Compuestos de vitamina D para pacientes con nefropatías crónicas que requieren diálisis
Referencias
Referencias de los estudios incluidos en esta revisión
Referencias de los estudios en curso
Referencias adicionales
Referencias de otras versiones publicadas de esta revisión
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods |
| |
| Participants |
Newer vitamin D group
Established vitamin D group
| |
| Interventions | Newer vitamin D group
Established vitamin D group
Cointerventions: NS | |
| Outcomes | Extractable and relevant to this review
| |
| Notes |
The study used a crossover design, with both interventions prescribed to each group randomly in reverse order, without washout period. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Central dynamic allocation method using serum calcium and iPTH concentrations during 8‐week observation period and institution as stratification variables |
| Allocation concealment? | Unclear risk | Not stated |
| Blinding? | High risk | No blinding of participants or investigators. Blinding of outcome assessors or data assessors not stated. |
| Intention‐to‐treat analysis ‐ was it performed? | High risk | ITT not performed |
| Was the study free of potential bias from the funding source? | High risk | Funded by a pharmaceutical company |
| Methods |
| |
| Participants |
Newer vitamin D group 1
Newer vitamin D group 2
Newer vitamin D group 3
Placebo group
| |
| Interventions | Newer vitamin D group 1
Newer vitamin D group 2
Newer vitamin D group 3
Placebo group
Cointerventions: Calcium carbonate or aluminium containing phosphate binders | |
| Outcomes | Extractable and relevant to this review
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Central dynamic allocation, stratified by iPTH concentration, corrected serum calcium level, age, and centre. |
| Allocation concealment? | Unclear risk | Not stated |
| Blinding? | Unclear risk | Blinding of participants or investigators. Blinding of outcome assessors or data assessors not stated. |
| Intention‐to‐treat analysis ‐ was it performed? | Low risk | ITT performed |
| Was the study free of potential bias from the funding source? | Unclear risk | Funding source not stated |
| Methods |
| |
| Participants |
IV group
Oral group
| |
| Interventions | IV group
Oral group
Cointerventions: Alteration of dialysate calcium concentration and oral calcium carbonate | |
| Outcomes | Extractable and relevant to this review
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Not stated |
| Allocation concealment? | Unclear risk | Not stated |
| Blinding? | Unclear risk | Not stated |
| Intention‐to‐treat analysis ‐ was it performed? | Unclear risk | Not stated |
| Was the study free of potential bias from the funding source? | Unclear risk | Funding source not stated |
| Methods |
| |
| Participants |
Established vitamin D group
Placebo group
| |
| Interventions | Established vitamin D group
Placebo group
Cointerventions: Aluminium containing phosphate binders, dialysis 15‐24 hours/week, dialysate calcium concentration 6.6 mg/dL (1.65 mmol/L), daily dietary calcium intake 48‐76 mg (12‐19 mmol) | |
| Outcomes | Extractable and relevant to this review
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Not stated, stratified by time on dialysis, age, plasma alkaline phosphatase, the presence or absence of kidneys, and centre of care. |
| Allocation concealment? | Unclear risk | Not stated |
| Blinding? | Unclear risk | Blinding of participants or investigators. Blinding of outcome assessors or data assessors not stated |
| Intention‐to‐treat analysis ‐ was it performed? | High risk | Not performed |
| Was the study free of potential bias from the funding source? | Unclear risk | Funding source not stated. |
| Methods |
| |
| Participants |
Established vitamin D group 1
Established vitamin D group 2
| |
| Interventions | Established vitamin D group 1
Established vitamin D group 2
Cointerventions: Stable dialysate calcium (6 mg/dL (1.5 mmol/L)), aluminium containing antacids to maintain serum phosphorus between 4.0‐7.0 mg/dL (1.3‐2.3 mmol/L) | |
| Outcomes | Extractable and relevant to this review
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Computed‐generated random sequence |
| Allocation concealment? | Unclear risk | Not stated |
| Blinding? | Low risk | Blinding of participants, investigators and outcomes assessors. Blinding of data assessors not stated. |
| Intention‐to‐treat analysis ‐ was it performed? | Unclear risk | Not stated |
| Was the study free of potential bias from the funding source? | High risk | Funded by pharmaceutical company |
| Methods |
| |
| Participants |
Oral group
IV group
| |
| Interventions | Oral group
IV group
Cointerventions: Dialysate calcium 6.8 mg/dL (1.7 mmol/L) | |
| Outcomes | Extractable and relevant to this review
| |
| Notes |
Ethics approval was not sought for study. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Not stated |
| Allocation concealment? | Unclear risk | Not stated |
| Blinding? | Unclear risk | Blinding of participants, investigators, outcome assessors or data assessors not stated. |
| Intention‐to‐treat analysis ‐ was it performed? | Low risk | ITT performed |
| Was the study free of potential bias from the funding source? | Unclear risk | Funding source not stated |
| Methods |
| |
| Participants |
Established vitamin D group 1
Established vitamin D group 2
| |
| Interventions | Established vitamin D group 1
Established vitamin D group 2
Cointerventions: NS | |
| Outcomes | Extractable and relevant to this review
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Not stated |
| Allocation concealment? | Unclear risk | Not stated |
| Blinding? | Unclear risk | Blinding of participants, investigators, outcome assessors or data assessors not stated. |
| Intention‐to‐treat analysis ‐ was it performed? | Unclear risk | Not stated |
| Was the study free of potential bias from the funding source? | Unclear risk | Funding source not stated |
| Methods |
| |
| Participants |
Intermittent group 1
Intermittent group 2
Daily group
| |
| Interventions | Intermittent group 1
Intermittent group 2
Daily group
Cointerventions: Calcium carbonate, calcium acetate, aluminium hydroxide | |
| Outcomes | Extractable and relevant to this review
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Not stated, stratified according to PTH |
| Allocation concealment? | Unclear risk | Not stated |
| Blinding? | Unclear risk | Blinding of participants, investigators, outcome assessors or data assessors not stated. |
| Intention‐to‐treat analysis ‐ was it performed? | High risk | Not performed |
| Was the study free of potential bias from the funding source? | Unclear risk | Funding source not stated |
| Methods |
| |
| Participants |
Intermittent group
Daily group
| |
| Interventions | Intermittent group
Daily group
Cointerventions: Calcium carbonate, calcium acetate, aluminium hydroxide | |
| Outcomes | Extractable and relevant to this review
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Not stated |
| Allocation concealment? | Unclear risk | Not stated |
| Blinding? | Unclear risk | Blinding of participants, investigators, outcome assessors or data assessors not stated. |
| Intention‐to‐treat analysis ‐ was it performed? | Unclear risk | Not stated |
| Was the study free of potential bias from the funding source? | Unclear risk | Funding source not stated |
| Methods |
| |
| Participants |
Established vitamin D group
No treatment group
| |
| Interventions | Established vitamin D group
No treatment group
Cointerventions: Calcium carbonate and aluminium hydroxide | |
| Outcomes | Extractable and relevant to this review
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | High risk | According to whether their patient number was even or odd. |
| Allocation concealment? | High risk | Inadequate |
| Blinding? | Unclear risk | Blinding of participants, investigators, outcome assessors or data assessors not stated. |
| Intention‐to‐treat analysis ‐ was it performed? | High risk | Not performed |
| Was the study free of potential bias from the funding source? | Unclear risk | Funding source not stated |
| Methods |
| |
| Participants |
Established vitamin D group 1
Established vitamin D group 2
| |
| Interventions | Established vitamin D group 1
Established vitamin D group 2
Cointerventions: NS | |
| Outcomes | Extractable and relevant to this review
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Not stated |
| Allocation concealment? | Unclear risk | Not stated |
| Blinding? | Unclear risk | Blinding of participants, investigators, outcome assessors or data assessors not stated. |
| Intention‐to‐treat analysis ‐ was it performed? | Unclear risk | Not stated |
| Was the study free of potential bias from the funding source? | Unclear risk | Funding source not stated |
| Methods |
| |
| Participants |
Established vitamin D group 1
Established vitamin D group 2
| |
| Interventions | Established vitamin D group 1
Established vitamin D group 2
Cointerventions: Calcium carbonate, aluminium hydroxide | |
| Outcomes | Extractable and relevant to this review
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Not stated |
| Allocation concealment? | Unclear risk | Not stated |
| Blinding? | Unclear risk | Blinding of participants, investigators, outcome assessors or data assessors not stated. |
| Intention‐to‐treat analysis ‐ was it performed? | Unclear risk | Not stated |
| Was the study free of potential bias from the funding source? | Unclear risk | Funding source not stated |
| Methods |
| |
| Participants |
Established vitamin D group 1
Established vitamin D group 2
| |
| Interventions | Established vitamin D group 1
Established vitamin D group 2
Cointerventions: Calcium gluconate PO and other unspecified phosphate binders | |
| Outcomes | Extractable and relevant to this review
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Not stated, matched for age, gender, and dialysis vintage |
| Allocation concealment? | Unclear risk | Not stated |
| Blinding? | Unclear risk | Blinding of participants, investigators, outcome assessors or data assessors not stated. |
| Intention‐to‐treat analysis ‐ was it performed? | Unclear risk | Not stated |
| Was the study free of potential bias from the funding source? | Unclear risk | Funding source not stated |
| Methods |
| |
| Participants |
IV group
Oral group
| |
| Interventions | IV group
Oral group
Cointerventions: Dialysate calcium reduction when hypercalcaemia occurred. No calcium containing phosphate binders were used. | |
| Outcomes | Extractable and relevant to this review
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Coin toss by nursing staff |
| Allocation concealment? | High risk | Inadequate |
| Blinding? | Unclear risk | No blinding of participants, investigators, outcome assessors or data assessors |
| Intention‐to‐treat analysis ‐ was it performed? | Low risk | ITT performed |
| Was the study free of potential bias from the funding source? | Unclear risk | Funding source not stated |
| Methods |
| |
| Participants |
Established vitamin D group
Calcium group
| |
| Interventions | Established vitamin D group
Calcium group
Cointerventions: Magnesium hydroxide | |
| Outcomes | Extractable and relevant to this review
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Not stated |
| Allocation concealment? | Unclear risk | Not stated |
| Blinding? | Unclear risk | Blinding of participants, investigators, outcome assessors or data assessors not stated. |
| Intention‐to‐treat analysis ‐ was it performed? | Unclear risk | Not stated |
| Was the study free of potential bias from the funding source? | Unclear risk | Funding source not stated |
| Methods |
| |
| Participants |
Newer vitamin D group
Placebo group
| |
| Interventions | Newer vitamin D group
Placebo group
Cointerventions: Calcium carbonate, calcium acetate | |
| Outcomes | Extractable and relevant to this review
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Remote randomisation (centrally located, unaffiliated statistician, who used a randomisation code). |
| Allocation concealment? | Low risk | Adequate |
| Blinding? | Unclear risk | Blinding of participants and investigators. Blinding of outcome assessors or data assessors not stated |
| Intention‐to‐treat analysis ‐ was it performed? | High risk | Not performed |
| Was the study free of potential bias from the funding source? | High risk | Funded by pharmaceutical company |
| Methods |
| |
| Participants |
IP group
Oral group
| |
| Interventions | IP group
Oral group
Cointerventions: Adjustment of dialysate calcium | |
| Outcomes | Extractable and relevant to this review
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | High risk | According to date of entry into dialysis program |
| Allocation concealment? | High risk | Inadequate |
| Blinding? | High risk | No blinding of participants, investigators, outcome assessors or data assessors. |
| Intention‐to‐treat analysis ‐ was it performed? | High risk | Not performed |
| Was the study free of potential bias from the funding source? | Unclear risk | Not stated |
| Methods |
| |
| Participants |
Thrice weekly group
Twice weekly group
| |
| Interventions | Thrice weekly group
Twice weekly group
Cointerventions: Aluminium‐containing phosphate binders | |
| Outcomes | Extractable and relevant to this review
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Not stated |
| Allocation concealment? | Unclear risk | Not stated |
| Blinding? | High risk | No blinding of participants, investigators, outcome assessors or data assessors |
| Intention‐to‐treat analysis ‐ was it performed? | Low risk | ITT performed |
| Was the study free of potential bias from the funding source? | Unclear risk | Funding source not stated |
| Methods |
| |
| Participants |
Newer vitamin D group
Placebo group
| |
| Interventions | Newer vitamin D group
Placebo group
Cointerventions: NS | |
| Outcomes | Extractable and relevant to this review
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Not stated |
| Allocation concealment? | Unclear risk | Not stated |
| Blinding? | Unclear risk | Blinding of participants and investigators. Blinding of outcome assessors or data assessors not stated. |
| Intention‐to‐treat analysis ‐ was it performed? | Unclear risk | Not stated |
| Was the study free of potential bias from the funding source? | High risk | Funded by Abbott Laboratories |
| Methods |
| |
| Participants |
Established vitamin D group
Placebo group
| |
| Interventions | Established vitamin D group
Placebo group
Cointerventions: Calcium‐containing phosphate binders | |
| Outcomes | Extractable and relevant to this review
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Not stated |
| Allocation concealment? | Unclear risk | Not stated |
| Blinding? | Unclear risk | Blinding of participants and investigators. Blinding of outcome assessors or data assessors not stated. |
| Intention‐to‐treat analysis ‐ was it performed? | Unclear risk | Not stated |
| Was the study free of potential bias from the funding source? | High risk | Funded by Abbott Laboratories |
| Methods |
| |
| Participants |
Newer vitamin D group
Placebo group
| |
| Interventions | Newer vitamin D group
Placebo group
Cointerventions: Calcium carbonate, calcium acetate, sevelamer hydrochloride, and maintain dialysate calcium levels at 2.5 mEg/L (1.25 mmol/L) | |
| Outcomes | Extractable and relevant to this review
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Not stated |
| Allocation concealment? | Unclear risk | Not stated |
| Blinding? | Unclear risk | Blinding of participants and investigators. Blinding of outcome assessors or data assessors not stated. |
| Intention‐to‐treat analysis ‐ was it performed? | Low risk | ITT performed |
| Was the study free of potential bias from the funding source? | High risk | Funded by Abbott Laboratories |
| Methods |
| |
| Participants |
IV group
Oral group
| |
| Interventions | IV group
Oral group
Cointerventions: NS | |
| Outcomes | Extractable and relevant to this review
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Not stated |
| Allocation concealment? | Unclear risk | Not stated |
| Blinding? | Unclear risk | Blinding of participants, investigators, outcomes assessors and data assessors not stated. |
| Intention‐to‐treat analysis ‐ was it performed? | High risk | Not performed |
| Was the study free of potential bias from the funding source? | High risk | Funded by a pharmaceutical company |
| Methods |
| |
| Participants |
Newer vitamin D group
Established vitamin D group
| |
| Interventions | Newer vitamin D group
Established vitamin D group
Cointerventions: Dialysate calcium, calcium carbonate | |
| Outcomes | Extractable and relevant to this review
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Not stated |
| Allocation concealment? | Low risk | Sealed envelopes |
| Blinding? | Unclear risk | No blinding of participants, investigators, outcomes assessors or data assessors. |
| Intention‐to‐treat analysis ‐ was it performed? | High risk | Not performed |
| Was the study free of potential bias from the funding source? | Unclear risk | Funding source not stated |
| Methods |
| |
| Participants |
Intermittent group
Continuous group
| |
| Interventions | Intermittent group
Continuous group
Cointerventions: Calcium carbonate, calcium acetate, aluminium hydroxide (one centre, protocol violation) | |
| Outcomes | Extractable and relevant to this review
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Random number generation |
| Allocation concealment? | Unclear risk | Not stated |
| Blinding? | Unclear risk | Blinding of participants, investigators, outcomes assessors or data assessors not stated |
| Intention‐to‐treat analysis ‐ was it performed? | Unclear risk | Not performed |
| Was the study free of potential bias from the funding source? | Unclear risk | Funding source not stated |
| Methods |
| |
| Participants |
IV group
Oral group
Calcium group
| |
| Interventions | IV group
Oral group
Calcium group
Cointerventions: Calcium carbonate, dietary advice, aluminium hydroxide | |
| Outcomes | Extractable and relevant to this review
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Permuted block randomisation |
| Allocation concealment? | Low risk | Adequate |
| Blinding? | Low risk | Blinding of outcome assessors. |
| Intention‐to‐treat analysis ‐ was it performed? | High risk | Not performed |
| Was the study free of potential bias from the funding source? | High risk | Funded by a pharmaceutical company |
| Methods |
| |
| Participants |
Oral group
IP group
| |
| Interventions | Oral group
IP group
Cointerventions: Dietary phosphorus restriction, calcium carbonate, dialysate calcium concentration | |
| Outcomes | Extractable and relevant to this review
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Not stated |
| Allocation concealment? | Unclear risk | Not stared |
| Blinding? | Low risk | Blinding of outcome assessors. |
| Intention‐to‐treat analysis ‐ was it performed? | Low risk | ITT performed |
| Was the study free of potential bias from the funding source? | Unclear risk | Funded by a pharmaceutical company |
| Methods |
| |
| Participants |
Established vitamin D group
Placebo group
| |
| Interventions | Established vitamin D group
Placebo group
Cointerventions: Calcium carbonate 4.5 g/d | |
| Outcomes | Extractable and relevant to this review
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Not stated |
| Allocation concealment? | Unclear risk | Not stated |
| Blinding? | Unclear risk | Not stated |
| Intention‐to‐treat analysis ‐ was it performed? | Unclear risk | Not stated |
| Was the study free of potential bias from the funding source? | Unclear risk | Funding source not stated |
| Methods |
| |
| Participants |
Newer vitamin D group
Established vitamin D group
| |
| Interventions | Newer vitamin D group
Established vitamin D group
Cointerventions: Calcium based phosphate binders | |
| Outcomes | Extractable and relevant to this review
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Not stated |
| Allocation concealment? | Unclear risk | Not stated |
| Blinding? | Unclear risk | Not stated |
| Intention‐to‐treat analysis ‐ was it performed? | Unclear risk | Not stated |
| Was the study free of potential bias from the funding source? | Unclear risk | Funding source not stated |
| Methods |
| |
| Participants |
Daily group
Intermittent group
| |
| Interventions | Daily group
Intermittent group
Cointerventions: Calcium‐containing phosphate binder | |
| Outcomes | Extractable and relevant to this review
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Not stated |
| Allocation concealment? | Unclear risk | Not stated |
| Blinding? | High risk | No blinding of participants, investigators, outcome assessors or data assessors |
| Intention‐to‐treat analysis ‐ was it performed? | High risk | Not performed |
| Was the study free of potential bias from the funding source? | Unclear risk | Funding source not stated |
| Methods |
| |
| Participants |
Established vitamin D group 1
Established vitamin D group 2
Established vitamin D group 3
Placebo group
| |
| Interventions | Established vitamin D group 1
Established vitamin D group 2
Established vitamin D group 3
Placebo group
Cointerventions: Calcium carbonate | |
| Outcomes | Extractable and relevant to this review
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Not stated |
| Allocation concealment? | Unclear risk | Not stated |
| Blinding? | Low risk | Blinding of participants, investigators, outcomes assessors and data assessors |
| Intention‐to‐treat analysis ‐ was it performed? | Unclear risk | Not performed |
| Was the study free of potential bias from the funding source? | Unclear risk | Funding source not stated |
| Methods |
| |
| Participants |
IV group
Oral group
| |
| Interventions | IV group
Oral group
Cointerventions: All received aluminium hydroxide 1.2‐4.2 g/d and/or calcium carbonate 2.5‐11.25 g/d; use of phosphate binders was adjusted to maintain a predialysis plasma phosphate < 6.2 mg/dL | |
| Outcomes | Extractable and relevant to this review
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Not stated |
| Allocation concealment? | Unclear risk | Not stated |
| Blinding? | High risk | No blinding of participants, investigators, outcomes assessors or data assessors |
| Intention‐to‐treat analysis ‐ was it performed? | Low risk | ITT performed |
| Was the study free of potential bias from the funding source? | High risk | Funded by a pharmaceutical company |
| Methods |
| |
| Participants |
IV group
Oral group
| |
| Interventions | IV group
Oral group
Cointerventions: Calcium supplementation, standardised dialysate calcium | |
| Outcomes | Extractable and relevant to this review
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Not stated |
| Allocation concealment? | Unclear risk | Not stated |
| Blinding? | Unclear risk | Not stated |
| Intention‐to‐treat analysis ‐ was it performed? | Unclear risk | Not stated |
| Was the study free of potential bias from the funding source? | High risk | Funded by pharmaceutical company |
| Methods |
| |
| Participants |
IV group
Oral group
| |
| Interventions | IV group
Oral group
Cointerventions: Dialysate calcium adjustment, calcium and aluminium‐containing phosphate binders | |
| Outcomes | Extractable and relevant to this review
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Not stated |
| Allocation concealment? | Unclear risk | Not stated |
| Blinding? | High risk | No blinding of participants, investigators, outcome assessors or data assessors |
| Intention‐to‐treat analysis ‐ was it performed? | High risk | Not performed |
| Was the study free of potential bias from the funding source? | High risk | Funded by a pharmaceutical company |
| Methods |
| |
| Participants |
Intermittent group
Daily group
| |
| Interventions | Intermittent group
Daily group
Cointerventions: Aluminium‐containing phosphate binders | |
| Outcomes | Extractable and relevant to this review
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Not stated |
| Allocation concealment? | Unclear risk | Not stated |
| Blinding? | Unclear risk | Not stated |
| Intention‐to‐treat analysis ‐ was it performed? | Unclear risk | Not stated |
| Was the study free of potential bias from the funding source? | High risk | Funded by a pharmaceutical company |
| Methods |
| |
| Participants |
SC group
Oral group
| |
| Interventions | SC group
Oral group
Cointerventions: NS | |
| Outcomes | Extractable and relevant to this review
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Not stated |
| Allocation concealment? | Unclear risk | Not stated |
| Blinding? | High risk | No blinding of participants or investigators. Blinding of outcome assessors or data assessors not stated. |
| Intention‐to‐treat analysis ‐ was it performed? | Unclear risk | Not stated |
| Was the study free of potential bias from the funding source? | High risk | Funded by a pharmaceutical company |
| Methods |
| |
| Participants |
Newer vitamin D group
Placebo group
| |
| Interventions | Newer vitamin D group
Placebo group
Cointerventions: NS | |
| Outcomes | Extractable and relevant to this review
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Not stated |
| Allocation concealment? | Unclear risk | Not stated |
| Blinding? | Unclear risk | Blinding of participants and investigators. Blinding of outcome assessors or data assessors not stated. |
| Intention‐to‐treat analysis ‐ was it performed? | Unclear risk | ITT performed |
| Was the study free of potential bias from the funding source? | Unclear risk | Funding source not stated |
| Methods |
| |
| Participants |
Newer vitamin D group
Placebo group
| |
| Interventions | Newer vitamin D group
Placebo group
Cointerventions: Calcium carbonate and calcium acetate based phosphate binders | |
| Outcomes | Extractable and relevant to this review
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Computer randomisation |
| Allocation concealment? | Unclear risk | Not stated |
| Blinding? | Low risk | Blinding of participants, investigators, outcomes assessors and data assessors |
| Intention‐to‐treat analysis ‐ was it performed? | Low risk | ITT performed |
| Was the study free of potential bias from the funding source? | Unclear risk | Funded by Abbott Laboratories |
| Methods |
| |
| Participants |
Newer vitamin D group 1
Newer vitamin D group 2
| |
| Interventions | Newer vitamin D group 1
Newer vitamin D group 2
Cointerventions: Calcium and aluminium containing phosphate binders | |
| Outcomes | Extractable and relevant to this review
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Not stated |
| Allocation concealment? | Unclear risk | Not stated |
| Blinding? | Low risk | Blinding of participants, investigators, outcomes assessors and data assessors |
| Intention‐to‐treat analysis ‐ was it performed? | Low risk | ITT performed |
| Was the study free of potential bias from the funding source? | High risk | Funded by a pharmaceutical company |
| Methods |
| |
| Participants |
Once weekly group
Thrice weekly group
| |
| Interventions | Once weekly group
Thrice weekly group
Cointerventions: Dialysate calcium, calcium‐containing phosphate binders | |
| Outcomes | Extractable and relevant to this review
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Not stated |
| Allocation concealment? | Unclear risk | Not stated |
| Blinding? | Unclear risk | Not stated |
| Intention‐to‐treat analysis ‐ was it performed? | Unclear risk | Not stated |
| Was the study free of potential bias from the funding source? | Unclear risk | Funding source not stated |
| Methods |
| |
| Participants |
Established vitamin D group 1
Established vitamin D group 2
| |
| Interventions | Established vitamin D group 1
Established vitamin D group 2
Cointerventions: Aluminium hydroxide and calcium gluconate | |
| Outcomes | Extractable and relevant to this review
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Not stated |
| Allocation concealment? | Unclear risk | Not stated |
| Blinding? | Unclear risk | Blinding of participants and investigators. Blinding of outcome assessors or data assessors not stated |
| Intention‐to‐treat analysis ‐ was it performed? | Unclear risk | Not stated |
| Was the study free of potential bias from the funding source? | High risk | Funded by a pharmaceutical company |
| Methods |
| |
| Participants |
Established vitamin D group
Placebo group
| |
| Interventions | Established vitamin D group
Placebo group
Cointerventions: Aluminium hydroxide phosphate binders | |
| Outcomes | Extractable and relevant to this review
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | By investigator (with no patient contact) allocated patients into 2 groups |
| Allocation concealment? | High risk | Inadequate |
| Blinding? | Low risk | Blinding of participants, investigators and outcomes assessors. Blinding of data assessors not stated |
| Intention‐to‐treat analysis ‐ was it performed? | High risk | ITT not performed |
| Was the study free of potential bias from the funding source? | Unclear risk | Funding source not stated |
| Methods |
| |
| Participants |
Newer vitamin D group 1
Newer vitamin D group 2
| |
| Interventions | Newer vitamin D group 1
Newer vitamin D group 2
Cointerventions: Dietary control of phosphorus, sevelamer hydrochloride, and calcium carbonate or aluminium hydroxide | |
| Outcomes | Extractable and relevant to this review
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Not stated |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Blinding? | Unclear risk | Blinding of participants and investigators. Blinding of outcome assessors or data assessors not stated |
| Intention‐to‐treat analysis ‐ was it performed? | Low risk | ITT performed |
| Was the study free of potential bias from the funding source? | Unclear risk | Funding source not stated |
| Methods |
| |
| Participants |
Newer vitamin D group
Established vitamin D group
| |
| Interventions | Newer vitamin D group
Established vitamin D group
| |
| Outcomes | Extractable and relevant to this review
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Not stated |
| Allocation concealment? | Unclear risk | Not stated |
| Blinding? | Unclear risk | Not stated |
| Intention‐to‐treat analysis ‐ was it performed? | Unclear risk | Not stated |
| Was the study free of potential bias from the funding source? | Unclear risk | Funding source not stated |
| Methods |
| |
| Participants |
Intermittent group
Daily group
| |
| Interventions | Intermittent group
Daily group Cointerventions: Low calcium dialysate, calcium acetate as phosphate binder. Calcium acetate to keep phosphorus < 6.0 mg/dL (1.94 mmol/L). Calcium carbonate if not tolerated. | |
| Outcomes | Extractable and relevant to this review
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Random number generator |
| Allocation concealment? | Unclear risk | Not stated |
| Blinding? | High risk | No blinding of participants, investigators, outcome assessors or data assessors |
| Intention‐to‐treat analysis ‐ was it performed? | High risk | ITT not performed |
| Was the study free of potential bias from the funding source? | Unclear risk | Funding source not stated |
| Methods |
| |
| Participants |
Newer vitamin D group
Placebo group
| |
| Interventions | Newer vitamin D group
Placebo group
Cointerventions: Sevelamer hydrochloride and adjustment of dialysate calcium | |
| Outcomes | Extractable and relevant to this review
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Not stated |
| Allocation concealment? | Unclear risk | Not stated |
| Blinding? | Unclear risk | Blinding of participants and investigators. Blinding of outcome assessors or data assessors not stated |
| Intention‐to‐treat analysis ‐ was it performed? | High risk | Not performed |
| Was the study free of potential bias from the funding source? | High risk | Funded by a pharmaceutical company |
| Methods |
| |
| Participants |
Established vitamin D group
No treatment group
| |
| Interventions | Established vitamin D group
No treatment group
Cointerventions: Calcium carbonate, aluminium hydroxide | |
| Outcomes | Extractable and relevant to this review
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Random number generator |
| Allocation concealment? | Unclear risk | Not stated |
| Blinding? | Low risk | Blinding of participants, outcomes assessors and data assessors, but not investigators |
| Intention‐to‐treat analysis ‐ was it performed? | Unclear risk | ITT performed |
| Was the study free of potential bias from the funding source? | Unclear risk | Funding source not stated |
| Methods |
| |
| Participants |
Established vitamin D group
No treatment group
| |
| Interventions | Established vitamin D group
No treatment group
Cointerventions: Dietary advice, calcium carbonate or aluminium hydroxide | |
| Outcomes | Extractable and relevant to this review
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Not stated |
| Allocation concealment? | Unclear risk | Not stated |
| Blinding? | Unclear risk | Not stated |
| Intention‐to‐treat analysis ‐ was it performed? | Unclear risk | Not stated |
| Was the study free of potential bias from the funding source? | Unclear risk | Funding source not stated |
| Methods |
| |
| Participants |
Established vitamin D group 1
Established vitamin D group 2
| |
| Interventions | Established vitamin D group 1
Established vitamin D group 2
Cointerventions: Aluminium hydroxide as phosphate binders + oral calcium gluconate supplements | |
| Outcomes | Extractable and relevant to this review
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Not stated |
| Allocation concealment? | Unclear risk | Not stated |
| Blinding? | Unclear risk | Blinding of participants, investigators or outcome assessors not stated. Data assessors were blinded. |
| Intention‐to‐treat analysis ‐ was it performed? | High risk | Not performed |
| Was the study free of potential bias from the funding source? | Unclear risk | Funding source not stated |
| Methods |
| |
| Participants |
Newer vitamin D group
Placebo group
| |
| Interventions | Newer vitamin D group
Placebo group
Cointerventions: NS | |
| Outcomes | Extractable and relevant to this review
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Not stated |
| Allocation concealment? | Unclear risk | Not stated |
| Blinding? | Unclear risk | Blinding of participants and investigators. Blinding of outcome assessors or data assessors not stated. |
| Intention‐to‐treat analysis ‐ was it performed? | High risk | Not performed |
| Was the study free of potential bias from the funding source? | High risk | Funded by Abbott Laboratories |
| Methods |
| |
| Participants |
IV group
Oral group
| |
| Interventions | IV group
Oral group
Cointerventions: Dietary calcium and phosphate restriction, calcium carbonate and aluminium containing phosphate binders | |
| Outcomes | Extractable and relevant to this review
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Table of random numbers, following pairing according to PTH concentration |
| Allocation concealment? | Low risk | Adequate |
| Blinding? | Low risk | Blinding of participants, investigators and outcomes assessors. Blinding of data assessors not stated. |
| Intention‐to‐treat analysis ‐ was it performed? | High risk | Not performed |
| Was the study free of potential bias from the funding source? | High risk | Funded by pharmaceutical company |
| Methods |
| |
| Participants |
IP group
Oral group
| |
| Interventions | IP group
Oral group
Cointerventions: Calcium carbonate, dietary modification, dialysate modification, aluminium‐containing phosphate binders | |
| Outcomes | Extractable and relevant to this review
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Biostatistician using 3 randomisation schedules, one for each skeletal lesion, that is, osteitis fibrosa, secondary hyperparathyroidism and normal bone formation. |
| Allocation concealment? | Unclear risk | Not stated |
| Blinding? | High risk | No blinding of participants or investigators. Blinding of outcome assessors or data assessors not stated |
| Intention‐to‐treat analysis ‐ was it performed? | High risk | ITT not performed |
| Was the study free of potential bias from the funding source? | Unclear risk | Funding source not stated |
| Methods |
| |
| Participants |
Weekly group
Thrice weekly group
| |
| Interventions | Weekly group
Thrice weekly group
Cointerventions: Calcium carbonate and aluminium‐containing phosphate binders | |
| Outcomes | Extractable and relevant to this review
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Not stated |
| Allocation concealment? | Unclear risk | Not stated |
| Blinding? | Unclear risk | Not stated |
| Intention‐to‐treat analysis ‐ was it performed? | Unclear risk | Not stated |
| Was the study free of potential bias from the funding source? | Unclear risk | Funding source not stated |
| Methods |
| |
| Participants |
Newer vitamin D group
Established vitamin D group
| |
| Interventions | Newer vitamin D group
Established vitamin D group
Cointerventions: Phosphate binders using calcium carbonate or calcium acetate | |
| Outcomes | Extractable and relevant to this review
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Not stated |
| Allocation concealment? | Unclear risk | Not stated |
| Blinding? | Unclear risk | Blinding of participants and investigators. Blinding of outcome assessors or data assessors not stated. |
| Intention‐to‐treat analysis ‐ was it performed? | Low risk | ITT performed |
| Was the study free of potential bias from the funding source? | High risk | Funded by Abbott Laboratories |
| Methods |
| |
| Participants |
Intermittent group
Continuous group
| |
| Interventions | Intermittent group
Continuous group
Cointerventions: Calcium carbonate | |
| Outcomes | Extractable and relevant to this review
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Not stated |
| Allocation concealment? | Unclear risk | Not stated |
| Blinding? | Unclear risk | Not stated |
| Intention‐to‐treat analysis ‐ was it performed? | Low risk | ITT performed |
| Was the study free of potential bias from the funding source? | Unclear risk | Funding source not stated |
| Methods |
| |
| Participants |
Morning group
Evening group
| |
| Interventions | Morning group
Evening group
Cointerventions: NS | |
| Outcomes | Extractable relevant to this review
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Not stated |
| Allocation concealment? | Unclear risk | Not stated |
| Blinding? | High risk | No blinding of participants, investigators, outcome assessors or data assessors. |
| Intention‐to‐treat analysis ‐ was it performed? | Unclear risk | Not stated |
| Was the study free of potential bias from the funding source? | Unclear risk | Funding source not stated |
| Methods |
| |
| Participants |
Oral group
IV group
| |
| Interventions | Oral group
IV group
Cointerventions: Calcium acetate and aluminium salts | |
| Outcomes | Extractable and relevant to this review
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | High risk | Alternating |
| Allocation concealment? | High risk | Inadequate |
| Blinding? | Unclear risk | Not stated |
| Intention‐to‐treat analysis ‐ was it performed? | Unclear risk | Not stated |
| Was the study free of potential bias from the funding source? | Unclear risk | Funding source not stated |
| Methods |
| |
| Participants |
Daily group
Intermittent group
| |
| Interventions | Daily group
Intermittent group
Cointerventions: NS | |
| Outcomes | Extractable and relevant to this review
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Not stated |
| Allocation concealment? | Unclear risk | Not stated |
| Blinding? | High risk | No blinding of participants or investigators. Blinding of outcome assessors or data assessors not stated. |
| Intention‐to‐treat analysis ‐ was it performed? | Low risk | ITT performed |
| Was the study free of potential bias from the funding source? | Unclear risk | Funding source not stated |
| Methods |
| |
| Participants |
Established vitamin D group
Placebo
Overall
| |
| Interventions | Established vitamin D group
Placebo group
Cointerventions: Phosphate binding antacids | |
| Outcomes | Extractable and relevant to this review
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Alternating envelopes |
| Allocation concealment? | Unclear risk | Not stated |
| Blinding? | Unclear risk | Not stated |
| Intention‐to‐treat analysis ‐ was it performed? | Unclear risk | Not stated |
| Was the study free of potential bias from the funding source? | Unclear risk | Funding source not stated |
| Methods |
| |
| Participants |
Established vitamin D group
No treatment group
| |
| Interventions | Established vitamin D group
No treatment group
Cointerventions: Restriction of dietary phosphorus, cholecalciferol 400 IU/d, calcium carbonate, vitamin B and C, aluminium hydroxide | |
| Outcomes | Extractable and relevant to this review
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Not stated |
| Allocation concealment? | Low risk | Adequate (sealed envelopes) |
| Blinding? | Low risk | No blinding of participant or investigators. Outcomes assessors and data assessors were blinded. |
| Intention‐to‐treat analysis ‐ was it performed? | Low risk | ITT performed |
| Was the study free of potential bias from the funding source? | High risk | Funded by Abbott Laboratories |
| Methods |
| |
| Participants |
Low dose group
Intermediate dose group
High dose group
| |
| Interventions | Low dose group
Intermediate dose group
High dose group
Cointerventions: Phosphate binders, dietary phosphorus restriction | |
| Outcomes | Extractable and relevant to this review
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Not stated |
| Allocation concealment? | Unclear risk | Not stated |
| Blinding? | High risk | No blinding of participants, investigators, outcome assessors or data assessors. |
| Intention‐to‐treat analysis ‐ was it performed? | Unclear risk | Not stated |
| Was the study free of potential bias from the funding source? | High risk | Funded by pharmaceutical company |
AKI ‐ acute kidney injury; CAPD ‐ continuous ambulatory peritoneal dialysis; CCPD ‐ continuous cyclic peritoneal dialysis; GFR ‐ glomerular filtration rate; HCT ‐ haematocrit; HD ‐ haemodialysis; IP ‐ intraperitoneal; IV‐ intravenous; NS ‐ not stated; PD ‐ peritoneal dialysis; SC‐ subcutaneous
Characteristics of ongoing studies [ordered by study ID]
Ir a:
| Trial name or title | A study to evaluate the effects of two vitamin D analogs, Zemplar injection and Hectorol injection, on intestinal absorption of calcium in patients with Stage 5 CKD |
| Methods | |
| Participants | Stage 5 CKD patients on HD three times a week for at least 2 months, iPTH level > 200 pg/mL |
| Interventions | Zemplar injection versus Hectorol injection |
| Outcomes | Intestinal calcium absorption |
| Starting date | May 2006 |
| Contact information | |
| Notes |
| Trial name or title | Evaluation of colecalciferol substitution in dialysis patients |
| Methods | |
| Participants | Dialysis patients, vitamin D levels < 60 ng/mL |
| Interventions | Colecalciferol versus placebo |
| Outcomes | Bone metabolism and immune system |
| Starting date | November 2006 |
| Contact information | Eric Seibert, MD |
| Notes |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All‐cause mortality Show forest plot | 5 | 233 | Risk Ratio (M‐H, Random, 95% CI) | 1.34 [0.34, 5.24] |
| Analysis 1.1  Comparison 1 Vitamin D compounds versus placebo/no treatment, Outcome 1 All‐cause mortality. | ||||
| 1.1 Established vitamin D compounds | 3 | 168 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [0.24, 5.05] |
| 1.2 Newer vitamin D compounds | 2 | 65 | Risk Ratio (M‐H, Random, 95% CI) | 3.00 [0.13, 68.26] |
| 2 Fracture Show forest plot | 4 | 181 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.06, 15.41] |
| Analysis 1.2  Comparison 1 Vitamin D compounds versus placebo/no treatment, Outcome 2 Fracture. | ||||
| 2.1 Established vitamin D compounds | 3 | 103 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.06, 15.41] |
| 2.2 Newer vitamin D compounds | 1 | 78 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Development of bone pain Show forest plot | 4 | 109 | Risk Ratio (M‐H, Random, 95% CI) | 0.29 [0.03, 2.63] |
| Analysis 1.3  Comparison 1 Vitamin D compounds versus placebo/no treatment, Outcome 3 Development of bone pain. | ||||
| 3.1 Established vitamin D compounds | 3 | 74 | Risk Ratio (M‐H, Random, 95% CI) | 0.41 [0.02, 9.25] |
| 3.2 Newer vitamin D compounds | 1 | 35 | Risk Ratio (M‐H, Random, 95% CI) | 0.20 [0.01, 4.65] |
| 4 Parathyroidectomy Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.4  Comparison 1 Vitamin D compounds versus placebo/no treatment, Outcome 4 Parathyroidectomy. | ||||
| 4.1 Established vitamin D compounds | 2 | 133 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.05, 12.47] |
| 4.2 Newer vitamin D compounds | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Development of subperiosteal erosions Show forest plot | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.5  Comparison 1 Vitamin D compounds versus placebo/no treatment, Outcome 5 Development of subperiosteal erosions. | ||||
| 5.1 Established vitamin D compounds | 3 | 120 | Risk Ratio (M‐H, Random, 95% CI) | 0.41 [0.07, 2.38] |
| 5.2 Newer vitamin D compounds | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Resolution of subperiosteal erosions Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 1.6  Comparison 1 Vitamin D compounds versus placebo/no treatment, Outcome 6 Resolution of subperiosteal erosions. | ||||
| 6.1 Established vitamin D compounds | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 Newer vitamin D compounds | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Development of vascular calcification Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.7  Comparison 1 Vitamin D compounds versus placebo/no treatment, Outcome 7 Development of vascular calcification. | ||||
| 7.1 Established vitamin D compounds | 2 | 103 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.45, 2.67] |
| 7.2 Newer vitamin D compounds | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Progression of vascular calcification Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 1.8  Comparison 1 Vitamin D compounds versus placebo/no treatment, Outcome 8 Progression of vascular calcification. | ||||
| 8.1 Established vitamin D compounds | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.2 Newer vitamin D compounds | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 9 Development of osteitis fibrosa Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.9  Comparison 1 Vitamin D compounds versus placebo/no treatment, Outcome 9 Development of osteitis fibrosa. | ||||
| 9.1 Established vitamin D compounds | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.2 Newer vitamin D compounds | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10 Development of osteomalacia Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.10  Comparison 1 Vitamin D compounds versus placebo/no treatment, Outcome 10 Development of osteomalacia. | ||||
| 10.1 Established vitamin D compounds | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.2 Newer vitamin D compounds | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 11 End of treatment parathyroid hormone (pg/mL) Show forest plot | 6 | 212 | Mean Difference (IV, Random, 95% CI) | ‐196.05 [‐298.43, ‐93.66] |
| Analysis 1.11  Comparison 1 Vitamin D compounds versus placebo/no treatment, Outcome 11 End of treatment parathyroid hormone (pg/mL). | ||||
| 11.1 Established vitamin D compounds | 4 | 104 | Mean Difference (IV, Random, 95% CI) | ‐220.54 [‐473.63, 32.55] |
| 11.2 Newer vitamin D compounds | 2 | 108 | Mean Difference (IV, Random, 95% CI) | ‐183.88 [‐217.88, ‐149.89] |
| 12 Reduction in parathyroid hormone level by 30% Show forest plot | 7 | 361 | Risk Ratio (M‐H, Random, 95% CI) | 5.90 [3.17, 10.96] |
| Analysis 1.12  Comparison 1 Vitamin D compounds versus placebo/no treatment, Outcome 12 Reduction in parathyroid hormone level by 30%. | ||||
| 12.1 Established vitamin D compounds | 1 | 47 | Risk Ratio (M‐H, Random, 95% CI) | 2.72 [1.12, 6.61] |
| 12.2 Newer vitamin D compounds | 6 | 314 | Risk Ratio (M‐H, Random, 95% CI) | 7.05 [3.82, 13.04] |
| 13 End of treatment serum phosphorus (mg/dL) Show forest plot | 2 | 105 | Mean Difference (IV, Random, 95% CI) | 0.70 [0.08, 1.33] |
| Analysis 1.13  Comparison 1 Vitamin D compounds versus placebo/no treatment, Outcome 13 End of treatment serum phosphorus (mg/dL). | ||||
| 13.1 Established vitamin D compounds | 1 | 27 | Mean Difference (IV, Random, 95% CI) | 0.60 [‐0.40, 1.60] |
| 13.2 Newer vitamin D compounds | 1 | 78 | Mean Difference (IV, Random, 95% CI) | 0.77 [‐0.04, 1.58] |
| 14 One or more episodes of hyperphosphataemia Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.14  Comparison 1 Vitamin D compounds versus placebo/no treatment, Outcome 14 One or more episodes of hyperphosphataemia. | ||||
| 14.1 Established vitamin D compound | 2 | 59 | Risk Ratio (M‐H, Random, 95% CI) | 1.57 [0.97, 2.54] |
| 14.2 Newer vitamin D compound | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15 End of treatment serum calcium (mg/dL) Show forest plot | 2 | 105 | Mean Difference (IV, Random, 95% CI) | 0.36 [‐0.26, 0.98] |
| Analysis 1.15  Comparison 1 Vitamin D compounds versus placebo/no treatment, Outcome 15 End of treatment serum calcium (mg/dL). | ||||
| 15.1 Established vitamin D compounds | 1 | 27 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐0.61, 0.61] |
| 15.2 Newer vitamin D compounds | 1 | 78 | Mean Difference (IV, Random, 95% CI) | 0.64 [0.22, 1.06] |
| 16 One or more episodes of hypercalcaemia Show forest plot | 5 | 182 | Risk Ratio (M‐H, Random, 95% CI) | 3.80 [0.90, 16.12] |
| Analysis 1.16  Comparison 1 Vitamin D compounds versus placebo/no treatment, Outcome 16 One or more episodes of hypercalcaemia. | ||||
| 16.1 Established vitamin D compounds | 3 | 74 | Risk Ratio (M‐H, Random, 95% CI) | 1.96 [0.62, 6.22] |
| 16.2 Newer vitamin D compounds | 2 | 108 | Risk Ratio (M‐H, Random, 95% CI) | 11.97 [1.48, 96.58] |
| 17 Withdrawal of treatment due to hypercalcaemia Show forest plot | 4 | 196 | Risk Ratio (M‐H, Random, 95% CI) | 4.17 [1.36, 12.77] |
| Analysis 1.17  Comparison 1 Vitamin D compounds versus placebo/no treatment, Outcome 17 Withdrawal of treatment due to hypercalcaemia. | ||||
| 17.1 Established vitamin D compounds | 2 | 88 | Risk Ratio (M‐H, Random, 95% CI) | 3.66 [0.96, 14.03] |
| 17.2 Newer vitamin D compounds | 2 | 108 | Risk Ratio (M‐H, Random, 95% CI) | 5.61 [0.74, 42.45] |
| 18 One or more episodes of elevated calcium x phosphorus product Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 1.18  Comparison 1 Vitamin D compounds versus placebo/no treatment, Outcome 18 One or more episodes of elevated calcium x phosphorus product. | ||||
| 18.1 Established vitamin D compounds | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 18.2 Newer vitamin D compounds | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 19 End of treatment alkaline phosphatase (U/L) Show forest plot | 3 | 135 | Mean Difference (IV, Random, 95% CI) | ‐24.34 [‐44.34, ‐4.33] |
| Analysis 1.19  Comparison 1 Vitamin D compounds versus placebo/no treatment, Outcome 19 End of treatment alkaline phosphatase (U/L). | ||||
| 19.1 Established vitamin D compounds | 1 | 27 | Mean Difference (IV, Random, 95% CI) | ‐16.0 [‐54.83, 22.83] |
| 19.2 Newer vitamin D compounds | 2 | 108 | Mean Difference (IV, Random, 95% CI) | ‐27.35 [‐50.69, ‐4.01] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All‐cause mortality Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 2.1  Comparison 2 Vitamin D compound versus vitamin D compound, Outcome 1 All‐cause mortality. | ||||
| 1.1 New versus established vitamin D compounds | 2 | 103 | Risk Ratio (M‐H, Random, 95% CI) | 2.0 [0.19, 21.21] |
| 2 Fracture Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 2.2  Comparison 2 Vitamin D compound versus vitamin D compound, Outcome 2 Fracture. | ||||
| 2.1 New versus established vitamin D compounds | 1 | 73 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Improvement in bone pain Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 2.3  Comparison 2 Vitamin D compound versus vitamin D compound, Outcome 3 Improvement in bone pain. | ||||
| 3.1 Active vitamin D compound versus vitamin D3 | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Improvement in bone histomorphometry Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 2.4  Comparison 2 Vitamin D compound versus vitamin D compound, Outcome 4 Improvement in bone histomorphometry. | ||||
| 4.1 Active vitamin D compound versus vitamin D3 | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 End of treatment parathyroid hormone (pg/mL) Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 2.5  Comparison 2 Vitamin D compound versus vitamin D compound, Outcome 5 End of treatment parathyroid hormone (pg/mL). | ||||
| 5.1 Active vitamin D compound versus vitamin D3 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 Calcitriol versus other established vitamin D compounds | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.3 New versus established vitamin D compounds | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 End of treatment serum phosphorus (mg/dL) Show forest plot | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 2.6  Comparison 2 Vitamin D compound versus vitamin D compound, Outcome 6 End of treatment serum phosphorus (mg/dL). | ||||
| 6.1 Active vitamin D compound versus vitamin D3 | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.2 Calcitriol versus another established vitamin D | 2 | 91 | Mean Difference (IV, Random, 95% CI) | 0.52 [‐0.40, 1.44] |
| 6.3 New versus established vitamin D compounds | 1 | 73 | Mean Difference (IV, Random, 95% CI) | ‐0.31 [‐1.05, 0.43] |
| 7 One or more episodes of hyperphosphataemia Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 2.7  Comparison 2 Vitamin D compound versus vitamin D compound, Outcome 7 One or more episodes of hyperphosphataemia. | ||||
| 7.1 Active vitamin D compound versus vitamin D3 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.2 Calcitriol versus other established vitamin D compounds | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.3 New versus established vitamin D compounds | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 End of treatment serum calcium (mg/dL) Show forest plot | 4 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 2.8  Comparison 2 Vitamin D compound versus vitamin D compound, Outcome 8 End of treatment serum calcium (mg/dL). | ||||
| 8.1 Active vitamin D compound versus vitamin D3 | 1 | 31 | Mean Difference (IV, Random, 95% CI) | 0.85 [0.35, 1.35] |
| 8.2 Calcitriol versus other established vitamin D compounds | 2 | 91 | Mean Difference (IV, Random, 95% CI) | 1.00 [‐0.56, 2.57] |
| 8.3 New versus established vitamin D compounds | 1 | 73 | Mean Difference (IV, Random, 95% CI) | 0.30 [‐0.11, 0.71] |
| 9 End of treatment alkaline phosphatase (U/L) | 0 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 9.1 Active vitamin D versus vitamin D3 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.2 Calcitriol versus other established vitamin D compounds | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.3 New versus established vitamin D compounds | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 10 One or more episodes of hypercalcaemia Show forest plot | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 2.10  Comparison 2 Vitamin D compound versus vitamin D compound, Outcome 10 One or more episodes of hypercalcaemia. | ||||
| 10.1 Active vitamin D compound versus vitamin D3 | 2 | 53 | Risk Ratio (M‐H, Random, 95% CI) | 9.56 [1.32, 69.04] |
| 10.2 Calcitriol versus other established vitamin D compounds | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.3 New versus established vitamin D compounds | 3 | 125 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.26, 2.54] |
| 11 Withdrawal of treatment due to hypercalcaemia Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 2.11  Comparison 2 Vitamin D compound versus vitamin D compound, Outcome 11 Withdrawal of treatment due to hypercalcaemia. | ||||
| 11.1 Active vitamin D compound versus vitamin D3 | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11.2 Calcitriol versus other established vitamin D compounds | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11.3 New versus established vitamin D compounds | 2 | 52 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.17, 2.22] |
| 12 Reduction in parathyroid hormone concentration by 50% Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 2.12  Comparison 2 Vitamin D compound versus vitamin D compound, Outcome 12 Reduction in parathyroid hormone concentration by 50%. | ||||
| 12.1 New versus established vitamin D compounds | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All‐cause mortality Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 3.1  Comparison 3 IV versus oral vitamin D compounds, Outcome 1 All‐cause mortality. | ||||
| 2 Fracture Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 3.2  Comparison 3 IV versus oral vitamin D compounds, Outcome 2 Fracture. | ||||
| 3 End of treatment absolute BMD femoral neck (g/cm²) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 3.3  Comparison 3 IV versus oral vitamin D compounds, Outcome 3 End of treatment absolute BMD femoral neck (g/cm²). | ||||
| 4 End of treatment absolute BMD lumbar spine (g/cm²) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 3.4  Comparison 3 IV versus oral vitamin D compounds, Outcome 4 End of treatment absolute BMD lumbar spine (g/cm²). | ||||
| 5 End of treatment parathyroid hormone (pg/mL) Show forest plot | 8 | 171 | Mean Difference (IV, Random, 95% CI) | ‐76.20 [‐150.92, ‐1.48] |
| Analysis 3.5  Comparison 3 IV versus oral vitamin D compounds, Outcome 5 End of treatment parathyroid hormone (pg/mL). | ||||
| 6 End of treatment serum phosphorus (mg/dL) Show forest plot | 5 | 112 | Mean Difference (IV, Random, 95% CI) | ‐0.30 [‐0.58, ‐0.03] |
| Analysis 3.6  Comparison 3 IV versus oral vitamin D compounds, Outcome 6 End of treatment serum phosphorus (mg/dL). | ||||
| 7 One or more episodes of hyperphosphataemia Show forest plot | 5 | 102 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.65, 1.48] |
| Analysis 3.7  Comparison 3 IV versus oral vitamin D compounds, Outcome 7 One or more episodes of hyperphosphataemia. | ||||
| 8 End of treatment serum calcium (mg/dL) Show forest plot | 6 | 146 | Mean Difference (IV, Random, 95% CI) | 0.08 [‐0.35, 0.52] |
| Analysis 3.8  Comparison 3 IV versus oral vitamin D compounds, Outcome 8 End of treatment serum calcium (mg/dL). | ||||
| 9 One of more episodes of hypercalcaemia Show forest plot | 6 | 128 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [0.80, 1.52] |
| Analysis 3.9  Comparison 3 IV versus oral vitamin D compounds, Outcome 9 One of more episodes of hypercalcaemia. | ||||
| 10 End of treatment alkaline phosphatase (U/L) Show forest plot | 4 | 116 | Mean Difference (IV, Random, 95% CI) | 3.61 [‐50.06, 57.28] |
| Analysis 3.10  Comparison 3 IV versus oral vitamin D compounds, Outcome 10 End of treatment alkaline phosphatase (U/L). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Improvement in bone histomorphometry Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 4.1  Comparison 4 Intraperitoneal (IP) versus oral vitamin D compounds, Outcome 1 Improvement in bone histomorphometry. | ||||
| 2 End of treatment parathyroid hormone (pg/mL) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 4.2  Comparison 4 Intraperitoneal (IP) versus oral vitamin D compounds, Outcome 2 End of treatment parathyroid hormone (pg/mL). | ||||
| 3 One or more episodes of hyperphosphataemia Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 4.3  Comparison 4 Intraperitoneal (IP) versus oral vitamin D compounds, Outcome 3 One or more episodes of hyperphosphataemia. | ||||
| 4 One or more episodes of hypercalcaemia Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 4.4  Comparison 4 Intraperitoneal (IP) versus oral vitamin D compounds, Outcome 4 One or more episodes of hypercalcaemia. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Fracture Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 5.1  Comparison 5 Intermittent versus daily vitamin D compounds, Outcome 1 Fracture. | ||||
| 2 End of treatment parathyroid hormone (pg/mL) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 5.2  Comparison 5 Intermittent versus daily vitamin D compounds, Outcome 2 End of treatment parathyroid hormone (pg/mL). | ||||
| 3 One or more episodes of hyperphosphataemia Show forest plot | 3 | 97 | Risk Ratio (M‐H, Random, 95% CI) | 1.74 [0.44, 6.79] |
| Analysis 5.3  Comparison 5 Intermittent versus daily vitamin D compounds, Outcome 3 One or more episodes of hyperphosphataemia. | ||||
| 4 End of treatment serum phosphorus (mg/dL) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 5.4  Comparison 5 Intermittent versus daily vitamin D compounds, Outcome 4 End of treatment serum phosphorus (mg/dL). | ||||
| 5 One of more episodes of hypercalcaemia Show forest plot | 4 | 118 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [0.36, 3.69] |
| Analysis 5.5  Comparison 5 Intermittent versus daily vitamin D compounds, Outcome 5 One of more episodes of hypercalcaemia. | ||||
| 6 End of treatment serum calcium (mg/dL) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 5.6  Comparison 5 Intermittent versus daily vitamin D compounds, Outcome 6 End of treatment serum calcium (mg/dL). | ||||
| 7 End of treatment alkaline phosphatase (U/L) | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |

Mechanisms for abnormal mineral metabolism in chronic kidney disease

Comparison 1 Vitamin D compounds versus placebo/no treatment, Outcome 1 All‐cause mortality.
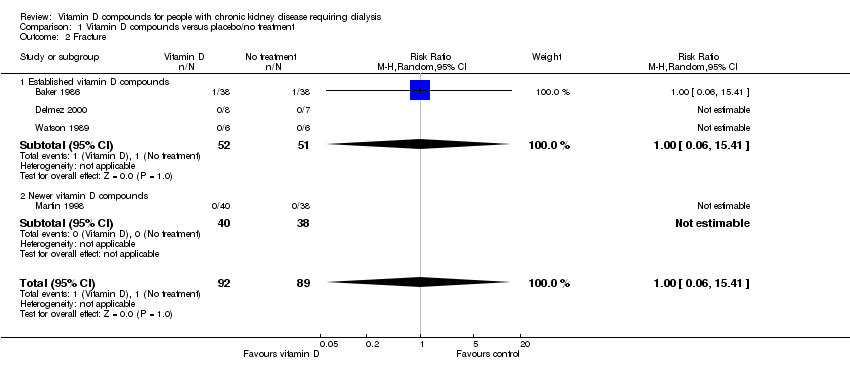
Comparison 1 Vitamin D compounds versus placebo/no treatment, Outcome 2 Fracture.

Comparison 1 Vitamin D compounds versus placebo/no treatment, Outcome 3 Development of bone pain.

Comparison 1 Vitamin D compounds versus placebo/no treatment, Outcome 4 Parathyroidectomy.
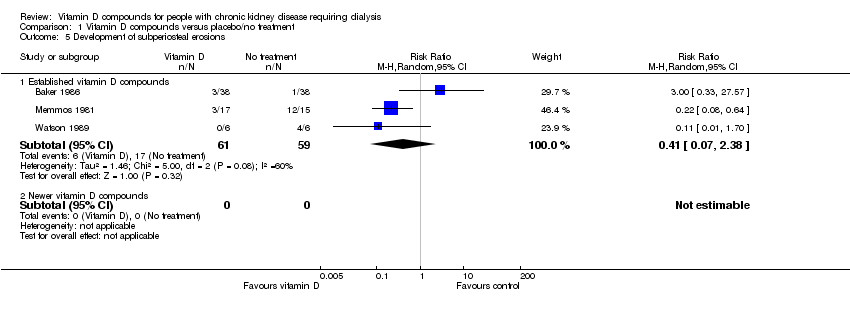
Comparison 1 Vitamin D compounds versus placebo/no treatment, Outcome 5 Development of subperiosteal erosions.
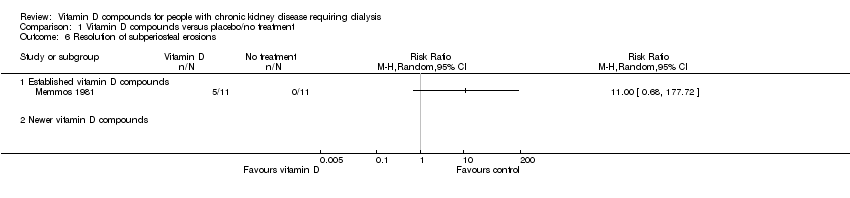
Comparison 1 Vitamin D compounds versus placebo/no treatment, Outcome 6 Resolution of subperiosteal erosions.

Comparison 1 Vitamin D compounds versus placebo/no treatment, Outcome 7 Development of vascular calcification.

Comparison 1 Vitamin D compounds versus placebo/no treatment, Outcome 8 Progression of vascular calcification.
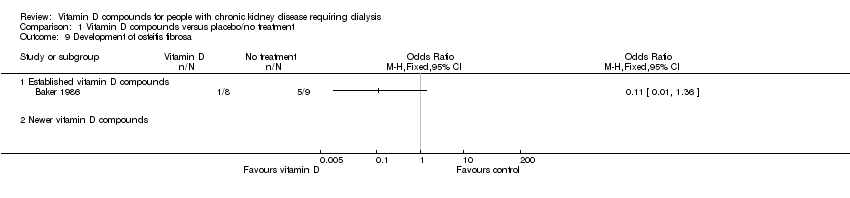
Comparison 1 Vitamin D compounds versus placebo/no treatment, Outcome 9 Development of osteitis fibrosa.

Comparison 1 Vitamin D compounds versus placebo/no treatment, Outcome 10 Development of osteomalacia.

Comparison 1 Vitamin D compounds versus placebo/no treatment, Outcome 11 End of treatment parathyroid hormone (pg/mL).
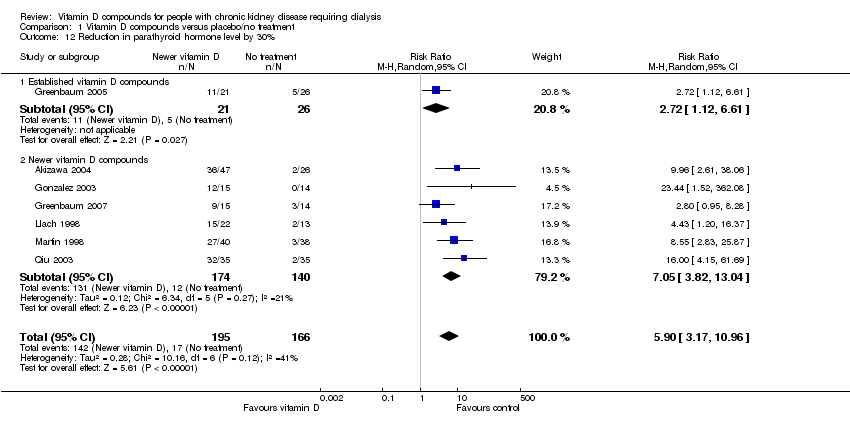
Comparison 1 Vitamin D compounds versus placebo/no treatment, Outcome 12 Reduction in parathyroid hormone level by 30%.
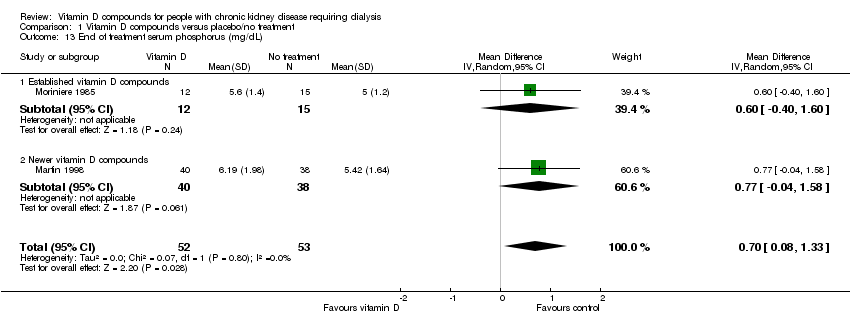
Comparison 1 Vitamin D compounds versus placebo/no treatment, Outcome 13 End of treatment serum phosphorus (mg/dL).
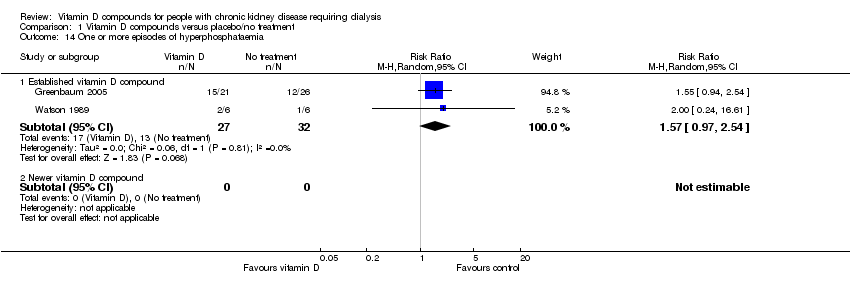
Comparison 1 Vitamin D compounds versus placebo/no treatment, Outcome 14 One or more episodes of hyperphosphataemia.

Comparison 1 Vitamin D compounds versus placebo/no treatment, Outcome 15 End of treatment serum calcium (mg/dL).
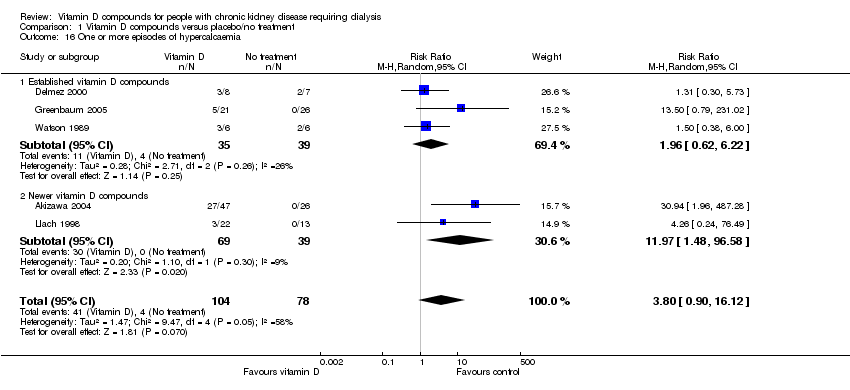
Comparison 1 Vitamin D compounds versus placebo/no treatment, Outcome 16 One or more episodes of hypercalcaemia.

Comparison 1 Vitamin D compounds versus placebo/no treatment, Outcome 17 Withdrawal of treatment due to hypercalcaemia.

Comparison 1 Vitamin D compounds versus placebo/no treatment, Outcome 18 One or more episodes of elevated calcium x phosphorus product.

Comparison 1 Vitamin D compounds versus placebo/no treatment, Outcome 19 End of treatment alkaline phosphatase (U/L).
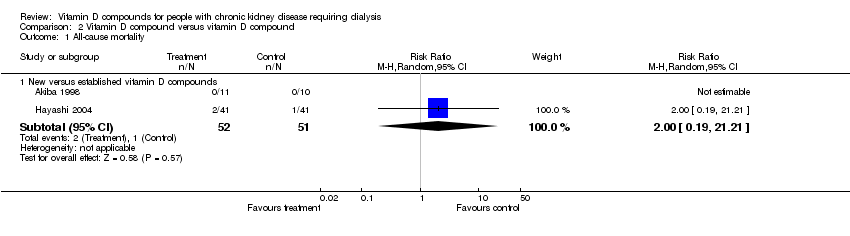
Comparison 2 Vitamin D compound versus vitamin D compound, Outcome 1 All‐cause mortality.
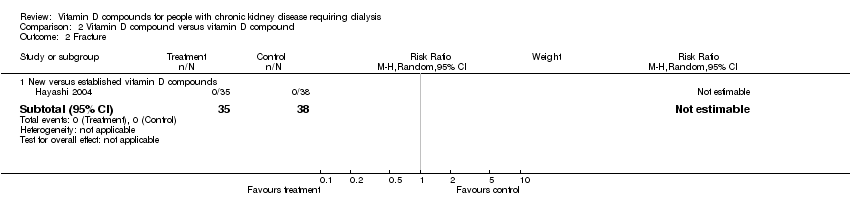
Comparison 2 Vitamin D compound versus vitamin D compound, Outcome 2 Fracture.

Comparison 2 Vitamin D compound versus vitamin D compound, Outcome 3 Improvement in bone pain.

Comparison 2 Vitamin D compound versus vitamin D compound, Outcome 4 Improvement in bone histomorphometry.

Comparison 2 Vitamin D compound versus vitamin D compound, Outcome 5 End of treatment parathyroid hormone (pg/mL).
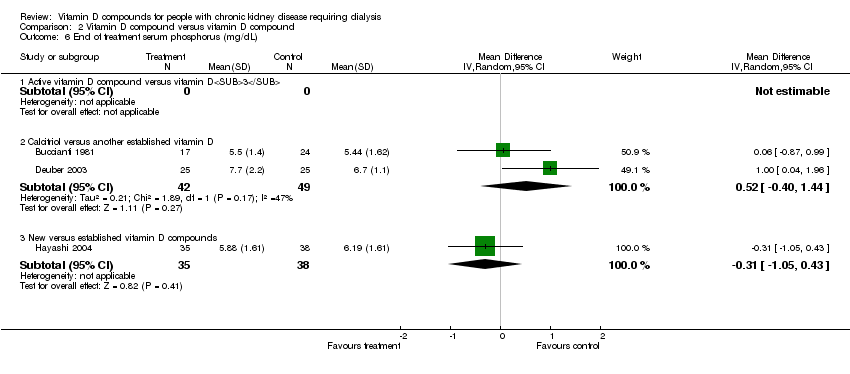
Comparison 2 Vitamin D compound versus vitamin D compound, Outcome 6 End of treatment serum phosphorus (mg/dL).

Comparison 2 Vitamin D compound versus vitamin D compound, Outcome 7 One or more episodes of hyperphosphataemia.

Comparison 2 Vitamin D compound versus vitamin D compound, Outcome 8 End of treatment serum calcium (mg/dL).

Comparison 2 Vitamin D compound versus vitamin D compound, Outcome 10 One or more episodes of hypercalcaemia.

Comparison 2 Vitamin D compound versus vitamin D compound, Outcome 11 Withdrawal of treatment due to hypercalcaemia.
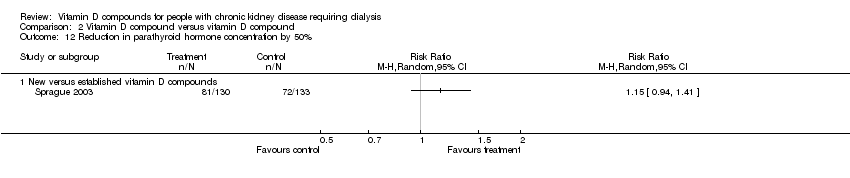
Comparison 2 Vitamin D compound versus vitamin D compound, Outcome 12 Reduction in parathyroid hormone concentration by 50%.

Comparison 3 IV versus oral vitamin D compounds, Outcome 1 All‐cause mortality.
Comparison 3 IV versus oral vitamin D compounds, Outcome 2 Fracture.

Comparison 3 IV versus oral vitamin D compounds, Outcome 3 End of treatment absolute BMD femoral neck (g/cm²).
Comparison 3 IV versus oral vitamin D compounds, Outcome 4 End of treatment absolute BMD lumbar spine (g/cm²).

Comparison 3 IV versus oral vitamin D compounds, Outcome 5 End of treatment parathyroid hormone (pg/mL).

Comparison 3 IV versus oral vitamin D compounds, Outcome 6 End of treatment serum phosphorus (mg/dL).

Comparison 3 IV versus oral vitamin D compounds, Outcome 7 One or more episodes of hyperphosphataemia.

Comparison 3 IV versus oral vitamin D compounds, Outcome 8 End of treatment serum calcium (mg/dL).
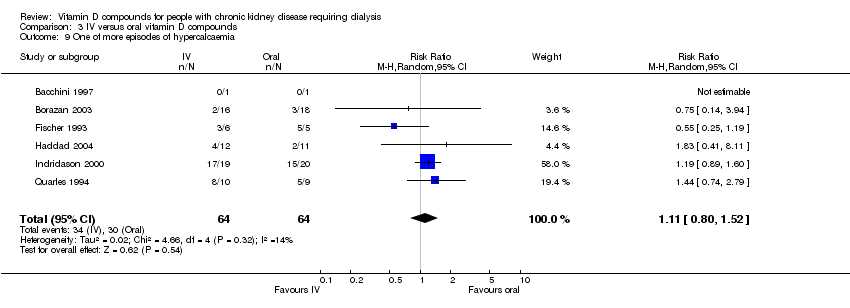
Comparison 3 IV versus oral vitamin D compounds, Outcome 9 One of more episodes of hypercalcaemia.

Comparison 3 IV versus oral vitamin D compounds, Outcome 10 End of treatment alkaline phosphatase (U/L).
Comparison 4 Intraperitoneal (IP) versus oral vitamin D compounds, Outcome 1 Improvement in bone histomorphometry.

Comparison 4 Intraperitoneal (IP) versus oral vitamin D compounds, Outcome 2 End of treatment parathyroid hormone (pg/mL).

Comparison 4 Intraperitoneal (IP) versus oral vitamin D compounds, Outcome 3 One or more episodes of hyperphosphataemia.
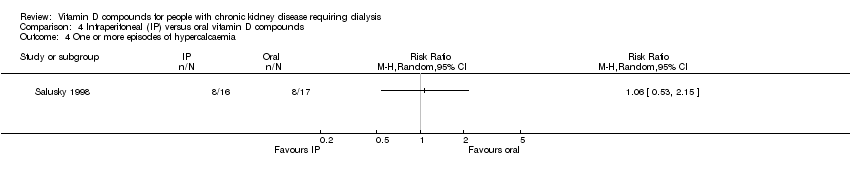
Comparison 4 Intraperitoneal (IP) versus oral vitamin D compounds, Outcome 4 One or more episodes of hypercalcaemia.
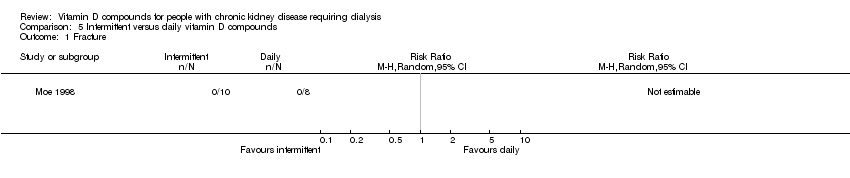
Comparison 5 Intermittent versus daily vitamin D compounds, Outcome 1 Fracture.

Comparison 5 Intermittent versus daily vitamin D compounds, Outcome 2 End of treatment parathyroid hormone (pg/mL).
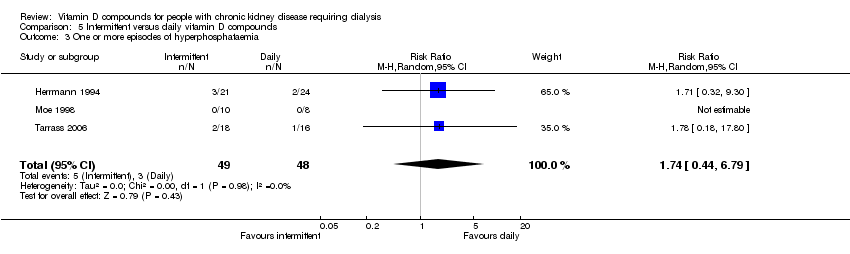
Comparison 5 Intermittent versus daily vitamin D compounds, Outcome 3 One or more episodes of hyperphosphataemia.
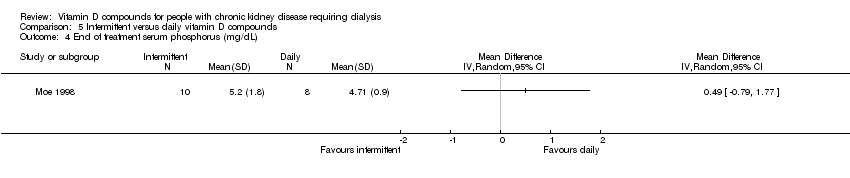
Comparison 5 Intermittent versus daily vitamin D compounds, Outcome 4 End of treatment serum phosphorus (mg/dL).

Comparison 5 Intermittent versus daily vitamin D compounds, Outcome 5 One of more episodes of hypercalcaemia.

Comparison 5 Intermittent versus daily vitamin D compounds, Outcome 6 End of treatment serum calcium (mg/dL).
| CKD stage | GFR range |
| 1 | ≥ 90 mL/min/1.73 m² with kidney damage for ≥3 months as defined by structural or functional abnormalities of the kidney |
| 2 | 60‐89 mL/min/1.73 m² |
| 3 | 30‐59 mL/min |
| 4 | 15‐29 mL/min |
| 5 | < 15 mL/min or dialysis |
| Comparison (studies) | Studies | Patients | Studies with efficacy data extractable for meta‐analysis | Studies with adverse event data available | Studies/meta‐analysis (range) |
| Vitamin D versus placebo (18) | |||||
| All studies combined | 18 | 1297 | 14 | 8 | 1‐7 |
| Established vitamin D | 10 | 850 | 7 | 5 | 1‐4 |
| Newer vitamin D | 8 | 447 | 7 | 3 | 1‐6 |
| Vitamin D versus vitamin D compound (13) | |||||
| Active vitamin D versus vitamin D3 | 2 | 53 | 1 | 2 | 1‐2 |
| Calcitriol versus another established vitamin D | 3 | 101 | 1 | 2 | 1‐2 |
| Established versus newer vitamin D | 5 | 414 | 2 | 3 | 1‐2 |
| Other comparisons | 3 | 59 | Meta‐analysis not possible | ||
| Vitamin D versus calcium (1) | |||||
| All studies combined | 1 | 47 | Meta‐analysis not possible | ||
| IV versus oral vitamin D (12) | |||||
| All studies combined | 12 | 720 | 8 | 8 | 1‐6 |
| Intraperitoneal versus oral vitamin D (3) | |||||
| All studies combined | 3 | 74 | Meta‐analysis not possible | ||
| Subcutaneous versus oral vitamin D (1) | |||||
| All studies combined | 1 | 6 | Meta‐analysis not possible | ||
| Intermittent versus daily vitamin D (5) | |||||
| All studies combined | 5 | 140 | 1 | 4 | 1‐4 |
| Differing dosing schedules (7) | |||||
| Differing frequency | 3 | 57 | Meta‐analysis not possible | ||
| Differing doses | 3 | 195 | Meta‐analysis not possible | ||
| Morning versus evening dosing | 1 | 13 | Meta‐analysis not possible | ||
| Guideline | Country | Year | Recommendation |
| Kidney Disease Outcomes Quality Initiative | USA | 2003 | Patients treated with HD or PD with serum levels of intact PTH levels > 300 pg/mL) (33.0 pmol/L) should receive an active vitamin D sterol (such as calcitriol, alfacalcidol, paricalcitol, or doxercalciferol) to reduce the serum levels of PTH to a target range of 150‐300 pg/mL (16.5‐33.0 pmol/L). (EVIDENCE) The intermittent, IV administration of calcitriol is more effective than daily oral calcitriol in lowering serum PTH levels. (EVIDENCE) In patients with corrected serum calcium and/or phosphorus levels above the target range, a study of alternative vitamin D analogs, such as paricalcitol or doxercalciferol may be warranted. (OPINION) When either HD or PD patients are treated with active vitamin D sterols, management should integrate the changes in serum calcium, serum phosphorus, and plasma PTH. |
| Caring for Australasians with Renal Impairment | Australasia | 2006 | Oral calcitriol (intermittent or pulsed) is effective at lowering parathyroid hormone levels in patients on PD (Level II evidence). Vitamin D and its analogs, either given orally daily, orally intermittently, or intravenously are effective at lowering PTH levels in patients on HD (Level I/II evidence). Oral calcitriol is effective for the prevention or treatment of hyperparathyroidism in most patients on HD or PD. IV calcitriol may be more effective at lowering PTH levels and be less likely to cause hypercalcaemia but the lack of well‐designed studies of sufficient size prevents a more definitive statement. Vitamin D analogs are effective at lowering PTH but clinical studies proving their effectiveness with fewer side‐effects prevents are either lacking or not definitive. On the basis of current evidence there is little reason to recommend their use over oral or IV calcitriol. |
| British Renal Society | UK | 2007 | The relative importance of hyperparathyroidism as a risk factor for premature vascular disease is difficult to determine from observational studies and no informative RCTs exist. There is no doubt that a serum PTH concentration over 4 times the normal limit is associated with increased risk of significant bone disease and should therefore be avoided by medical (or if necessary surgical) management of hyperparathyroidism. In the absence of firm evidence, individual clinicians should decide on the degree to which hyperparathyroidism should be corrects and how this should be achieved. |
| European Best Practice Guidelines (EBPG) | Europe | 2002 | No guideline |
| Europe ‐‐ expert panel (including members from Fresenius Medical Care) (not EBPG) | Europe | 2001 | PTH above 9‐18 pmol/L (78‐156 pg/mL) may well be treated with small daily doses of active vitamin D. IV pulse administration does not have any advantage over oral route. No evidence from direct comparative studies to show any superiority of maxacalcitol, doxercalciferol, or paricalcitol over calcitriol or alfacalcidol. |
| Canadian Society of Nephrology | Canada | 2006 | Avoid intact PTH (iPTH) levels < 100 pg/mL (10.6 pmol/L (Grade C); iPTH levels > 500 pg/mL (53 pmol/L) should be treated if accompanied by symptoms or clinical signs of hyperparathyroidism. Vitamin D analogs should be used in conjunction with a specialist. There is insufficient evidence to recommend use of novel vitamin D analogs (grade D, opinion) |
| HD ‐ haemodialysis; PD ‐ peritoneal dialysis | |||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All‐cause mortality Show forest plot | 5 | 233 | Risk Ratio (M‐H, Random, 95% CI) | 1.34 [0.34, 5.24] |
| 1.1 Established vitamin D compounds | 3 | 168 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [0.24, 5.05] |
| 1.2 Newer vitamin D compounds | 2 | 65 | Risk Ratio (M‐H, Random, 95% CI) | 3.00 [0.13, 68.26] |
| 2 Fracture Show forest plot | 4 | 181 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.06, 15.41] |
| 2.1 Established vitamin D compounds | 3 | 103 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.06, 15.41] |
| 2.2 Newer vitamin D compounds | 1 | 78 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Development of bone pain Show forest plot | 4 | 109 | Risk Ratio (M‐H, Random, 95% CI) | 0.29 [0.03, 2.63] |
| 3.1 Established vitamin D compounds | 3 | 74 | Risk Ratio (M‐H, Random, 95% CI) | 0.41 [0.02, 9.25] |
| 3.2 Newer vitamin D compounds | 1 | 35 | Risk Ratio (M‐H, Random, 95% CI) | 0.20 [0.01, 4.65] |
| 4 Parathyroidectomy Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 Established vitamin D compounds | 2 | 133 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.05, 12.47] |
| 4.2 Newer vitamin D compounds | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Development of subperiosteal erosions Show forest plot | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 Established vitamin D compounds | 3 | 120 | Risk Ratio (M‐H, Random, 95% CI) | 0.41 [0.07, 2.38] |
| 5.2 Newer vitamin D compounds | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Resolution of subperiosteal erosions Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 6.1 Established vitamin D compounds | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 Newer vitamin D compounds | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Development of vascular calcification Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 7.1 Established vitamin D compounds | 2 | 103 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.45, 2.67] |
| 7.2 Newer vitamin D compounds | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Progression of vascular calcification Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 8.1 Established vitamin D compounds | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.2 Newer vitamin D compounds | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 9 Development of osteitis fibrosa Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 9.1 Established vitamin D compounds | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.2 Newer vitamin D compounds | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10 Development of osteomalacia Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 10.1 Established vitamin D compounds | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.2 Newer vitamin D compounds | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 11 End of treatment parathyroid hormone (pg/mL) Show forest plot | 6 | 212 | Mean Difference (IV, Random, 95% CI) | ‐196.05 [‐298.43, ‐93.66] |
| 11.1 Established vitamin D compounds | 4 | 104 | Mean Difference (IV, Random, 95% CI) | ‐220.54 [‐473.63, 32.55] |
| 11.2 Newer vitamin D compounds | 2 | 108 | Mean Difference (IV, Random, 95% CI) | ‐183.88 [‐217.88, ‐149.89] |
| 12 Reduction in parathyroid hormone level by 30% Show forest plot | 7 | 361 | Risk Ratio (M‐H, Random, 95% CI) | 5.90 [3.17, 10.96] |
| 12.1 Established vitamin D compounds | 1 | 47 | Risk Ratio (M‐H, Random, 95% CI) | 2.72 [1.12, 6.61] |
| 12.2 Newer vitamin D compounds | 6 | 314 | Risk Ratio (M‐H, Random, 95% CI) | 7.05 [3.82, 13.04] |
| 13 End of treatment serum phosphorus (mg/dL) Show forest plot | 2 | 105 | Mean Difference (IV, Random, 95% CI) | 0.70 [0.08, 1.33] |
| 13.1 Established vitamin D compounds | 1 | 27 | Mean Difference (IV, Random, 95% CI) | 0.60 [‐0.40, 1.60] |
| 13.2 Newer vitamin D compounds | 1 | 78 | Mean Difference (IV, Random, 95% CI) | 0.77 [‐0.04, 1.58] |
| 14 One or more episodes of hyperphosphataemia Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 14.1 Established vitamin D compound | 2 | 59 | Risk Ratio (M‐H, Random, 95% CI) | 1.57 [0.97, 2.54] |
| 14.2 Newer vitamin D compound | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15 End of treatment serum calcium (mg/dL) Show forest plot | 2 | 105 | Mean Difference (IV, Random, 95% CI) | 0.36 [‐0.26, 0.98] |
| 15.1 Established vitamin D compounds | 1 | 27 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐0.61, 0.61] |
| 15.2 Newer vitamin D compounds | 1 | 78 | Mean Difference (IV, Random, 95% CI) | 0.64 [0.22, 1.06] |
| 16 One or more episodes of hypercalcaemia Show forest plot | 5 | 182 | Risk Ratio (M‐H, Random, 95% CI) | 3.80 [0.90, 16.12] |
| 16.1 Established vitamin D compounds | 3 | 74 | Risk Ratio (M‐H, Random, 95% CI) | 1.96 [0.62, 6.22] |
| 16.2 Newer vitamin D compounds | 2 | 108 | Risk Ratio (M‐H, Random, 95% CI) | 11.97 [1.48, 96.58] |
| 17 Withdrawal of treatment due to hypercalcaemia Show forest plot | 4 | 196 | Risk Ratio (M‐H, Random, 95% CI) | 4.17 [1.36, 12.77] |
| 17.1 Established vitamin D compounds | 2 | 88 | Risk Ratio (M‐H, Random, 95% CI) | 3.66 [0.96, 14.03] |
| 17.2 Newer vitamin D compounds | 2 | 108 | Risk Ratio (M‐H, Random, 95% CI) | 5.61 [0.74, 42.45] |
| 18 One or more episodes of elevated calcium x phosphorus product Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 18.1 Established vitamin D compounds | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 18.2 Newer vitamin D compounds | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 19 End of treatment alkaline phosphatase (U/L) Show forest plot | 3 | 135 | Mean Difference (IV, Random, 95% CI) | ‐24.34 [‐44.34, ‐4.33] |
| 19.1 Established vitamin D compounds | 1 | 27 | Mean Difference (IV, Random, 95% CI) | ‐16.0 [‐54.83, 22.83] |
| 19.2 Newer vitamin D compounds | 2 | 108 | Mean Difference (IV, Random, 95% CI) | ‐27.35 [‐50.69, ‐4.01] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All‐cause mortality Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 New versus established vitamin D compounds | 2 | 103 | Risk Ratio (M‐H, Random, 95% CI) | 2.0 [0.19, 21.21] |
| 2 Fracture Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 New versus established vitamin D compounds | 1 | 73 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Improvement in bone pain Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3.1 Active vitamin D compound versus vitamin D3 | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Improvement in bone histomorphometry Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4.1 Active vitamin D compound versus vitamin D3 | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 End of treatment parathyroid hormone (pg/mL) Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 5.1 Active vitamin D compound versus vitamin D3 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 Calcitriol versus other established vitamin D compounds | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.3 New versus established vitamin D compounds | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 End of treatment serum phosphorus (mg/dL) Show forest plot | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 6.1 Active vitamin D compound versus vitamin D3 | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.2 Calcitriol versus another established vitamin D | 2 | 91 | Mean Difference (IV, Random, 95% CI) | 0.52 [‐0.40, 1.44] |
| 6.3 New versus established vitamin D compounds | 1 | 73 | Mean Difference (IV, Random, 95% CI) | ‐0.31 [‐1.05, 0.43] |
| 7 One or more episodes of hyperphosphataemia Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 7.1 Active vitamin D compound versus vitamin D3 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.2 Calcitriol versus other established vitamin D compounds | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.3 New versus established vitamin D compounds | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 End of treatment serum calcium (mg/dL) Show forest plot | 4 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 8.1 Active vitamin D compound versus vitamin D3 | 1 | 31 | Mean Difference (IV, Random, 95% CI) | 0.85 [0.35, 1.35] |
| 8.2 Calcitriol versus other established vitamin D compounds | 2 | 91 | Mean Difference (IV, Random, 95% CI) | 1.00 [‐0.56, 2.57] |
| 8.3 New versus established vitamin D compounds | 1 | 73 | Mean Difference (IV, Random, 95% CI) | 0.30 [‐0.11, 0.71] |
| 9 End of treatment alkaline phosphatase (U/L) | 0 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 9.1 Active vitamin D versus vitamin D3 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.2 Calcitriol versus other established vitamin D compounds | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.3 New versus established vitamin D compounds | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 10 One or more episodes of hypercalcaemia Show forest plot | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 10.1 Active vitamin D compound versus vitamin D3 | 2 | 53 | Risk Ratio (M‐H, Random, 95% CI) | 9.56 [1.32, 69.04] |
| 10.2 Calcitriol versus other established vitamin D compounds | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.3 New versus established vitamin D compounds | 3 | 125 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.26, 2.54] |
| 11 Withdrawal of treatment due to hypercalcaemia Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 11.1 Active vitamin D compound versus vitamin D3 | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11.2 Calcitriol versus other established vitamin D compounds | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11.3 New versus established vitamin D compounds | 2 | 52 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.17, 2.22] |
| 12 Reduction in parathyroid hormone concentration by 50% Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 12.1 New versus established vitamin D compounds | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All‐cause mortality Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2 Fracture Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3 End of treatment absolute BMD femoral neck (g/cm²) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4 End of treatment absolute BMD lumbar spine (g/cm²) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 5 End of treatment parathyroid hormone (pg/mL) Show forest plot | 8 | 171 | Mean Difference (IV, Random, 95% CI) | ‐76.20 [‐150.92, ‐1.48] |
| 6 End of treatment serum phosphorus (mg/dL) Show forest plot | 5 | 112 | Mean Difference (IV, Random, 95% CI) | ‐0.30 [‐0.58, ‐0.03] |
| 7 One or more episodes of hyperphosphataemia Show forest plot | 5 | 102 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.65, 1.48] |
| 8 End of treatment serum calcium (mg/dL) Show forest plot | 6 | 146 | Mean Difference (IV, Random, 95% CI) | 0.08 [‐0.35, 0.52] |
| 9 One of more episodes of hypercalcaemia Show forest plot | 6 | 128 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [0.80, 1.52] |
| 10 End of treatment alkaline phosphatase (U/L) Show forest plot | 4 | 116 | Mean Difference (IV, Random, 95% CI) | 3.61 [‐50.06, 57.28] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Improvement in bone histomorphometry Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2 End of treatment parathyroid hormone (pg/mL) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3 One or more episodes of hyperphosphataemia Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4 One or more episodes of hypercalcaemia Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Fracture Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2 End of treatment parathyroid hormone (pg/mL) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3 One or more episodes of hyperphosphataemia Show forest plot | 3 | 97 | Risk Ratio (M‐H, Random, 95% CI) | 1.74 [0.44, 6.79] |
| 4 End of treatment serum phosphorus (mg/dL) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 5 One of more episodes of hypercalcaemia Show forest plot | 4 | 118 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [0.36, 3.69] |
| 6 End of treatment serum calcium (mg/dL) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 7 End of treatment alkaline phosphatase (U/L) | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |

