Coronas metálicas preformadas para los molares primarios cariados
Información
- DOI:
- https://doi.org/10.1002/14651858.CD005512.pub3Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 31 diciembre 2015see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Salud oral
- Copyright:
-
- Copyright © 2015 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
The review was conceived and co‐ordinated by Nicola Innes (NI). All review authors (NI, David Ricketts (DR), Ruth Santamaria (RS), Alex Keightley (AK), Thomas Lamont (TL), Lee Yee Chong (LC)) participated in updating the review, developing the search strategy and the screening the search results and retrieved papers. All authors contributed to screening retrieved papers against inclusion criteria and assessing risk of bias. All review authors contributed to writing and revising the review.
Sources of support
Internal sources
-
School of Dentistry, The University of Manchester, UK.
External sources
-
Cochrane Oral Health Group Global Alliance, Other.
Through our Global Alliance (http://ohg.cochrane.org/partnerships‐alliances), the Cochrane Oral Health Group has received support from: British Association for the Study of Community Dentistry, UK; British Association of Oral Surgeons, UK; British Orthodontic Society, UK; British Society of Paediatric Dentistry, UK; British Society of Periodontology, UK; Canadian Dental Hygienists Association, Canada; Mayo Clinic, USA; National Center for Dental Hygiene Research & Practice, USA; New York University College of Dentistry, USA; and Royal College of Surgeons of Edinburgh, UK.
-
National Institute for Health Research (NIHR), UK.
This project was supported by the NIHR, via Cochrane Infrastructure funding to the Cochrane Oral Health Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Declarations of interest
Original 2007 review
Whilst there is no conflict of interest with regard to one of the review authors (David Ricketts (DR)), two of the review authors (Nicola Innes (NI) and Dafydd Evans (DE)) received partial sponsorship in 2000, from 3M/ESPE, for a clinical trial investigating the use of preformed metal crowns to seal carious tissues into primary molar teeth using a different technique (the Hall Technique) to that investigated in this review. These authors have not taken part in the decision to include the study into the review or assessment of risk of bias of the study.
2015 update
Nicola PT Innes: received partial sponsorship in 2000 from 3M/ESPE for a clinical trial investigating the use of preformed metal crowns to seal carious tissues into primary molar teeth using the Hall Technique. She was an author on another included study. She did not take part in the decision to include these studies (Innes 2011; Santamaria 2014), nor did she conduct the risk of bias assessment or data extraction for them.
David Ricketts: none known
Lee Yee Chong: none known
Alexander J Keightley: none known
Thomas Lamont: none known
Ruth Santamaria: was an author on one of the included studies (Santamaria 2014), but did not have any involvement in study selection, risk of bias assessment or data extraction for that study.
Acknowledgements
The review authors would like to thank Sylvia Bickley and Anne Littlewood for assistance with searching, the peer referees who commented on this review as part of the pre‐publication process (David Manton, Nicky Kilpatrick, Barbara Chadwick), Laura MacDonald for her assistance with editorial processing and Jan Clarkson and Helen Worthington for insight into the implications of the methodological inadequacies of some of the clinical trials.
We would also like to thank Dafydd Evans, one of the authors of version one of this review who also conceived the original review, and contributed to its search strategy, screening of search results and retrieved papers, screening of retrieved papers against inclusion criteria and resolving conflicts related to risk of bias.
Version history
| Published | Title | Stage | Authors | Version |
| 2015 Dec 31 | Preformed crowns for decayed primary molar teeth | Review | Nicola PT Innes, David Ricketts, Lee Yee Chong, Alexander J Keightley, Thomas Lamont, Ruth M Santamaria | |
| 2007 Jan 24 | Preformed metal crowns for decayed primary molar teeth | Review | Nicola P T Innes, David Ricketts, Dafydd J P Evans | |
| 2005 Oct 19 | Preformed metal crowns for decayed primary molar teeth | Protocol | Nicola P T Innes, Dafydd J P Evans, David Ricketts | |
Differences between protocol and review
Following a prioritisation project carried out by the Cochrane Oral Health Group, a decision was taken to expand the scope of the review. The earlier protocol only included studies with preformed metal crowns (PMCs). The current protocol includes studies using any type of crown material, compared to usual restoration methods or non‐restorative caries treatment.
We have broadened the objectives for this updated review compared to the 2008 version (Innes 2007). In the previous version, in the first objective we compared only PMCs with commonly used filling materials. This updated version has widened to include all preformed crowns (metal and aesthetic) compared to the same commonly used filling materials, and also non‐restorative caries treatment, as well as comparisons between different types of crowns and different methods for placing crowns. The second objective of the previous version of the review compared whether the extent of decay had an effect on outcome; this has not changed apart from now including all of the interventions in our first objective. The third objective in the original version of the review, dealt with adverse events and safety and this has now been incorporated into our first objective in this review.
We have also updated the outcomes. In the original review the primary outcomes were: freedom from clinical or radiographic signs or symptoms of pulp pathology including pain/pulp infection/discharging sinus/swelling; time until filling or crown needs to be replaced or requires further intervention; and proportion of filled or crowned teeth retained until appropriate age of shedding. We had included other measures of success: absence of clinical or radiographic evidence of secondary caries; other clinical signs of pathology (fracture of tooth or filling, wear of crown, inflammation of gingival (gum) tissue); patient satisfaction; costs to patient and provider; and adverse events. To reduce the number of outcomes but still maintain the applicability of the findings, we have used 'major failure', as a primary outcome. This is a composite outcome measure made up of different clinical and radiographic findings and comprises a number of outcomes from the previous review. Clinically, this is a reasonable outcome, as it requires the same treatment to manage. Our other two primary outcomes are pain and satisfaction with treatment (including satisfaction with aesthetics). The secondary outcomes were rationalised to: time to restoration failure/retreatment; peri/postoperative discomfort/pain; cost; and adverse events (e.g. bone loss, gingival inflammation or others).
In our protocol, we had planned to conducted adjustments for the study designs where within‐patient randomisation was conducted and investigate any impact of clustering. We conducted these analyses in our review using generic inverse variance as planned, but decided to present the results without adjustment. This decision is based on the fact that the adjustments did not make a real difference to the effect estimates obtained, and took into account the easier interpretability of unadjusted results in the usual dichotomous outcome format for clinicians. In future versions of this review we will revisit this decision on the basis of the data obtained.
We did not calculate the number needed to treat for an additional beneficial outcome (NNTB) as we had planned to.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
Medical Subject Headings Check Words
Child; Child, Preschool; Female; Humans; Male;
PICO

Study flow diagram

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies
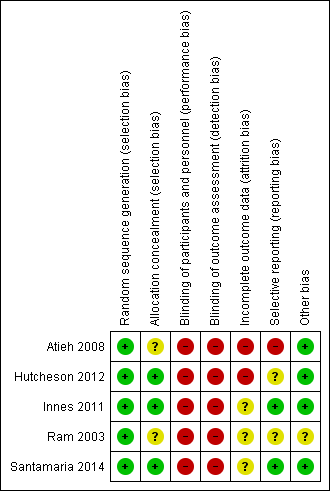
Risk of bias summary: review authors' judgements about each risk of bias item for each included study
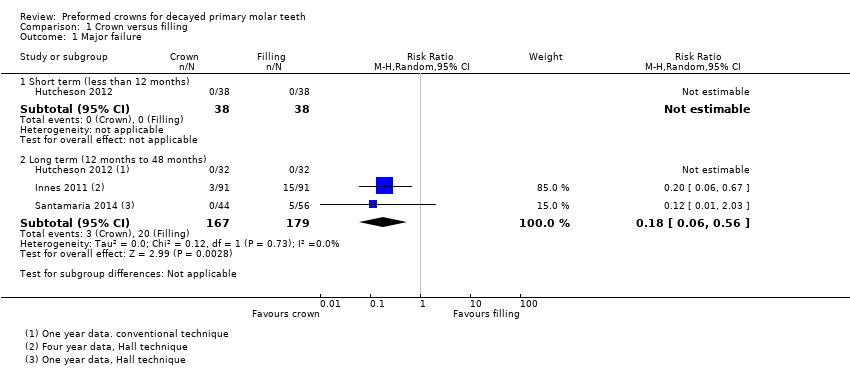
Comparison 1 Crown versus filling, Outcome 1 Major failure.

Comparison 1 Crown versus filling, Outcome 2 Pain.

Comparison 1 Crown versus filling, Outcome 3 Discomfort associated with the procedure.
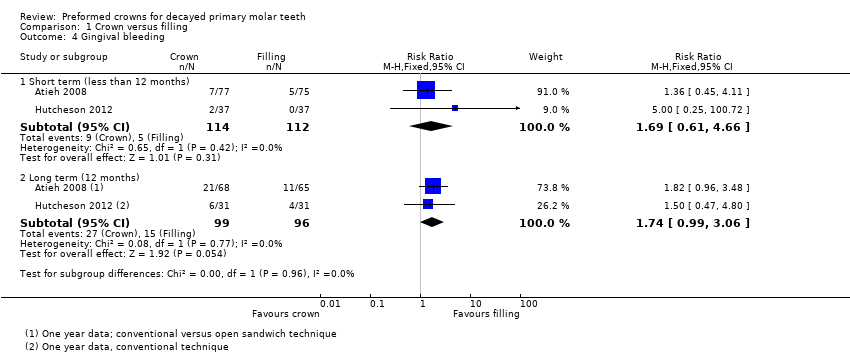
Comparison 1 Crown versus filling, Outcome 4 Gingival bleeding.
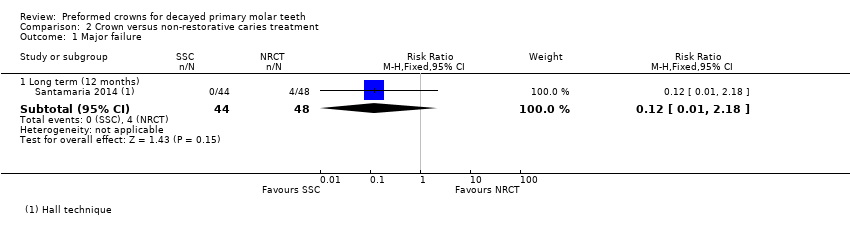
Comparison 2 Crown versus non‐restorative caries treatment, Outcome 1 Major failure.

Comparison 2 Crown versus non‐restorative caries treatment, Outcome 2 Discomfort associated with the procedure.

Comparison 2 Crown versus non‐restorative caries treatment, Outcome 3 Gingival bleeding.

Comparison 3 Stainless steel crown vs aesthetic crown, Outcome 1 Gingival bleeding.
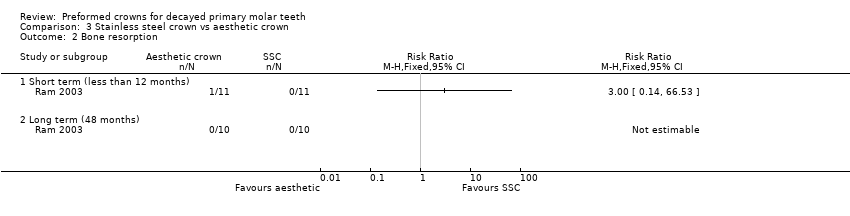
Comparison 3 Stainless steel crown vs aesthetic crown, Outcome 2 Bone resorption.
| Preformed crowns compared to fillings for decayed primary molar teeth | ||||||
| Patient or population: decayed primary molar teeth | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Filling | Preformed crown | |||||
| Major failure ‐ long term (12 months to 48 months) | Study population | RR 0.18 | 346 | ⊕⊕⊕⊝ | ||
| 112 per 1000 | 20 per 1000 | |||||
| Pain ‐ long term (12 months to 24 months) | Study population | RR 0.15 | 312 | ⊕⊕⊕⊝ | This was based on patient and/or parent reports | |
| 83 per 1000 | 12 per 1000 | |||||
| Satisfaction with treatment | ||||||
| Discomfort associated with the procedure | Study population | RR 0.56 | 381 | ⊕⊕⊕⊝ | This was patient‐reported in one study, and dentist‐reported in another study. Outcomes were recorded using different 5‐point scales, but dichotomised for analyses, with all partients who scored 'moderate' or more severe levels of discomfort considered as having experienced discomfort | |
| 239 per 1000 | 134 per 1000 | |||||
| Gingival bleeding ‐ long term (12 months) | Study population | RR 1.74 | 195 | ⊕⊕⊝⊝ | ||
| 156 per 1000 | 272 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Intervention and comparison look different. Blinding of outcome assessor, patients and the person doing the procedures was not possible. Outcomes have subjective elements. Although pain was not measured using validated tools, there was no further downgrading for this. 2 One of the studies only had data from 87% of randomised participants from one country (from a multinational study of three countries); the study is still ongoing at the time of publication. 3 Small sample size; event rates were low. Confidence intervals were wide. | ||||||
| Preformed crowns compared to non‐restorative caries treatment for decayed primary molar teeth | ||||||
| Patient or population: children with decayed primary molar teeth | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Non‐restorative caries treatment | Preformed crown | |||||
| Major failure (12 months) | Study population | RR 0.12 | 92 | ⊕⊝⊝⊝ | ||
| 83 per 1000 | 10 per 1000 | |||||
| Pain | No evidence available | |||||
| Satisfaction with treatment | No evidence available | |||||
| Discomfort associated with the procedure | Study population | RR 1.67 | 104 | ⊕⊝⊝⊝ | Data were measured on different 5‐point scales, but dichotomised for analyses, with all patients who scored 'moderate' or more severe levels of discomfort considered as having experienced discomfort | |
| 115 per 1000 | 193 per 1000 | |||||
| Gingival bleeding ‐ long term (12 months) | Study population | RR 1.09 | 92 | ⊕⊝⊝⊝ | ||
| 146 per 1000 | 159 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 There was a very serious risk of bias for this trial. Blinding was impossible as the intervention and comparison looked different, and the outcome had subjective elements in its assessment. Futhermore only 87% of the data from one country (this was a multinational study with three countries) were available. Although pain was not measured using validated tools, there was no further downgrading for this. The study is still ongoing. 2 Very serious imprecision; small sample size; wide confidence intervals. | ||||||
| Outcome (Analysis) | With all studies included | Excluding Atieh 2008 | ||||
| Studies | Participants | Effect estimate | Studies | Participants | Effect estimate | |
| 1.1 Major failure | ||||||
| 1.1.1 Short term (less than 12 months) | 1 | 76 | Not estimable | Not affected | ||
| 1.1.2 Long term 12 months or more | 3 | 346 | RR 0.18 (CI 0.06 to 0.56) | Not affected | ||
| 1.2 Pain | ||||||
| 1.2.1 Short term (less than 12 months) | 1 | 64 | Not estimable | Not affected | ||
| 1.2.2 Long term (12 months or more) | 2 | 312 | RR 0.15 (0.04 to 0.67) | Not affected | ||
| 1.3. Peri/postoperative discomfort/pain | 2 | 381 | RR 0.56 (0.36 to 0.87) | Not affected | ||
| 1.4 Gingival bleeding | ||||||
| 1.4.2 Short term (less than 12 months) | 2 | 226 | RR 1.69 (0.61, 4.66) | 1 | 76 | 5.00 (0.25, 100.80) |
| 1.4.3 Long term (12 months or more) | 2 | 195 | 1.74 (0.99, 3.06) | 1 | 62 | 1.50 (0.47, 4.80) |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Major failure Show forest plot | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Short term (less than 12 months) | 1 | 76 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Long term (12 months to 48 months) | 3 | 346 | Risk Ratio (M‐H, Random, 95% CI) | 0.18 [0.06, 0.56] |
| 2 Pain Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Short term (less than 12 months) | 1 | 64 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Long term (12 months to 24 months) | 2 | 312 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.15 [0.04, 0.67] |
| 3 Discomfort associated with the procedure Show forest plot | 2 | 381 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.36, 0.87] |
| 4 Gingival bleeding Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Short term (less than 12 months) | 2 | 226 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.69 [0.61, 4.66] |
| 4.2 Long term (12 months) | 2 | 195 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.74 [0.99, 3.06] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Major failure Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Long term (12 months) | 1 | 92 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.12 [0.01, 2.18] |
| 2 Discomfort associated with the procedure Show forest plot | 1 | 104 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.67 [0.65, 4.25] |
| 3 Gingival bleeding Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Long term (12 months) | 1 | 92 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.42, 2.86] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Gingival bleeding Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Short term (less than 12 months) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Long term (48 months) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Bone resorption Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 Short term (less than 12 months) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Long term (48 months) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |

