge from hospital Mortality during hospitalisation | |
| Notes | There were more women in intervention group (75.3%) than in the control group (67.1%). Participants were more likely to be married in the intervention group (43.6%) than the control group (27.8%). | |
| Study characteristics | ||
| Methods | Design: quasi‐RCT Baseline time point (T1): within 72 hours of admission Outcome time point (T2): day of discharge | |
| Participants | Inclusion criteria: aged ≥ 65 years and admitted for an acute medical pathology to a geriatric care unit Exclusion criteria: dependent in all basic ADL before admission; admitted to intensive care; transferred from other services; intubated patients; severe dementia; terminal cancer; severe aphasia; discharged in < 24 hours; admitted for specific procedures; patients from the internal medicine service to the geriatric care team for treatment Exercise arm
Control arm
| |
| Interventions | Exercise arm
Control arm
| |
| Outcomes | Katz ADL (score 0–6) at T2 (categorised scores only) Length of hospital stay | |
| Notes | Intervention group was older (79.6 years in intervention vs 76.1 years in control), was more likely to be admitted with renal conditions (14.7% in intervention vs 6.7% in control) and less likely to have cardiovascular problems (9.9% in intervention vs 25.3% in control). | |
ADL: activities of daily living; AGW: acute geriatric ward; CAM: Confusion Assessment Method; CGA: comprehensive geriatric assessment; CI: confidence interval; CIRACT: Community In‐Reach and Care Transition; COPD: chronic obstructive pulmonary disease; EQ‐5D: EuroQol 5 Dimensions; EQ‐5D‐5L: EuroQol 5 Dimensions 5 Levels; IADL: Instrumental Activities of Daily Living; IQR: interquartile range; MDT: multidisciplinary team; n: number; NA: not applicable; OT: occupational therapy; PT: physiotherapy; RCT: randomised controlled trial; SD: standard deviation; T: time point (e.g. T1: time point 1); THB‐Rehab: traditional hospital‐based rehabilitation service; VAS: visual analogue scale.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Critical care setting | |
| Exercise not part of the intervention | |
| Participants randomised after 72 hours of hospital admission | |
| No outcome data | |
| Community setting | |
| Exercise intervention started after discharge from hospital | |
| Non‐general medical population | |
| Non‐general medical population | |
| Participants randomised after 72 hours of hospital admission | |
| Protocol, full manuscript identified | |
| Non‐general medical population | |
| Control group received additional exercise | |
| Non‐general medical population | |
| Inpatient rehabilitation setting | |
| Inpatient rehabilitation setting | |
| Abstract/letter to editor, full manuscript identified | |
| < 95% of the study participants were aged ≥ 65 years | |
| Exercise not part of the intervention | |
| Did not measure outcomes at time points of interest. | |
| Non‐general medical population | |
| < 95% of the study participants were aged ≥ 65 years | |
| < 95% of the study participants were aged ≥ 65 years | |
| Non‐general medical population | |
| Non‐randomised study | |
| Inpatient rehabilitation setting | |
| Protocol, full manuscript identified | |
| Inpatient rehabilitation setting | |
| Non‐general medical population | |
| Participants randomised after 72 hours of hospital admission | |
| Abstract/letter to editor, full manuscript identified | |
| Abstract/letter to editor, full manuscript identified | |
| Abstract/letter to editor, full manuscript not identified | |
| Abstract/letter to editor, full manuscript identified | |
| Abstract/letter to editor, full manuscript not identified | |
| < 95% of the study participants were aged ≥ 65 years | |
| Protocol, full manuscript identified | |
| Non‐randomised study | |
| Community setting | |
| Inpatient rehabilitation setting | |
| Protocol, < 95% of the study participants expected to be aged ≥ 65 years | |
| Inpatient rehabilitation setting | |
| < 95% of the study participants were aged ≥ 65 years | |
| Exercise not part of the intervention | |
| Inpatient rehabilitation setting | |
| Abstract/letter to editor, full manuscript not identified | |
| Inpatient rehabilitation setting | |
| Exercise not part of the intervention | |
| Participants randomised after 72 hours of hospital admission | |
| Non‐randomised study | |
| Participants randomised after 72 hours of hospital admission | |
| Abstract/letter to editor, full manuscript identified | |
| Inpatient rehabilitation setting | |
| Participants randomised after 72 hours of hospital admission | |
| Participants randomised after 72 hours of hospital admission | |
| Participants randomised after 72 hours of hospital admission | |
| Inpatient rehabilitation setting | |
| Community setting | |
| Non‐general medical population | |
| Community setting | |
| Non‐randomised study | |
| Community setting | |
| Inpatient rehabilitation setting | |
| Exercise intervention started after discharge from hospital | |
| Exercise intervention started after discharge from hospital | |
| Abstract/letter to editor, full manuscript identified | |
| Inpatient rehabilitation setting | |
| Inpatient rehabilitation setting | |
| Non‐randomised study | |
| Inpatient rehabilitation setting | |
| Exercise not part of the intervention |
Characteristics of studies awaiting classification [ordered by study ID]
| Methods | Design: randomised controlled trial Baseline time point (T1): admission to hospital Outcome time point (T2): discharge from hospital |
| Participants | Inclusion criteria: aged ≥ 70 years who are admitted to the internal medicine wards, with ≥ 1 risk factor for delirium at admission (visual impairment, hearing impairment, cognitive impairment, impaired sleep, mobility impairment or dehydration). Expected length of stay > 7 days; able to communicate verbally or in writing Exclusion criteria: diagnosis of delirium at hospital admission, coma, mechanical ventilation, aphasia (expressive or receptive), severely impaired communication ability, terminal/end‐stage conditions, imminent death, combative or dangerous behaviours, a severe psychotic disorder that prevents from participation in interventions, severe dementia (being unable to communicate based on Short Portable Mental Status Questionnaire 10 errors), airborne precautions (e.g. tuberculosis), being isolated, droplet precautions (e.g. influenza), neutropenic precautions, being discharged around 48 hours after admission, refusal to participate in study, and patient's family members or physician's refusal to allow participation in study in the case of incompetent patients |
| Interventions | Intervention: care programme based on the Hospitalized Elder Life Program (HELP) provided by nursing students. The intervention includes an early mobilisation programme in which all patients will be enrolled. The mobilisation programme includes ambulation or active range‐of‐motion exercises 3 times daily. Control group: usual care consisting of standard hospital care for the setting, provided by physicians, nurses and support staff (e.g. dietitians, physiotherapists). |
| Outcomes | Incidence of delirium (as assessed with the Confusion Assessment Method tool) Independence with activities of daily living using the Barthel Index Number of falls during hospitalisation. Mortality |
| Notes | en.irct.ir/trial/33830 |
Characteristics of ongoing studies [ordered by study ID]
| Study name | Physical Training and Health Education in Hospitalized Elderly |
| Methods | Design: randomised controlled trial Baseline time point (T1): day of hospital admission Outcome time point (T2): day of hospital discharge Follow‐up time point (T3): 3 months after hospital discharge |
| Participants | Inclusion criteria: aged ≥ 75 years, admitted into the Geriatrics Department of the Hospital General Universitario Gregorio Marañón (Madrid, Spain); able to ambulate, with or without personal/technical assistance; able to communicate; provide informed consent Exclusion criteria: duration of hospitalisation < 72 hours; any factor precluding performance of the physical training programme or testing procedures as determined by the attending physician (including but not limited to: terminal illness, incapable of ambulation, unstable cardiovascular disease or other medical condition, severe dementia, unwillingness to either complete the study requirements or to be randomised into control or intervention group) |
| Interventions | Exercise arm: training programme (30 minutes per session, 2 sessions per day, lower limb strength training, balance training, walking and inspiratory muscle training) and also health education. Health education consists of several informational activities. Each activity session will teach the patient and carer how to perform the exercises to ensure they will continue to be performed at home and before discharge the entire session will be devoted to reviewing the entire programme. The type, frequency and progression of the exercises to be carried out will be reviewed; they will be explained how to perform them at home and given personalised written instructions with illustrations of the exercises. Also, after 1 and 2 months of discharge, the professional with whom they have completed the training will call them to insist on the completion of the programme or to clarify any doubts that may exist Control arm: usual care for the setting |
| Outcomes | Independence with ADL (range 0–6) at T3 Barthel Index at T3 Functional Ambulation Classification at T3 Short Physical Performance Battery at T2 Alusti Test at T2 |
| Starting date | July 2018 |
| Contact information | Dr Jose Antonio [email protected] |
| Notes | clinicaltrials.gov/ct2/show/NCT03604640 |
| Study name | Prevention of functional and cognitive impairment through a multicomponent exercise program in hospitalized elderly |
| Methods | Design: randomised controlled trial Baseline time point (T1): admission to hospital Outcome time point (T2): discharge from hospital Follow‐up time point (T3): 3 years after hospital discharge |
| Participants | Inclusion criteria: aged > 75 years; Barthel Index ≥ 60 points; able to ambulate (with/without assistance); provide informed consent; able to communicate Exclusion criteria: expected length of stay < 6 days; terminal illness; very severe cognitive decline (i.e. Global Deterioration Scale 7); uncontrolled arrhythmias, acute pulmonary embolism and myocardial infarction, or extremity bone fracture in the past 3 months Target sample size: 240 |
| Interventions | Exercise arm: multicomponent exercise training programme composed of supervised progressive resistance exercise training, balance‐training and walking for 4 consecutive days. During the training period, participants will be trained in 20‐minute sessions twice per day (morning and evening). The supervised multicomponent exercise training programme will comprise upper and lower body strengthening exercises, tailored to the individual's functional capacity, using weight machines and aiming for 2–3 sets of 8–10 repetitions at an intensity of 40–60% of 1 repetition maximum combined with balance and gait retraining exercises that progressed in difficulty and functional exercises, such as rises from a chair. The second part of the session will consist of functional exercises such as knee extension and flexion, hip abduction, balance movements, and daily walking in the hospital Control arm: usual care for setting |
| Outcomes | Short Physical Performance Battery EuroQol‐5 Dimension (EQ‐5D) visual analogue scale Incidence of delirium as assessed with the Confusion Assessment Method 3‐year mortality Total use of health‐related resources (number of readmissions, visits to accident and emergency department, visits to outpatient clinics) |
| Starting date | October 2020 |
| Contact information | Nicolas Martinez Velilla [email protected] |
| Notes | clinicaltrials.gov/ct2/show/NCT04600453 |
ADL: activities of daily living.
Riesgo de sesgo
Click on one or more cells to see and compare the Support for judgement for that bias, or click on a bias header to open all bias in that column.
Legend: Low risk of bias
Low risk of bias  High risk of bias
High risk of bias  Some concerns
Some concerns
| Bias | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study | Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | ||||||
| Subgroup 1.1.1 Rehabilitation‐related activities | ||||||||||||
| Abizanda 2011 |  |  |  |  |  |  | ||||||
| Allocation sequence was random (computer generated random numbers). Allocation occurred after enrolment by an investigator not involved in the participants' clinical management. Higher number of participants admitted with a stroke in the intervention group (39 vs. 16), in addition, the 'Others' subgroup the Barthel index is higher in the control group than the intervention group (29.1 vs. 23.1). This is thought to be compatible with chance. | ||||||||||||
| Its assumed participants and the Occupational Therapist (OT) delivering the intervention were aware of the assigned intervention allocation. All participants received their allocated intervention. Therefore, even though intention to treat analysis not specified, analysis deemed appropriate. | ||||||||||||
| Given the nature of this population (older adults during and after acute hospitalisation) we considered a threshold of 90% of participants with data as sufficient. We felt this would be consistent with measurements outside of a trial context. When accounting for mortality, 95% and 94% assessed at discharge in intervention and control arms respectively. | ||||||||||||
| The use of the Barthel Index to measure independence with ADLs is considered appropriate, and there were no differences in measurement or ascertainment of the outcomes between groups. The OT assessor was blinded. | ||||||||||||
| The data analyst was blinded, and followed a pre‐specified statistical plan. It is not thought that results were from multiple outcome measurements or multiple analyses. | ||||||||||||
| The study is judged to be at low risk of bias in all domains for this outcome. | ||||||||||||
| Counsell 2000 |  |  |  |  |  |  | ||||||
| Allocation sequence was random (computer generated random numbers) and sequence concealed (opaque sealed envelope). Participant characteristics were balanced. | ||||||||||||
| Participants and those delivering the interventions were aware of intervention assignments. Seventy‐nine participants were not admitted to the unit to which they were assigned. Deviations may have affected the effect estimate, however they were well‐balanced between groups. Intention to treat analysis was used. | ||||||||||||
| Functional data was obtained in 1476 of 1483 of surviving patients (99.5%) at discharge. | ||||||||||||
| The Katz ADL score is considered appropriate, and there were no differences in the measurement or ascertainment between groups. The assessors were not blinded, and it was therefore considered likely that knowledge of the intervention could influence the outcome, given the likely strong belief in the benefits of the intervention ward. | ||||||||||||
| A pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to be at high risk of bias due to measurement of the outcome. | ||||||||||||
| Ekerstad 2017 |  |  |  |  |  |  | ||||||
| Randomisation was based on the availability of beds, and there was no allocation concealment as participants were allocated a ward prior to enrolment. Participant characteristics appeared balanced. | ||||||||||||
| Participants and clinicians delivering care were aware of treatment assignments. There was no evidence of deviations from intended interventions and intention to treat analysis was used. | ||||||||||||
| No missing data at hospital discharge. | ||||||||||||
| The ADL Staircase is considered appropriate, and there were no differences in the measurement or ascertainment between groups. The assessors were not blinded, and it was therefore considered likely that knowledge of the intervention could influence the outcome, given the likely strong belief in the benefits of the intervention ward. | ||||||||||||
| A detailed pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to be at high risk of bias due to bias in the measurement of the outcome and bias arising from the randomisation process. | ||||||||||||
| Landefeld 1995 |  |  |  |  |  |  | ||||||
| Allocation sequence was random (computer generated random numbers) and balanced participant characteristics, but no information was provided regarding allocation concealment. | ||||||||||||
| It is assumed participants and clinicians were aware of the treatment assignments. There was no evidence of deviations from the assigned interventions and evidence of an intention to treat approach being used for analysis. | ||||||||||||
| Accounting for mortality (24 died in each arm) all participants had outcome data at discharge. | ||||||||||||
| The Katz ADL score is considered appropriate, and there were no differences in the measurement or ascertainment between groups. The assessors were not blinded, and it was therefore considered likely that knowledge of the intervention could influence the outcome, given the likely strong belief in the benefits of the intervention ward. | ||||||||||||
| A pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to be at high risk of bias in measurement of the outcome for this outcome. | ||||||||||||
| Subgroup 1.1.2 Structured exercise | ||||||||||||
| Blanc‐Bisson 2008 |  |  |  |  |  |  | ||||||
| No information provided on randomisation methods or sequence concealment other than to say participants were randomised. Differences in participant characteristics appear compatible with chance. | ||||||||||||
| It is assumed that participants and those delivering the interventions were aware of intervention assignments. However, all participants received their intended intervention and intention to treat was specified in the analysis. | ||||||||||||
| When accounting for mortality, data was available for only 72% and 81% of intervention and control group respectively at T1. Some missing data is likely to be dependent on its true value, as the main reason for missing data was adverse events, and we consider participants who experience adverse events to be more likely to have a lower ADL score. | ||||||||||||
| The Katz ADL score is considered appropriate, and there were no differences in the measurement or ascertainment between groups. The assessors were not blinded, and it was therefore considered likely that knowledge of the intervention could influence the outcome, given the likely strong belief in the benefits of the intervention ward. | ||||||||||||
| A pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to be at high risk of bias due to missing outcome data and measurement of the outcome. | ||||||||||||
| Brown 2016 |  |  |  |  |  |  | ||||||
| No description of randomisation sequence generation, but the paper describes a block randomisation strategy and sequence concealed (sealed envelopes). The patient characteristics appear well‐balanced between groups. | ||||||||||||
| We assume both participants and those delivering the interventions were aware of intervention assignments. 6 participants did not receive allocated intervention, this is considered to be consistent with what could occur outside the trial context, and intention to treat analysis was used. | ||||||||||||
| No missing data. | ||||||||||||
| The Katz ADL score is considered appropriate, and there were no differences in the measurement or ascertainment between groups. The assessors were blinded. | ||||||||||||
| The study protocol details statistical analysis plan which is in accordance with results presented, and it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to be at low risk of bias in all domains for this outcome. | ||||||||||||
| Gazineo 2021 |  |  |  |  |  |  | ||||||
| Allocation sequence was random (generated using an online system) and the allocation sequence was concealed until participants were enrolled (opaque concealed and sealed envelopes). Participant characteristics were well‐balanced. | ||||||||||||
| Participants and those delivering the interventions were aware of intervention allocations. There was no evidence of deviations from the intended interventions and intention to treat analysis used. | ||||||||||||
| No missing data after accounting for mortality. | ||||||||||||
| The Barthel Index is considered appropriate, and there were no differences in the measurement or ascertainment between groups. The assessors were not blinded, and it was therefore considered likely that knowledge of the intervention could influence the outcome, given the likely strong belief in the benefits of the intervention ward. | ||||||||||||
| A pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to be at high risk of bias due to measurement of the outcome. | ||||||||||||
| Hu 2020 |  |  |  |  |  |  | ||||||
| Allocation sequence was random (computer generated random numbers) and sequence concealed (blinded project coordinator allocated participants). Participant characteristics were balanced. | ||||||||||||
| Participants and those delivering the interventions were aware of intervention allocations. There was no evidence of deviations from the intended interventions and as such it is presumed intention to treat analysis was used. | ||||||||||||
| At discharge data was available for 76% of reablement group, (78% of reminder group) and 80% of the control group. Only participants who had complete data (i.e. data collected at baseline, discharge and follow‐up) had outcomes reported. Although missing data is well‐balanced between all three groups, it is considered likely that reason for missing data is related to its true value. | ||||||||||||
| The Katz ADL score is considered appropriate, and there were no differences in the measurement or ascertainment between groups. The assessors were blinded. | ||||||||||||
| A pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to be at high risk of bias due to missing outcome data. | ||||||||||||
| Killey 2006 |  |  |  |  |  |  | ||||||
| Allocation not random and sequence predictable as based on alternation. There is limited data on participant characteristics, but available data suggests balanced characteristics between groups. | ||||||||||||
| Participants and those delivering the interventions were aware of intervention allocations. There was no evidence of deviations from the intended interventions. Per‐protocol analysis appears to have been used. 2 participants in the intervention group were excluded from analysis as they completed less than 70% of their walks. The 2 participants that were excluded accounted for 7% of the remaining sample, it is therefore considered that there was potential for a significant impact on the results. | ||||||||||||
| Only 71% of intervention and 74% of control group had outcome data. A significant proportion of missing data was related to early discharge from hospital. These participants could be expected to have a higher functional level than those who remained in hospital. However, the missing data was well‐balanced between the two groups. | ||||||||||||
| The Barthel Index is considered appropriate, and there were no differences in the measurement or ascertainment between groups. The assessors were not blinded, and it was therefore considered likely that knowledge of the intervention could influence the outcome, given the likely strong belief in the benefits of the intervention ward. | ||||||||||||
| A pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to be at high risk of bias in three domains for this outcome. | ||||||||||||
| Subgroup 1.1.3 Progressive resistance exercise | ||||||||||||
| de Morton 2007 |  |  |  |  |  |  | ||||||
| Allocation of wards was random (coin toss) and the allocating officer unaware of study. Baseline differences between groups were thought to be compatible with chance. | ||||||||||||
| Participants and clinicians delivering care were believed to be aware of treatment assignments. There was no evidence of deviations from intended interventions and intention to treat analysis was used. | ||||||||||||
| After accounting for mortality, discharge Barthel Index (BI) scores were available for 75% in intervention group and 70% in control group. Although it is feasible that following discharge patients with lower levels of functional ability are more likely to be missing, we do not think this applies in this situation as assessment prior to discharge. We do not see a situation where it would be more likely to miss an assessment due to high/low level of disability at discharge. | ||||||||||||
| The use of the BI to measure independence with ADLs is considered appropriate, and there were no differences in the measurement or ascertainment between groups. The assessors were not blinded, and it is considered likely that knowledge of the intervention could influence the outcome, given the likely strong belief in the benefits of the intervention. | ||||||||||||
| A pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to be at high risk of bias in measurement of the outcome. | ||||||||||||
| Jeffs 2013 |  |  |  |  |  |  | ||||||
| Sequence concealment was achieved via a member of the research team not involved in recruitment and using sealed envelopes. There was no information regarding method of generating random sequence, other than to say that there was randomisation, given details of allocation concealment, assumption made that appropriate method used. Patient characteristics between groups appear well‐balanced. | ||||||||||||
| Participants and those delivering the interventions were likely to be aware of intervention allocations. One participant was not allocated to a group due to an administrative error and 35 participants did not receive the intervention as planned (17 in the intervention group). This is thought to be consistent with what could occur outside of the trial context. Intention to treat analysis was used. | ||||||||||||
| No missing data. | ||||||||||||
| The Barthel Index is considered appropriate, and there were no differences in the measurement or ascertainment between groups. The assessors were blinded. | ||||||||||||
| A pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to raise some bias concerns in selection of the reported result. | ||||||||||||
| Jones 2006 |  |  |  |  |  |  | ||||||
| Allocation sequence was random (computer generated random numbers). Sequence allocation was not concealed, but performed by a member of staff independent of the enrolment procedures. ). Participant characteristics were balanced between groups and differences compatible with chance. | ||||||||||||
| It is assumed that both participants and clinicians delivering care were aware of assigned interventions. There is no evidence of deviations from intended interventions and intention to treat analysis used. | ||||||||||||
| Admission and discharge modified Barthel Index (mBI) scores were available for 78.8% of patients (126/160). Although we felt that patients with lower levels of functional ability were more likely to be missing at follow‐up, we do not think this applies in this situation as assessment was prior to discharge. We do not see a situation where it would be more likely to miss an assessment due to high/low level of disability at discharge | ||||||||||||
| The use of the mBI to measure independence with ADLs is considered appropriate, and there were no differences in the measurement or ascertainment between groups. The assessors were blinded. | ||||||||||||
| A pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to raise some concerns due to missing outcome data and in selection of the reported results. | ||||||||||||
| Martinez‐Velilla 2019 |  |  |  |  |  |  | ||||||
| Allocation sequence was random (www.randomizer.org) and personal communication with author confirms allocation concealment. Baseline differences between groups are thought to be compatible with chance. | ||||||||||||
| Both participants and clinicians delivering care were aware of assigned interventions. There is no evidence of deviations from intended interventions and intention to treat analysis used. | ||||||||||||
| Assuming that those that discontinued the study did not provide Barthel Index (BI) outcome data, and accounting for mortality, 80% of intervention group and 78% of control group had outcome data. Although there were participants who discontinued the study due to medical deterioration, which could have and is considered likely to have biased the results (i.e. medical deterioration being associated with poor functional outcomes), the numbers were relatively low (only 6% of the sample) and were well‐balanced between groups. We therefore feel that this was unlikely to bias the results. | ||||||||||||
| The use of the BI to measure independence with ADLs is considered appropriate, and there were no differences in the measurement or ascertainment between groups. The assessors were blinded. | ||||||||||||
| The trial protocol reflects the analyses as that were conducted, and it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to raise some concerns due to missing outcome data. | ||||||||||||
| Mudge 2008 |  |  |  |  |  |  | ||||||
| Pseudo‐randomisation, allocation was based on admitting unit and bed availability, and admitting unit was determined by a rotating roster. No evidence of baseline differences in patient characteristics other than those thought to be compatible with chance. | ||||||||||||
| Both participants and clinicians delivering care were aware of assigned interventions. There is no evidence of deviations from intended interventions and intention to treat analysis used. | ||||||||||||
| No missing data. | ||||||||||||
| The use of the modified Barthel Index to measure independence with ADLs is considered appropriate, and there were no differences in the measurement or ascertainment between groups. The assessors were blinded. | ||||||||||||
| A pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to be at high risk of bias due to the randomisation process. | ||||||||||||
| Ortiz‐Alonso 2020 |  |  |  |  |  |  | ||||||
| The allocation sequence was based on recruitment blocks of 4‐8 weeks. Activity of daily living (ADL) scores at baseline and admission were significantly lower in the intervention group than the control group, this is thought to be an important prognostic factor and therefore a concern regarding the randomisation process. | ||||||||||||
| Methods were designed to blind participants from group assignments however due to the nature of the intervention and lack of sham intervention the success of this blinding is felt unlikely. Ward staff and research staff were not blinded to the participant's assigned intervention. There were no deviations from the intended interventions due to trial context described and although intention to treat analysis not specified given the lack of deviations it is assumed. | ||||||||||||
| After adjusting for mortality 97% in intervention group and 97% in control group had outcome data. | ||||||||||||
| The Katz ADL score is considered appropriate, and there were no differences in the measurement or ascertainment between groups. The assessors were not blinded, and it was therefore considered likely that knowledge of the intervention could influence the outcome, given the likely strong belief in the benefits of the intervention ward. | ||||||||||||
| Analysis reflects plan in trial registration and study protocol, and results are not thought to be from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to be at high risk of bias due to the randomisation process and measurement of the outcome. | ||||||||||||
| Pedersen 2019 |  |  |  |  |  |  | ||||||
| Allocation sequence was random (computer‐generated block randomisation), and allocation sequence concealed from the investigators. Participant characteristics were well‐balanced between groups. | ||||||||||||
| Participants and clinicians were aware of the treatment assignments. There was no evidence of deviations from the assigned interventions and an intention to treat approach was used for analysis. | ||||||||||||
| When accounting for mortality only 93% of participants in intervention group and 67% in control group completed measures at discharge. The difference in the proportion of missing data, and that the assessments were carried out home after discharge, it was judged likely that the reasons for missing data may have depended on its true value. | ||||||||||||
| The Barthel Index is considered appropriate, and there were no differences in the measurement or ascertainment between groups. The assessors were blinded. | ||||||||||||
| The analysis is in accordance with a pre‐specified analysis in a published protocol, and results are not thought to be from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to be at high risk of bias due to missing outcome data. | ||||||||||||
| Bias | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study | Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | ||||||
| Subgroup 1.2.1 Rehabilitation‐related activities | ||||||||||||
| Counsell 2000 |  |  |  |  |  |  | ||||||
| Allocation sequence was random (computer generated random numbers) and sequence concealed (opaque sealed envelope). Participant characteristics were balanced. | ||||||||||||
| Participants and those delivering the interventions were aware of intervention assignments. Seventy‐nine participants were not admitted to the unit to which they were assigned. Deviations may have affected the effect estimate, however the were well‐balanced between groups. Intention to treat analysis was used. | ||||||||||||
| Functional data was obtained in 1476 of 1483 of surviving patients (99.5%) at discharge. | ||||||||||||
| The Physical Performance and Mobility Examination (PPME) is considered appropriate, and there were no differences in the measurement or ascertainment between groups. The assessors were not blinded, and it was therefore considered likely that knowledge of the intervention could influence the outcome, given the likely strong belief in the benefits of the intervention ward. | ||||||||||||
| A pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to be at high risk of bias due to measurement of the outcome. | ||||||||||||
| Subgroup 1.2.2 Structured exercise | ||||||||||||
| Gazineo 2021 |  |  |  |  |  |  | ||||||
| Allocation sequence was random (generated using an online system) and the allocation sequence was concealed until participants were enrolled (opaque concealed and sealed envelopes). Participant characteristics were well‐balanced. | ||||||||||||
| Participants and those delivering the interventions were aware of intervention allocations. There was no evidence of deviations from the intended interventions and intention to treat analysis used. | ||||||||||||
| No missing data after accounting for mortality. | ||||||||||||
| The Barden Activity Subscale to measure functional mobility is considered appropriate, and there were no differences in the measurement or ascertainment between groups. The assessors were not blinded, and it was therefore considered likely that knowledge of the intervention could influence the outcome, given the likely strong belief in the benefits of the intervention ward. | ||||||||||||
| A pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to be at high risk of bias due to measurement of the outcome. | ||||||||||||
| McGowan 2018a |  |  |  |  |  |  | ||||||
| Allocation sequence was random (www.randomization.com) and the allocation sequence was only known by the chief investigator who was not involved with screening patients. Baseline differences between groups are thought to be compatible with chance. | ||||||||||||
| Both participants and clinicians delivering care were aware of assigned interventions. There is no evidence of deviations from intended interventions and intention to treat analysis not explicitly stated, but all participants appear to have been analysed in the group to which they were randomised. | ||||||||||||
| 96% of participants had complete data sets. | ||||||||||||
| The use of the Elderly Mobility Scale (EMS) to measure functional mobility is considered appropriate, and there were no differences in the measurement or ascertainment between groups. The assessors were not blinded, and it is considered likely that knowledge of the intervention could influence the outcome, given the likely strong belief in the benefits of the intervention. | ||||||||||||
| The trial protocol and registration reflects the analyses as that were conducted, and it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to raise some concerns due to the measurement of the outcome. | ||||||||||||
| Subgroup 1.2.3 Progressive resistance exercise | ||||||||||||
| de Morton 2007 |  |  |  |  |  |  | ||||||
| Allocation of wards was random (coin toss) and the allocating officer unaware of study. Baseline differences between groups were thought to be compatible with chance. | ||||||||||||
| Participants and clinicians delivering care were believed to be aware of treatment assignments. There was no evidence of deviations from intended interventions and intention to treat analysis was used. | ||||||||||||
| After accounting for mortality, discharge Functional Ambulation Classification (FAC) scores were available for 75% in intervention group and 70% in control group. Although it is feasible that following discharge patients with lower levels of functional ability are more likely to be missing, we do not think this applies in this situation as assessment prior to discharge. We do not see a situation where it would be more likely to miss an assessment due to high/low level of disability at discharge. | ||||||||||||
| The use of the FAC to measure functional mobility is considered appropriate, and there were no differences in the measurement or ascertainment between groups. The assessors were not blinded, and it is considered likely that knowledge of the intervention could influence the outcome, given the likely strong belief in the benefits of the intervention. | ||||||||||||
| A pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to be at high risk of bias in measurement of the outcome. | ||||||||||||
| Martinez‐Velilla 2019 |  |  |  |  |  |  | ||||||
| Allocation sequence was random (www.randomizer.org) and personal communication with author confirms allocation concealment. Baseline differences between groups are thought to be compatible with chance. | ||||||||||||
| Both participants and clinicians delivering care were aware of assigned interventions. There is no evidence of deviations from intended interventions and intention to treat analysis used. | ||||||||||||
| Assuming that those that discontinued the study did not provide Short Physical Performance Battery (SPPB) outcome data, and accounting for mortality, 83% of intervention group and 86% of control group had outcome data. Although there were participants who discontinued the study due to medical deterioration, which could have and is considered likely to have biased the results (i.e. medical deterioration being associated with poor functional outcomes), the numbers were relatively low (only 6% of the sample) and were well‐balanced between groups. We therefore feel that this was unlikely to bias the results. | ||||||||||||
| The use of the SPPB to measure functional mobility is considered appropriate, and there were no differences in the measurement or ascertainment between groups. The assessors were blinded. | ||||||||||||
| The trial protocol reflects the analyses as that were conducted, and it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to raise some concerns due to missing outcome data. | ||||||||||||
| McCullagh 2020 |  |  |  |  |  |  | ||||||
| Allocation sequence was random (computer‐generated) and allocation sequence was concealed. Baseline differences are thought to be compatible with chance. | ||||||||||||
| Both participants and clinicians delivering care were aware of assigned interventions. There is no evidence of deviations from intended interventions and intention to treat analysis used. | ||||||||||||
| 90% of intervention group and 94% of the control group had Short Physical Performance Battery (SPPB) data. Given the nature of this population (older adults during and after acute hospitalisation) we considered a threshold of 90% of participants with data as sufficient. | ||||||||||||
| The use of SPPB to measure functional mobility is considered appropriate, and there were no differences in the measurement or ascertainment between groups. The assessors were blinded. | ||||||||||||
| The trial protocol reflects the analyses as that were conducted, and it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to be at low risk of bias for all domains for this outcome. | ||||||||||||
| Ortiz‐Alonso 2020 |  |  |  |  |  |  | ||||||
| The allocation sequence was based on recruitment blocks of 4‐8 weeks. Activity of daily living (ADL) scores at baseline and admission were significantly lower in the intervention group than the control group, this is thought to be an important prognostic factor and therefore a concern regarding the randomisation process. | ||||||||||||
| Methods were designed to blind participants from group assignments however due to the nature of the intervention and lack of sham intervention the success of this blinding is felt unlikely. Ward staff and research staff were not blinded to the participant's assigned intervention. There were no deviations from the intended interventions due to trial context described and although intention to treat analysis not specified given the lack of deviations it is assumed. | ||||||||||||
| After adjusting for mortality 97% in intervention group and 97% in control group had outcome data. | ||||||||||||
| The use of the Short Physical Performance Battery (SPPB) for measuring functional mobility is considered appropriate, and there were no differences in the measurement or ascertainment between groups. The assessors were not blinded, and it was therefore considered likely that knowledge of the intervention could influence the outcome, given the likely strong belief in the benefits of the intervention ward. | ||||||||||||
| Analysis reflects plan in trial registration and study protocol, and results are not thought to be from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to be at high risk of bias due to the randomisation process and measurement of the outcome. | ||||||||||||
| Pedersen 2019 |  |  |  |  |  |  | ||||||
| Allocation sequence was random (computer‐generated block randomisation), and allocation sequence concealed from the investigators. Participant characteristics were well‐balanced between groups. | ||||||||||||
| Participants and clinicians were aware of the treatment assignments. There was no evidence of deviations from the assigned interventions and an intention to treat approach was used for analysis. | ||||||||||||
| When accounting for mortality only 93% of participants in intervention group and 67% in control group completed measures at discharge. The difference in the proportion of missing data, and that the assessments were carried out home after discharge, it was judged likely that the reasons for missing data may have depended on its true value. | ||||||||||||
| The use of the de Morton Mobility Index (DEMMI) is considered appropriate, and there were no differences in the measurement or ascertainment between groups. The assessors were blinded. | ||||||||||||
| The analysis is in accordance with a pre‐specified analysis in a published protocol, and results are not thought to be from multiple outcome measures or multiple analyses. | ||||||||||||
| As per RoB2 algorithm, the study is judged to be at high risk of bias due to missing outcome data. | ||||||||||||
| Bias | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study | Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | ||||||
| Subgroup 1.3.1 Rehabilitation‐related activities | ||||||||||||
| Abizanda 2011 |  |  |  |  |  |  | ||||||
| Allocation sequence was random (computer generated random numbers). Allocation occurred after enrolment by an investigator not involved in the participants' clinical management. Higher number of participants admitted with a stroke in the intervention group (39 vs. 16), in addition, the 'Others' subgroup the Barthel index is higher in the control group than the intervention group (29.1 vs. 23.1). This is thought to be compatible with chance. | ||||||||||||
| Its assumed participants and the Occupational Therapist (OT) delivering the intervention were aware of the assigned intervention allocation. All participants received their allocated intervention. Therefore, even though intention to treat analysis not specified, analysis deemed appropriate. | ||||||||||||
| Given the nature of this population (older adults during and after acute hospitalisation) we considered a threshold of 90% of participants with data as sufficient. We felt this would be consistent with measurements outside of a trial context. When accounting for mortality, 93% and 91% assessed at discharge in intervention and control arms respectively. | ||||||||||||
| The use of the Confusion Assessment Method (CAM) to assess for delirium is considered appropriate, and there were no differences in measurement or ascertainment of the outcomes between groups. The OT assessor was blinded. | ||||||||||||
| The data analyst was blinded, and followed a pre‐specified statistical plan. It is not thought that results were from multiple outcome measurements or multiple analyses. | ||||||||||||
| The study is judged to be at low risk of bias in all domains for this outcome. | ||||||||||||
| Asplund 2000 |  |  |  |  |  |  | ||||||
| The method of allocation sequence is not described, but the use of sealed envelopes suggests a random component was used. Participant characteristics were balanced. | ||||||||||||
| Participants and those delivering the interventions were aware of intervention assignments. Twenty‐five patients were excluded due to them not meeting the set eligibility criteria, however, these: protocol violations are not expected to influence the effect estimate of the outcome as per protocol analyses used. The per protocol analyses was not thought to have a substantial impact on the result given the main reason for exclusion was inappropriate recruitment. Excluding this 25, the other 6 exclusions represent approximately 1% of the total sample size. | ||||||||||||
| Data at discharge from hospital was available for 98% of participants. | ||||||||||||
| The confusion assessment method (CAM) instrument was considered appropriate, and there were no differences in the measurement or ascertainment between groups. The assessors were not blinded, and it was therefore considered likely that knowledge of the intervention could influence the outcome, given the likely strong belief in the benefits of the intervention ward. | ||||||||||||
| A pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to be at high risk of bias due to measurement of the outcome. | ||||||||||||
| Subgroup 1.3.2 Structured exercise | ||||||||||||
| Brown 2016 |  |  |  |  |  |  | ||||||
| No description of randomisation sequence generation, but the paper describes a block randomisation strategy and sequence concealed (sealed envelopes). The patient characteristics appear well‐balanced between groups. | ||||||||||||
| We assume both participants and those delivering the interventions were aware of intervention assignments. 6 participants did not receive allocated intervention, this is considered to be consistent with what could occur outside the trial context, and intention to treat analysis was used. | ||||||||||||
| No missing data. | ||||||||||||
| The Confusion Assessment Method (CAM) score is considered appropriate, and there were no differences in the measurement or ascertainment between groups. The assessors were blinded at admission, discharge and follow up, but not the research assistants as they were delivering the intervention. “The research assistants also used the CAM at each patient visit throughout the hospital stay to ensure that patients in either group did not develop incident delirium.” Therefore, assessors not considered to be blinded, and it is likely given that there is judgement involved in scoring of the CAM, that knowledge of the intervention could influence the scoring of the CAM given the likely strong beliefs in the benefits of the intervention ward. | ||||||||||||
| The study protocol details statistical analysis plan which did not refer to assessment of delirium after baseline assessments, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to be at high risk of bias in measurement of the outcome. | ||||||||||||
| Subgroup 1.3.3 Progressive resistance exercise | ||||||||||||
| Jeffs 2013 |  |  |  |  |  |  | ||||||
| Sequence concealment was achieved via a member of the research team not involved in recruitment and using sealed envelopes. There was no information regarding method of generating random sequence, other than to say that there was randomisation, given details of allocation concealment, assumption made that appropriate method used. Patient characteristics between groups appear well‐balanced. | ||||||||||||
| Participants and those delivering the interventions were likely to be aware of intervention allocations. One participant was not allocated to a group due to an administrative error and 35 participants did not receive the intervention as planned (17 in the intervention group). This is thought to be consistent with what could occur outside of the trial context. Intention to treat analysis was used. | ||||||||||||
| No missing data. | ||||||||||||
| The Confusion Assessment Method (CAM) is considered appropriate, and there were no differences in the measurement or ascertainment between groups. The assessors were blinded. | ||||||||||||
| A pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to raise some concerns in the selection of the reported results. | ||||||||||||
| Martinez‐Velilla 2019 |  |  |  |  |  |  | ||||||
| Allocation sequence was random (www.randomizer.org) and personal communication with author confirms allocation concealment. Baseline differences between groups are thought to be compatible with chance. | ||||||||||||
| Both participants and clinicians delivering care were aware of assigned interventions. There is no evidence of deviations from intended interventions and intention to treat analysis used. | ||||||||||||
| Assuming that those that discontinued the study did not provide Barthel Index (BI) outcome data, and accounting for mortality, 84% of intervention group and 86% of control group had outcome data. Although there were participants who discontinued the study due to medical deterioration, which could have and is considered likely to have biased the results (i.e. medical deterioration being associated with poor functional outcomes), the numbers were relatively low (only 6% of the sample) and were well‐balanced between groups. We therefore feel that this was unlikely to bias the results. | ||||||||||||
| The use of the Confusion Assessment Method (CAM) to measure delirium is considered appropriate, and there were no differences in the measurement or ascertainment between groups. The assessors were blinded. | ||||||||||||
| The trial protocol reflects the analyses as that were conducted, and it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to raise some concerns due to missing outcome data. | ||||||||||||
| McCullagh 2020 |  |  |  |  |  |  | ||||||
| Allocation sequence was random (computer‐generated) and allocation sequence was concealed. Baseline differences are thought to be compatible with chance. | ||||||||||||
| Both participants and clinicians delivering care were aware of assigned interventions. There is no evidence of deviations from intended interventions and intention to treat analysis used. | ||||||||||||
| 90% of intervention group and 94% of the control group had outcome data. Given the nature of this population (older adults during and after acute hospitalisation) we considered a threshold of 90% of participants with data as sufficient. | ||||||||||||
| The use of the Six‐item Cognitive Impairment Test (6CIT) to assess for delirium is considered appropriate, and there were no differences in the measurement or ascertainment between groups. The assessors were blinded. | ||||||||||||
| The trial protocol reflects the analyses as that were conducted, and it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to be at low risk of bias in all domains for this outcome. | ||||||||||||
| Mudge 2008 |  |  |  |  |  |  | ||||||
| Pseudo‐randomisation, allocation was based on admitting unit and bed availability, and admitting unit was determined by a rotating roster. No evidence of baseline differences in patient characteristics other than those thought to be compatible with chance. | ||||||||||||
| Both participants and clinicians delivering care were aware of assigned interventions. There is no evidence of deviations from intended interventions and intention to treat analysis used. | ||||||||||||
| No missing data. | ||||||||||||
| Identifying delirium according to chart review using validated methodology is considered appropriate, and there were no differences in the measurement or ascertainment between groups. The assessors were blinded. | ||||||||||||
| A pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to be at high risk of bias due to the randomisation process. | ||||||||||||
| Bias | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study | Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | ||||||
| Subgroup 1.4.1 Rehabilitation‐related activities | ||||||||||||
| Ekerstad 2017 |  |  |  |  |  |  | ||||||
| Randomisation was based on the availability of beds, and there was no allocation concealment as participants were allocated a ward prior to enrolment. Participant characteristics appeared balanced. | ||||||||||||
| Participants and clinicians delivering care were aware of treatment assignments. There was no evidence of deviations from intended interventions and intention to treat analysis was used. | ||||||||||||
| No missing data. | ||||||||||||
| The EQ‐VAS is considered appropriate, and there were no differences in the measurement or ascertainment between groups. The assessors were not blinded, and it was therefore considered likely that knowledge of the intervention could influence the outcome, given the likely strong belief in the benefits of the intervention ward. | ||||||||||||
| A detailed pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to be at high risk of bias due to bias in the measurement of the outcome and bias arising from the randomisation process. | ||||||||||||
| Subgroup 1.4.2 Structured exercise | ||||||||||||
| Hu 2020 |  |  |  |  |  |  | ||||||
| Allocation sequence was random (computer generated random numbers) and sequence concealed (blinded project coordinator allocated participants). Participant characteristics were balanced. | ||||||||||||
| Participants and those delivering the interventions were aware of intervention allocations. There was no evidence of deviations from the intended interventions and as such it is presumed intention to treat analysis was used. | ||||||||||||
| At discharge data was available for 76% of reablement group, (78% of reminder group) and 80% of the control group. Only participants who had complete data (i.e. data collected at baseline, discharge and follow‐up) had outcomes reported. Although missing data is well‐balanced between all three groups, it is considered likely that reason for missing data is related to its true value. | ||||||||||||
| The EQ5D is considered appropriate, and there were no differences in the measurement or ascertainment between groups. The assessors were blinded. | ||||||||||||
| A pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to be at high risk of bias due to missing outcome data. | ||||||||||||
| Subgroup 1.4.3 Progressive resistance exercise | ||||||||||||
| Martinez‐Velilla 2019 |  |  |  |  |  |  | ||||||
| Allocation sequence was random (www.randomizer.org) and personal communication with author confirms allocation concealment. Baseline differences between groups are thought to be compatible with chance. | ||||||||||||
| Both participants and clinicians delivering care were aware of assigned interventions. There is no evidence of deviations from intended interventions and intention to treat analysis used. | ||||||||||||
| Assuming that those that discontinued the study did not provide e EQ‐5D outcome data, and accounting for mortality, 83% of intervention group and 86% of control group had outcome data. Although there were participants who discontinued the study due to medical deterioration, which could have and is considered likely to have biased the results (i.e. medical deterioration being associated with poor functional outcomes), the numbers were relatively low (only 6% of the sample) and were well‐balanced between groups. We therefore feel that this was unlikely to bias the results. | ||||||||||||
| The EQ‐5D is considered appropriate, and there were no differences in the measurement or ascertainment between groups. The assessors were blinded. | ||||||||||||
| The trial protocol reflects the analyses as that were conducted, and it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to raise some concerns due to missing outcome data. | ||||||||||||
| McCullagh 2020 |  |  |  |  |  |  | ||||||
| Allocation sequence was random (computer‐generated) and allocation sequence was concealed. Baseline differences are thought to be compatible with chance. | ||||||||||||
| Both participants and clinicians delivering care were aware of assigned interventions. There is no evidence of deviations from intended interventions and intention to treat analysis used. | ||||||||||||
| 90% of intervention group and 94% of the control group had outcome data. Given the nature of this population (older adults during and after acute hospitalisation) we considered a threshold of 90% of participants with data as sufficient. | ||||||||||||
| The use of EQ‐5D5L to measure quality of life is considered appropriate, and there were no differences in the measurement or ascertainment between groups. The assessors were blinded. | ||||||||||||
| The trial protocol reflects the analyses as that were conducted, and it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to be at low risk of bias for all domains for this outcome. | ||||||||||||
| Bias | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study | Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | ||||||
| Subgroup 1.5.1 Rehabilitation‐related activities | ||||||||||||
| Sahota 2017 |  |  |  |  |  |  | ||||||
| No information on randomisation methods other than to say participants were randomised, however, patient characteristics appear well‐balanced between groups and differences compatible with chance. | ||||||||||||
| It is assumed that participants and those delivering the interventions were aware of intervention assignments. There were 15 protocol deviations in the CIRACT group and 8 in the THB‐Rehab group. The deviations were thought to be in keeping with what would be expected outside of a trial context. Intention to treat not explicitly stated though it appears that the 1 participant who did not receive the intended intervention was analysed within the group to which they were assigned. | ||||||||||||
| No missing data | ||||||||||||
| Methods of measuring the number of falls was considered appropriate and there were no differences in measurement or ascertainment of the outcomes between groups. The assessor was not blinded, but it is thought that due to the lack of judgement in the measurement, that the knowledge of the intervention did not influence the outcomes. | ||||||||||||
| A pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to raise some concerns in the selection of the reported results and the randomisation process. | ||||||||||||
| Subgroup 1.5.2 Structured exercise | ||||||||||||
| Brown 2016 |  |  |  |  |  |  | ||||||
| No description of randomisation sequence generation, but the paper describes a block randomisation strategy and sequence concealed (sealed envelopes). The patient characteristics appear well‐balanced between groups. | ||||||||||||
| We assume both participants and those delivering the interventions were aware of intervention assignments. 6 participants did not receive allocated intervention, this is considered to be consistent with what could occur outside the trial context, and intention to treat analysis was used. | ||||||||||||
| No missing data. | ||||||||||||
| Methods of measuring the number of falls was considered appropriate and there were no differences in measurement or ascertainment of the outcomes between groups. The assessor was blinded. | ||||||||||||
| The study protocol details statistical analysis plan which is in accordance with results presented, and it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to be at low risk of bias in all domains for this outcome. | ||||||||||||
| Gazineo 2021 |  |  |  |  |  |  | ||||||
| Allocation sequence was random (generated using an online system) and the allocation sequence was concealed until participants were enrolled (opaque concealed and sealed envelopes). Participant characteristics were well‐balanced. | ||||||||||||
| Participants and those delivering the interventions were aware of intervention allocations. There was no evidence of deviations from the intended interventions and intention to treat analysis used. | ||||||||||||
| No missing data after accounting for mortality. | ||||||||||||
| Methods of measuring the number of falls was considered appropriate and there were no differences in measurement or ascertainment of the outcomes between groups. The assessor was not blinded, but it is thought that due to the lack of judgement in the measurement, that the knowledge of the intervention did not influence the outcomes. | ||||||||||||
| A pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to raise some concerns in the selection of the reported results . | ||||||||||||
| Killey 2006 |  |  |  |  |  |  | ||||||
| Allocation not random and sequence predictable as based on alternation. There is limited data on participant characteristics, but available data suggests balanced characteristics between groups. | ||||||||||||
| Participants and those delivering the interventions were aware of intervention allocations. There was no evidence of deviations from the intended interventions. Per‐protocol analysis appears to have been used. 2 participants in the intervention group were excluded from analysis as they completed less than 70% of their walks. The 2 participants that were excluded accounted for 7% of the remaining sample, it is therefore considered that there was potential for a significant impact on the results. | ||||||||||||
| Only 71% of intervention and 74% of control group had outcome data. A significant proportion of missing data was related to early discharge from hospital. These participants could be expected to have a higher functional level than those who remained in hospital. However, the missing data was well‐balanced between the two groups. | ||||||||||||
| Methods of measuring the number of falls was considered appropriate and there were no differences in measurement or ascertainment of the outcomes between groups. The assessor was not blinded, but it is thought that due to the lack of judgement in the measurement, that the knowledge of the intervention did not influence the outcomes. | ||||||||||||
| A pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to be at high risk of bias arising from the randomisation process and due to deviations from the intended interventions. | ||||||||||||
| Subgroup 1.5.3 Progressive resistance exercise | ||||||||||||
| de Morton 2007 |  |  |  |  |  |  | ||||||
| Allocation of wards was random (coin toss) and the allocating officer unaware of study. Baseline differences between groups were thought to be compatible with chance. | ||||||||||||
| Participants and clinicians delivering care were believed to be aware of treatment assignments. There was no evidence of deviations from intended interventions and intention to treat analysis was used. | ||||||||||||
| No missing data. | ||||||||||||
| Methods of measuring the number of falls was considered appropriate and there were no differences in measurement or ascertainment of the outcomes between groups. The assessor was not blinded, but it is thought that due to the lack of judgement in the measurement, that the knowledge of the intervention did not influence the outcomes. | ||||||||||||
| A pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to raise some concerns in the selection of the reported result. | ||||||||||||
| Jones 2006 |  |  |  |  |  |  | ||||||
| Allocation sequence was random (computer generated random numbers). Sequence allocation was not concealed, but performed by a member of staff independent of the enrolment procedures. ). Participant characteristics were balanced between groups and differences compatible with chance. | ||||||||||||
| It is assumed that both participants and clinicians delivering care were aware of assigned interventions. There is no evidence of deviations from intended interventions and intention to treat analysis used. | ||||||||||||
| After accounting for mortality, falls data available for 99% of control group and 93% of intervention group. Given the nature of this population (older adults during and after acute hospitalisation) we considered a threshold of 90% of participants with data as sufficient. | ||||||||||||
| Methods of measuring the number of falls was considered appropriate and there were no differences in measurement or ascertainment of the outcomes between groups. The assessor was blinded. | ||||||||||||
| A pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to raise some concerns due to the selection of the reported results. | ||||||||||||
| Martinez‐Velilla 2019 |  |  |  |  |  |  | ||||||
| Allocation sequence was random (www.randomizer.org) and personal communication with author confirms allocation concealment. Baseline differences between groups are thought to be compatible with chance. | ||||||||||||
| Both participants and clinicians delivering care were aware of assigned interventions. There is no evidence of deviations from intended interventions and intention to treat analysis used. | ||||||||||||
| No missing data | ||||||||||||
| Methods of measuring the number of falls was considered appropriate and there were no differences in measurement or ascertainment of the outcomes between groups. The assessor was blinded. | ||||||||||||
| The trial protocol reflects the analyses as that were conducted, and it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to be at low risk of bias in all domains for this outcome. | ||||||||||||
| McCullagh 2020 |  |  |  |  |  |  | ||||||
| Allocation sequence was random (computer‐generated) and allocation sequence was concealed. Baseline differences are thought to be compatible with chance. | ||||||||||||
| Both participants and clinicians delivering care were aware of assigned interventions. There is no evidence of deviations from intended interventions and intention to treat analysis used. | ||||||||||||
| No missing data. | ||||||||||||
| Methods of measuring the number of falls was considered appropriate and there were no differences in measurement or ascertainment of the outcomes between groups. The assessor was blinded. | ||||||||||||
| The trial protocol reflects the analyses as that were conducted, and it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| As per RoB2 algorithm, the study is judged to be at low risk of bias for all domains for this outcome. | ||||||||||||
| Mudge 2008 |  |  |  |  |  |  | ||||||
| Pseudo‐randomisation, allocation was based on admitting unit and bed availability, and admitting unit was determined by a rotating roster. No evidence of baseline differences in patient characteristics other than those thought to be compatible with chance. | ||||||||||||
| Both participants and clinicians delivering care were aware of assigned interventions. There is no evidence of deviations from intended interventions and intention to treat analysis used. | ||||||||||||
| No missing data. | ||||||||||||
| Methods of measuring the number of falls was considered appropriate and there were no differences in measurement or ascertainment of the outcomes between groups. The assessor was blinded. | ||||||||||||
| A pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to be at high risk of bias due to the randomisation process. | ||||||||||||
| Bias | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study | Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | ||||||
| Subgroup 1.6.1 Rehabilitation‐related activities | ||||||||||||
| Abizanda 2011 |  |  |  |  |  |  | ||||||
| Allocation sequence was random (computer generated random numbers). Allocation occurred after enrolment by an investigator not involved in the participants' clinical management. Higher number of participants admitted with a stroke in the intervention group (39 vs. 16), in addition, the 'Others' subgroup the Barthel index is higher in the control group than the intervention group (29.1 vs. 23.1). This is thought to be compatible with chance. | ||||||||||||
| Its assumed participants and the Occupational Therapist (OT) delivering the intervention were aware of the assigned intervention allocation. All participants received their allocated intervention. Therefore, even though intention to treat analysis not specified, analysis deemed appropriate. | ||||||||||||
| Given the nature of this population (older adults during and after acute hospitalisation) we considered a threshold of 90% of participants with data as sufficient. We felt this would be consistent with measurements outside of a trial context. When accounting for mortality, 93% and 91% assessed at discharge for confusion in intervention and control arms respectively. | ||||||||||||
| The use of the Confusion Assessment Method (CAM) to assess for delirium is considered appropriate, and there were no differences in measurement or ascertainment of the outcomes between groups. The OT assessor was blinded. | ||||||||||||
| The data analyst was blinded, and followed a pre‐specified statistical plan. It is not thought that results were from multiple outcome measurements or multiple analyses. | ||||||||||||
| The study is judged to be at low risk of bias in all domains for this outcome. | ||||||||||||
| Asplund 2000 |  |  |  |  |  |  | ||||||
| The method of allocation sequence is not described, but the use of sealed envelopes suggests a random component was used. Participant characteristics were balanced. | ||||||||||||
| Participants and those delivering the interventions were aware of intervention assignments. Twenty‐five patients were excluded due to them not meeting the set eligibility criteria, however, these: protocol violations are not expected to influence the effect estimate of the outcome as per protocol analyses used. The per protocol analyses was not thought to have a substantial impact on the result given the main reason for exclusion was inappropriate recruitment. Excluding this 25, the other 6 exclusions represent approximately 1% of the total sample size. | ||||||||||||
| Data at discharge from hospital was available for 98% of participants. | ||||||||||||
| The confusion assessment method (CAM) instrument was considered appropriate, and there were no differences in the measurement or ascertainment between groups. The assessors were not blinded, and it was therefore considered likely that knowledge of the intervention could influence the outcome, given the likely strong belief in the benefits of the intervention ward. | ||||||||||||
| A pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to be at high risk of bias due to measurement of the outcome. | ||||||||||||
| Subgroup 1.6.2 Structured exercise | ||||||||||||
| Brown 2016 |  |  |  |  |  |  | ||||||
| No description of randomisation sequence generation, but the paper describes a block randomisation strategy and sequence concealed (sealed envelopes). The patient characteristics appear well‐balanced between groups. | ||||||||||||
| We assume both participants and those delivering the interventions were aware of intervention assignments. Six participants did not receive allocated intervention, this is considered to be consistent with what could occur outside the trial context, and intention to treat analysis was used. | ||||||||||||
| No missing data. | ||||||||||||
| Measures used to measure medical deterioration are considered appropriate and there and there were no differences in the measurement or ascertainment between groups. The assessors were blinded. | ||||||||||||
| The study protocol details statistical analysis plan which is in line with the analyses conducted, and it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to be at low risk of bias in all domains for this outcome. | ||||||||||||
| Hu 2020 |  |  |  |  |  |  | ||||||
| Allocation sequence was random (computer generated random numbers) and sequence concealed (blinded project coordinator allocated participants). Participant characteristics were balanced. | ||||||||||||
| Participants and those delivering the interventions were aware of intervention allocations. There was no evidence of deviations from the intended interventions and as such it is presumed intention to treat analysis was used. | ||||||||||||
| No missing data for medical deterioration during hospitalisation. | ||||||||||||
| The measures are considered appropriate, and there were no differences in the measurement or ascertainment between groups. The assessors were blinded. | ||||||||||||
| A pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to have some concerns in the selection of the reported result. | ||||||||||||
| Subgroup 1.6.3 Progressive resistance exercise | ||||||||||||
| de Morton 2007 |  |  |  |  |  |  | ||||||
| Allocation of wards was random (coin toss) and the allocating officer unaware of study. Baseline differences between groups were thought to be compatible with chance. | ||||||||||||
| Participants and clinicians delivering care were believed to be aware of treatment assignments. There was no evidence of deviations from intended interventions and intention to treat analysis was used. | ||||||||||||
| No missing data. | ||||||||||||
| The measures are considered appropriate, and there were no differences in the measurement or ascertainment between groups. The assessors were not blinded, but it is thought that due to the lack of judgement in the outcome, that the knowledge of the intervention did not influence the outcomes. | ||||||||||||
| A pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to raise some concerns in the selection of the reported result. | ||||||||||||
| Jeffs 2013 |  |  |  |  |  |  | ||||||
| Sequence concealment was achieved via a member of the research team not involved in recruitment and using sealed envelopes. There was no information regarding method of generating random sequence, other than to say that there was randomisation, given details of allocation concealment, assumption made that appropriate method used. Patient characteristics between groups appear well‐balanced. | ||||||||||||
| Participants and those delivering the interventions were likely to be aware of intervention allocations. One participant was not allocated to a group due to an administrative error and 35 participants did not receive the intervention as planned (17 in the intervention group). This is thought to be consistent with what could occur outside of the trial context. Intention to treat analysis was used. | ||||||||||||
| No missing data. | ||||||||||||
| The Confusion Assessment Method (CAM) is considered appropriate, and there were no differences in the measurement or ascertainment between groups. The assessors were blinded. | ||||||||||||
| A pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to raise some concerns in the selection of the reported results. | ||||||||||||
| Jones 2006 |  |  |  |  |  |  | ||||||
| Allocation sequence was random (computer generated random numbers). Sequence allocation was not concealed, but performed by a member of staff independent of the enrolment procedures. ). Participant characteristics were balanced between groups and differences compatible with chance. | ||||||||||||
| It is assumed that both participants and clinicians delivering care were aware of assigned interventions. There is no evidence of deviations from intended interventions and intention to treat analysis used. | ||||||||||||
| No missing data. | ||||||||||||
| Measurement methods are considered appropriate, and there were no differences in the measurement or ascertainment between groups. The assessors were blinded. | ||||||||||||
| A pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to raise some concerns in the selection of the reported results. | ||||||||||||
| Martinez‐Velilla 2019 |  |  |  |  |  |  | ||||||
| Allocation sequence was random (www.randomizer.org) and personal communication with author confirms allocation concealment. Baseline differences between groups are thought to be compatible with chance. | ||||||||||||
| Both participants and clinicians delivering care were aware of assigned interventions. There is no evidence of deviations from intended interventions and intention to treat analysis used. | ||||||||||||
| The methods of measurement are considered appropriate, and there were no differences in the measurement or ascertainment between groups. The assessors were blinded. | ||||||||||||
| The trial protocol reflects the analyses as that were conducted, and it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to be at low risk of bias in all domains for this outcome. | ||||||||||||
| The study is judged to be at low risk of bias in all domains for this outcome. | ||||||||||||
| McCullagh 2020 |  |  |  |  |  |  | ||||||
| Allocation sequence was random (computer‐generated) and allocation sequence was concealed. Baseline differences are thought to be compatible with chance. | ||||||||||||
| Both participants and clinicians delivering care were aware of assigned interventions. There is no evidence of deviations from intended interventions and intention to treat analysis used. | ||||||||||||
| 90% of intervention group and 94% of the control group had outcome data. Given the nature of this population (older adults during and after acute hospitalisation) we considered a threshold of 90% of participants with data as sufficient. | ||||||||||||
| The use of the Six‐item Cognitive Impairment Test (6CIT) to assess for delirium is considered appropriate, and there were no differences in the measurement or ascertainment between groups. The assessors were blinded. | ||||||||||||
| The trial protocol reflects the analyses as that were conducted, and it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to be at low risk of bias in all domains for this outcome. | ||||||||||||
| Mudge 2008 |  |  |  |  |  |  | ||||||
| Pseudo‐randomisation, allocation was based on admitting unit and bed availability, and admitting unit was determined by a rotating roster. No evidence of baseline differences in patient characteristics other than those thought to be compatible with chance. | ||||||||||||
| Both participants and clinicians delivering care were aware of assigned interventions. There is no evidence of deviations from intended interventions and intention to treat analysis used. | ||||||||||||
| No missing data. | ||||||||||||
| Identifying delirium according to chart review using validated methodology is considered appropriate, and there were no differences in the measurement or ascertainment between groups. The assessors were blinded. | ||||||||||||
| A pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to be at high risk of bias due to the randomisation process. | ||||||||||||
| Pedersen 2019 |  |  |  |  |  |  | ||||||
| Allocation sequence was random (computer‐generated block randomisation), and allocation sequence concealed from the investigators. Participant characteristics were well‐balanced between groups. | ||||||||||||
| Participants and clinicians were aware of the treatment assignments. There was no evidence of deviations from the assigned interventions and an intention to treat approach was used for analysis. | ||||||||||||
| No missing data. | ||||||||||||
| The measure is considered appropriate, and there were no differences in the measurement or ascertainment between groups. The assessors were blinded. | ||||||||||||
| The analysis is in accordance with a pre‐specified analysis in a published protocol, and results are not thought to be from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to be at low risk of bias in all domains for this outcome. | ||||||||||||
| Bias | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study | Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | ||||||
| Subgroup 2.2.1 Rehabilitation‐related activities | ||||||||||||
| Abizanda 2011 |  |  |  |  |  |  | ||||||
| Allocation sequence was random (computer generated random numbers). Allocation occurred after enrolment by an investigator not involved in the participants' clinical management. Higher number of participants admitted with a stroke in the intervention group (39 vs. 16), in addition, the 'Others' subgroup the Barthel index is higher in the control group than the intervention group (29.1 vs. 23.1). This is thought to be compatible with chance. | ||||||||||||
| Its assumed participants and the Occupational Therapist (OT) delivering the intervention were aware of the assigned intervention allocation. All participants received their allocated intervention. Therefore, even though intention to treat analysis not specified, analysis deemed appropriate. | ||||||||||||
| No missing data. | ||||||||||||
| The method is considered appropriates, and there were no differences in measurement or ascertainment of the outcomes between groups. The OT assessor was blinded. | ||||||||||||
| The data analyst was blinded, and followed a pre‐specified statistical plan. It is not thought that results were from multiple outcome measurements or multiple analyses. | ||||||||||||
| The study is judged to be at low risk of bias in all domains for this outcome. | ||||||||||||
| Asplund 2000 |  |  |  |  |  |  | ||||||
| The method of allocation sequence is not described, but the use of sealed envelopes suggests a random component was used. Participant characteristics were balanced. | ||||||||||||
| Participants and those delivering the interventions were aware of intervention assignments. Twenty‐five patients were excluded due to them not meeting the set eligibility criteria, however, these: protocol violations are not expected to influence the effect estimate of the outcome as per protocol analyses used. The per protocol analyses was not thought to have a substantial impact on the result given the main reason for exclusion was inappropriate recruitment. Excluding this 25, the other 6 exclusions represent approximately 1% of the total sample size. | ||||||||||||
| Data at discharge from hospital complete for 98% of participants. | ||||||||||||
| The measurement of length of stay is considered appropriate, and there were no differences in measurement or ascertainment between groups. Assessors were not blinded, but is thought that due to the lack of judgement in scoring a 'hard outcome' like length of stay, that it is unlikely that the knowledge of the intervention could influence the outcome. | ||||||||||||
| A pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to raise some concerns due to deviations from intended interventions and the selection of the reported results. | ||||||||||||
| Counsell 2000 |  |  |  |  |  |  | ||||||
| Allocation sequence was random (computer generated random numbers) and sequence concealed (opaque sealed envelope). Participant characteristics were balanced. | ||||||||||||
| Participants and those delivering the interventions were aware of intervention assignments. Seventy‐nine participants were not admitted to the unit to which they were assigned. Deviations may have affected the effect estimate, however they were well‐balanced between groups. Intention to treat analysis was used. | ||||||||||||
| No missing data. | ||||||||||||
| The methods of measuring length of stay are considered appropriate, and there were no differences in measurement or ascertainment between groups. Assessors were not blinded, but is thought that due to the lack of judgement in scoring a 'hard outcome' like length of hospital stay, that it is unlikely that the knowledge of the intervention could influence the outcome. | ||||||||||||
| A pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to raise some concerns in the selection of the reported results and deviations from the intended interventions. | ||||||||||||
| Ekerstad 2017 |  |  |  |  |  |  | ||||||
| Randomisation was based on the availability of beds, and there was no allocation concealment as participants were allocated a ward prior to enrolment. Participant characteristics appeared balanced. | ||||||||||||
| Participants and clinicians delivering care were aware of treatment assignments. There was no evidence of deviations from intended interventions and intention to treat analysis was used. | ||||||||||||
| No missing data. | ||||||||||||
| The methods of measuring length of hospital stay are considered appropriate, and there were no differences in measurement or ascertainment between groups. Assessors were not blinded, but is thought that due to the lack of judgement in scoring a 'hard outcome' like length of hospital stay, that it is unlikely that the knowledge of the intervention could influence the outcome. | ||||||||||||
| A detailed pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to be at high risk of bias arising from the randomisation process. | ||||||||||||
| Fretwell 1990 |  |  |  |  |  |  | ||||||
| “Patients were randomised only when both a treatment and control bed were available”. Although not stated, we believe the necessity for available beds on both wards indicate that a random component was likely used. Sequence allocation concealment is not discussed, but no significant differences in patient characteristics other than those compatible with chance were observed. | ||||||||||||
| Participants and those delivering the interventions were aware of intervention assignments. There were 30 randomisation errors which has been interpreted to mean 'allocated to the ward that they were not randomised to' however there is no explanation of this term. These deviations were thought likely to have affected the outcome, though were relative well‐balanced. It appears a per protocol analyses was used, though given the low number (7%) and relative balance between groups, the omission of the participants randomised incorrectly probably did not have substantial impact on the results. | ||||||||||||
| All participants accounted for at discharge. | ||||||||||||
| The methods of measuring length of stay are considered appropriate, and there were no differences in measurement or ascertainment between groups. Assessors were not blinded, but is thought that due to the lack of judgement in scoring a 'hard outcome' like length of stay, that it is unlikely that the knowledge of the intervention could influence the outcome. | ||||||||||||
| A detailed pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to raise some concerns in deviations from the intended interventions, methods of randomisation and selection of the reported result. | ||||||||||||
| Landefeld 1995 |  |  |  |  |  |  | ||||||
| Allocation sequence was random (computer generated random numbers) and balanced participant characteristics, but no information was provided regarding allocation concealment. | ||||||||||||
| It is assumed participants and clinicians were aware of the treatment assignments. There was no evidence of deviations from the assigned interventions and evidence of an intention to treat approach being used for analysis. | ||||||||||||
| Accounting for mortality (24 died in each arm) all participants had outcome data at discharge. | ||||||||||||
| The methods of measuring length of hospital stay are considered appropriate, and there were no differences in measurement or ascertainment between groups. Assessors were not blinded, but is thought that due to the lack of judgement in scoring a 'hard outcome' like length of hospital stay, that it is unlikely that the knowledge of the intervention could influence the outcome. | ||||||||||||
| A pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to have some concerns due to the methods of randomisation and in the selection of the reported result. | ||||||||||||
| Sahota 2017 |  |  |  |  |  |  | ||||||
| No information on randomisation methods other than to say participants were randomised, however, patient characteristics appear well‐balanced between groups and differences compatible with chance. | ||||||||||||
| It is assumed that participants and those delivering the interventions were aware of intervention assignments. There were 15 protocol deviations in the CIRACT group and 8 in the THB‐Rehab group. The deviations were thought to be in keeping with what would be expected outside of a trial context. Intention to treat not explicitly stated though it appears that the 1 participant who did not receive the intended intervention was analysed within the group to which they were assigned. | ||||||||||||
| No missing data | ||||||||||||
| The methods of measuring length of hospital stay are considered appropriate, and there were no differences in measurement or ascertainment between groups. Assessors were not blinded, but is thought that due to the lack of judgement in scoring a 'hard outcome' like length of hospital stay, that it is unlikely that the knowledge of the intervention could influence the outcome. | ||||||||||||
| A pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to raise some concerns in the selection of the reported results and the randomisation process. | ||||||||||||
| Slaets 1997 |  |  |  |  |  |  | ||||||
| Randomisation methods are described only as: “an alternating randomisation procedure”. However, there was a centrally administered method to allocate interventions, and baseline differences did not appear to show a significant problem with randomisation process other than those thought to be compatible with chance. | ||||||||||||
| Participants and clinicians delivering care were aware of allocated interventions. There was no evidence of deviations from intended intervention, and although intention to treat analysis was not specified, there was no information to suggest participants were not assessed in the group to which they were randomised. | ||||||||||||
| After accounting for mortality (6 patients died), data was available for 91% of participants. | ||||||||||||
| The methods of measuring length of hospital stay in hospital are considered appropriate, and there were no differences in measurement or ascertainment between groups. Assessors were not blinded, but is thought that due to the lack of judgement in scoring a 'hard outcome' like length of stay, that it is unlikely that the knowledge of the intervention could influence the outcome. | ||||||||||||
| A pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to raise some concerns in the randomisation process and in the selection of the reported results. | ||||||||||||
| Zelada 2009 |  |  |  |  |  |  | ||||||
| The allocation sequence was based on bed availability, and therefore not random, and sequence is believed to be predictable. However, baseline differences did not appear to show a significant problem with randomisation process other than those thought to be compatible with chance. | ||||||||||||
| Participants and clinicians delivering care were aware of treatment allocations. There is no evidence of deviations from intended intervention, or that participants moved between the intervention and usual care units during the study period. Intention to treat analysis was not specified, however there was no information to suggest participants were not assessed in the group to which they were randomised. | ||||||||||||
| No missing data. | ||||||||||||
| The methods of measuring length of stay are considered appropriate, and there were no differences in measurement or ascertainment between groups. Assessors were not blinded, but is thought that due to the lack of judgement in scoring a 'hard outcome' like length of stay, that it is unlikely that the knowledge of the intervention could influence the outcome. | ||||||||||||
| A pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to be at high risk of bias due to bias arising from the randomisation process. | ||||||||||||
| Subgroup 2.2.2 Structured exercise | ||||||||||||
| Brown 2016 |  |  |  |  |  |  | ||||||
| No description of randomisation sequence generation, but the paper describes a block randomisation strategy and sequence concealed (sealed envelopes). The patient characteristics appear well‐balanced between groups. | ||||||||||||
| We assume both participants and those delivering the interventions were aware of intervention assignments. 6 participants did not receive allocated intervention, this is considered to be consistent with what could occur outside the trial context, and intention to treat analysis was used. | ||||||||||||
| No missing data. | ||||||||||||
| The methods of measuring length of hospital stay are considered appropriate, and there were no differences in measurement or ascertainment between groups. The assessor was blinded. | ||||||||||||
| The study protocol details statistical analysis plan which is in accordance with results presented, and it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to be at low risk of bias in all domains for this outcome. | ||||||||||||
| Gazineo 2021 |  |  |  |  |  |  | ||||||
| Allocation sequence was random (generated using an online system) and the allocation sequence was concealed until participants were enrolled (opaque concealed and sealed envelopes). Participant characteristics were well‐balanced. | ||||||||||||
| Participants and those delivering the interventions were aware of intervention allocations. There was no evidence of deviations from the intended interventions and intention to treat analysis used. | ||||||||||||
| No missing data after accounting for mortality. | ||||||||||||
| Methods of measuring length of hospital stay are considered appropriate and there were no differences in measurement or ascertainment of the outcomes between groups. The assessor was not blinded, but it is thought that due to the lack of judgement in the measurement, that the knowledge of the intervention did not influence the outcomes. | ||||||||||||
| A pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to raise some concerns in the selection of the reported results . | ||||||||||||
| Hu 2020 |  |  |  |  |  |  | ||||||
| Allocation sequence was random (computer generated random numbers) and sequence concealed (blinded project coordinator allocated participants). Participant characteristics were balanced. | ||||||||||||
| Participants and those delivering the interventions were aware of intervention allocations. There was no evidence of deviations from the intended interventions and as such it is presumed intention to treat analysis was used. | ||||||||||||
| No missing data for length of hospital stay. | ||||||||||||
| The methods of measuring legnth of hospital stay are considered appropriate, and there were no differences in measurement or ascertainment between groups. The assessors were blinded. | ||||||||||||
| A pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to have some concerns in the selection of the reported result. | ||||||||||||
| McGowan 2018a |  |  |  |  |  |  | ||||||
| Allocation sequence was random (www.randomization.com) and the allocation sequence was only known by the chief investigator who was not involved with screening patients. Baseline differences between groups are thought to be compatible with chance. | ||||||||||||
| Both participants and clinicians delivering care were aware of assigned interventions. There is no evidence of deviations from intended interventions and intention to treat analysis not explicitly stated, but all participants appear to have been analysed in the group to which they were randomised. | ||||||||||||
| 96% of participants had complete data sets. | ||||||||||||
| The measurement of length of hospital stay is considered appropriate, and there were no differences in the measurement or ascertainment between groups. The assessors were not blinded, but is thought that due to the lack of judgement in scoring a 'hard outcome' like length of hospital stay, that it is unlikely that the knowledge of the intervention could influence the outcome. | ||||||||||||
| The trial protocol and registration reflects the analyses as that were conducted, and it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to raise some concerns due to the measurement of the outcome. | ||||||||||||
| Subgroup 2.2.3 Progressive resistance exercise | ||||||||||||
| Courtney 2009 |  |  |  |  |  |  | ||||||
| Allocation sequence was random (computer generated random numbers) and the allocation sequence concealed until participants were enrolled (via project coordinator blinded to baseline data). Participant characteristics appear well‐balanced. | ||||||||||||
| Participants and clinicians delivering care were believed to be aware of treatment assignments. 6/64 participants in the intervention group did not receive allocated treatment, the reasons are considered consistent with what could occur outside the trial context, and intention to treat analysis was used. | ||||||||||||
| No missing length of stay data. | ||||||||||||
| The measurement of hospital length of stay is considered appropriate, and there were no differences in measurement or ascertainment between groups. The assessors are believed to be blinded to treatment allocation. | ||||||||||||
| A pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to raise some concerns in the selection of the reported result. | ||||||||||||
| de Morton 2007 |  |  |  |  |  |  | ||||||
| Allocation of wards was random (coin toss) and the allocating officer unaware of study. Baseline differences between groups were thought to be compatible with chance. | ||||||||||||
| Participants and clinicians delivering care were believed to be aware of treatment assignments. There was no evidence of deviations from intended interventions and intention to treat analysis was used. | ||||||||||||
| No missing data. | ||||||||||||
| The measure of hospital length of stay considered appropriate, and there were no differences in measurement or ascertainment between groups. Assessors were not blinded, but is thought that due to the lack of judgement in scoring a 'hard outcome' like length of stay, that it is unlikely that the knowledge of the intervention could influence the outcome. | ||||||||||||
| A pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to raise some concerns in the selection of the reported result. | ||||||||||||
| Jeffs 2013 |  |  |  |  |  |  | ||||||
| Sequence concealment was achieved via a member of the research team not involved in recruitment and using sealed envelopes. There was no information regarding method of generating random sequence, other than to say that there was randomisation, given details of allocation concealment, assumption made that appropriate method used. Patient characteristics between groups appear well‐balanced. | ||||||||||||
| Participants and those delivering the interventions were likely to be aware of intervention allocations. One participant was not allocated to a group due to an administrative error and 35 participants did not receive the intervention as planned (17 in the intervention group). This is thought to be consistent with what could occur outside the trial context. Intention to treat analysis was used. | ||||||||||||
| No missing data. | ||||||||||||
| Measurement of length of hospital stay is considered appropriate, and there were no differences in the measurement or ascertainment between groups. The assessors were blinded. | ||||||||||||
| A pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to raise some concerns in the selection of the reported results. | ||||||||||||
| Jones 2006 |  |  |  |  |  |  | ||||||
| Allocation sequence was random (computer generated random numbers). Sequence allocation was not concealed, but performed by a member of staff independent of the enrolment procedures. ). Participant characteristics were balanced between groups and differences compatible with chance. | ||||||||||||
| It is assumed that both participants and clinicians delivering care were aware of assigned interventions. There is no evidence of deviations from intended interventions and intention to treat analysis used. | ||||||||||||
| After accounting for mortality, length of stay data available for 99% of control group and 93% of intervention group. Given the nature of this population (older adults during and after acute hospitalisation) we considered a threshold of 90% of participants with data as sufficient. | ||||||||||||
| Measurement methods are considered appropriate, and there were no differences in the measurement or ascertainment between groups. The assessors were blinded. | ||||||||||||
| A pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to raise some concerns in the selection of the reported results. | ||||||||||||
| Martinez‐Velilla 2019 |  |  |  |  |  |  | ||||||
| Allocation sequence was random (www.randomizer.org) and personal communication with author confirms allocation concealment. Baseline differences between groups are thought to be compatible with chance. | ||||||||||||
| Both participants and clinicians delivering care were aware of assigned interventions. There is no evidence of deviations from intended interventions and intention to treat analysis used. | ||||||||||||
| No missing data. | ||||||||||||
| The methods of measurement are considered appropriate, and there were no differences in the measurement or ascertainment between groups. The assessors were blinded. | ||||||||||||
| The trial protocol reflects the analyses as that were conducted, and it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to be at low risk of bias in all domains for this outcome. | ||||||||||||
| McCullagh 2020 |  |  |  |  |  |  | ||||||
| Allocation sequence was random (computer‐generated) and allocation sequence was concealed. Baseline differences are thought to be compatible with chance. | ||||||||||||
| Both participants and clinicians delivering care were aware of assigned interventions. There is no evidence of deviations from intended interventions and intention to treat analysis used. | ||||||||||||
| No missing data. | ||||||||||||
| The measure is considered appropriate, and there were no differences in the measurement or ascertainment between groups. The assessors were blinded. | ||||||||||||
| The trial protocol reflects the analyses as that were conducted, and it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to be at low risk of bias in all domains for this outcome. | ||||||||||||
| Mudge 2008 |  |  |  |  |  |  | ||||||
| Pseudo‐randomisation, allocation was based on admitting unit and bed availability, and admitting unit was determined by a rotating roster. No evidence of baseline differences in patient characteristics other than those thought to be compatible with chance. | ||||||||||||
| Both participants and clinicians delivering care were aware of assigned interventions. There is no evidence of deviations from intended interventions and intention to treat analysis used. | ||||||||||||
| No missing data. | ||||||||||||
| The method is considered appropriate, and there were no differences in the measurement or ascertainment between groups. The assessors were blinded. | ||||||||||||
| A pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to be at high risk of bias due to the randomisation process. | ||||||||||||
| Ortiz‐Alonso 2020 |  |  |  |  |  |  | ||||||
| The allocation sequence was based on recruitment blocks of 4‐8 weeks. Activity of daily living (ADL) scores at baseline and admission were significantly lower in the intervention group than the control group, this is thought to be an important prognostic factor and therefore a concern regarding the randomisation process. | ||||||||||||
| Methods were designed to blind participants from group assignments however due to the nature of the intervention and lack of sham intervention the success of this blinding is felt unlikely. Ward staff and research staff were not blinded to the participant's assigned intervention. There were no deviations from the intended interventions due to trial context described and although intention to treat analysis not specified given the lack of deviations it is assumed. | ||||||||||||
| No missing outcome data. | ||||||||||||
| The measure is considered appropriate, and there were no differences in the measurement or ascertainment between groups. The assessors were not blinded, but it is thought that due to the lack of judgement in the outcome, that the knowledge of the intervention did not influence the outcome. | ||||||||||||
| Analysis reflects plan in trial registration and study protocol, and results are not thought to be from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to be at high risk of bias due to the randomisation process. | ||||||||||||
| Pedersen 2019 |  |  |  |  |  |  | ||||||
| Allocation sequence was random (computer‐generated block randomisation), and allocation sequence concealed from the investigators. Participant characteristics were well‐balanced between groups. | ||||||||||||
| Participants and clinicians were aware of the treatment assignments. There was no evidence of deviations from the assigned interventions and an intention to treat approach was used for analysis. | ||||||||||||
| No missing data | ||||||||||||
| The measure is considered appropriate, and there were no differences in the measurement or ascertainment between groups. The assessors were blinded. | ||||||||||||
| The analysis is in accordance with a pre‐specified analysis in a published protocol, and results are not thought to be from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to be at low risk of bias for all domains for this outcome. | ||||||||||||
| Bias | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study | Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | ||||||
| Subgroup 2.3.1 Rehabilitation‐related activities | ||||||||||||
| Asplund 2000 |  |  |  |  |  |  | ||||||
| The method of allocation sequence is not described, but the use of sealed envelopes suggests a random component was used. Participant characteristics were balanced. | ||||||||||||
| Participants and those delivering the interventions were aware of intervention assignments. Twenty‐five patients were excluded due to them not meeting the set eligibility criteria, however, these: protocol violations are not expected to influence the effect estimate of the outcome as per protocol analyses used. The per protocol analyses was not thought to have a substantial impact on the result given the main reason for exclusion was inappropriate recruitment. Excluding this 25, the other 6 exclusions represent approximately 1% of the total sample size. | ||||||||||||
| Data at discharge from hospital complete for 98% of participants. | ||||||||||||
| The measurement of new institutionalisation is considered appropriate, and there were no differences in measurement or ascertainment between groups. Assessors were not blinded, but is thought that due to the lack of judgement in scoring a 'hard outcome' like new institutionalisation, that it is unlikely that the knowledge of the intervention could influence the outcome. | ||||||||||||
| A pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to raise some concerns due to deviations from intended interventions and the selection of the reported results. | ||||||||||||
| Counsell 2000 |  |  |  |  |  |  | ||||||
| Allocation sequence was random (computer generated random numbers) and sequence concealed (opaque sealed envelope). Participant characteristics were balanced. | ||||||||||||
| Participants and those delivering the interventions were aware of intervention assignments. Seventy‐nine participants were not admitted to the unit to which they were assigned. Deviations may have affected the effect estimate, however they were well‐balanced between groups. Intention to treat analysis was used. | ||||||||||||
| No missing data. | ||||||||||||
| The methods of measuring length of stay are considered appropriate, and there were no differences in measurement or ascertainment between groups. Assessors were not blinded, but is thought that due to the lack of judgement in scoring a 'hard outcome' like length of hospital stay, that it is unlikely that the knowledge of the intervention could influence the outcome. | ||||||||||||
| A pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to raise some concerns in the selection of the reported results and deviations from the intended interventions. | ||||||||||||
| Fretwell 1990 |  |  |  |  |  |  | ||||||
| “Patients were randomised only when both a treatment and control bed were available”. Although not stated, we believe the necessity for available beds on both wards indicate that a random component was likely used. Sequence allocation concealment is not discussed, but no significant differences in patient characteristics other than those compatible with chance were observed. | ||||||||||||
| Participants and those delivering the interventions were aware of intervention assignments. There were 30 randomisation errors which has been interpreted to mean 'allocated to the ward that they were not randomised to' however there is no explanation of this term. These deviations were thought likely to have affected the outcome, though were relative well‐balanced. It appears a per protocol analyses was used, though given the low number (7%) and relative balance between groups, the omission of the participants randomised incorrectly probably did not have substantial impact on the results. | ||||||||||||
| All participants accounted for at discharge. | ||||||||||||
| The methods of measuring new institutionalisation are considered appropriate, and there were no differences in measurement or ascertainment between groups. Assessors were not blinded, but is thought that due to the lack of judgement in scoring a 'hard outcome' like new institutionalisation, that it is unlikely that the knowledge of the intervention could influence the outcome. | ||||||||||||
| A detailed pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to raise some concerns in deviations from the intended interventions, methods of randomisation and selection of the reported result. | ||||||||||||
| Subgroup 2.3.2 Progressive resistance exercise | ||||||||||||
| de Morton 2007 |  |  |  |  |  |  | ||||||
| Allocation of wards was random (coin toss) and the allocating officer unaware of study. Baseline differences between groups were thought to be compatible with chance. | ||||||||||||
| Participants and clinicians delivering care were believed to be aware of treatment assignments. There was no evidence of deviations from intended interventions and intention to treat analysis was used. | ||||||||||||
| No missing data. | ||||||||||||
| The measure of new institutionalisation considered appropriate, and there were no differences in measurement or ascertainment between groups. Assessors were not blinded, but is thought that due to the lack of judgement in scoring a 'hard outcome' like new institutionalisation, that it is unlikely that the knowledge of the intervention could influence the outcome. | ||||||||||||
| A pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to raise some concerns in the selection of the reported result. | ||||||||||||
| Mudge 2008 |  |  |  |  |  |  | ||||||
| Pseudo‐randomisation, allocation was based on admitting unit and bed availability, and admitting unit was determined by a rotating roster. No evidence of baseline differences in patient characteristics other than those thought to be compatible with chance. | ||||||||||||
| Both participants and clinicians delivering care were aware of assigned interventions. There is no evidence of deviations from intended interventions and intention to treat analysis used. | ||||||||||||
| No missing data. | ||||||||||||
| The method is considered appropriate, and there were no differences in the measurement or ascertainment between groups. The assessors were blinded. | ||||||||||||
| A pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to be at high risk of bias due to the randomisation process. | ||||||||||||
| Bias | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study | Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | ||||||
| Subgroup 2.4.1 Rehabilitation‐related activities | ||||||||||||
| Asplund 2000 |  |  |  |  |  |  | ||||||
| The method of allocation sequence is not described, but the use of sealed envelopes suggests a random component was used. Participant characteristics were balanced. | ||||||||||||
| Participants and those delivering the interventions were aware of intervention assignments. Twenty‐five patients were excluded due to them not meeting the set eligibility criteria, however, these: protocol violations are not expected to influence the effect estimate of the outcome as per protocol analyses used. The per protocol analyses was not thought to have a substantial impact on the result given the main reason for exclusion was inappropriate recruitment. Excluding this 25, the other 6 exclusions represent approximately 1% of the total sample size. | ||||||||||||
| Data for readmissions complete for 98% of participants. | ||||||||||||
| The measurement of hospital readmissions is considered appropriate, and there were no differences in measurement or ascertainment between groups. Assessors were not blinded, but is thought that due to the lack of judgement in scoring a 'hard outcome' like hospital readmissions, that it is unlikely that the knowledge of the intervention could influence the outcome. | ||||||||||||
| A pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to raise some concerns due to deviations from intended interventions and the selection of the reported results. | ||||||||||||
| Counsell 2000 |  |  |  |  |  |  | ||||||
| Allocation sequence was random (computer generated random numbers) and sequence concealed (opaque sealed envelope). Participant characteristics were balanced. | ||||||||||||
| Participants and those delivering the interventions were aware of intervention assignments. Seventy‐nine participants were not admitted to the unit to which they were assigned. Deviations may have affected the effect estimate, however they were well‐balanced between groups. Intention to treat analysis was used. | ||||||||||||
| Given the nature of this population (older adults during and after acute hospitalisation) we considered a threshold of 90% of participants with data as sufficient, data was available for approximately 93% at 6 month follow‐up participants at discharge. | ||||||||||||
| The methods of measuring length of stay are considered appropriate, and there were no differences in measurement or ascertainment between groups. Assessors were not blinded, but is thought that due to the lack of judgement in scoring a 'hard outcome' like length of hospital stay, that it is unlikely that the knowledge of the intervention could influence the outcome. | ||||||||||||
| A pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to raise some concerns in the selection of the reported results and deviations from the intended interventions. | ||||||||||||
| Ekerstad 2017 |  |  |  |  |  |  | ||||||
| Randomisation was based on the availability of beds, and there was no allocation concealment as participants were allocated a ward prior to enrolment. Participant characteristics appeared balanced. | ||||||||||||
| Participants and clinicians delivering care were aware of treatment assignments. There was no evidence of deviations from intended interventions and intention to treat analysis was used. | ||||||||||||
| No missing data. | ||||||||||||
| The methods of measuring length of hospital stay are considered appropriate, and there were no differences in measurement or ascertainment between groups. Assessors were not blinded, but is thought that due to the lack of judgement in scoring a 'hard outcome' like length of hospital stay, that it is unlikely that the knowledge of the intervention could influence the outcome. | ||||||||||||
| A detailed pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to be at high risk of bias arising from the randomisation process. | ||||||||||||
| Landefeld 1995 |  |  |  |  |  |  | ||||||
| Allocation sequence was random (computer generated random numbers) and balanced participant characteristics, but no information was provided regarding allocation concealment. | ||||||||||||
| It is assumed participants and clinicians were aware of the treatment assignments. There was no evidence of deviations from the assigned interventions and evidence of an intention to treat approach being used for analysis. | ||||||||||||
| Accounting for mortality (24 died in each arm) all participants had outcome data at discharge. At follow up (a further 42, and 40 in intervention and control group had died), data was missing for just 6 participants (1%).at follow up (a further 42, and 40 in intervention and control group had died), data was missing for just 6 participants (1%). | ||||||||||||
| The measure is considered appropriate, and there were no differences in measurement or ascertainment between groups. Assessors were not blinded, but is thought that due to the lack of judgement in scoring a 'hard outcome' like hospital readmissions, that it is unlikely that the knowledge of the intervention could influence the outcome. | ||||||||||||
| A pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to have some concerns due to the methods of randomisation and in the selection of the reported result. | ||||||||||||
| Sahota 2017 |  |  |  |  |  |  | ||||||
| No information on randomisation methods other than to say participants were randomised, however, patient characteristics appear well‐balanced between groups and differences compatible with chance. | ||||||||||||
| It is assumed that participants and those delivering the interventions were aware of intervention assignments. There were 15 protocol deviations in the CIRACT group and 8 in the THB‐Rehab group. The deviations were thought to be in keeping with what would be expected outside of a trial context. Intention to treat not explicitly stated though it appears that the 1 participant who did not receive the intended intervention was analysed within the group to which they were assigned. | ||||||||||||
| No missing data. | ||||||||||||
| The measure is considered appropriate, and there were no differences in measurement or ascertainment between groups. Assessors were not blinded, but is thought that due to the lack of judgement in scoring a 'hard outcome' like hospital readmissions, that it is unlikely that the knowledge of the intervention could influence the outcome. | ||||||||||||
| A pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to raise some concerns in the selection of the reported results and the randomisation process. | ||||||||||||
| Slaets 1997 |  |  |  |  |  |  | ||||||
| Randomisation methods are described only as: “an alternating randomisation procedure”. However, there was a centrally administered method to allocate interventions, and baseline differences did not appear to show a significant problem with randomisation process other than those thought to be compatible with chance. | ||||||||||||
| Participants and clinicians delivering care were aware of allocated interventions. There was no evidence of deviations from intended intervention, and although intention to treat analysis was not specified, there was no information to suggest participants were not assessed in the group to which they were randomised. | ||||||||||||
| After accounting for mortality (6 patients died), data was available for 91% of participants. | ||||||||||||
| The methods of measuring hospital readmissions are considered appropriate, and there were no differences in measurement or ascertainment between groups. Assessors were not blinded, but is thought that due to the lack of judgement in scoring a 'hard outcome' like hospital readmissions, that it is unlikely that the knowledge of the intervention could influence the outcome. | ||||||||||||
| A pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to raise some concerns in the randomisation process and in the selection of the reported results. | ||||||||||||
| Subgroup 2.4.2 Structured exercise | ||||||||||||
| Gazineo 2021 |  |  |  |  |  |  | ||||||
| Allocation sequence was random (generated using an online system) and the allocation sequence was concealed until participants were enrolled (opaque concealed and sealed envelopes). Participant characteristics were well‐balanced. | ||||||||||||
| Participants and those delivering the interventions were aware of intervention allocations. There was no evidence of deviations from the intended interventions and intention to treat analysis used. | ||||||||||||
| No missing data after accounting for mortality. | ||||||||||||
| Methods of measuring hospital readmissions are considered appropriate and there were no differences in measurement or ascertainment of the outcomes between groups. The assessor was not blinded, but it is thought that due to the lack of judgement in the measurement, that the knowledge of the intervention did not influence the outcomes. | ||||||||||||
| A pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to raise some concerns in the selection of the reported results. | ||||||||||||
| Subgroup 2.4.3 Progressive resistance exercise | ||||||||||||
| Courtney 2009 |  |  |  |  |  |  | ||||||
| Allocation sequence was random (computer generated random numbers) and the allocation sequence concealed until participants were enrolled (via project coordinator blinded to baseline data). Participant characteristics appear well‐balanced. | ||||||||||||
| Participants and clinicians delivering care were believed to be aware of treatment assignments. 6/64 participants in the intervention group did not receive allocated treatment, the reasons are considered consistent with what could occur outside the trial context, and intention to treat analysis was used. | ||||||||||||
| Data regarding readmissions available for 92% of intervention and 98% of control group participants. Given the nature of this population (older adults during and after acute hospitalisation) we considered a threshold of 90% of participants with data as sufficient. | ||||||||||||
| The measurement of hospital readmissions is considered appropriate, and there were no differences in measurement or ascertainment between groups. The assessors are believed to be blinded to treatment allocation. | ||||||||||||
| A pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to raise some concerns in the selection of the reported result. | ||||||||||||
| de Morton 2007 |  |  |  |  |  |  | ||||||
| Allocation of wards was random (coin toss) and the allocating officer unaware of study. Baseline differences between groups were thought to be compatible with chance. | ||||||||||||
| Participants and clinicians delivering care were believed to be aware of treatment assignments. There was no evidence of deviations from intended interventions and intention to treat analysis was used. | ||||||||||||
| No missing data. | ||||||||||||
| The measure of hospital readmissions is considered appropriate, and there were no differences in measurement or ascertainment between groups. Assessors were not blinded, but is thought that due to the lack of judgement in scoring a 'hard outcome' like hospital readmissions, that it is unlikely that the knowledge of the intervention could influence the outcome. | ||||||||||||
| A pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to raise some concerns in the selection of the reported result. | ||||||||||||
| Martinez‐Velilla 2019 |  |  |  |  |  |  | ||||||
| Allocation sequence was random (www.randomizer.org) and personal communication with author confirms allocation concealment. Baseline differences between groups are thought to be compatible with chance. | ||||||||||||
| Both participants and clinicians delivering care were aware of assigned interventions. There is no evidence of deviations from intended interventions and intention to treat analysis used. | ||||||||||||
| No missing data. | ||||||||||||
| The methods of measurement are considered appropriate, and there were no differences in the measurement or ascertainment between groups. The assessors were blinded. | ||||||||||||
| The trial protocol reflects the analyses as that were conducted, and it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to be at low risk of bias in all domains for this outcome. | ||||||||||||
| McCullagh 2020 |  |  |  |  |  |  | ||||||
| Allocation sequence was random (computer‐generated) and allocation sequence was concealed. Baseline differences are thought to be compatible with chance. | ||||||||||||
| Both participants and clinicians delivering care were aware of assigned interventions. There is no evidence of deviations from intended interventions and intention to treat analysis used. | ||||||||||||
| No missing data. | ||||||||||||
| The measure is considered appropriate, and there were no differences in the measurement or ascertainment between groups. The assessors were blinded. | ||||||||||||
| The trial protocol reflects the analyses as that were conducted, and it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to be at low risk of bias in all domains for this outcome. | ||||||||||||
| Mudge 2008 |  |  |  |  |  |  | ||||||
| Pseudo‐randomisation, allocation was based on admitting unit and bed availability, and admitting unit was determined by a rotating roster. No evidence of baseline differences in patient characteristics other than those thought to be compatible with chance. | ||||||||||||
| Both participants and clinicians delivering care were aware of assigned interventions. There is no evidence of deviations from intended interventions and intention to treat analysis used. | ||||||||||||
| No missing data. | ||||||||||||
| The method is considered appropriate, and there were no differences in the measurement or ascertainment between groups. The assessors were blinded. | ||||||||||||
| A pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to be at high risk of bias due to the randomisation process. | ||||||||||||
| Ortiz‐Alonso 2020 |  |  |  |  |  |  | ||||||
| The allocation sequence was based on recruitment blocks of 4‐8 weeks. Activity of daily living (ADL) scores at baseline and admission were significantly lower in the intervention group than the control group, this is thought to be an important prognostic factor and therefore a concern regarding the randomisation process. | ||||||||||||
| Methods were designed to blind participants from group assignments however due to the nature of the intervention and lack of sham intervention the success of this blinding is felt unlikely. Ward staff and research staff were not blinded to the participant's assigned intervention. There were no deviations from the intended interventions due to trial context described and although intention to treat analysis not specified given the lack of deviations it is assumed. | ||||||||||||
| No missing outcome data. | ||||||||||||
| The measure is considered appropriate, and there were no differences in the measurement or ascertainment between groups. The assessors were not blinded, but it is thought that due to the lack of judgement in the outcome, that the knowledge of the intervention did not influence the outcome. | ||||||||||||
| Analysis reflects plan in trial registration and study protocol, and results are not thought to be from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to be at high risk of bias due to the randomisation process. | ||||||||||||
| Pedersen 2019 |  |  |  |  |  |  | ||||||
| Allocation sequence was random (computer‐generated block randomisation), and allocation sequence concealed from the investigators. Participant characteristics were well‐balanced between groups. | ||||||||||||
| Participants and clinicians were aware of the treatment assignments. There was no evidence of deviations from the assigned interventions and an intention to treat approach was used for analysis. | ||||||||||||
| No missing data | ||||||||||||
| The measure is considered appropriate, and there were no differences in the measurement or ascertainment between groups. The assessors were blinded. | ||||||||||||
| The analysis is in accordance with a pre‐specified analysis in a published protocol, and results are not thought to be from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to be at low risk of bias for all domains for this outcome. | ||||||||||||
| Bias | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study | Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | ||||||
| Subgroup 2.5.1 Rehabilitation‐related activities | ||||||||||||
| Ekerstad 2017 |  |  |  |  |  |  | ||||||
| Randomisation was based on the availability of beds, and there was no allocation concealment as participants were allocated a ward prior to enrolment. Participant characteristics appeared balanced. | ||||||||||||
| Participants and clinicians delivering care were aware of treatment assignments. There was no evidence of deviations from intended interventions and intention to treat analysis was used. | ||||||||||||
| The timed up and go (TUG) (when accounting for mortality) was available for 74% and 49% of participants in intervention and control groups at hospital discharge respectively. The difference in missing data for the TUG acompared to the activity of daily living data may be due to participants being unable to complete the walking tasks. Therefore a large proportion of missing data is thought to be due to the 'true' value. | ||||||||||||
| The use of the TUG to measure walking performance is considered appropriate and there was no differences in measurement between groups. The assessors were not blinded, and it was therefore considered likely that knowledge of the intervention could influence the outcome, given the likely strong belief in the benefits of the intervention ward. | ||||||||||||
| A detailed pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to be at high risk of bias due to bias in the measurement of the outcome and bias arising from the randomisation process. | ||||||||||||
| Subgroup 2.5.2 Structured exercise | ||||||||||||
| Hu 2020 |  |  |  |  |  |  | ||||||
| Allocation sequence was random (computer generated random numbers) and sequence concealed (blinded project coordinator allocated participants). Participant characteristics were balanced. | ||||||||||||
| Participants and those delivering the interventions were aware of intervention allocations. There was no evidence of deviations from the intended interventions and as such it is presumed intention to treat analysis was used. | ||||||||||||
| At discharge data was available for 76% of reablement group, (78% of reminder group) and 80% of the control group. Only participants who had complete data (i.e. data collected at baseline, discharge and follow‐up) had outcomes reported. Although missing data is well‐balanced between all three groups, it is considered likely that reason for missing data is related to its true value. | ||||||||||||
| The use of the timed up and go (TUG) is considered appropriate, and there were no differences in the measurement or ascertainment between groups. The assessors were blinded. | ||||||||||||
| A pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to be at high risk of bias due to missing outcome data. | ||||||||||||
| Killey 2006 |  |  |  |  |  |  | ||||||
| Allocation not random and sequence predictable as based on alternation. There is limited data on participant characteristics, but available data suggests balanced characteristics between groups. | ||||||||||||
| Participants and those delivering the interventions were aware of intervention allocations. There was no evidence of deviations from the intended interventions. Per‐protocol analysis appears to have been used. 2 participants in the intervention group were excluded from analysis as they completed less than 70% of their walks. The 2 participants that were excluded accounted for 7% of the remaining sample, it is therefore considered that there was potential for a significant impact on the results. | ||||||||||||
| Only 71% of intervention and 74% of control group had outcome data. A significant proportion of missing data was related to early discharge from hospital. These participants could be expected to have a higher functional level than those who remained in hospital. However, the missing data was well‐balanced between the two groups. | ||||||||||||
| The total distance able to walk is considered appropriate, and there were no differences in the measurement or ascertainment between groups. The assessors were not blinded, and it was therefore considered likely that knowledge of the intervention could influence the outcome, given the likely strong belief in the benefits of the intervention ward. | ||||||||||||
| A pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to be at high risk of bias in three domains for this outcome. | ||||||||||||
| Subgroup 2.5.3 Progressive resistance exercise | ||||||||||||
| de Morton 2007 |  |  |  |  |  |  | ||||||
| Allocation of wards was random (coin toss) and the allocating officer unaware of study. Baseline differences between groups were thought to be compatible with chance. | ||||||||||||
| Participants and clinicians delivering care were believed to be aware of treatment assignments. There was no evidence of deviations from intended interventions and intention to treat analysis was used. | ||||||||||||
| After accounting for mortality, at discharge, only 70% in intervention arm and 60% in control arm had timed up and go (TUG) assessed. There is a higher amount of missing data for the TUG than the Barthel Index which may indicate that some of the missing data is due to participants being unable to complete the TUG task. Therefore a proportion of missing data is believed to be due to the 'true' value. | ||||||||||||
| The use of the TUG to measure walking performance is considered appropriate, and there were no differences in the measurement or ascertainment between groups. The assessors were not blinded, and it is considered likely that knowledge of the intervention could influence the outcome, given the likely strong belief in the benefits of the intervention. | ||||||||||||
| A pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to be at high risk of bias in measurement of the outcome and missing data. | ||||||||||||
| Jones 2006 |  |  |  |  |  |  | ||||||
| Allocation sequence was random (computer generated random numbers). Sequence allocation was not concealed, but performed by a member of staff independent of the enrolment procedures. ). Participant characteristics were balanced between groups and differences compatible with chance. | ||||||||||||
| It is assumed that both participants and clinicians delivering care were aware of assigned interventions. There is no evidence of deviations from intended interventions and intention to treat analysis used. | ||||||||||||
| After accounting for mortality, timed up and go (TUG) data was available for 31% of control group and 51% of intervention group. "39.4% (63/160) were unable to complete the TUG either on admission or on discharge and therefore a change score could not be calculated." Therefore, a large proportion of missing data is due to the 'true' value. | ||||||||||||
| The use of the TUG to measure walking performance is considered appropriate, and there were no differences in the measurement or ascertainment between groups. The assessors were blinded. | ||||||||||||
| A pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to be at high risk of bias due to missing outcome data. | ||||||||||||
| Pedersen 2019 |  |  |  |  |  |  | ||||||
| Allocation sequence was random (computer‐generated block randomisation), and allocation sequence concealed from the investigators. Participant characteristics were well‐balanced between groups. | ||||||||||||
| Participants and clinicians were aware of the treatment assignments. There was no evidence of deviations from the assigned interventions and an intention to treat approach was used for analysis. | ||||||||||||
| When accounting for mortality only 93% of participants in intervention group and 67% in control group completed measures at discharge. The difference in the proportion of missing data, and that the assessments were carried out home after discharge, it was judged likely that the reasons for missing data may have depended on its true value. | ||||||||||||
| The use of the 4m timed walk for measuring gait speed/walking performance is considered appropriate, and there were no differences in the measurement or ascertainment between groups. The assessors were blinded. | ||||||||||||
| The analysis is in accordance with a pre‐specified analysis in a published protocol, and results are not thought to be from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to be at high risk of bias due to missing outcome data. | ||||||||||||
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1.1 Functional ability: independence with activities of daily living at discharge from hospital Show forest plot | 16 | 5174 | Std. Mean Difference (IV, Random, 95% CI) | 0.09 [‐0.02, 0.19] |
| Analysis 1.1  Comparison 1: Major outcomes, Outcome 1: Functional ability: independence with activities of daily living at discharge from hospital | ||||
| 1.1.1 Rehabilitation‐related activities | 4 | 2838 | Std. Mean Difference (IV, Random, 95% CI) | 0.00 [‐0.12, 0.13] |
| 1.1.2 Structured exercise | 5 | 648 | Std. Mean Difference (IV, Random, 95% CI) | 0.12 [‐0.21, 0.45] |
| 1.1.3 Progressive resistance exercise | 7 | 1688 | Std. Mean Difference (IV, Random, 95% CI) | 0.14 [‐0.05, 0.32] |
| 1.2 Functional ability: functional mobility at discharge from hospital Show forest plot | 8 | 2369 | Mean Difference (IV, Random, 95% CI) | 0.54 [0.09, 0.99] |
| Analysis 1.2  Comparison 1: Major outcomes, Outcome 2: Functional ability: functional mobility at discharge from hospital | ||||
| 1.2.1 Rehabilitation‐related activities | 1 | 975 | Mean Difference (IV, Random, 95% CI) | 0.60 [0.06, 1.14] |
| 1.2.2 Structured exercise | 2 | 416 | Mean Difference (IV, Random, 95% CI) | 0.30 [‐0.96, 1.57] |
| 1.2.3 Progressive resistance exercise | 5 | 978 | Mean Difference (IV, Random, 95% CI) | 0.63 [‐0.28, 1.55] |
| 1.3 Functional ability: new incidence of delirium during hospitalisation Show forest plot | 7 | 2088 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.58, 1.41] |
| Analysis 1.3  Comparison 1: Major outcomes, Outcome 3: Functional ability: new incidence of delirium during hospitalisation | ||||
| 1.3.1 Rehabilitation‐related activities | 2 | 732 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.30, 2.50] |
| 1.3.2 Structured exercise | 1 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 3.00 [0.13, 71.92] |
| 1.3.3 Progressive resistance exercise | 4 | 1256 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.55, 1.68] |
| 1.4 Quality of life at discharge from hospital Show forest plot | 4 | 875 | Mean Difference (IV, Random, 95% CI) | 6.04 [0.90, 11.18] |
| Analysis 1.4  Comparison 1: Major outcomes, Outcome 4: Quality of life at discharge from hospital | ||||
| 1.4.1 Rehabilitation‐related activities | 1 | 350 | Mean Difference (IV, Random, 95% CI) | 2.20 [‐1.90, 6.30] |
| 1.4.2 Structured exercise | 1 | 76 | Mean Difference (IV, Random, 95% CI) | 3.74 [‐6.32, 13.80] |
| 1.4.3 Progressive resistance exercise | 2 | 449 | Mean Difference (IV, Random, 95% CI) | 8.90 [2.35, 15.45] |
| 1.5 Falls during hospitalisation Show forest plot | 9 | 1787 | Risk Ratio (IV, Random, 95% CI) | 0.99 [0.59, 1.65] |
| Analysis 1.5  Comparison 1: Major outcomes, Outcome 5: Falls during hospitalisation | ||||
| 1.5.1 Rehabilitation‐related activities | 1 | 250 | Risk Ratio (IV, Random, 95% CI) | 1.33 [0.30, 5.84] |
| 1.5.2 Structured exercise | 3 | 542 | Risk Ratio (IV, Random, 95% CI) | 0.76 [0.23, 2.53] |
| 1.5.3 Progressive resistance exercise | 5 | 995 | Risk Ratio (IV, Random, 95% CI) | 0.96 [0.48, 1.91] |
| 1.6 Medical deterioration during hospitalisation Show forest plot | 11 | 2730 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.62, 1.68] |
| Analysis 1.6  Comparison 1: Major outcomes, Outcome 6: Medical deterioration during hospitalisation | ||||
| 1.6.1 Rehabilitation‐related activities | 2 | 732 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.30, 2.50] |
| 1.6.2 Structured exercise | 2 | 200 | Risk Ratio (M‐H, Random, 95% CI) | 2.56 [0.48, 13.54] |
| 1.6.3 Progressive resistance exercise | 7 | 1798 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.52, 1.87] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 2.1 Death during hospitalisation Show forest plot | 20 | 6822 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.79, 1.22] |
| Analysis 2.1  Comparison 2: Minor outcomes, Outcome 1: Death during hospitalisation | ||||
| 2.1.1 Rehabilitation‐related activities | 7 | 3926 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.78, 1.34] |
| 2.1.2 Structured exercise | 5 | 740 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.54, 1.56] |
| 2.1.3 Progressive resistance exercise | 8 | 2156 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.54, 1.48] |
| 2.2 Hospital length of stay (days) Show forest plot | 22 | 7182 | Mean Difference (IV, Random, 95% CI) | ‐0.25 [‐0.62, 0.12] |
| Analysis 2.2  Comparison 2: Minor outcomes, Outcome 2: Hospital length of stay (days) | ||||
| 2.2.1 Rehabilitation‐related activities | 9 | 4388 | Mean Difference (IV, Random, 95% CI) | ‐0.55 [‐1.42, 0.32] |
| 2.2.2 Structured exercise | 4 | 635 | Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.93, 0.89] |
| 2.2.3 Progressive resistance exercise | 9 | 2159 | Mean Difference (IV, Random, 95% CI) | ‐0.24 [‐0.63, 0.16] |
| 2.3 New institutionalisation at hospital discharge Show forest plot | 5 | 2364 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.74, 1.12] |
| Analysis 2.3  Comparison 2: Minor outcomes, Outcome 3: New institutionalisation at hospital discharge | ||||
| 2.3.1 Rehabilitation‐related activities | 3 | 2004 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.74, 1.13] |
| 2.3.2 Progressive resistance exercise | 2 | 360 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.37, 2.01] |
| 2.4 Hospital readmission Show forest plot | 14 | 4689 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.81, 1.11] |
| Analysis 2.4  Comparison 2: Minor outcomes, Outcome 4: Hospital readmission | ||||
| 2.4.1 Rehabilitation‐related activities | 6 | 2960 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.78, 1.16] |
| 2.4.2 Structured exercise | 1 | 339 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.52, 1.18] |
| 2.4.3 Progressive resistance exercise | 7 | 1390 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.72, 1.36] |
| 2.5 Walking performance at discharge from hospital Show forest plot | 6 | 682 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.13 [‐0.35, 0.09] |
| Analysis 2.5  Comparison 2: Minor outcomes, Outcome 5: Walking performance at discharge from hospital | ||||
| 2.5.1 Rehabilitation‐related activities | 1 | 273 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.29 [‐0.53, ‐0.05] |
| 2.5.2 Structured exercise | 2 | 131 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.32 [‐0.80, 0.16] |
| 2.5.3 Progressive resistance exercise | 3 | 278 | Std. Mean Difference (IV, Random, 95% CI) | 0.09 [‐0.15, 0.33] |
Study flow diagram

Funnel plot: independence with activities of daily living at discharge from hospital.
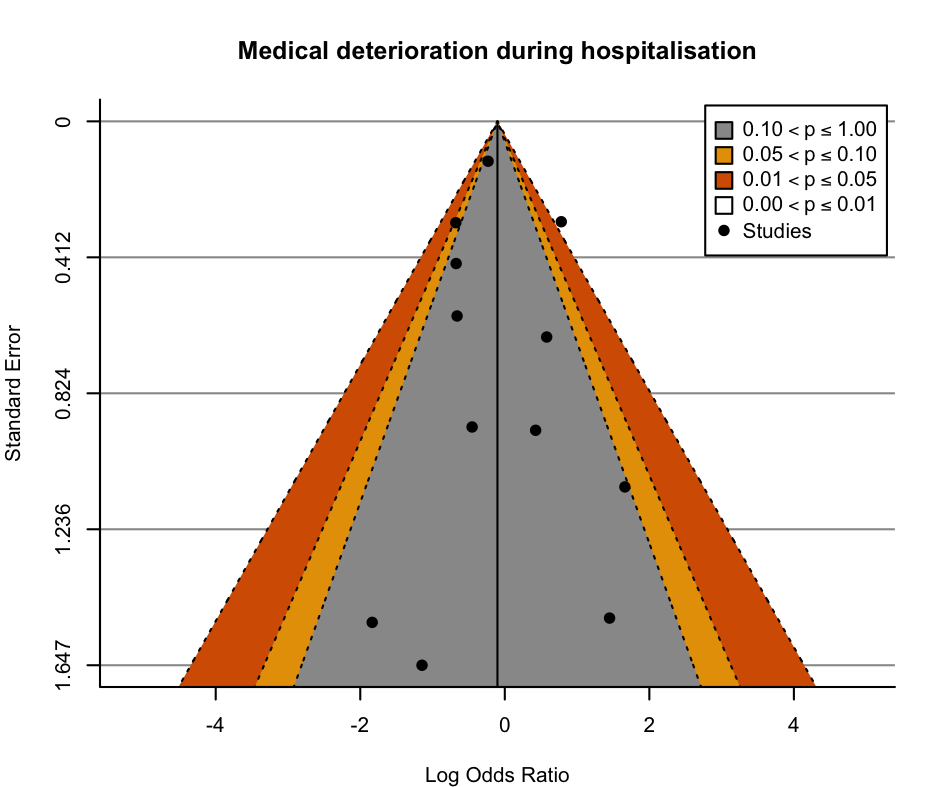
Funnel plot: medical deterioration during hospitalisation.

Funnel plot: mortality during hospitalisation.
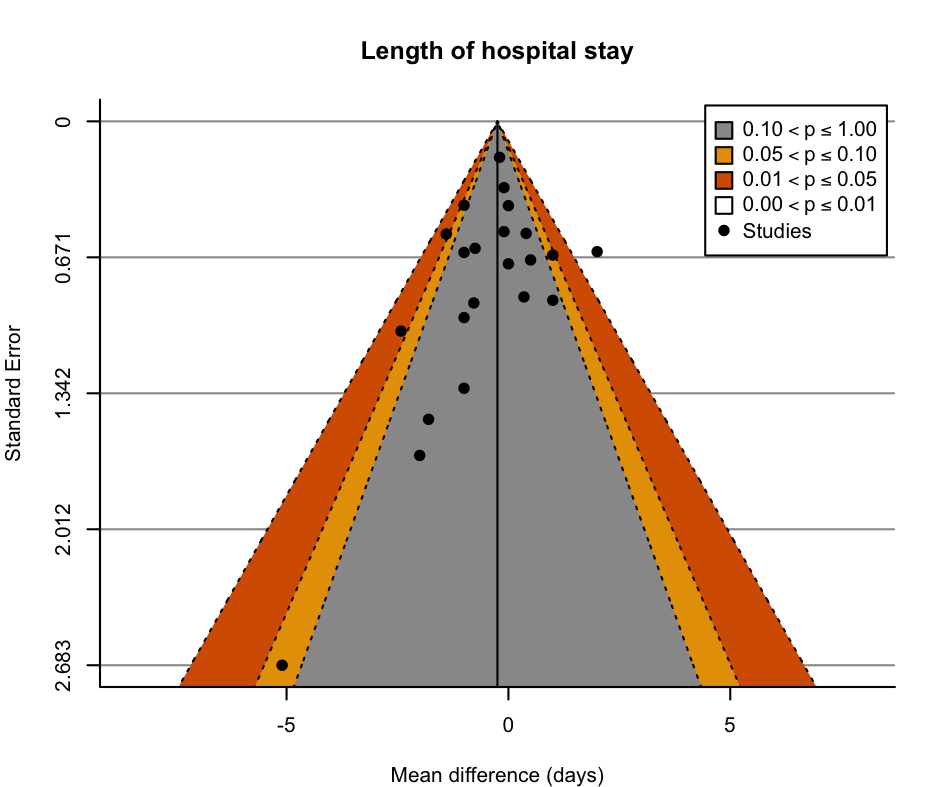
Funnel plot: length of hospital stay.

Funnel plot: readmissions to hospital.

Comparison 1: Major outcomes, Outcome 1: Functional ability: independence with activities of daily living at discharge from hospital

Comparison 1: Major outcomes, Outcome 2: Functional ability: functional mobility at discharge from hospital

Comparison 1: Major outcomes, Outcome 3: Functional ability: new incidence of delirium during hospitalisation

Comparison 1: Major outcomes, Outcome 4: Quality of life at discharge from hospital

Comparison 1: Major outcomes, Outcome 5: Falls during hospitalisation

Comparison 1: Major outcomes, Outcome 6: Medical deterioration during hospitalisation

Comparison 2: Minor outcomes, Outcome 1: Death during hospitalisation

Comparison 2: Minor outcomes, Outcome 2: Hospital length of stay (days)

Comparison 2: Minor outcomes, Outcome 3: New institutionalisation at hospital discharge

Comparison 2: Minor outcomes, Outcome 4: Hospital readmission

Comparison 2: Minor outcomes, Outcome 5: Walking performance at discharge from hospital
| Exercise interventions compared to usual care with or without sham interventions for acutely hospitalised older medical patients | ||||||
| Patient or population: acutely hospitalised medical patients | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Risk with usual care ± sham interventions | Risk with exercise interventions | |||||
| Functional ability: independence with activities of daily living at discharge from hospital | The mean functional ability: independence with activities of daily living at discharge from hospital ranged from 42 to 96 points on the Barthel Indexa | MD 1.8 points on the Barthel Index higher | ‐ | 5174 | ⊕⊕⊝⊝ | Exercise interventions may result in little to no difference in independence with activities of daily living at discharge from hospital (SMD 0.09, 95% CI −0.02 to 0.19). A change of 11 points on the Barthel Index is thought to represent a minimally clinically important difference (MCID). |
| Functional ability: functional mobility at discharge from hospital | The mean functional ability: functional mobility at discharge from hospital ranged from 3.7 to 4.9 points on the Short Physical Performance Battery e | MD 0.78 points on the Short Physical Performance Battery higher | ‐ | 2369 | ⊕⊝⊝⊝ | The evidence is very uncertain about the effect of exercise on functional mobility at discharge from hospital (SMD 0.28, 95% CI −0.01 to 0.56). A change of 1.0 points on the Short Physical Performance Battery is thought to represent an MCID. |
| Functional ability: new incidence of delirium during hospitalisation | 81 per 1000 | 73 per 1000 | RR 0.90 | 2088 | ⊕⊝⊝⊝ | The evidence suggests that exercise results in little to no difference in incidence of delirium during hospitalisation. |
| Quality of life at discharge from hospital | The mean quality of life at discharge from hospital ranged from 48.7 to 64.7 points on the EQ‐5D VAS | MD 6.04 points on the EQ‐5D VAS higher | ‐ | 875 | ⊕⊕⊝⊝ | Exercise interventions may result in a small clinically unimportant improvement in quality of life at discharge from hospital. A change of 10 points on the EQ‐5D VAS is thought to represent a MCID. |
| Falls during hospitalisation | 34 per 1000 | 34 per 1000 | RR 0.99 | 1787 | ⊕⊕⊕⊝ | Exercise interventions probably result in little to no difference in falls during hospitalisation. |
| Medical deterioration during hospitalisation | 71 per 1000 | 73 per 1000 | RR 1.02 | 2730 | ⊕⊝⊝⊝ | Exercise interventions may have no effect on medical deterioration during hospitalisation. |
| Participant global assessment of success | Not pooled | Not pooled | Not pooled | (0 studies) | ‐ | No studies reported participant global assessment of success. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| See interactive version of this table: https://gdt.gradepro.org/presentations/#/isof/isof_question_revman_web_423027971375700878. | ||||||
| a Range based on the seven studies that measured activities of daily living using a Barthel Index (range 0–100). | ||||||
| Rehabilitation‐related activity interventions compared to usual care with or without sham interventions for acutely hospitalised older medical patients | ||||||
| Patient or population: acutely hospitalised older medical patients | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Risk with usual care ± sham interventions | Risk with rehabilitation‐related activities | |||||
| Functional ability: independence with activities of daily living at discharge from hospital | The mean functional ability: independence with activities of daily living at discharge from hospital was 42 points on the Barthel Indexa | MD 0 points on the Barthel Index | ‐ | 2838 | ⊕⊕⊝⊝ | Rehabilitation‐related activities may result in little to no difference in independence with activities of daily living at discharge from hospital (standardised mean difference (SMD) 0.00, 95% CI −0.12 to 0.13). A change of 11 points on the Barthel Index is thought to represent a minimally clinically important difference (MCID). |
| Functional ability: functional mobility at discharge from hospital | The mean functional ability: functional mobility at discharge from hospital was 5 points on the Physical Performance and Mobility Examination | MD 0.14 points on the Physical Performance and Mobility Examination higher | ‐ | 975 | ‐ | Included only 1 study categorised as delivering a rehabilitation‐related activity intervention. The effect of rehabilitation‐related activities on functional mobility at discharge from hospital was very uncertain. |
| Incidence of new delirium during hospitalisation | 107 per 1000 | 92 per 1000 | RR 0.86 | 732 | ⊕⊝⊝⊝ | The evidence was very uncertain with regard to the effect of rehabilitation‐related activity interventions on incidence of delirium during hospitalisation. |
| Falls during hospitalisation | 24 per 1000 | 32 per 1000 | RR 1.33 | 250 | ‐ | Only 1 study categorised as delivering a rehabilitation‐related activity intervention was included. The effect of rehabilitation‐related activities on falls during hospitalisation was very uncertain. |
| Quality of life at discharge from hospital | The mean quality of life at discharge from hospital was 48.9 points on the EQ‐5D VAS | MD 2.2 points on the EQ‐5D VAS higher | ‐ | 350 | ‐ | Only 1 study reported a quality‐of‐life outcome at hospital discharge. The effect of rehabilitation‐related activities on the incidence of delirium during hospitalisation was very uncertain. |
| Medical deterioration during hospitalisation | 107 per 1000 | 92 per 1000 | RR 0.86 | 732 | ⊕⊝⊝⊝ | The evidence was very uncertain with regard to the effect of rehabilitation‐related activity interventions on incidence of medical deterioration during hospitalisation. |
| Participant global assessment of success | Not pooled | Not pooled | Not pooled | (0 studies) | ‐ | No studies reported participant global assessment of success. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| See interactive version of this table: https://gdt.gradepro.org/presentations/#/isof/isof_question_revman_web_423062461138566957. | ||||||
| a Based on the one study that measured activities of daily living using a Barthel Index (range of possible scores 0–100). | ||||||
| Structured exercise interventions compared to usual care with or without sham interventions for acutely hospitalised older medical patients | ||||||
| Patient or population: acutely hospitalised older medical patients | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Risk with usual care ± sham interventions | Risk with structured exercise interventions | |||||
| Functional ability: independence with activities of daily living at discharge from hospital | The mean functional ability: independence with activities of daily living at discharge from hospital ranged from 55 to 56 points on the Barthel Indexa | MD 2.6 points on the Barthel Index higher | ‐ | 648 | ⊕⊕⊝⊝ | Structured exercise may result in little to no difference in independence with activities of daily living at discharge from hospital (standardised mean difference (SMD) 0.12, 95% CI −0.21 to 0.45). A change of 11 points on the Barthel Index is thought to represent a minimally clinically important difference (MCID). |
| Functional ability: functional mobility at discharge from hospital | The mean functional ability: functional mobility at discharge from hospital was 14.13 units on the Elderly Mobility Scalee | MD 1.79 units on the Elderly Mobility Scale higher | ‐ | 416 | ⊕⊝⊝⊝ | The evidence was very uncertain with regard to the effect of structured exercise programmes on functional mobility at discharge from hospital (SMD 0.30 95% CI, ‐0.96, 1.57). A change of 2 points on the Elderly Mobility Scale is thought to represent an MCID. |
| Functional ability: new incidence of delirium during hospitalisation | Only 1 study reported the outcome. The study found only 1 incidence of delirium in the intervention group and 0 in the control group. | 100 | ‐ | Included only 1 study categorised as delivering a structured exercise intervention. The effect of structured exercise on the incidence of new delirium during hospitalisation was very uncertain. | ||
| Quality of life at discharge from hospital | The mean quality of life at discharge from hospital was 64.74 points on the EQ‐5D VAS | MD 3.74 points on the EQ‐5D VAS higher | ‐ | 76 | ‐ | Only 1 study reported a quality‐of‐life outcome at hospital discharge. The effect of structured exercise interventions on quality of life at discharge from hospital was very uncertain. |
| Falls during hospitalisation | 40 per 1000 | 31 per 1000 | RR 0.76 | 542 | ⊕⊕⊝⊝ | Structured exercise interventions may result in little to no difference in falls during hospitalisation. |
| Medical deterioration during hospitalisation | 20 per 1000 | 51 per 1000 | RR 2.56 | 200 | ⊕⊝⊝⊝ | The evidence was very uncertain with regard to the effect of structured exercise programmes on medical deterioration during hospitalisation. |
| Participant global assessment of success | Not pooled | Not pooled | Not pooled | (0 studies) | ‐ | No studies reported participant global assessment of success. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| See interactive version of this table: https://gdt.gradepro.org/presentations/#/isof/isof_question_revman_web_423064120727928815. | ||||||
| a Range based on the two studies that measured activities of daily living using a Barthel Index (range of possible scores 0–100). | ||||||
| Progressive resistance exercise interventions compared to usual care with or without sham interventions for acutely hospitalised older medical patients | ||||||
| Patient or population: acutely hospitalised older medical patients | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Risk with usual care ± sham interventions | Risk with progressive resistance exercise | |||||
| Functional ability: independence with activities of daily living at discharge from hospital | The mean functional ability: independence with activities of daily living at discharge from hospital ranged from 75 to 96 points on the Barthel Indexa | MD 0.14 points on the Barthel Index higher | ‐ | 1688 | ⊕⊕⊝⊝ | The evidence is classified as very uncertain with regard to the effect of progressive resistance exercise on independence with activities of daily living at discharge from hospital (SMD 0.14, 95% CI −0.05 to 0.32). A change of 11 points on the Barthel Index is thought to represent a minimally clinically important difference (MCID). |
| Functional ability: functional mobility at discharge from hospital | The mean functional ability: functional mobility at discharge from hospital ranged from 3.7 to 4.9 points on the Short Physical Performance Battery e | MD 0.24 points on the Short Physical Performance Battery higher | ‐ | 978 | ⊕⊝⊝⊝ | The evidence is classified as very uncertain with regard to the effect of progressive resistance exercise on functional mobility at discharge from hospital. (SMD 0.63, 95% CI‐0.28, 1.55). A change of 1.0 points on the Short Physical Performance Battery is thought to represent a MCID. |
| Functional ability: new incidence of delirium during hospitalisation | 71 per 1000 | 68 per 1000 | RR 0.96 | 1256 | ⊕⊕⊝⊝ | The evidence is classified as very uncertain with regard to the effect of progressive resistance exercise on incidence of delirium during hospitalisation. |
| Quality of life at discharge from hospital | The mean quality of life at discharge from hospital ranged from 57.5 to 62.4 points on the EQ‐5D VAS | MD 8.9 points on the EQ‐5D VAS higher | ‐ | 449 | ⊕⊕⊕⊝ | Progressive resistance exercise probably increases quality of life at discharge from hospital slightly. A change of 10 points on the EQ‐5D VAS is thought to represent a MCID. |
| Falls during hospitalisation | 34 per 1000 | 33 per 1000 | RR 0.96 | 995 | ⊕⊕⊝⊝ | Progressive resistance exercise may result in little to no difference in falls during hospitalisation. |
| Medical deterioration during hospitalisation | 62 per 1000 | 61 per 1000 | RR 0.99 | 1798 | ⊕⊝⊝⊝ | The evidence is classified as very uncertain with regard to the effect of progressive resistance exercise on medical deterioration during hospitalisation. |
| Participant global assessment of success | Not pooled | Not pooled | Not pooled | (0 studies) | ‐ | This outcome was not measured by any of the included studies. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| See interactive version of this table: https://gdt.gradepro.org/presentations/#/isof/isof_question_revman_web_423057317911555615. | ||||||
| a Range based on the four studies that measured activities of daily living using a Barthel Index (range of possible scores 0–100). | ||||||
| Study ID | Usual care setting and description | Control/sham intervention | Intervention group setting and description | Intervention subgroup category | Exercise component of intervention | Exercise dose prescription | Exercise intervention adherence |
|---|---|---|---|---|---|---|---|
| Acute geriatric unit. Geriatrician‐led care, physiotherapy requested by the geriatrician as required. | None. | Usual care conditions with additional occupational therapy interventions. | Rehabilitation‐related activities. | Occupational therapy including practice of activities of daily living. | 45 minutes, 5 times per week (Monday–Friday), for the duration of hospital admission. | Mean 5 sessions per participant. | |
| Medical ward. Internist‐led care, physiotherapy and occupational therapy not routinely available. No geriatrician. | None. | Acute geriatric ward. Care provided by both geriatricians and internists. Multidisciplinary team included physiotherapists, occupational therapists and dietitians. Emphasis on interdisciplinary care. | Rehabilitation‐related activities. | Exercise component not specifically described, intervention included early start of rehabilitation and routine physiotherapy and occupational therapy assessments. | No information. | No information. | |
| Acute care geriatric medicine unit. Physiotherapy provided from day 3 of admission, for 3 sessions per week until discharge. | None. | Usual care conditions with additional physiotherapy. | Structured exercise. | Early physiotherapy starting from day 1–2 of admission consisting of bed and standing exercises. | 30 minutes, 2 times per day, 5 times per week, until deemed clinically stable. | No information. | |
| Medical ward. Physicians could order physiotherapy services. | Usual care with daily 15‐ to 20‐minute visits from research assistants, up to twice daily, 7 days per week. Participants requested to keep a diary of their visitors. | Usual care conditions + mobility programme, and encouragement to increase time out of bed. | Structured exercise | Assisted/ supervised mobility programme with behavioural intervention to encourage additional physical activity outside the supervised intervention. | 15–20 minutes, up to twice per day, 7 days per week, for the duration of hospital admission. | 122/238 (51.3%) potential walks were completed. | |
| Usual care units. Not described.
| None. | Acute Care for Elders Unit Renovated ward with a physiotherapy room. Daily interdisciplinary team rounds provided by geriatrician medical director and geriatric clinical nurse specialist who created care plans. Care processes designed to promote functional independence. | Rehabilitation related activities. | Exercise component not specifically described, intervention included a mobility protocol and physiotherapy. | No information. | No information. | |
| Medical ward. Routine care, discharge planning and rehabilitation advice normally provided. | None. | Usual care with additional exercise. | Progressive resistance exercise. | With 72 hours of admission a care plan was produced by a nurse and physiotherapist which included: facilitated stretching, balance training, walking and strengthening exercises. | Walking for up to 15 minutes (duration of other exercise not specified), up to 2–4 times per week, for the duration of the hospital admission. | No information regarding in‐hospital adherence. | |
| Medical wards. Daily medical assessment, and allied health service on referral. | None. | Usual care with additional exercise. | Progressive resistance exercise. | Supervised strengthening and mobility exercise. | 20–30 minutes, twice per day, 5 days per week, for the duration of the hospital admission. | No information. | |
| Acute medical care unit. Care led by physicians specialising in internal medicine. Physiotherapy/ occupational therapy available for counselling only. | None. | Comprehensive geriatric assessment unit. Structured comprehensive geriatric assessment and care led by physicians specialising in internal medicine, family medicine, geriatrics or a combination. Unit staff included physiotherapists and occupational therapists. | Rehabilitation‐related activities. | Exercise component not specifically described, intervention included routine physiotherapy and occupational therapy. | No information. | No information. | |
| Medical or surgical floors. Description not provided other than 'standard medical care'. | None. | Senior Care Unit Functional assessment on admission, 3 clinical team meetings and 1 administration meeting weekly. Geriatric assessment team included nurse co‐ordinators and a physiotherapist. Emphasise interdisciplinary comprehensive geriatric assessment and intervention. | Rehabilitation‐related activities. | Exercise component not specifically described, intervention included routine functional assessment and physiotherapy. | No information. | No information. | |
| Geriatric unit. Care led by a geriatrician and provided by multidisciplinary team. | None. | Usual care with a walking intervention guided by geriatrician, delivered by nurses. | Structured exercise. | Assisted walking programme. | 20–30 minutes, daily, 5 days per week, for the duration of hospitalisation. | A mean time of 32 minutes per session (range 10–67), with a mean distance of 89 m (range 0–260). Mean number of intervention days for each participant was 5.8. | |
| Medical wards. Not described. | None. | Usual care conditions with mobility programme. | Structured exercise. | Assisted or supervised exercise, including balance, pedalling and mobility activities. | Up to 30 minutes per day, for the duration of hospital admission. | No information. | |
| Medical unit. Daily medical assessment and allied health professionals available via referral. | None. | Usual care conditions with additional exercise and orientation. | Progressive resistance exercise. | Progressive resistance exercise and mobility training. | 20–30 minutes per day (Monday–Friday), twice per day, for the duration of hospitalisation. | Median of 1.4 therapy sessions per day or 38 minutes per day (including weekends and routine therapy). This was equivalent to approximately 1.4 sessions or 42 minutes of additional therapy per weekday compared to the control group. | |
| General medical wards. Allied health interventions including physiotherapy available. | None. | Usual care conditions with additional exercise. | Progressive resistance exercise. | Individualised assisted or supervised strength, balance and functional exercises. | 30 minutes, twice per day for the duration of hospitalisation. | Median of 160 minutes (IQR 120–360) participating in the exercise intervention. | |
| Medical units. Physiotherapy available. | None. | Usual care conditions with additional assisted/ supervised walking. | Structured exercise. | Assisted or supervised walking programme. | Twice per day, 7 days per week, for 7 days. The distance walked was the maximum distance able to be comfortably walked as decided by that individual at that time. | No information. | |
| General medical unit. Care led by attending physician, nursing:participant ratio approximately 1:2. Access to hospital wide support services including physiotherapy. | None. | Acute Care for Elders Unit Care led by medical and nursing directors. Increased funded multidisciplinary team hours compared to usual care (including physiotherapy) with care protocols and ward environment designed to promote independence and early discharge. | Rehabilitation‐related activities | Exercise component not specifically described, intervention included a mobility protocol and physiotherapy. | No information. | No information. | |
| Acute Care for the Elderly Unit. Care led by a geriatrician with routine physiotherapy available when needed. | None | Usual care conditions with additional exercise. | Progressive resistance exercise | Supervised morning sessions included progressive resistance, balance and walking exercises. Unsupervised functional exercises in evenings. | 20 minutes, twice per day for 5–7 consecutive days (including weekends). | The mean number of completed morning sessions per participant was 5 (SD 1) and evening sessions was 4 (SD 1). Adherence to the intervention was 95.8% for the morning sessions (i.e. 806 successfully completed sessions of 841 total possible sessions) and 83.4% in the evening sessions (574 of 688 successfully completed sessions). | |
| All wards admitting older medical patients. Physiotherapy available to all participants (mean 3 sessions per week).
| Usual care with twice‐daily sessions (Monday–Friday) each 20–30 minutes of stretching and relaxation exercises in lying or sitting. Participants encouraged to talk about their condition and exercise, none given education, encouragement or assisted to exercise or walk more. | Usual care with additional exercise. | Progressive resistance exercise. | Assisted or supervised tailored strengthening, balance and gait exercises. | Up to 30 minutes, 2 times per day (Monday–Friday) for the duration of hospital admission. | 63/95 participants completed ≥ 75% of possible exercise sessions; 16/95 participants completed 50–74% of possible exercise sessions. 13/95 participants completed 25–49% of possible exercise sessions. 3/95 participants completed < 25% of possible exercise sessions. | |
| Acute medical wards for older people. Not described. | None. | Usual care with additional pedalling exercise. | Structured exercise. | Unsupervised pedalling exercise. | 5 minutes, 3 times per day. | The median number of revolutions cycled throughout the entire study period with the pedal exerciser was 152 (IQR 43.5–464.5) revolutions. The median time spent on the pedal exerciser was 5.08 (IQR 2.03–20.05) minutes across the whole study period. | |
| Medical ward. Multidisciplinary care included daily discussion of participant progress and discharge plan. Referrals made to physiotherapy or occupational therapy when needed. | None. | Medical ward Usual care with additional exercise and cognitive group therapy to encourage mobility. Intervention ward staff, participants and carers educated to encourage mobility and functional independence. | Progressive resistance exercise. | Graduated and tailored supervised exercise programme. | Twice per day for the duration of hospital admission. | 92% of participants in the intervention group received an exercise diary and made some record of exercise; 1/3 completed their diary every day. | |
| Acute care of older patient units Not described. | None. | Usual care with additional exercise. | Progressive resistance exercise. | Supervised walking and sit to stand exercises. | 1–3 sessions per day, with a total duration of approximately 20 minutes per day (Monday–Friday). | Participants performed a median of 3 training days (IQR 2) and 2 training sessions per day (IQR 2), with a mean total exercise time per day of 20 minutes (for each session, the median duration of the walking part was 5 minutes (IQR 4, range 0–10), and participants performed a mean of 9 (SD 6, range 0 to 30) sit‐to‐stands). | |
| Acute medical ward and internal medicine ward. National targets to assess function and nutrition and make an appropriate plan within 24–48 hours of admission. Rehabilitation often started during hospitalisation. | None. | Usual care with additional exercise and protein supplements. | Progressive resistance exercise. | Supervised progressive strength training based on sit to stand exercises. | 20 minutes daily (Monday–Friday) for the duration of hospital admission. | 78.8% of participants started the intervention 0–2 days after admission. Overall (during and after hospitalisation), 43% (18/42) of the participants randomised to the intervention group were very compliant with the intervention (80% of sessions performed with 2 sets of 8 repetitions). | |
| General medical elderly care wards. Therapy provided by ward occupational therapist and physiotherapist on weekdays only. | None. | General medical elderly care wards. Therapy provided by community therapy team including occupational therapist and physiotherapist 7 days per week if appropriate. | Rehabilitation related activities. | Exercise component not specifically described, intervention included daily rehabilitation with a physiotherapist or occupational therapist. | Daily, duration dependent on needs. | No information. | |
| General medical unit. Description not provided. | None. | General Medical Unit. In addition to usual care, a geriatric team consisting of a geriatrician, physiotherapist and liaison nurse provided care including daily physiotherapy. The aim of the team was to optimise function and mobility. | Rehabilitation related activities | Exercise component not specifically described, intervention included daily physiotherapy. | No information. | No information. | |
| Internal medical care unit. Care led by internist physician and had access to physical and occupational therapy by referral. | None. | Geriatric care unit. Care led by geriatrician and ward team included physiotherapist and occupational therapist. | Rehabilitation‐related activities | Exercise component not specifically described, intervention included a mobility protocol and physiotherapy. | No information. | No information. | |
| IQR: interquartile range; SD: standard deviation. | |||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1.1 Functional ability: independence with activities of daily living at discharge from hospital Show forest plot | 16 | 5174 | Std. Mean Difference (IV, Random, 95% CI) | 0.09 [‐0.02, 0.19] |
| 1.1.1 Rehabilitation‐related activities | 4 | 2838 | Std. Mean Difference (IV, Random, 95% CI) | 0.00 [‐0.12, 0.13] |
| 1.1.2 Structured exercise | 5 | 648 | Std. Mean Difference (IV, Random, 95% CI) | 0.12 [‐0.21, 0.45] |
| 1.1.3 Progressive resistance exercise | 7 | 1688 | Std. Mean Difference (IV, Random, 95% CI) | 0.14 [‐0.05, 0.32] |
| 1.2 Functional ability: functional mobility at discharge from hospital Show forest plot | 8 | 2369 | Mean Difference (IV, Random, 95% CI) | 0.54 [0.09, 0.99] |
| 1.2.1 Rehabilitation‐related activities | 1 | 975 | Mean Difference (IV, Random, 95% CI) | 0.60 [0.06, 1.14] |
| 1.2.2 Structured exercise | 2 | 416 | Mean Difference (IV, Random, 95% CI) | 0.30 [‐0.96, 1.57] |
| 1.2.3 Progressive resistance exercise | 5 | 978 | Mean Difference (IV, Random, 95% CI) | 0.63 [‐0.28, 1.55] |
| 1.3 Functional ability: new incidence of delirium during hospitalisation Show forest plot | 7 | 2088 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.58, 1.41] |
| 1.3.1 Rehabilitation‐related activities | 2 | 732 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.30, 2.50] |
| 1.3.2 Structured exercise | 1 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 3.00 [0.13, 71.92] |
| 1.3.3 Progressive resistance exercise | 4 | 1256 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.55, 1.68] |
| 1.4 Quality of life at discharge from hospital Show forest plot | 4 | 875 | Mean Difference (IV, Random, 95% CI) | 6.04 [0.90, 11.18] |
| 1.4.1 Rehabilitation‐related activities | 1 | 350 | Mean Difference (IV, Random, 95% CI) | 2.20 [‐1.90, 6.30] |
| 1.4.2 Structured exercise | 1 | 76 | Mean Difference (IV, Random, 95% CI) | 3.74 [‐6.32, 13.80] |
| 1.4.3 Progressive resistance exercise | 2 | 449 | Mean Difference (IV, Random, 95% CI) | 8.90 [2.35, 15.45] |
| 1.5 Falls during hospitalisation Show forest plot | 9 | 1787 | Risk Ratio (IV, Random, 95% CI) | 0.99 [0.59, 1.65] |
| 1.5.1 Rehabilitation‐related activities | 1 | 250 | Risk Ratio (IV, Random, 95% CI) | 1.33 [0.30, 5.84] |
| 1.5.2 Structured exercise | 3 | 542 | Risk Ratio (IV, Random, 95% CI) | 0.76 [0.23, 2.53] |
| 1.5.3 Progressive resistance exercise | 5 | 995 | Risk Ratio (IV, Random, 95% CI) | 0.96 [0.48, 1.91] |
| 1.6 Medical deterioration during hospitalisation Show forest plot | 11 | 2730 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.62, 1.68] |
| 1.6.1 Rehabilitation‐related activities | 2 | 732 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.30, 2.50] |
| 1.6.2 Structured exercise | 2 | 200 | Risk Ratio (M‐H, Random, 95% CI) | 2.56 [0.48, 13.54] |
| 1.6.3 Progressive resistance exercise | 7 | 1798 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.52, 1.87] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 2.1 Death during hospitalisation Show forest plot | 20 | 6822 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.79, 1.22] |
| 2.1.1 Rehabilitation‐related activities | 7 | 3926 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.78, 1.34] |
| 2.1.2 Structured exercise | 5 | 740 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.54, 1.56] |
| 2.1.3 Progressive resistance exercise | 8 | 2156 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.54, 1.48] |
| 2.2 Hospital length of stay (days) Show forest plot | 22 | 7182 | Mean Difference (IV, Random, 95% CI) | ‐0.25 [‐0.62, 0.12] |
| 2.2.1 Rehabilitation‐related activities | 9 | 4388 | Mean Difference (IV, Random, 95% CI) | ‐0.55 [‐1.42, 0.32] |
| 2.2.2 Structured exercise | 4 | 635 | Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.93, 0.89] |
| 2.2.3 Progressive resistance exercise | 9 | 2159 | Mean Difference (IV, Random, 95% CI) | ‐0.24 [‐0.63, 0.16] |
| 2.3 New institutionalisation at hospital discharge Show forest plot | 5 | 2364 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.74, 1.12] |
| 2.3.1 Rehabilitation‐related activities | 3 | 2004 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.74, 1.13] |
| 2.3.2 Progressive resistance exercise | 2 | 360 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.37, 2.01] |
| 2.4 Hospital readmission Show forest plot | 14 | 4689 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.81, 1.11] |
| 2.4.1 Rehabilitation‐related activities | 6 | 2960 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.78, 1.16] |
| 2.4.2 Structured exercise | 1 | 339 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.52, 1.18] |
| 2.4.3 Progressive resistance exercise | 7 | 1390 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.72, 1.36] |
| 2.5 Walking performance at discharge from hospital Show forest plot | 6 | 682 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.13 [‐0.35, 0.09] |
| 2.5.1 Rehabilitation‐related activities | 1 | 273 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.29 [‐0.53, ‐0.05] |
| 2.5.2 Structured exercise | 2 | 131 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.32 [‐0.80, 0.16] |
| 2.5.3 Progressive resistance exercise | 3 | 278 | Std. Mean Difference (IV, Random, 95% CI) | 0.09 [‐0.15, 0.33] |
| Bias | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study | Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | ||||||
| Subgroup 1.1.1 Rehabilitation‐related activities | ||||||||||||
| Abizanda 2011 |  |  |  |  |  |  | ||||||
| Allocation sequence was random (computer generated random numbers). Allocation occurred after enrolment by an investigator not involved in the participants' clinical management. Higher number of participants admitted with a stroke in the intervention group (39 vs. 16), in addition, the 'Others' subgroup the Barthel index is higher in the control group than the intervention group (29.1 vs. 23.1). This is thought to be compatible with chance. | ||||||||||||
| Its assumed participants and the Occupational Therapist (OT) delivering the intervention were aware of the assigned intervention allocation. All participants received their allocated intervention. Therefore, even though intention to treat analysis not specified, analysis deemed appropriate. | ||||||||||||
| Given the nature of this population (older adults during and after acute hospitalisation) we considered a threshold of 90% of participants with data as sufficient. We felt this would be consistent with measurements outside of a trial context. When accounting for mortality, 95% and 94% assessed at discharge in intervention and control arms respectively. | ||||||||||||
| The use of the Barthel Index to measure independence with ADLs is considered appropriate, and there were no differences in measurement or ascertainment of the outcomes between groups. The OT assessor was blinded. | ||||||||||||
| The data analyst was blinded, and followed a pre‐specified statistical plan. It is not thought that results were from multiple outcome measurements or multiple analyses. | ||||||||||||
| The study is judged to be at low risk of bias in all domains for this outcome. | ||||||||||||
| Counsell 2000 |  |  |  |  |  |  | ||||||
| Allocation sequence was random (computer generated random numbers) and sequence concealed (opaque sealed envelope). Participant characteristics were balanced. | ||||||||||||
| Participants and those delivering the interventions were aware of intervention assignments. Seventy‐nine participants were not admitted to the unit to which they were assigned. Deviations may have affected the effect estimate, however they were well‐balanced between groups. Intention to treat analysis was used. | ||||||||||||
| Functional data was obtained in 1476 of 1483 of surviving patients (99.5%) at discharge. | ||||||||||||
| The Katz ADL score is considered appropriate, and there were no differences in the measurement or ascertainment between groups. The assessors were not blinded, and it was therefore considered likely that knowledge of the intervention could influence the outcome, given the likely strong belief in the benefits of the intervention ward. | ||||||||||||
| A pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to be at high risk of bias due to measurement of the outcome. | ||||||||||||
| Ekerstad 2017 |  |  |  |  |  |  | ||||||
| Randomisation was based on the availability of beds, and there was no allocation concealment as participants were allocated a ward prior to enrolment. Participant characteristics appeared balanced. | ||||||||||||
| Participants and clinicians delivering care were aware of treatment assignments. There was no evidence of deviations from intended interventions and intention to treat analysis was used. | ||||||||||||
| No missing data at hospital discharge. | ||||||||||||
| The ADL Staircase is considered appropriate, and there were no differences in the measurement or ascertainment between groups. The assessors were not blinded, and it was therefore considered likely that knowledge of the intervention could influence the outcome, given the likely strong belief in the benefits of the intervention ward. | ||||||||||||
| A detailed pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to be at high risk of bias due to bias in the measurement of the outcome and bias arising from the randomisation process. | ||||||||||||
| Landefeld 1995 |  |  |  |  |  |  | ||||||
| Allocation sequence was random (computer generated random numbers) and balanced participant characteristics, but no information was provided regarding allocation concealment. | ||||||||||||
| It is assumed participants and clinicians were aware of the treatment assignments. There was no evidence of deviations from the assigned interventions and evidence of an intention to treat approach being used for analysis. | ||||||||||||
| Accounting for mortality (24 died in each arm) all participants had outcome data at discharge. | ||||||||||||
| The Katz ADL score is considered appropriate, and there were no differences in the measurement or ascertainment between groups. The assessors were not blinded, and it was therefore considered likely that knowledge of the intervention could influence the outcome, given the likely strong belief in the benefits of the intervention ward. | ||||||||||||
| A pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to be at high risk of bias in measurement of the outcome for this outcome. | ||||||||||||
| Subgroup 1.1.2 Structured exercise | ||||||||||||
| Blanc‐Bisson 2008 |  |  |  |  |  |  | ||||||
| No information provided on randomisation methods or sequence concealment other than to say participants were randomised. Differences in participant characteristics appear compatible with chance. | ||||||||||||
| It is assumed that participants and those delivering the interventions were aware of intervention assignments. However, all participants received their intended intervention and intention to treat was specified in the analysis. | ||||||||||||
| When accounting for mortality, data was available for only 72% and 81% of intervention and control group respectively at T1. Some missing data is likely to be dependent on its true value, as the main reason for missing data was adverse events, and we consider participants who experience adverse events to be more likely to have a lower ADL score. | ||||||||||||
| The Katz ADL score is considered appropriate, and there were no differences in the measurement or ascertainment between groups. The assessors were not blinded, and it was therefore considered likely that knowledge of the intervention could influence the outcome, given the likely strong belief in the benefits of the intervention ward. | ||||||||||||
| A pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to be at high risk of bias due to missing outcome data and measurement of the outcome. | ||||||||||||
| Brown 2016 |  |  |  |  |  |  | ||||||
| No description of randomisation sequence generation, but the paper describes a block randomisation strategy and sequence concealed (sealed envelopes). The patient characteristics appear well‐balanced between groups. | ||||||||||||
| We assume both participants and those delivering the interventions were aware of intervention assignments. 6 participants did not receive allocated intervention, this is considered to be consistent with what could occur outside the trial context, and intention to treat analysis was used. | ||||||||||||
| No missing data. | ||||||||||||
| The Katz ADL score is considered appropriate, and there were no differences in the measurement or ascertainment between groups. The assessors were blinded. | ||||||||||||
| The study protocol details statistical analysis plan which is in accordance with results presented, and it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to be at low risk of bias in all domains for this outcome. | ||||||||||||
| Gazineo 2021 |  |  |  |  |  |  | ||||||
| Allocation sequence was random (generated using an online system) and the allocation sequence was concealed until participants were enrolled (opaque concealed and sealed envelopes). Participant characteristics were well‐balanced. | ||||||||||||
| Participants and those delivering the interventions were aware of intervention allocations. There was no evidence of deviations from the intended interventions and intention to treat analysis used. | ||||||||||||
| No missing data after accounting for mortality. | ||||||||||||
| The Barthel Index is considered appropriate, and there were no differences in the measurement or ascertainment between groups. The assessors were not blinded, and it was therefore considered likely that knowledge of the intervention could influence the outcome, given the likely strong belief in the benefits of the intervention ward. | ||||||||||||
| A pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to be at high risk of bias due to measurement of the outcome. | ||||||||||||
| Hu 2020 |  |  |  |  |  |  | ||||||
| Allocation sequence was random (computer generated random numbers) and sequence concealed (blinded project coordinator allocated participants). Participant characteristics were balanced. | ||||||||||||
| Participants and those delivering the interventions were aware of intervention allocations. There was no evidence of deviations from the intended interventions and as such it is presumed intention to treat analysis was used. | ||||||||||||
| At discharge data was available for 76% of reablement group, (78% of reminder group) and 80% of the control group. Only participants who had complete data (i.e. data collected at baseline, discharge and follow‐up) had outcomes reported. Although missing data is well‐balanced between all three groups, it is considered likely that reason for missing data is related to its true value. | ||||||||||||
| The Katz ADL score is considered appropriate, and there were no differences in the measurement or ascertainment between groups. The assessors were blinded. | ||||||||||||
| A pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to be at high risk of bias due to missing outcome data. | ||||||||||||
| Killey 2006 |  |  |  |  |  |  | ||||||
| Allocation not random and sequence predictable as based on alternation. There is limited data on participant characteristics, but available data suggests balanced characteristics between groups. | ||||||||||||
| Participants and those delivering the interventions were aware of intervention allocations. There was no evidence of deviations from the intended interventions. Per‐protocol analysis appears to have been used. 2 participants in the intervention group were excluded from analysis as they completed less than 70% of their walks. The 2 participants that were excluded accounted for 7% of the remaining sample, it is therefore considered that there was potential for a significant impact on the results. | ||||||||||||
| Only 71% of intervention and 74% of control group had outcome data. A significant proportion of missing data was related to early discharge from hospital. These participants could be expected to have a higher functional level than those who remained in hospital. However, the missing data was well‐balanced between the two groups. | ||||||||||||
| The Barthel Index is considered appropriate, and there were no differences in the measurement or ascertainment between groups. The assessors were not blinded, and it was therefore considered likely that knowledge of the intervention could influence the outcome, given the likely strong belief in the benefits of the intervention ward. | ||||||||||||
| A pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to be at high risk of bias in three domains for this outcome. | ||||||||||||
| Subgroup 1.1.3 Progressive resistance exercise | ||||||||||||
| de Morton 2007 |  |  |  |  |  |  | ||||||
| Allocation of wards was random (coin toss) and the allocating officer unaware of study. Baseline differences between groups were thought to be compatible with chance. | ||||||||||||
| Participants and clinicians delivering care were believed to be aware of treatment assignments. There was no evidence of deviations from intended interventions and intention to treat analysis was used. | ||||||||||||
| After accounting for mortality, discharge Barthel Index (BI) scores were available for 75% in intervention group and 70% in control group. Although it is feasible that following discharge patients with lower levels of functional ability are more likely to be missing, we do not think this applies in this situation as assessment prior to discharge. We do not see a situation where it would be more likely to miss an assessment due to high/low level of disability at discharge. | ||||||||||||
| The use of the BI to measure independence with ADLs is considered appropriate, and there were no differences in the measurement or ascertainment between groups. The assessors were not blinded, and it is considered likely that knowledge of the intervention could influence the outcome, given the likely strong belief in the benefits of the intervention. | ||||||||||||
| A pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to be at high risk of bias in measurement of the outcome. | ||||||||||||
| Jeffs 2013 |  |  |  |  |  |  | ||||||
| Sequence concealment was achieved via a member of the research team not involved in recruitment and using sealed envelopes. There was no information regarding method of generating random sequence, other than to say that there was randomisation, given details of allocation concealment, assumption made that appropriate method used. Patient characteristics between groups appear well‐balanced. | ||||||||||||
| Participants and those delivering the interventions were likely to be aware of intervention allocations. One participant was not allocated to a group due to an administrative error and 35 participants did not receive the intervention as planned (17 in the intervention group). This is thought to be consistent with what could occur outside of the trial context. Intention to treat analysis was used. | ||||||||||||
| No missing data. | ||||||||||||
| The Barthel Index is considered appropriate, and there were no differences in the measurement or ascertainment between groups. The assessors were blinded. | ||||||||||||
| A pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to raise some bias concerns in selection of the reported result. | ||||||||||||
| Jones 2006 |  |  |  |  |  |  | ||||||
| Allocation sequence was random (computer generated random numbers). Sequence allocation was not concealed, but performed by a member of staff independent of the enrolment procedures. ). Participant characteristics were balanced between groups and differences compatible with chance. | ||||||||||||
| It is assumed that both participants and clinicians delivering care were aware of assigned interventions. There is no evidence of deviations from intended interventions and intention to treat analysis used. | ||||||||||||
| Admission and discharge modified Barthel Index (mBI) scores were available for 78.8% of patients (126/160). Although we felt that patients with lower levels of functional ability were more likely to be missing at follow‐up, we do not think this applies in this situation as assessment was prior to discharge. We do not see a situation where it would be more likely to miss an assessment due to high/low level of disability at discharge | ||||||||||||
| The use of the mBI to measure independence with ADLs is considered appropriate, and there were no differences in the measurement or ascertainment between groups. The assessors were blinded. | ||||||||||||
| A pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to raise some concerns due to missing outcome data and in selection of the reported results. | ||||||||||||
| Martinez‐Velilla 2019 |  |  |  |  |  |  | ||||||
| Allocation sequence was random (www.randomizer.org) and personal communication with author confirms allocation concealment. Baseline differences between groups are thought to be compatible with chance. | ||||||||||||
| Both participants and clinicians delivering care were aware of assigned interventions. There is no evidence of deviations from intended interventions and intention to treat analysis used. | ||||||||||||
| Assuming that those that discontinued the study did not provide Barthel Index (BI) outcome data, and accounting for mortality, 80% of intervention group and 78% of control group had outcome data. Although there were participants who discontinued the study due to medical deterioration, which could have and is considered likely to have biased the results (i.e. medical deterioration being associated with poor functional outcomes), the numbers were relatively low (only 6% of the sample) and were well‐balanced between groups. We therefore feel that this was unlikely to bias the results. | ||||||||||||
| The use of the BI to measure independence with ADLs is considered appropriate, and there were no differences in the measurement or ascertainment between groups. The assessors were blinded. | ||||||||||||
| The trial protocol reflects the analyses as that were conducted, and it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to raise some concerns due to missing outcome data. | ||||||||||||
| Mudge 2008 |  |  |  |  |  |  | ||||||
| Pseudo‐randomisation, allocation was based on admitting unit and bed availability, and admitting unit was determined by a rotating roster. No evidence of baseline differences in patient characteristics other than those thought to be compatible with chance. | ||||||||||||
| Both participants and clinicians delivering care were aware of assigned interventions. There is no evidence of deviations from intended interventions and intention to treat analysis used. | ||||||||||||
| No missing data. | ||||||||||||
| The use of the modified Barthel Index to measure independence with ADLs is considered appropriate, and there were no differences in the measurement or ascertainment between groups. The assessors were blinded. | ||||||||||||
| A pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to be at high risk of bias due to the randomisation process. | ||||||||||||
| Ortiz‐Alonso 2020 |  |  |  |  |  |  | ||||||
| The allocation sequence was based on recruitment blocks of 4‐8 weeks. Activity of daily living (ADL) scores at baseline and admission were significantly lower in the intervention group than the control group, this is thought to be an important prognostic factor and therefore a concern regarding the randomisation process. | ||||||||||||
| Methods were designed to blind participants from group assignments however due to the nature of the intervention and lack of sham intervention the success of this blinding is felt unlikely. Ward staff and research staff were not blinded to the participant's assigned intervention. There were no deviations from the intended interventions due to trial context described and although intention to treat analysis not specified given the lack of deviations it is assumed. | ||||||||||||
| After adjusting for mortality 97% in intervention group and 97% in control group had outcome data. | ||||||||||||
| The Katz ADL score is considered appropriate, and there were no differences in the measurement or ascertainment between groups. The assessors were not blinded, and it was therefore considered likely that knowledge of the intervention could influence the outcome, given the likely strong belief in the benefits of the intervention ward. | ||||||||||||
| Analysis reflects plan in trial registration and study protocol, and results are not thought to be from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to be at high risk of bias due to the randomisation process and measurement of the outcome. | ||||||||||||
| Pedersen 2019 |  |  |  |  |  |  | ||||||
| Allocation sequence was random (computer‐generated block randomisation), and allocation sequence concealed from the investigators. Participant characteristics were well‐balanced between groups. | ||||||||||||
| Participants and clinicians were aware of the treatment assignments. There was no evidence of deviations from the assigned interventions and an intention to treat approach was used for analysis. | ||||||||||||
| When accounting for mortality only 93% of participants in intervention group and 67% in control group completed measures at discharge. The difference in the proportion of missing data, and that the assessments were carried out home after discharge, it was judged likely that the reasons for missing data may have depended on its true value. | ||||||||||||
| The Barthel Index is considered appropriate, and there were no differences in the measurement or ascertainment between groups. The assessors were blinded. | ||||||||||||
| The analysis is in accordance with a pre‐specified analysis in a published protocol, and results are not thought to be from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to be at high risk of bias due to missing outcome data. | ||||||||||||
| Bias | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study | Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | ||||||
| Subgroup 1.2.1 Rehabilitation‐related activities | ||||||||||||
| Counsell 2000 |  |  |  |  |  |  | ||||||
| Allocation sequence was random (computer generated random numbers) and sequence concealed (opaque sealed envelope). Participant characteristics were balanced. | ||||||||||||
| Participants and those delivering the interventions were aware of intervention assignments. Seventy‐nine participants were not admitted to the unit to which they were assigned. Deviations may have affected the effect estimate, however the were well‐balanced between groups. Intention to treat analysis was used. | ||||||||||||
| Functional data was obtained in 1476 of 1483 of surviving patients (99.5%) at discharge. | ||||||||||||
| The Physical Performance and Mobility Examination (PPME) is considered appropriate, and there were no differences in the measurement or ascertainment between groups. The assessors were not blinded, and it was therefore considered likely that knowledge of the intervention could influence the outcome, given the likely strong belief in the benefits of the intervention ward. | ||||||||||||
| A pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to be at high risk of bias due to measurement of the outcome. | ||||||||||||
| Subgroup 1.2.2 Structured exercise | ||||||||||||
| Gazineo 2021 |  |  |  |  |  |  | ||||||
| Allocation sequence was random (generated using an online system) and the allocation sequence was concealed until participants were enrolled (opaque concealed and sealed envelopes). Participant characteristics were well‐balanced. | ||||||||||||
| Participants and those delivering the interventions were aware of intervention allocations. There was no evidence of deviations from the intended interventions and intention to treat analysis used. | ||||||||||||
| No missing data after accounting for mortality. | ||||||||||||
| The Barden Activity Subscale to measure functional mobility is considered appropriate, and there were no differences in the measurement or ascertainment between groups. The assessors were not blinded, and it was therefore considered likely that knowledge of the intervention could influence the outcome, given the likely strong belief in the benefits of the intervention ward. | ||||||||||||
| A pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to be at high risk of bias due to measurement of the outcome. | ||||||||||||
| McGowan 2018a |  |  |  |  |  |  | ||||||
| Allocation sequence was random (www.randomization.com) and the allocation sequence was only known by the chief investigator who was not involved with screening patients. Baseline differences between groups are thought to be compatible with chance. | ||||||||||||
| Both participants and clinicians delivering care were aware of assigned interventions. There is no evidence of deviations from intended interventions and intention to treat analysis not explicitly stated, but all participants appear to have been analysed in the group to which they were randomised. | ||||||||||||
| 96% of participants had complete data sets. | ||||||||||||
| The use of the Elderly Mobility Scale (EMS) to measure functional mobility is considered appropriate, and there were no differences in the measurement or ascertainment between groups. The assessors were not blinded, and it is considered likely that knowledge of the intervention could influence the outcome, given the likely strong belief in the benefits of the intervention. | ||||||||||||
| The trial protocol and registration reflects the analyses as that were conducted, and it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to raise some concerns due to the measurement of the outcome. | ||||||||||||
| Subgroup 1.2.3 Progressive resistance exercise | ||||||||||||
| de Morton 2007 |  |  |  |  |  |  | ||||||
| Allocation of wards was random (coin toss) and the allocating officer unaware of study. Baseline differences between groups were thought to be compatible with chance. | ||||||||||||
| Participants and clinicians delivering care were believed to be aware of treatment assignments. There was no evidence of deviations from intended interventions and intention to treat analysis was used. | ||||||||||||
| After accounting for mortality, discharge Functional Ambulation Classification (FAC) scores were available for 75% in intervention group and 70% in control group. Although it is feasible that following discharge patients with lower levels of functional ability are more likely to be missing, we do not think this applies in this situation as assessment prior to discharge. We do not see a situation where it would be more likely to miss an assessment due to high/low level of disability at discharge. | ||||||||||||
| The use of the FAC to measure functional mobility is considered appropriate, and there were no differences in the measurement or ascertainment between groups. The assessors were not blinded, and it is considered likely that knowledge of the intervention could influence the outcome, given the likely strong belief in the benefits of the intervention. | ||||||||||||
| A pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to be at high risk of bias in measurement of the outcome. | ||||||||||||
| Martinez‐Velilla 2019 |  |  |  |  |  |  | ||||||
| Allocation sequence was random (www.randomizer.org) and personal communication with author confirms allocation concealment. Baseline differences between groups are thought to be compatible with chance. | ||||||||||||
| Both participants and clinicians delivering care were aware of assigned interventions. There is no evidence of deviations from intended interventions and intention to treat analysis used. | ||||||||||||
| Assuming that those that discontinued the study did not provide Short Physical Performance Battery (SPPB) outcome data, and accounting for mortality, 83% of intervention group and 86% of control group had outcome data. Although there were participants who discontinued the study due to medical deterioration, which could have and is considered likely to have biased the results (i.e. medical deterioration being associated with poor functional outcomes), the numbers were relatively low (only 6% of the sample) and were well‐balanced between groups. We therefore feel that this was unlikely to bias the results. | ||||||||||||
| The use of the SPPB to measure functional mobility is considered appropriate, and there were no differences in the measurement or ascertainment between groups. The assessors were blinded. | ||||||||||||
| The trial protocol reflects the analyses as that were conducted, and it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to raise some concerns due to missing outcome data. | ||||||||||||
| McCullagh 2020 |  |  |  |  |  |  | ||||||
| Allocation sequence was random (computer‐generated) and allocation sequence was concealed. Baseline differences are thought to be compatible with chance. | ||||||||||||
| Both participants and clinicians delivering care were aware of assigned interventions. There is no evidence of deviations from intended interventions and intention to treat analysis used. | ||||||||||||
| 90% of intervention group and 94% of the control group had Short Physical Performance Battery (SPPB) data. Given the nature of this population (older adults during and after acute hospitalisation) we considered a threshold of 90% of participants with data as sufficient. | ||||||||||||
| The use of SPPB to measure functional mobility is considered appropriate, and there were no differences in the measurement or ascertainment between groups. The assessors were blinded. | ||||||||||||
| The trial protocol reflects the analyses as that were conducted, and it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to be at low risk of bias for all domains for this outcome. | ||||||||||||
| Ortiz‐Alonso 2020 |  |  |  |  |  |  | ||||||
| The allocation sequence was based on recruitment blocks of 4‐8 weeks. Activity of daily living (ADL) scores at baseline and admission were significantly lower in the intervention group than the control group, this is thought to be an important prognostic factor and therefore a concern regarding the randomisation process. | ||||||||||||
| Methods were designed to blind participants from group assignments however due to the nature of the intervention and lack of sham intervention the success of this blinding is felt unlikely. Ward staff and research staff were not blinded to the participant's assigned intervention. There were no deviations from the intended interventions due to trial context described and although intention to treat analysis not specified given the lack of deviations it is assumed. | ||||||||||||
| After adjusting for mortality 97% in intervention group and 97% in control group had outcome data. | ||||||||||||
| The use of the Short Physical Performance Battery (SPPB) for measuring functional mobility is considered appropriate, and there were no differences in the measurement or ascertainment between groups. The assessors were not blinded, and it was therefore considered likely that knowledge of the intervention could influence the outcome, given the likely strong belief in the benefits of the intervention ward. | ||||||||||||
| Analysis reflects plan in trial registration and study protocol, and results are not thought to be from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to be at high risk of bias due to the randomisation process and measurement of the outcome. | ||||||||||||
| Pedersen 2019 |  |  |  |  |  |  | ||||||
| Allocation sequence was random (computer‐generated block randomisation), and allocation sequence concealed from the investigators. Participant characteristics were well‐balanced between groups. | ||||||||||||
| Participants and clinicians were aware of the treatment assignments. There was no evidence of deviations from the assigned interventions and an intention to treat approach was used for analysis. | ||||||||||||
| When accounting for mortality only 93% of participants in intervention group and 67% in control group completed measures at discharge. The difference in the proportion of missing data, and that the assessments were carried out home after discharge, it was judged likely that the reasons for missing data may have depended on its true value. | ||||||||||||
| The use of the de Morton Mobility Index (DEMMI) is considered appropriate, and there were no differences in the measurement or ascertainment between groups. The assessors were blinded. | ||||||||||||
| The analysis is in accordance with a pre‐specified analysis in a published protocol, and results are not thought to be from multiple outcome measures or multiple analyses. | ||||||||||||
| As per RoB2 algorithm, the study is judged to be at high risk of bias due to missing outcome data. | ||||||||||||
| Bias | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study | Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | ||||||
| Subgroup 1.3.1 Rehabilitation‐related activities | ||||||||||||
| Abizanda 2011 |  |  |  |  |  |  | ||||||
| Allocation sequence was random (computer generated random numbers). Allocation occurred after enrolment by an investigator not involved in the participants' clinical management. Higher number of participants admitted with a stroke in the intervention group (39 vs. 16), in addition, the 'Others' subgroup the Barthel index is higher in the control group than the intervention group (29.1 vs. 23.1). This is thought to be compatible with chance. | ||||||||||||
| Its assumed participants and the Occupational Therapist (OT) delivering the intervention were aware of the assigned intervention allocation. All participants received their allocated intervention. Therefore, even though intention to treat analysis not specified, analysis deemed appropriate. | ||||||||||||
| Given the nature of this population (older adults during and after acute hospitalisation) we considered a threshold of 90% of participants with data as sufficient. We felt this would be consistent with measurements outside of a trial context. When accounting for mortality, 93% and 91% assessed at discharge in intervention and control arms respectively. | ||||||||||||
| The use of the Confusion Assessment Method (CAM) to assess for delirium is considered appropriate, and there were no differences in measurement or ascertainment of the outcomes between groups. The OT assessor was blinded. | ||||||||||||
| The data analyst was blinded, and followed a pre‐specified statistical plan. It is not thought that results were from multiple outcome measurements or multiple analyses. | ||||||||||||
| The study is judged to be at low risk of bias in all domains for this outcome. | ||||||||||||
| Asplund 2000 |  |  |  |  |  |  | ||||||
| The method of allocation sequence is not described, but the use of sealed envelopes suggests a random component was used. Participant characteristics were balanced. | ||||||||||||
| Participants and those delivering the interventions were aware of intervention assignments. Twenty‐five patients were excluded due to them not meeting the set eligibility criteria, however, these: protocol violations are not expected to influence the effect estimate of the outcome as per protocol analyses used. The per protocol analyses was not thought to have a substantial impact on the result given the main reason for exclusion was inappropriate recruitment. Excluding this 25, the other 6 exclusions represent approximately 1% of the total sample size. | ||||||||||||
| Data at discharge from hospital was available for 98% of participants. | ||||||||||||
| The confusion assessment method (CAM) instrument was considered appropriate, and there were no differences in the measurement or ascertainment between groups. The assessors were not blinded, and it was therefore considered likely that knowledge of the intervention could influence the outcome, given the likely strong belief in the benefits of the intervention ward. | ||||||||||||
| A pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to be at high risk of bias due to measurement of the outcome. | ||||||||||||
| Subgroup 1.3.2 Structured exercise | ||||||||||||
| Brown 2016 |  |  |  |  |  |  | ||||||
| No description of randomisation sequence generation, but the paper describes a block randomisation strategy and sequence concealed (sealed envelopes). The patient characteristics appear well‐balanced between groups. | ||||||||||||
| We assume both participants and those delivering the interventions were aware of intervention assignments. 6 participants did not receive allocated intervention, this is considered to be consistent with what could occur outside the trial context, and intention to treat analysis was used. | ||||||||||||
| No missing data. | ||||||||||||
| The Confusion Assessment Method (CAM) score is considered appropriate, and there were no differences in the measurement or ascertainment between groups. The assessors were blinded at admission, discharge and follow up, but not the research assistants as they were delivering the intervention. “The research assistants also used the CAM at each patient visit throughout the hospital stay to ensure that patients in either group did not develop incident delirium.” Therefore, assessors not considered to be blinded, and it is likely given that there is judgement involved in scoring of the CAM, that knowledge of the intervention could influence the scoring of the CAM given the likely strong beliefs in the benefits of the intervention ward. | ||||||||||||
| The study protocol details statistical analysis plan which did not refer to assessment of delirium after baseline assessments, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to be at high risk of bias in measurement of the outcome. | ||||||||||||
| Subgroup 1.3.3 Progressive resistance exercise | ||||||||||||
| Jeffs 2013 |  |  |  |  |  |  | ||||||
| Sequence concealment was achieved via a member of the research team not involved in recruitment and using sealed envelopes. There was no information regarding method of generating random sequence, other than to say that there was randomisation, given details of allocation concealment, assumption made that appropriate method used. Patient characteristics between groups appear well‐balanced. | ||||||||||||
| Participants and those delivering the interventions were likely to be aware of intervention allocations. One participant was not allocated to a group due to an administrative error and 35 participants did not receive the intervention as planned (17 in the intervention group). This is thought to be consistent with what could occur outside of the trial context. Intention to treat analysis was used. | ||||||||||||
| No missing data. | ||||||||||||
| The Confusion Assessment Method (CAM) is considered appropriate, and there were no differences in the measurement or ascertainment between groups. The assessors were blinded. | ||||||||||||
| A pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to raise some concerns in the selection of the reported results. | ||||||||||||
| Martinez‐Velilla 2019 |  |  |  |  |  |  | ||||||
| Allocation sequence was random (www.randomizer.org) and personal communication with author confirms allocation concealment. Baseline differences between groups are thought to be compatible with chance. | ||||||||||||
| Both participants and clinicians delivering care were aware of assigned interventions. There is no evidence of deviations from intended interventions and intention to treat analysis used. | ||||||||||||
| Assuming that those that discontinued the study did not provide Barthel Index (BI) outcome data, and accounting for mortality, 84% of intervention group and 86% of control group had outcome data. Although there were participants who discontinued the study due to medical deterioration, which could have and is considered likely to have biased the results (i.e. medical deterioration being associated with poor functional outcomes), the numbers were relatively low (only 6% of the sample) and were well‐balanced between groups. We therefore feel that this was unlikely to bias the results. | ||||||||||||
| The use of the Confusion Assessment Method (CAM) to measure delirium is considered appropriate, and there were no differences in the measurement or ascertainment between groups. The assessors were blinded. | ||||||||||||
| The trial protocol reflects the analyses as that were conducted, and it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to raise some concerns due to missing outcome data. | ||||||||||||
| McCullagh 2020 |  |  |  |  |  |  | ||||||
| Allocation sequence was random (computer‐generated) and allocation sequence was concealed. Baseline differences are thought to be compatible with chance. | ||||||||||||
| Both participants and clinicians delivering care were aware of assigned interventions. There is no evidence of deviations from intended interventions and intention to treat analysis used. | ||||||||||||
| 90% of intervention group and 94% of the control group had outcome data. Given the nature of this population (older adults during and after acute hospitalisation) we considered a threshold of 90% of participants with data as sufficient. | ||||||||||||
| The use of the Six‐item Cognitive Impairment Test (6CIT) to assess for delirium is considered appropriate, and there were no differences in the measurement or ascertainment between groups. The assessors were blinded. | ||||||||||||
| The trial protocol reflects the analyses as that were conducted, and it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to be at low risk of bias in all domains for this outcome. | ||||||||||||
| Mudge 2008 |  |  |  |  |  |  | ||||||
| Pseudo‐randomisation, allocation was based on admitting unit and bed availability, and admitting unit was determined by a rotating roster. No evidence of baseline differences in patient characteristics other than those thought to be compatible with chance. | ||||||||||||
| Both participants and clinicians delivering care were aware of assigned interventions. There is no evidence of deviations from intended interventions and intention to treat analysis used. | ||||||||||||
| No missing data. | ||||||||||||
| Identifying delirium according to chart review using validated methodology is considered appropriate, and there were no differences in the measurement or ascertainment between groups. The assessors were blinded. | ||||||||||||
| A pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to be at high risk of bias due to the randomisation process. | ||||||||||||
| Bias | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study | Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | ||||||
| Subgroup 1.4.1 Rehabilitation‐related activities | ||||||||||||
| Ekerstad 2017 |  |  |  |  |  |  | ||||||
| Randomisation was based on the availability of beds, and there was no allocation concealment as participants were allocated a ward prior to enrolment. Participant characteristics appeared balanced. | ||||||||||||
| Participants and clinicians delivering care were aware of treatment assignments. There was no evidence of deviations from intended interventions and intention to treat analysis was used. | ||||||||||||
| No missing data. | ||||||||||||
| The EQ‐VAS is considered appropriate, and there were no differences in the measurement or ascertainment between groups. The assessors were not blinded, and it was therefore considered likely that knowledge of the intervention could influence the outcome, given the likely strong belief in the benefits of the intervention ward. | ||||||||||||
| A detailed pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to be at high risk of bias due to bias in the measurement of the outcome and bias arising from the randomisation process. | ||||||||||||
| Subgroup 1.4.2 Structured exercise | ||||||||||||
| Hu 2020 |  |  |  |  |  |  | ||||||
| Allocation sequence was random (computer generated random numbers) and sequence concealed (blinded project coordinator allocated participants). Participant characteristics were balanced. | ||||||||||||
| Participants and those delivering the interventions were aware of intervention allocations. There was no evidence of deviations from the intended interventions and as such it is presumed intention to treat analysis was used. | ||||||||||||
| At discharge data was available for 76% of reablement group, (78% of reminder group) and 80% of the control group. Only participants who had complete data (i.e. data collected at baseline, discharge and follow‐up) had outcomes reported. Although missing data is well‐balanced between all three groups, it is considered likely that reason for missing data is related to its true value. | ||||||||||||
| The EQ5D is considered appropriate, and there were no differences in the measurement or ascertainment between groups. The assessors were blinded. | ||||||||||||
| A pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to be at high risk of bias due to missing outcome data. | ||||||||||||
| Subgroup 1.4.3 Progressive resistance exercise | ||||||||||||
| Martinez‐Velilla 2019 |  |  |  |  |  |  | ||||||
| Allocation sequence was random (www.randomizer.org) and personal communication with author confirms allocation concealment. Baseline differences between groups are thought to be compatible with chance. | ||||||||||||
| Both participants and clinicians delivering care were aware of assigned interventions. There is no evidence of deviations from intended interventions and intention to treat analysis used. | ||||||||||||
| Assuming that those that discontinued the study did not provide e EQ‐5D outcome data, and accounting for mortality, 83% of intervention group and 86% of control group had outcome data. Although there were participants who discontinued the study due to medical deterioration, which could have and is considered likely to have biased the results (i.e. medical deterioration being associated with poor functional outcomes), the numbers were relatively low (only 6% of the sample) and were well‐balanced between groups. We therefore feel that this was unlikely to bias the results. | ||||||||||||
| The EQ‐5D is considered appropriate, and there were no differences in the measurement or ascertainment between groups. The assessors were blinded. | ||||||||||||
| The trial protocol reflects the analyses as that were conducted, and it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to raise some concerns due to missing outcome data. | ||||||||||||
| McCullagh 2020 |  |  |  |  |  |  | ||||||
| Allocation sequence was random (computer‐generated) and allocation sequence was concealed. Baseline differences are thought to be compatible with chance. | ||||||||||||
| Both participants and clinicians delivering care were aware of assigned interventions. There is no evidence of deviations from intended interventions and intention to treat analysis used. | ||||||||||||
| 90% of intervention group and 94% of the control group had outcome data. Given the nature of this population (older adults during and after acute hospitalisation) we considered a threshold of 90% of participants with data as sufficient. | ||||||||||||
| The use of EQ‐5D5L to measure quality of life is considered appropriate, and there were no differences in the measurement or ascertainment between groups. The assessors were blinded. | ||||||||||||
| The trial protocol reflects the analyses as that were conducted, and it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to be at low risk of bias for all domains for this outcome. | ||||||||||||
| Bias | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study | Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | ||||||
| Subgroup 1.5.1 Rehabilitation‐related activities | ||||||||||||
| Sahota 2017 |  |  |  |  |  |  | ||||||
| No information on randomisation methods other than to say participants were randomised, however, patient characteristics appear well‐balanced between groups and differences compatible with chance. | ||||||||||||
| It is assumed that participants and those delivering the interventions were aware of intervention assignments. There were 15 protocol deviations in the CIRACT group and 8 in the THB‐Rehab group. The deviations were thought to be in keeping with what would be expected outside of a trial context. Intention to treat not explicitly stated though it appears that the 1 participant who did not receive the intended intervention was analysed within the group to which they were assigned. | ||||||||||||
| No missing data | ||||||||||||
| Methods of measuring the number of falls was considered appropriate and there were no differences in measurement or ascertainment of the outcomes between groups. The assessor was not blinded, but it is thought that due to the lack of judgement in the measurement, that the knowledge of the intervention did not influence the outcomes. | ||||||||||||
| A pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to raise some concerns in the selection of the reported results and the randomisation process. | ||||||||||||
| Subgroup 1.5.2 Structured exercise | ||||||||||||
| Brown 2016 |  |  |  |  |  |  | ||||||
| No description of randomisation sequence generation, but the paper describes a block randomisation strategy and sequence concealed (sealed envelopes). The patient characteristics appear well‐balanced between groups. | ||||||||||||
| We assume both participants and those delivering the interventions were aware of intervention assignments. 6 participants did not receive allocated intervention, this is considered to be consistent with what could occur outside the trial context, and intention to treat analysis was used. | ||||||||||||
| No missing data. | ||||||||||||
| Methods of measuring the number of falls was considered appropriate and there were no differences in measurement or ascertainment of the outcomes between groups. The assessor was blinded. | ||||||||||||
| The study protocol details statistical analysis plan which is in accordance with results presented, and it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to be at low risk of bias in all domains for this outcome. | ||||||||||||
| Gazineo 2021 |  |  |  |  |  |  | ||||||
| Allocation sequence was random (generated using an online system) and the allocation sequence was concealed until participants were enrolled (opaque concealed and sealed envelopes). Participant characteristics were well‐balanced. | ||||||||||||
| Participants and those delivering the interventions were aware of intervention allocations. There was no evidence of deviations from the intended interventions and intention to treat analysis used. | ||||||||||||
| No missing data after accounting for mortality. | ||||||||||||
| Methods of measuring the number of falls was considered appropriate and there were no differences in measurement or ascertainment of the outcomes between groups. The assessor was not blinded, but it is thought that due to the lack of judgement in the measurement, that the knowledge of the intervention did not influence the outcomes. | ||||||||||||
| A pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to raise some concerns in the selection of the reported results . | ||||||||||||
| Killey 2006 |  |  |  |  |  |  | ||||||
| Allocation not random and sequence predictable as based on alternation. There is limited data on participant characteristics, but available data suggests balanced characteristics between groups. | ||||||||||||
| Participants and those delivering the interventions were aware of intervention allocations. There was no evidence of deviations from the intended interventions. Per‐protocol analysis appears to have been used. 2 participants in the intervention group were excluded from analysis as they completed less than 70% of their walks. The 2 participants that were excluded accounted for 7% of the remaining sample, it is therefore considered that there was potential for a significant impact on the results. | ||||||||||||
| Only 71% of intervention and 74% of control group had outcome data. A significant proportion of missing data was related to early discharge from hospital. These participants could be expected to have a higher functional level than those who remained in hospital. However, the missing data was well‐balanced between the two groups. | ||||||||||||
| Methods of measuring the number of falls was considered appropriate and there were no differences in measurement or ascertainment of the outcomes between groups. The assessor was not blinded, but it is thought that due to the lack of judgement in the measurement, that the knowledge of the intervention did not influence the outcomes. | ||||||||||||
| A pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to be at high risk of bias arising from the randomisation process and due to deviations from the intended interventions. | ||||||||||||
| Subgroup 1.5.3 Progressive resistance exercise | ||||||||||||
| de Morton 2007 |  |  |  |  |  |  | ||||||
| Allocation of wards was random (coin toss) and the allocating officer unaware of study. Baseline differences between groups were thought to be compatible with chance. | ||||||||||||
| Participants and clinicians delivering care were believed to be aware of treatment assignments. There was no evidence of deviations from intended interventions and intention to treat analysis was used. | ||||||||||||
| No missing data. | ||||||||||||
| Methods of measuring the number of falls was considered appropriate and there were no differences in measurement or ascertainment of the outcomes between groups. The assessor was not blinded, but it is thought that due to the lack of judgement in the measurement, that the knowledge of the intervention did not influence the outcomes. | ||||||||||||
| A pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to raise some concerns in the selection of the reported result. | ||||||||||||
| Jones 2006 |  |  |  |  |  |  | ||||||
| Allocation sequence was random (computer generated random numbers). Sequence allocation was not concealed, but performed by a member of staff independent of the enrolment procedures. ). Participant characteristics were balanced between groups and differences compatible with chance. | ||||||||||||
| It is assumed that both participants and clinicians delivering care were aware of assigned interventions. There is no evidence of deviations from intended interventions and intention to treat analysis used. | ||||||||||||
| After accounting for mortality, falls data available for 99% of control group and 93% of intervention group. Given the nature of this population (older adults during and after acute hospitalisation) we considered a threshold of 90% of participants with data as sufficient. | ||||||||||||
| Methods of measuring the number of falls was considered appropriate and there were no differences in measurement or ascertainment of the outcomes between groups. The assessor was blinded. | ||||||||||||
| A pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to raise some concerns due to the selection of the reported results. | ||||||||||||
| Martinez‐Velilla 2019 |  |  |  |  |  |  | ||||||
| Allocation sequence was random (www.randomizer.org) and personal communication with author confirms allocation concealment. Baseline differences between groups are thought to be compatible with chance. | ||||||||||||
| Both participants and clinicians delivering care were aware of assigned interventions. There is no evidence of deviations from intended interventions and intention to treat analysis used. | ||||||||||||
| No missing data | ||||||||||||
| Methods of measuring the number of falls was considered appropriate and there were no differences in measurement or ascertainment of the outcomes between groups. The assessor was blinded. | ||||||||||||
| The trial protocol reflects the analyses as that were conducted, and it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to be at low risk of bias in all domains for this outcome. | ||||||||||||
| McCullagh 2020 |  |  |  |  |  |  | ||||||
| Allocation sequence was random (computer‐generated) and allocation sequence was concealed. Baseline differences are thought to be compatible with chance. | ||||||||||||
| Both participants and clinicians delivering care were aware of assigned interventions. There is no evidence of deviations from intended interventions and intention to treat analysis used. | ||||||||||||
| No missing data. | ||||||||||||
| Methods of measuring the number of falls was considered appropriate and there were no differences in measurement or ascertainment of the outcomes between groups. The assessor was blinded. | ||||||||||||
| The trial protocol reflects the analyses as that were conducted, and it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| As per RoB2 algorithm, the study is judged to be at low risk of bias for all domains for this outcome. | ||||||||||||
| Mudge 2008 |  |  |  |  |  |  | ||||||
| Pseudo‐randomisation, allocation was based on admitting unit and bed availability, and admitting unit was determined by a rotating roster. No evidence of baseline differences in patient characteristics other than those thought to be compatible with chance. | ||||||||||||
| Both participants and clinicians delivering care were aware of assigned interventions. There is no evidence of deviations from intended interventions and intention to treat analysis used. | ||||||||||||
| No missing data. | ||||||||||||
| Methods of measuring the number of falls was considered appropriate and there were no differences in measurement or ascertainment of the outcomes between groups. The assessor was blinded. | ||||||||||||
| A pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to be at high risk of bias due to the randomisation process. | ||||||||||||
| Bias | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study | Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | ||||||
| Subgroup 1.6.1 Rehabilitation‐related activities | ||||||||||||
| Abizanda 2011 |  |  |  |  |  |  | ||||||
| Allocation sequence was random (computer generated random numbers). Allocation occurred after enrolment by an investigator not involved in the participants' clinical management. Higher number of participants admitted with a stroke in the intervention group (39 vs. 16), in addition, the 'Others' subgroup the Barthel index is higher in the control group than the intervention group (29.1 vs. 23.1). This is thought to be compatible with chance. | ||||||||||||
| Its assumed participants and the Occupational Therapist (OT) delivering the intervention were aware of the assigned intervention allocation. All participants received their allocated intervention. Therefore, even though intention to treat analysis not specified, analysis deemed appropriate. | ||||||||||||
| Given the nature of this population (older adults during and after acute hospitalisation) we considered a threshold of 90% of participants with data as sufficient. We felt this would be consistent with measurements outside of a trial context. When accounting for mortality, 93% and 91% assessed at discharge for confusion in intervention and control arms respectively. | ||||||||||||
| The use of the Confusion Assessment Method (CAM) to assess for delirium is considered appropriate, and there were no differences in measurement or ascertainment of the outcomes between groups. The OT assessor was blinded. | ||||||||||||
| The data analyst was blinded, and followed a pre‐specified statistical plan. It is not thought that results were from multiple outcome measurements or multiple analyses. | ||||||||||||
| The study is judged to be at low risk of bias in all domains for this outcome. | ||||||||||||
| Asplund 2000 |  |  |  |  |  |  | ||||||
| The method of allocation sequence is not described, but the use of sealed envelopes suggests a random component was used. Participant characteristics were balanced. | ||||||||||||
| Participants and those delivering the interventions were aware of intervention assignments. Twenty‐five patients were excluded due to them not meeting the set eligibility criteria, however, these: protocol violations are not expected to influence the effect estimate of the outcome as per protocol analyses used. The per protocol analyses was not thought to have a substantial impact on the result given the main reason for exclusion was inappropriate recruitment. Excluding this 25, the other 6 exclusions represent approximately 1% of the total sample size. | ||||||||||||
| Data at discharge from hospital was available for 98% of participants. | ||||||||||||
| The confusion assessment method (CAM) instrument was considered appropriate, and there were no differences in the measurement or ascertainment between groups. The assessors were not blinded, and it was therefore considered likely that knowledge of the intervention could influence the outcome, given the likely strong belief in the benefits of the intervention ward. | ||||||||||||
| A pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to be at high risk of bias due to measurement of the outcome. | ||||||||||||
| Subgroup 1.6.2 Structured exercise | ||||||||||||
| Brown 2016 |  |  |  |  |  |  | ||||||
| No description of randomisation sequence generation, but the paper describes a block randomisation strategy and sequence concealed (sealed envelopes). The patient characteristics appear well‐balanced between groups. | ||||||||||||
| We assume both participants and those delivering the interventions were aware of intervention assignments. Six participants did not receive allocated intervention, this is considered to be consistent with what could occur outside the trial context, and intention to treat analysis was used. | ||||||||||||
| No missing data. | ||||||||||||
| Measures used to measure medical deterioration are considered appropriate and there and there were no differences in the measurement or ascertainment between groups. The assessors were blinded. | ||||||||||||
| The study protocol details statistical analysis plan which is in line with the analyses conducted, and it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to be at low risk of bias in all domains for this outcome. | ||||||||||||
| Hu 2020 |  |  |  |  |  |  | ||||||
| Allocation sequence was random (computer generated random numbers) and sequence concealed (blinded project coordinator allocated participants). Participant characteristics were balanced. | ||||||||||||
| Participants and those delivering the interventions were aware of intervention allocations. There was no evidence of deviations from the intended interventions and as such it is presumed intention to treat analysis was used. | ||||||||||||
| No missing data for medical deterioration during hospitalisation. | ||||||||||||
| The measures are considered appropriate, and there were no differences in the measurement or ascertainment between groups. The assessors were blinded. | ||||||||||||
| A pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to have some concerns in the selection of the reported result. | ||||||||||||
| Subgroup 1.6.3 Progressive resistance exercise | ||||||||||||
| de Morton 2007 |  |  |  |  |  |  | ||||||
| Allocation of wards was random (coin toss) and the allocating officer unaware of study. Baseline differences between groups were thought to be compatible with chance. | ||||||||||||
| Participants and clinicians delivering care were believed to be aware of treatment assignments. There was no evidence of deviations from intended interventions and intention to treat analysis was used. | ||||||||||||
| No missing data. | ||||||||||||
| The measures are considered appropriate, and there were no differences in the measurement or ascertainment between groups. The assessors were not blinded, but it is thought that due to the lack of judgement in the outcome, that the knowledge of the intervention did not influence the outcomes. | ||||||||||||
| A pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to raise some concerns in the selection of the reported result. | ||||||||||||
| Jeffs 2013 |  |  |  |  |  |  | ||||||
| Sequence concealment was achieved via a member of the research team not involved in recruitment and using sealed envelopes. There was no information regarding method of generating random sequence, other than to say that there was randomisation, given details of allocation concealment, assumption made that appropriate method used. Patient characteristics between groups appear well‐balanced. | ||||||||||||
| Participants and those delivering the interventions were likely to be aware of intervention allocations. One participant was not allocated to a group due to an administrative error and 35 participants did not receive the intervention as planned (17 in the intervention group). This is thought to be consistent with what could occur outside of the trial context. Intention to treat analysis was used. | ||||||||||||
| No missing data. | ||||||||||||
| The Confusion Assessment Method (CAM) is considered appropriate, and there were no differences in the measurement or ascertainment between groups. The assessors were blinded. | ||||||||||||
| A pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to raise some concerns in the selection of the reported results. | ||||||||||||
| Jones 2006 |  |  |  |  |  |  | ||||||
| Allocation sequence was random (computer generated random numbers). Sequence allocation was not concealed, but performed by a member of staff independent of the enrolment procedures. ). Participant characteristics were balanced between groups and differences compatible with chance. | ||||||||||||
| It is assumed that both participants and clinicians delivering care were aware of assigned interventions. There is no evidence of deviations from intended interventions and intention to treat analysis used. | ||||||||||||
| No missing data. | ||||||||||||
| Measurement methods are considered appropriate, and there were no differences in the measurement or ascertainment between groups. The assessors were blinded. | ||||||||||||
| A pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to raise some concerns in the selection of the reported results. | ||||||||||||
| Martinez‐Velilla 2019 |  |  |  |  |  |  | ||||||
| Allocation sequence was random (www.randomizer.org) and personal communication with author confirms allocation concealment. Baseline differences between groups are thought to be compatible with chance. | ||||||||||||
| Both participants and clinicians delivering care were aware of assigned interventions. There is no evidence of deviations from intended interventions and intention to treat analysis used. | ||||||||||||
| The methods of measurement are considered appropriate, and there were no differences in the measurement or ascertainment between groups. The assessors were blinded. | ||||||||||||
| The trial protocol reflects the analyses as that were conducted, and it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to be at low risk of bias in all domains for this outcome. | ||||||||||||
| The study is judged to be at low risk of bias in all domains for this outcome. | ||||||||||||
| McCullagh 2020 |  |  |  |  |  |  | ||||||
| Allocation sequence was random (computer‐generated) and allocation sequence was concealed. Baseline differences are thought to be compatible with chance. | ||||||||||||
| Both participants and clinicians delivering care were aware of assigned interventions. There is no evidence of deviations from intended interventions and intention to treat analysis used. | ||||||||||||
| 90% of intervention group and 94% of the control group had outcome data. Given the nature of this population (older adults during and after acute hospitalisation) we considered a threshold of 90% of participants with data as sufficient. | ||||||||||||
| The use of the Six‐item Cognitive Impairment Test (6CIT) to assess for delirium is considered appropriate, and there were no differences in the measurement or ascertainment between groups. The assessors were blinded. | ||||||||||||
| The trial protocol reflects the analyses as that were conducted, and it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to be at low risk of bias in all domains for this outcome. | ||||||||||||
| Mudge 2008 |  |  |  |  |  |  | ||||||
| Pseudo‐randomisation, allocation was based on admitting unit and bed availability, and admitting unit was determined by a rotating roster. No evidence of baseline differences in patient characteristics other than those thought to be compatible with chance. | ||||||||||||
| Both participants and clinicians delivering care were aware of assigned interventions. There is no evidence of deviations from intended interventions and intention to treat analysis used. | ||||||||||||
| No missing data. | ||||||||||||
| Identifying delirium according to chart review using validated methodology is considered appropriate, and there were no differences in the measurement or ascertainment between groups. The assessors were blinded. | ||||||||||||
| A pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to be at high risk of bias due to the randomisation process. | ||||||||||||
| Pedersen 2019 |  |  |  |  |  |  | ||||||
| Allocation sequence was random (computer‐generated block randomisation), and allocation sequence concealed from the investigators. Participant characteristics were well‐balanced between groups. | ||||||||||||
| Participants and clinicians were aware of the treatment assignments. There was no evidence of deviations from the assigned interventions and an intention to treat approach was used for analysis. | ||||||||||||
| No missing data. | ||||||||||||
| The measure is considered appropriate, and there were no differences in the measurement or ascertainment between groups. The assessors were blinded. | ||||||||||||
| The analysis is in accordance with a pre‐specified analysis in a published protocol, and results are not thought to be from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to be at low risk of bias in all domains for this outcome. | ||||||||||||
| Bias | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study | Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | ||||||
| Subgroup 2.2.1 Rehabilitation‐related activities | ||||||||||||
| Abizanda 2011 |  |  |  |  |  |  | ||||||
| Allocation sequence was random (computer generated random numbers). Allocation occurred after enrolment by an investigator not involved in the participants' clinical management. Higher number of participants admitted with a stroke in the intervention group (39 vs. 16), in addition, the 'Others' subgroup the Barthel index is higher in the control group than the intervention group (29.1 vs. 23.1). This is thought to be compatible with chance. | ||||||||||||
| Its assumed participants and the Occupational Therapist (OT) delivering the intervention were aware of the assigned intervention allocation. All participants received their allocated intervention. Therefore, even though intention to treat analysis not specified, analysis deemed appropriate. | ||||||||||||
| No missing data. | ||||||||||||
| The method is considered appropriates, and there were no differences in measurement or ascertainment of the outcomes between groups. The OT assessor was blinded. | ||||||||||||
| The data analyst was blinded, and followed a pre‐specified statistical plan. It is not thought that results were from multiple outcome measurements or multiple analyses. | ||||||||||||
| The study is judged to be at low risk of bias in all domains for this outcome. | ||||||||||||
| Asplund 2000 |  |  |  |  |  |  | ||||||
| The method of allocation sequence is not described, but the use of sealed envelopes suggests a random component was used. Participant characteristics were balanced. | ||||||||||||
| Participants and those delivering the interventions were aware of intervention assignments. Twenty‐five patients were excluded due to them not meeting the set eligibility criteria, however, these: protocol violations are not expected to influence the effect estimate of the outcome as per protocol analyses used. The per protocol analyses was not thought to have a substantial impact on the result given the main reason for exclusion was inappropriate recruitment. Excluding this 25, the other 6 exclusions represent approximately 1% of the total sample size. | ||||||||||||
| Data at discharge from hospital complete for 98% of participants. | ||||||||||||
| The measurement of length of stay is considered appropriate, and there were no differences in measurement or ascertainment between groups. Assessors were not blinded, but is thought that due to the lack of judgement in scoring a 'hard outcome' like length of stay, that it is unlikely that the knowledge of the intervention could influence the outcome. | ||||||||||||
| A pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to raise some concerns due to deviations from intended interventions and the selection of the reported results. | ||||||||||||
| Counsell 2000 |  |  |  |  |  |  | ||||||
| Allocation sequence was random (computer generated random numbers) and sequence concealed (opaque sealed envelope). Participant characteristics were balanced. | ||||||||||||
| Participants and those delivering the interventions were aware of intervention assignments. Seventy‐nine participants were not admitted to the unit to which they were assigned. Deviations may have affected the effect estimate, however they were well‐balanced between groups. Intention to treat analysis was used. | ||||||||||||
| No missing data. | ||||||||||||
| The methods of measuring length of stay are considered appropriate, and there were no differences in measurement or ascertainment between groups. Assessors were not blinded, but is thought that due to the lack of judgement in scoring a 'hard outcome' like length of hospital stay, that it is unlikely that the knowledge of the intervention could influence the outcome. | ||||||||||||
| A pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to raise some concerns in the selection of the reported results and deviations from the intended interventions. | ||||||||||||
| Ekerstad 2017 |  |  |  |  |  |  | ||||||
| Randomisation was based on the availability of beds, and there was no allocation concealment as participants were allocated a ward prior to enrolment. Participant characteristics appeared balanced. | ||||||||||||
| Participants and clinicians delivering care were aware of treatment assignments. There was no evidence of deviations from intended interventions and intention to treat analysis was used. | ||||||||||||
| No missing data. | ||||||||||||
| The methods of measuring length of hospital stay are considered appropriate, and there were no differences in measurement or ascertainment between groups. Assessors were not blinded, but is thought that due to the lack of judgement in scoring a 'hard outcome' like length of hospital stay, that it is unlikely that the knowledge of the intervention could influence the outcome. | ||||||||||||
| A detailed pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to be at high risk of bias arising from the randomisation process. | ||||||||||||
| Fretwell 1990 |  |  |  |  |  |  | ||||||
| “Patients were randomised only when both a treatment and control bed were available”. Although not stated, we believe the necessity for available beds on both wards indicate that a random component was likely used. Sequence allocation concealment is not discussed, but no significant differences in patient characteristics other than those compatible with chance were observed. | ||||||||||||
| Participants and those delivering the interventions were aware of intervention assignments. There were 30 randomisation errors which has been interpreted to mean 'allocated to the ward that they were not randomised to' however there is no explanation of this term. These deviations were thought likely to have affected the outcome, though were relative well‐balanced. It appears a per protocol analyses was used, though given the low number (7%) and relative balance between groups, the omission of the participants randomised incorrectly probably did not have substantial impact on the results. | ||||||||||||
| All participants accounted for at discharge. | ||||||||||||
| The methods of measuring length of stay are considered appropriate, and there were no differences in measurement or ascertainment between groups. Assessors were not blinded, but is thought that due to the lack of judgement in scoring a 'hard outcome' like length of stay, that it is unlikely that the knowledge of the intervention could influence the outcome. | ||||||||||||
| A detailed pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to raise some concerns in deviations from the intended interventions, methods of randomisation and selection of the reported result. | ||||||||||||
| Landefeld 1995 |  |  |  |  |  |  | ||||||
| Allocation sequence was random (computer generated random numbers) and balanced participant characteristics, but no information was provided regarding allocation concealment. | ||||||||||||
| It is assumed participants and clinicians were aware of the treatment assignments. There was no evidence of deviations from the assigned interventions and evidence of an intention to treat approach being used for analysis. | ||||||||||||
| Accounting for mortality (24 died in each arm) all participants had outcome data at discharge. | ||||||||||||
| The methods of measuring length of hospital stay are considered appropriate, and there were no differences in measurement or ascertainment between groups. Assessors were not blinded, but is thought that due to the lack of judgement in scoring a 'hard outcome' like length of hospital stay, that it is unlikely that the knowledge of the intervention could influence the outcome. | ||||||||||||
| A pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to have some concerns due to the methods of randomisation and in the selection of the reported result. | ||||||||||||
| Sahota 2017 |  |  |  |  |  |  | ||||||
| No information on randomisation methods other than to say participants were randomised, however, patient characteristics appear well‐balanced between groups and differences compatible with chance. | ||||||||||||
| It is assumed that participants and those delivering the interventions were aware of intervention assignments. There were 15 protocol deviations in the CIRACT group and 8 in the THB‐Rehab group. The deviations were thought to be in keeping with what would be expected outside of a trial context. Intention to treat not explicitly stated though it appears that the 1 participant who did not receive the intended intervention was analysed within the group to which they were assigned. | ||||||||||||
| No missing data | ||||||||||||
| The methods of measuring length of hospital stay are considered appropriate, and there were no differences in measurement or ascertainment between groups. Assessors were not blinded, but is thought that due to the lack of judgement in scoring a 'hard outcome' like length of hospital stay, that it is unlikely that the knowledge of the intervention could influence the outcome. | ||||||||||||
| A pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to raise some concerns in the selection of the reported results and the randomisation process. | ||||||||||||
| Slaets 1997 |  |  |  |  |  |  | ||||||
| Randomisation methods are described only as: “an alternating randomisation procedure”. However, there was a centrally administered method to allocate interventions, and baseline differences did not appear to show a significant problem with randomisation process other than those thought to be compatible with chance. | ||||||||||||
| Participants and clinicians delivering care were aware of allocated interventions. There was no evidence of deviations from intended intervention, and although intention to treat analysis was not specified, there was no information to suggest participants were not assessed in the group to which they were randomised. | ||||||||||||
| After accounting for mortality (6 patients died), data was available for 91% of participants. | ||||||||||||
| The methods of measuring length of hospital stay in hospital are considered appropriate, and there were no differences in measurement or ascertainment between groups. Assessors were not blinded, but is thought that due to the lack of judgement in scoring a 'hard outcome' like length of stay, that it is unlikely that the knowledge of the intervention could influence the outcome. | ||||||||||||
| A pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to raise some concerns in the randomisation process and in the selection of the reported results. | ||||||||||||
| Zelada 2009 |  |  |  |  |  |  | ||||||
| The allocation sequence was based on bed availability, and therefore not random, and sequence is believed to be predictable. However, baseline differences did not appear to show a significant problem with randomisation process other than those thought to be compatible with chance. | ||||||||||||
| Participants and clinicians delivering care were aware of treatment allocations. There is no evidence of deviations from intended intervention, or that participants moved between the intervention and usual care units during the study period. Intention to treat analysis was not specified, however there was no information to suggest participants were not assessed in the group to which they were randomised. | ||||||||||||
| No missing data. | ||||||||||||
| The methods of measuring length of stay are considered appropriate, and there were no differences in measurement or ascertainment between groups. Assessors were not blinded, but is thought that due to the lack of judgement in scoring a 'hard outcome' like length of stay, that it is unlikely that the knowledge of the intervention could influence the outcome. | ||||||||||||
| A pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to be at high risk of bias due to bias arising from the randomisation process. | ||||||||||||
| Subgroup 2.2.2 Structured exercise | ||||||||||||
| Brown 2016 |  |  |  |  |  |  | ||||||
| No description of randomisation sequence generation, but the paper describes a block randomisation strategy and sequence concealed (sealed envelopes). The patient characteristics appear well‐balanced between groups. | ||||||||||||
| We assume both participants and those delivering the interventions were aware of intervention assignments. 6 participants did not receive allocated intervention, this is considered to be consistent with what could occur outside the trial context, and intention to treat analysis was used. | ||||||||||||
| No missing data. | ||||||||||||
| The methods of measuring length of hospital stay are considered appropriate, and there were no differences in measurement or ascertainment between groups. The assessor was blinded. | ||||||||||||
| The study protocol details statistical analysis plan which is in accordance with results presented, and it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to be at low risk of bias in all domains for this outcome. | ||||||||||||
| Gazineo 2021 |  |  |  |  |  |  | ||||||
| Allocation sequence was random (generated using an online system) and the allocation sequence was concealed until participants were enrolled (opaque concealed and sealed envelopes). Participant characteristics were well‐balanced. | ||||||||||||
| Participants and those delivering the interventions were aware of intervention allocations. There was no evidence of deviations from the intended interventions and intention to treat analysis used. | ||||||||||||
| No missing data after accounting for mortality. | ||||||||||||
| Methods of measuring length of hospital stay are considered appropriate and there were no differences in measurement or ascertainment of the outcomes between groups. The assessor was not blinded, but it is thought that due to the lack of judgement in the measurement, that the knowledge of the intervention did not influence the outcomes. | ||||||||||||
| A pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to raise some concerns in the selection of the reported results . | ||||||||||||
| Hu 2020 |  |  |  |  |  |  | ||||||
| Allocation sequence was random (computer generated random numbers) and sequence concealed (blinded project coordinator allocated participants). Participant characteristics were balanced. | ||||||||||||
| Participants and those delivering the interventions were aware of intervention allocations. There was no evidence of deviations from the intended interventions and as such it is presumed intention to treat analysis was used. | ||||||||||||
| No missing data for length of hospital stay. | ||||||||||||
| The methods of measuring legnth of hospital stay are considered appropriate, and there were no differences in measurement or ascertainment between groups. The assessors were blinded. | ||||||||||||
| A pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to have some concerns in the selection of the reported result. | ||||||||||||
| McGowan 2018a |  |  |  |  |  |  | ||||||
| Allocation sequence was random (www.randomization.com) and the allocation sequence was only known by the chief investigator who was not involved with screening patients. Baseline differences between groups are thought to be compatible with chance. | ||||||||||||
| Both participants and clinicians delivering care were aware of assigned interventions. There is no evidence of deviations from intended interventions and intention to treat analysis not explicitly stated, but all participants appear to have been analysed in the group to which they were randomised. | ||||||||||||
| 96% of participants had complete data sets. | ||||||||||||
| The measurement of length of hospital stay is considered appropriate, and there were no differences in the measurement or ascertainment between groups. The assessors were not blinded, but is thought that due to the lack of judgement in scoring a 'hard outcome' like length of hospital stay, that it is unlikely that the knowledge of the intervention could influence the outcome. | ||||||||||||
| The trial protocol and registration reflects the analyses as that were conducted, and it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to raise some concerns due to the measurement of the outcome. | ||||||||||||
| Subgroup 2.2.3 Progressive resistance exercise | ||||||||||||
| Courtney 2009 |  |  |  |  |  |  | ||||||
| Allocation sequence was random (computer generated random numbers) and the allocation sequence concealed until participants were enrolled (via project coordinator blinded to baseline data). Participant characteristics appear well‐balanced. | ||||||||||||
| Participants and clinicians delivering care were believed to be aware of treatment assignments. 6/64 participants in the intervention group did not receive allocated treatment, the reasons are considered consistent with what could occur outside the trial context, and intention to treat analysis was used. | ||||||||||||
| No missing length of stay data. | ||||||||||||
| The measurement of hospital length of stay is considered appropriate, and there were no differences in measurement or ascertainment between groups. The assessors are believed to be blinded to treatment allocation. | ||||||||||||
| A pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to raise some concerns in the selection of the reported result. | ||||||||||||
| de Morton 2007 |  |  |  |  |  |  | ||||||
| Allocation of wards was random (coin toss) and the allocating officer unaware of study. Baseline differences between groups were thought to be compatible with chance. | ||||||||||||
| Participants and clinicians delivering care were believed to be aware of treatment assignments. There was no evidence of deviations from intended interventions and intention to treat analysis was used. | ||||||||||||
| No missing data. | ||||||||||||
| The measure of hospital length of stay considered appropriate, and there were no differences in measurement or ascertainment between groups. Assessors were not blinded, but is thought that due to the lack of judgement in scoring a 'hard outcome' like length of stay, that it is unlikely that the knowledge of the intervention could influence the outcome. | ||||||||||||
| A pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to raise some concerns in the selection of the reported result. | ||||||||||||
| Jeffs 2013 |  |  |  |  |  |  | ||||||
| Sequence concealment was achieved via a member of the research team not involved in recruitment and using sealed envelopes. There was no information regarding method of generating random sequence, other than to say that there was randomisation, given details of allocation concealment, assumption made that appropriate method used. Patient characteristics between groups appear well‐balanced. | ||||||||||||
| Participants and those delivering the interventions were likely to be aware of intervention allocations. One participant was not allocated to a group due to an administrative error and 35 participants did not receive the intervention as planned (17 in the intervention group). This is thought to be consistent with what could occur outside the trial context. Intention to treat analysis was used. | ||||||||||||
| No missing data. | ||||||||||||
| Measurement of length of hospital stay is considered appropriate, and there were no differences in the measurement or ascertainment between groups. The assessors were blinded. | ||||||||||||
| A pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to raise some concerns in the selection of the reported results. | ||||||||||||
| Jones 2006 |  |  |  |  |  |  | ||||||
| Allocation sequence was random (computer generated random numbers). Sequence allocation was not concealed, but performed by a member of staff independent of the enrolment procedures. ). Participant characteristics were balanced between groups and differences compatible with chance. | ||||||||||||
| It is assumed that both participants and clinicians delivering care were aware of assigned interventions. There is no evidence of deviations from intended interventions and intention to treat analysis used. | ||||||||||||
| After accounting for mortality, length of stay data available for 99% of control group and 93% of intervention group. Given the nature of this population (older adults during and after acute hospitalisation) we considered a threshold of 90% of participants with data as sufficient. | ||||||||||||
| Measurement methods are considered appropriate, and there were no differences in the measurement or ascertainment between groups. The assessors were blinded. | ||||||||||||
| A pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to raise some concerns in the selection of the reported results. | ||||||||||||
| Martinez‐Velilla 2019 |  |  |  |  |  |  | ||||||
| Allocation sequence was random (www.randomizer.org) and personal communication with author confirms allocation concealment. Baseline differences between groups are thought to be compatible with chance. | ||||||||||||
| Both participants and clinicians delivering care were aware of assigned interventions. There is no evidence of deviations from intended interventions and intention to treat analysis used. | ||||||||||||
| No missing data. | ||||||||||||
| The methods of measurement are considered appropriate, and there were no differences in the measurement or ascertainment between groups. The assessors were blinded. | ||||||||||||
| The trial protocol reflects the analyses as that were conducted, and it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to be at low risk of bias in all domains for this outcome. | ||||||||||||
| McCullagh 2020 |  |  |  |  |  |  | ||||||
| Allocation sequence was random (computer‐generated) and allocation sequence was concealed. Baseline differences are thought to be compatible with chance. | ||||||||||||
| Both participants and clinicians delivering care were aware of assigned interventions. There is no evidence of deviations from intended interventions and intention to treat analysis used. | ||||||||||||
| No missing data. | ||||||||||||
| The measure is considered appropriate, and there were no differences in the measurement or ascertainment between groups. The assessors were blinded. | ||||||||||||
| The trial protocol reflects the analyses as that were conducted, and it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to be at low risk of bias in all domains for this outcome. | ||||||||||||
| Mudge 2008 |  |  |  |  |  |  | ||||||
| Pseudo‐randomisation, allocation was based on admitting unit and bed availability, and admitting unit was determined by a rotating roster. No evidence of baseline differences in patient characteristics other than those thought to be compatible with chance. | ||||||||||||
| Both participants and clinicians delivering care were aware of assigned interventions. There is no evidence of deviations from intended interventions and intention to treat analysis used. | ||||||||||||
| No missing data. | ||||||||||||
| The method is considered appropriate, and there were no differences in the measurement or ascertainment between groups. The assessors were blinded. | ||||||||||||
| A pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to be at high risk of bias due to the randomisation process. | ||||||||||||
| Ortiz‐Alonso 2020 |  |  |  |  |  |  | ||||||
| The allocation sequence was based on recruitment blocks of 4‐8 weeks. Activity of daily living (ADL) scores at baseline and admission were significantly lower in the intervention group than the control group, this is thought to be an important prognostic factor and therefore a concern regarding the randomisation process. | ||||||||||||
| Methods were designed to blind participants from group assignments however due to the nature of the intervention and lack of sham intervention the success of this blinding is felt unlikely. Ward staff and research staff were not blinded to the participant's assigned intervention. There were no deviations from the intended interventions due to trial context described and although intention to treat analysis not specified given the lack of deviations it is assumed. | ||||||||||||
| No missing outcome data. | ||||||||||||
| The measure is considered appropriate, and there were no differences in the measurement or ascertainment between groups. The assessors were not blinded, but it is thought that due to the lack of judgement in the outcome, that the knowledge of the intervention did not influence the outcome. | ||||||||||||
| Analysis reflects plan in trial registration and study protocol, and results are not thought to be from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to be at high risk of bias due to the randomisation process. | ||||||||||||
| Pedersen 2019 |  |  |  |  |  |  | ||||||
| Allocation sequence was random (computer‐generated block randomisation), and allocation sequence concealed from the investigators. Participant characteristics were well‐balanced between groups. | ||||||||||||
| Participants and clinicians were aware of the treatment assignments. There was no evidence of deviations from the assigned interventions and an intention to treat approach was used for analysis. | ||||||||||||
| No missing data | ||||||||||||
| The measure is considered appropriate, and there were no differences in the measurement or ascertainment between groups. The assessors were blinded. | ||||||||||||
| The analysis is in accordance with a pre‐specified analysis in a published protocol, and results are not thought to be from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to be at low risk of bias for all domains for this outcome. | ||||||||||||
| Bias | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study | Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | ||||||
| Subgroup 2.3.1 Rehabilitation‐related activities | ||||||||||||
| Asplund 2000 |  |  |  |  |  |  | ||||||
| The method of allocation sequence is not described, but the use of sealed envelopes suggests a random component was used. Participant characteristics were balanced. | ||||||||||||
| Participants and those delivering the interventions were aware of intervention assignments. Twenty‐five patients were excluded due to them not meeting the set eligibility criteria, however, these: protocol violations are not expected to influence the effect estimate of the outcome as per protocol analyses used. The per protocol analyses was not thought to have a substantial impact on the result given the main reason for exclusion was inappropriate recruitment. Excluding this 25, the other 6 exclusions represent approximately 1% of the total sample size. | ||||||||||||
| Data at discharge from hospital complete for 98% of participants. | ||||||||||||
| The measurement of new institutionalisation is considered appropriate, and there were no differences in measurement or ascertainment between groups. Assessors were not blinded, but is thought that due to the lack of judgement in scoring a 'hard outcome' like new institutionalisation, that it is unlikely that the knowledge of the intervention could influence the outcome. | ||||||||||||
| A pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to raise some concerns due to deviations from intended interventions and the selection of the reported results. | ||||||||||||
| Counsell 2000 |  |  |  |  |  |  | ||||||
| Allocation sequence was random (computer generated random numbers) and sequence concealed (opaque sealed envelope). Participant characteristics were balanced. | ||||||||||||
| Participants and those delivering the interventions were aware of intervention assignments. Seventy‐nine participants were not admitted to the unit to which they were assigned. Deviations may have affected the effect estimate, however they were well‐balanced between groups. Intention to treat analysis was used. | ||||||||||||
| No missing data. | ||||||||||||
| The methods of measuring length of stay are considered appropriate, and there were no differences in measurement or ascertainment between groups. Assessors were not blinded, but is thought that due to the lack of judgement in scoring a 'hard outcome' like length of hospital stay, that it is unlikely that the knowledge of the intervention could influence the outcome. | ||||||||||||
| A pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to raise some concerns in the selection of the reported results and deviations from the intended interventions. | ||||||||||||
| Fretwell 1990 |  |  |  |  |  |  | ||||||
| “Patients were randomised only when both a treatment and control bed were available”. Although not stated, we believe the necessity for available beds on both wards indicate that a random component was likely used. Sequence allocation concealment is not discussed, but no significant differences in patient characteristics other than those compatible with chance were observed. | ||||||||||||
| Participants and those delivering the interventions were aware of intervention assignments. There were 30 randomisation errors which has been interpreted to mean 'allocated to the ward that they were not randomised to' however there is no explanation of this term. These deviations were thought likely to have affected the outcome, though were relative well‐balanced. It appears a per protocol analyses was used, though given the low number (7%) and relative balance between groups, the omission of the participants randomised incorrectly probably did not have substantial impact on the results. | ||||||||||||
| All participants accounted for at discharge. | ||||||||||||
| The methods of measuring new institutionalisation are considered appropriate, and there were no differences in measurement or ascertainment between groups. Assessors were not blinded, but is thought that due to the lack of judgement in scoring a 'hard outcome' like new institutionalisation, that it is unlikely that the knowledge of the intervention could influence the outcome. | ||||||||||||
| A detailed pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to raise some concerns in deviations from the intended interventions, methods of randomisation and selection of the reported result. | ||||||||||||
| Subgroup 2.3.2 Progressive resistance exercise | ||||||||||||
| de Morton 2007 |  |  |  |  |  |  | ||||||
| Allocation of wards was random (coin toss) and the allocating officer unaware of study. Baseline differences between groups were thought to be compatible with chance. | ||||||||||||
| Participants and clinicians delivering care were believed to be aware of treatment assignments. There was no evidence of deviations from intended interventions and intention to treat analysis was used. | ||||||||||||
| No missing data. | ||||||||||||
| The measure of new institutionalisation considered appropriate, and there were no differences in measurement or ascertainment between groups. Assessors were not blinded, but is thought that due to the lack of judgement in scoring a 'hard outcome' like new institutionalisation, that it is unlikely that the knowledge of the intervention could influence the outcome. | ||||||||||||
| A pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to raise some concerns in the selection of the reported result. | ||||||||||||
| Mudge 2008 |  |  |  |  |  |  | ||||||
| Pseudo‐randomisation, allocation was based on admitting unit and bed availability, and admitting unit was determined by a rotating roster. No evidence of baseline differences in patient characteristics other than those thought to be compatible with chance. | ||||||||||||
| Both participants and clinicians delivering care were aware of assigned interventions. There is no evidence of deviations from intended interventions and intention to treat analysis used. | ||||||||||||
| No missing data. | ||||||||||||
| The method is considered appropriate, and there were no differences in the measurement or ascertainment between groups. The assessors were blinded. | ||||||||||||
| A pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to be at high risk of bias due to the randomisation process. | ||||||||||||
| Bias | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study | Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | ||||||
| Subgroup 2.4.1 Rehabilitation‐related activities | ||||||||||||
| Asplund 2000 |  |  |  |  |  |  | ||||||
| The method of allocation sequence is not described, but the use of sealed envelopes suggests a random component was used. Participant characteristics were balanced. | ||||||||||||
| Participants and those delivering the interventions were aware of intervention assignments. Twenty‐five patients were excluded due to them not meeting the set eligibility criteria, however, these: protocol violations are not expected to influence the effect estimate of the outcome as per protocol analyses used. The per protocol analyses was not thought to have a substantial impact on the result given the main reason for exclusion was inappropriate recruitment. Excluding this 25, the other 6 exclusions represent approximately 1% of the total sample size. | ||||||||||||
| Data for readmissions complete for 98% of participants. | ||||||||||||
| The measurement of hospital readmissions is considered appropriate, and there were no differences in measurement or ascertainment between groups. Assessors were not blinded, but is thought that due to the lack of judgement in scoring a 'hard outcome' like hospital readmissions, that it is unlikely that the knowledge of the intervention could influence the outcome. | ||||||||||||
| A pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to raise some concerns due to deviations from intended interventions and the selection of the reported results. | ||||||||||||
| Counsell 2000 |  |  |  |  |  |  | ||||||
| Allocation sequence was random (computer generated random numbers) and sequence concealed (opaque sealed envelope). Participant characteristics were balanced. | ||||||||||||
| Participants and those delivering the interventions were aware of intervention assignments. Seventy‐nine participants were not admitted to the unit to which they were assigned. Deviations may have affected the effect estimate, however they were well‐balanced between groups. Intention to treat analysis was used. | ||||||||||||
| Given the nature of this population (older adults during and after acute hospitalisation) we considered a threshold of 90% of participants with data as sufficient, data was available for approximately 93% at 6 month follow‐up participants at discharge. | ||||||||||||
| The methods of measuring length of stay are considered appropriate, and there were no differences in measurement or ascertainment between groups. Assessors were not blinded, but is thought that due to the lack of judgement in scoring a 'hard outcome' like length of hospital stay, that it is unlikely that the knowledge of the intervention could influence the outcome. | ||||||||||||
| A pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to raise some concerns in the selection of the reported results and deviations from the intended interventions. | ||||||||||||
| Ekerstad 2017 |  |  |  |  |  |  | ||||||
| Randomisation was based on the availability of beds, and there was no allocation concealment as participants were allocated a ward prior to enrolment. Participant characteristics appeared balanced. | ||||||||||||
| Participants and clinicians delivering care were aware of treatment assignments. There was no evidence of deviations from intended interventions and intention to treat analysis was used. | ||||||||||||
| No missing data. | ||||||||||||
| The methods of measuring length of hospital stay are considered appropriate, and there were no differences in measurement or ascertainment between groups. Assessors were not blinded, but is thought that due to the lack of judgement in scoring a 'hard outcome' like length of hospital stay, that it is unlikely that the knowledge of the intervention could influence the outcome. | ||||||||||||
| A detailed pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to be at high risk of bias arising from the randomisation process. | ||||||||||||
| Landefeld 1995 |  |  |  |  |  |  | ||||||
| Allocation sequence was random (computer generated random numbers) and balanced participant characteristics, but no information was provided regarding allocation concealment. | ||||||||||||
| It is assumed participants and clinicians were aware of the treatment assignments. There was no evidence of deviations from the assigned interventions and evidence of an intention to treat approach being used for analysis. | ||||||||||||
| Accounting for mortality (24 died in each arm) all participants had outcome data at discharge. At follow up (a further 42, and 40 in intervention and control group had died), data was missing for just 6 participants (1%).at follow up (a further 42, and 40 in intervention and control group had died), data was missing for just 6 participants (1%). | ||||||||||||
| The measure is considered appropriate, and there were no differences in measurement or ascertainment between groups. Assessors were not blinded, but is thought that due to the lack of judgement in scoring a 'hard outcome' like hospital readmissions, that it is unlikely that the knowledge of the intervention could influence the outcome. | ||||||||||||
| A pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to have some concerns due to the methods of randomisation and in the selection of the reported result. | ||||||||||||
| Sahota 2017 |  |  |  |  |  |  | ||||||
| No information on randomisation methods other than to say participants were randomised, however, patient characteristics appear well‐balanced between groups and differences compatible with chance. | ||||||||||||
| It is assumed that participants and those delivering the interventions were aware of intervention assignments. There were 15 protocol deviations in the CIRACT group and 8 in the THB‐Rehab group. The deviations were thought to be in keeping with what would be expected outside of a trial context. Intention to treat not explicitly stated though it appears that the 1 participant who did not receive the intended intervention was analysed within the group to which they were assigned. | ||||||||||||
| No missing data. | ||||||||||||
| The measure is considered appropriate, and there were no differences in measurement or ascertainment between groups. Assessors were not blinded, but is thought that due to the lack of judgement in scoring a 'hard outcome' like hospital readmissions, that it is unlikely that the knowledge of the intervention could influence the outcome. | ||||||||||||
| A pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to raise some concerns in the selection of the reported results and the randomisation process. | ||||||||||||
| Slaets 1997 |  |  |  |  |  |  | ||||||
| Randomisation methods are described only as: “an alternating randomisation procedure”. However, there was a centrally administered method to allocate interventions, and baseline differences did not appear to show a significant problem with randomisation process other than those thought to be compatible with chance. | ||||||||||||
| Participants and clinicians delivering care were aware of allocated interventions. There was no evidence of deviations from intended intervention, and although intention to treat analysis was not specified, there was no information to suggest participants were not assessed in the group to which they were randomised. | ||||||||||||
| After accounting for mortality (6 patients died), data was available for 91% of participants. | ||||||||||||
| The methods of measuring hospital readmissions are considered appropriate, and there were no differences in measurement or ascertainment between groups. Assessors were not blinded, but is thought that due to the lack of judgement in scoring a 'hard outcome' like hospital readmissions, that it is unlikely that the knowledge of the intervention could influence the outcome. | ||||||||||||
| A pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to raise some concerns in the randomisation process and in the selection of the reported results. | ||||||||||||
| Subgroup 2.4.2 Structured exercise | ||||||||||||
| Gazineo 2021 |  |  |  |  |  |  | ||||||
| Allocation sequence was random (generated using an online system) and the allocation sequence was concealed until participants were enrolled (opaque concealed and sealed envelopes). Participant characteristics were well‐balanced. | ||||||||||||
| Participants and those delivering the interventions were aware of intervention allocations. There was no evidence of deviations from the intended interventions and intention to treat analysis used. | ||||||||||||
| No missing data after accounting for mortality. | ||||||||||||
| Methods of measuring hospital readmissions are considered appropriate and there were no differences in measurement or ascertainment of the outcomes between groups. The assessor was not blinded, but it is thought that due to the lack of judgement in the measurement, that the knowledge of the intervention did not influence the outcomes. | ||||||||||||
| A pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to raise some concerns in the selection of the reported results. | ||||||||||||
| Subgroup 2.4.3 Progressive resistance exercise | ||||||||||||
| Courtney 2009 |  |  |  |  |  |  | ||||||
| Allocation sequence was random (computer generated random numbers) and the allocation sequence concealed until participants were enrolled (via project coordinator blinded to baseline data). Participant characteristics appear well‐balanced. | ||||||||||||
| Participants and clinicians delivering care were believed to be aware of treatment assignments. 6/64 participants in the intervention group did not receive allocated treatment, the reasons are considered consistent with what could occur outside the trial context, and intention to treat analysis was used. | ||||||||||||
| Data regarding readmissions available for 92% of intervention and 98% of control group participants. Given the nature of this population (older adults during and after acute hospitalisation) we considered a threshold of 90% of participants with data as sufficient. | ||||||||||||
| The measurement of hospital readmissions is considered appropriate, and there were no differences in measurement or ascertainment between groups. The assessors are believed to be blinded to treatment allocation. | ||||||||||||
| A pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to raise some concerns in the selection of the reported result. | ||||||||||||
| de Morton 2007 |  |  |  |  |  |  | ||||||
| Allocation of wards was random (coin toss) and the allocating officer unaware of study. Baseline differences between groups were thought to be compatible with chance. | ||||||||||||
| Participants and clinicians delivering care were believed to be aware of treatment assignments. There was no evidence of deviations from intended interventions and intention to treat analysis was used. | ||||||||||||
| No missing data. | ||||||||||||
| The measure of hospital readmissions is considered appropriate, and there were no differences in measurement or ascertainment between groups. Assessors were not blinded, but is thought that due to the lack of judgement in scoring a 'hard outcome' like hospital readmissions, that it is unlikely that the knowledge of the intervention could influence the outcome. | ||||||||||||
| A pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to raise some concerns in the selection of the reported result. | ||||||||||||
| Martinez‐Velilla 2019 |  |  |  |  |  |  | ||||||
| Allocation sequence was random (www.randomizer.org) and personal communication with author confirms allocation concealment. Baseline differences between groups are thought to be compatible with chance. | ||||||||||||
| Both participants and clinicians delivering care were aware of assigned interventions. There is no evidence of deviations from intended interventions and intention to treat analysis used. | ||||||||||||
| No missing data. | ||||||||||||
| The methods of measurement are considered appropriate, and there were no differences in the measurement or ascertainment between groups. The assessors were blinded. | ||||||||||||
| The trial protocol reflects the analyses as that were conducted, and it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to be at low risk of bias in all domains for this outcome. | ||||||||||||
| McCullagh 2020 |  |  |  |  |  |  | ||||||
| Allocation sequence was random (computer‐generated) and allocation sequence was concealed. Baseline differences are thought to be compatible with chance. | ||||||||||||
| Both participants and clinicians delivering care were aware of assigned interventions. There is no evidence of deviations from intended interventions and intention to treat analysis used. | ||||||||||||
| No missing data. | ||||||||||||
| The measure is considered appropriate, and there were no differences in the measurement or ascertainment between groups. The assessors were blinded. | ||||||||||||
| The trial protocol reflects the analyses as that were conducted, and it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to be at low risk of bias in all domains for this outcome. | ||||||||||||
| Mudge 2008 |  |  |  |  |  |  | ||||||
| Pseudo‐randomisation, allocation was based on admitting unit and bed availability, and admitting unit was determined by a rotating roster. No evidence of baseline differences in patient characteristics other than those thought to be compatible with chance. | ||||||||||||
| Both participants and clinicians delivering care were aware of assigned interventions. There is no evidence of deviations from intended interventions and intention to treat analysis used. | ||||||||||||
| No missing data. | ||||||||||||
| The method is considered appropriate, and there were no differences in the measurement or ascertainment between groups. The assessors were blinded. | ||||||||||||
| A pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to be at high risk of bias due to the randomisation process. | ||||||||||||
| Ortiz‐Alonso 2020 |  |  |  |  |  |  | ||||||
| The allocation sequence was based on recruitment blocks of 4‐8 weeks. Activity of daily living (ADL) scores at baseline and admission were significantly lower in the intervention group than the control group, this is thought to be an important prognostic factor and therefore a concern regarding the randomisation process. | ||||||||||||
| Methods were designed to blind participants from group assignments however due to the nature of the intervention and lack of sham intervention the success of this blinding is felt unlikely. Ward staff and research staff were not blinded to the participant's assigned intervention. There were no deviations from the intended interventions due to trial context described and although intention to treat analysis not specified given the lack of deviations it is assumed. | ||||||||||||
| No missing outcome data. | ||||||||||||
| The measure is considered appropriate, and there were no differences in the measurement or ascertainment between groups. The assessors were not blinded, but it is thought that due to the lack of judgement in the outcome, that the knowledge of the intervention did not influence the outcome. | ||||||||||||
| Analysis reflects plan in trial registration and study protocol, and results are not thought to be from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to be at high risk of bias due to the randomisation process. | ||||||||||||
| Pedersen 2019 |  |  |  |  |  |  | ||||||
| Allocation sequence was random (computer‐generated block randomisation), and allocation sequence concealed from the investigators. Participant characteristics were well‐balanced between groups. | ||||||||||||
| Participants and clinicians were aware of the treatment assignments. There was no evidence of deviations from the assigned interventions and an intention to treat approach was used for analysis. | ||||||||||||
| No missing data | ||||||||||||
| The measure is considered appropriate, and there were no differences in the measurement or ascertainment between groups. The assessors were blinded. | ||||||||||||
| The analysis is in accordance with a pre‐specified analysis in a published protocol, and results are not thought to be from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to be at low risk of bias for all domains for this outcome. | ||||||||||||
| Bias | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study | Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | ||||||
| Subgroup 2.5.1 Rehabilitation‐related activities | ||||||||||||
| Ekerstad 2017 |  |  |  |  |  |  | ||||||
| Randomisation was based on the availability of beds, and there was no allocation concealment as participants were allocated a ward prior to enrolment. Participant characteristics appeared balanced. | ||||||||||||
| Participants and clinicians delivering care were aware of treatment assignments. There was no evidence of deviations from intended interventions and intention to treat analysis was used. | ||||||||||||
| The timed up and go (TUG) (when accounting for mortality) was available for 74% and 49% of participants in intervention and control groups at hospital discharge respectively. The difference in missing data for the TUG acompared to the activity of daily living data may be due to participants being unable to complete the walking tasks. Therefore a large proportion of missing data is thought to be due to the 'true' value. | ||||||||||||
| The use of the TUG to measure walking performance is considered appropriate and there was no differences in measurement between groups. The assessors were not blinded, and it was therefore considered likely that knowledge of the intervention could influence the outcome, given the likely strong belief in the benefits of the intervention ward. | ||||||||||||
| A detailed pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to be at high risk of bias due to bias in the measurement of the outcome and bias arising from the randomisation process. | ||||||||||||
| Subgroup 2.5.2 Structured exercise | ||||||||||||
| Hu 2020 |  |  |  |  |  |  | ||||||
| Allocation sequence was random (computer generated random numbers) and sequence concealed (blinded project coordinator allocated participants). Participant characteristics were balanced. | ||||||||||||
| Participants and those delivering the interventions were aware of intervention allocations. There was no evidence of deviations from the intended interventions and as such it is presumed intention to treat analysis was used. | ||||||||||||
| At discharge data was available for 76% of reablement group, (78% of reminder group) and 80% of the control group. Only participants who had complete data (i.e. data collected at baseline, discharge and follow‐up) had outcomes reported. Although missing data is well‐balanced between all three groups, it is considered likely that reason for missing data is related to its true value. | ||||||||||||
| The use of the timed up and go (TUG) is considered appropriate, and there were no differences in the measurement or ascertainment between groups. The assessors were blinded. | ||||||||||||
| A pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to be at high risk of bias due to missing outcome data. | ||||||||||||
| Killey 2006 |  |  |  |  |  |  | ||||||
| Allocation not random and sequence predictable as based on alternation. There is limited data on participant characteristics, but available data suggests balanced characteristics between groups. | ||||||||||||
| Participants and those delivering the interventions were aware of intervention allocations. There was no evidence of deviations from the intended interventions. Per‐protocol analysis appears to have been used. 2 participants in the intervention group were excluded from analysis as they completed less than 70% of their walks. The 2 participants that were excluded accounted for 7% of the remaining sample, it is therefore considered that there was potential for a significant impact on the results. | ||||||||||||
| Only 71% of intervention and 74% of control group had outcome data. A significant proportion of missing data was related to early discharge from hospital. These participants could be expected to have a higher functional level than those who remained in hospital. However, the missing data was well‐balanced between the two groups. | ||||||||||||
| The total distance able to walk is considered appropriate, and there were no differences in the measurement or ascertainment between groups. The assessors were not blinded, and it was therefore considered likely that knowledge of the intervention could influence the outcome, given the likely strong belief in the benefits of the intervention ward. | ||||||||||||
| A pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to be at high risk of bias in three domains for this outcome. | ||||||||||||
| Subgroup 2.5.3 Progressive resistance exercise | ||||||||||||
| de Morton 2007 |  |  |  |  |  |  | ||||||
| Allocation of wards was random (coin toss) and the allocating officer unaware of study. Baseline differences between groups were thought to be compatible with chance. | ||||||||||||
| Participants and clinicians delivering care were believed to be aware of treatment assignments. There was no evidence of deviations from intended interventions and intention to treat analysis was used. | ||||||||||||
| After accounting for mortality, at discharge, only 70% in intervention arm and 60% in control arm had timed up and go (TUG) assessed. There is a higher amount of missing data for the TUG than the Barthel Index which may indicate that some of the missing data is due to participants being unable to complete the TUG task. Therefore a proportion of missing data is believed to be due to the 'true' value. | ||||||||||||
| The use of the TUG to measure walking performance is considered appropriate, and there were no differences in the measurement or ascertainment between groups. The assessors were not blinded, and it is considered likely that knowledge of the intervention could influence the outcome, given the likely strong belief in the benefits of the intervention. | ||||||||||||
| A pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to be at high risk of bias in measurement of the outcome and missing data. | ||||||||||||
| Jones 2006 |  |  |  |  |  |  | ||||||
| Allocation sequence was random (computer generated random numbers). Sequence allocation was not concealed, but performed by a member of staff independent of the enrolment procedures. ). Participant characteristics were balanced between groups and differences compatible with chance. | ||||||||||||
| It is assumed that both participants and clinicians delivering care were aware of assigned interventions. There is no evidence of deviations from intended interventions and intention to treat analysis used. | ||||||||||||
| After accounting for mortality, timed up and go (TUG) data was available for 31% of control group and 51% of intervention group. "39.4% (63/160) were unable to complete the TUG either on admission or on discharge and therefore a change score could not be calculated." Therefore, a large proportion of missing data is due to the 'true' value. | ||||||||||||
| The use of the TUG to measure walking performance is considered appropriate, and there were no differences in the measurement or ascertainment between groups. The assessors were blinded. | ||||||||||||
| A pre‐specified statistical plan was not found, but it is not thought that the results were from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to be at high risk of bias due to missing outcome data. | ||||||||||||
| Pedersen 2019 |  |  |  |  |  |  | ||||||
| Allocation sequence was random (computer‐generated block randomisation), and allocation sequence concealed from the investigators. Participant characteristics were well‐balanced between groups. | ||||||||||||
| Participants and clinicians were aware of the treatment assignments. There was no evidence of deviations from the assigned interventions and an intention to treat approach was used for analysis. | ||||||||||||
| When accounting for mortality only 93% of participants in intervention group and 67% in control group completed measures at discharge. The difference in the proportion of missing data, and that the assessments were carried out home after discharge, it was judged likely that the reasons for missing data may have depended on its true value. | ||||||||||||
| The use of the 4m timed walk for measuring gait speed/walking performance is considered appropriate, and there were no differences in the measurement or ascertainment between groups. The assessors were blinded. | ||||||||||||
| The analysis is in accordance with a pre‐specified analysis in a published protocol, and results are not thought to be from multiple outcome measures or multiple analyses. | ||||||||||||
| The study is judged to be at high risk of bias due to missing outcome data. | ||||||||||||

