| 1 Global state: 1. Not improved (CGI) Show forest plot | 7 | | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only |
|
| 1.1 not 'mildly ill or normal' | 2 | 114 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.55, 1.66] |
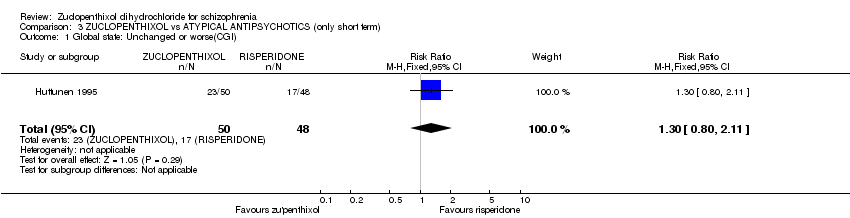
| 1.2 unchanged or worse | 7 | 357 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.53, 0.98] |
| 2 Global state: 2. Average score (CGI, high score = poor) Show forest plot | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐1.30, 0.90] |
|
| 3 Mental state: 1. Average scores (BPRS, high score=poor) Show forest plot | 1 | 41 | Mean Difference (IV, Fixed, 95% CI) | ‐2.66 [‐9.09, 3.77] |
|
| 4 Mental state: 2. Average scores (data skewed, high score=poor) Show forest plot | | | Other data | No numeric data |
|
| 4.1 BPRS | | | Other data | No numeric data |
| 4.2 CPRS | | | Other data | No numeric data |
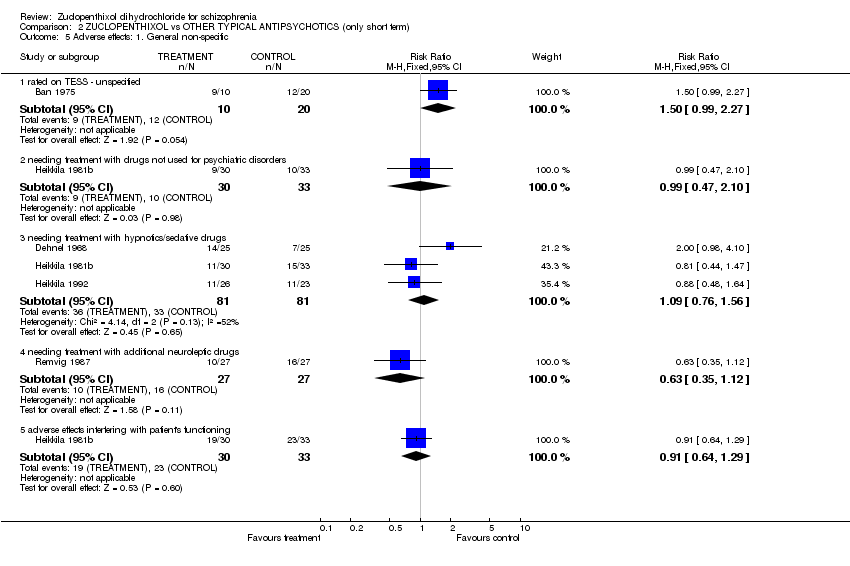
| 5 Adverse effects: 1. General non‐specific Show forest plot | 5 | | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only |
|
| 5.1 rated on TESS ‐ unspecified | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.5 [0.99, 2.27] |
| 5.2 needing treatment with drugs not used for psychiatric disorders | 1 | 63 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.47, 2.10] |
| 5.3 needing treatment with hypnotics/sedative drugs | 3 | 162 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.76, 1.56] |
| 5.4 needing treatment with additional neuroleptic drugs | 1 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.35, 1.12] |
| 5.5 adverse effects interfering with patient's functioning | 1 | 63 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.64, 1.29] |
| 6 Adverse effects: 2. Autonomic side effects Show forest plot | 2 | 92 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.35, 2.24] |
|
| 6.1 general ‐ autonomic side effects(UKU side effect rating scale) | 1 | 49 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.36 [0.71, 7.85] |
| 6.2 specific ‐ orthostatic side effects | 1 | 43 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.11 [0.01, 1.73] |
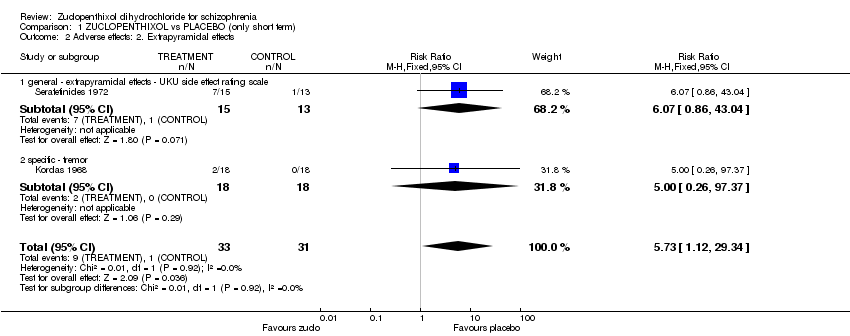
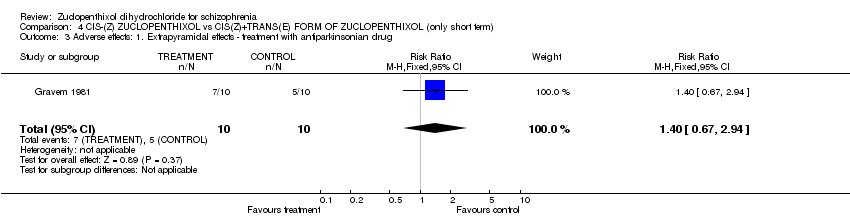
| 7 Adverse effects: 3. Extrapyramidal effects Show forest plot | 8 | | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only |
|
| 7.1 general ‐ needing antiparkinsonian medication | 6 | 280 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.86, 1.33] |
| 7.2 general ‐ extrapyramidal effects ‐ UKU side effect rating scale | 2 | 92 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.56, 1.09] |
| 7.3 specific ‐ akathisia | 1 | 49 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.06 [0.60, 7.07] |
| 7.4 specific ‐ hypokinesia / akinesia | 1 | 49 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.30, 1.93] |
| 7.5 specific ‐ dystonia | 1 | 49 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.30 [0.01, 6.94] |
| 7.6 specific ‐ tremor | 2 | 85 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.29, 1.73] |
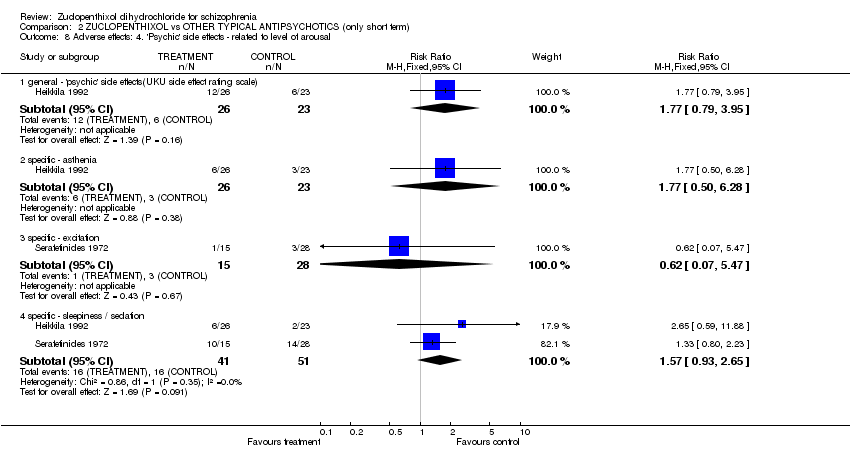
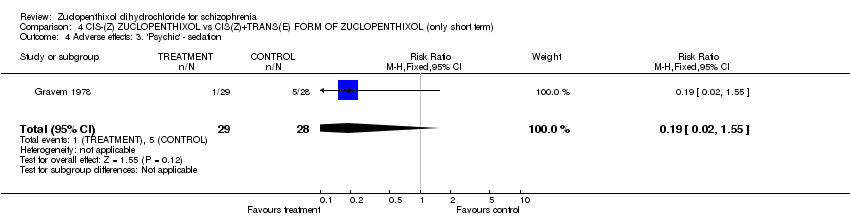
| 8 Adverse effects: 4. 'Psychic' side effects ‐ related to level of arousal Show forest plot | 2 | | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only |
|
| 8.1 general ‐ 'psychic' side effects(UKU side effect rating scale) | 1 | 49 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.77 [0.79, 3.95] |
| 8.2 specific ‐ asthenia | 1 | 49 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.77 [0.50, 6.28] |
| 8.3 specific ‐ excitation | 1 | 43 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.07, 5.47] |
| 8.4 specific ‐ sleepiness / sedation | 2 | 92 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.57 [0.93, 2.65] |
| 9 Adverse effects: 5a. Weight ‐ change (kg) Show forest plot | 1 | 43 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.20, 1.23] |
|
| 9.1 loss or gain of weight of 10 pounds (high change=poor) | 1 | 43 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.20, 1.23] |
| 10 Adverse effects: 5b. Weight ‐ total weight increase (kg) Show forest plot | 1 | 83 | Mean Difference (IV, Fixed, 95% CI) | ‐1.89 [‐7.89, 4.11] |
|
| 11 Leaving the study early Show forest plot | 10 | 488 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.52, 0.97] |
|
| 11.1 any reason | 8 | 424 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.51, 0.95] |
| 11.2 due to adverse effects | 2 | 64 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.28 [0.18, 8.82] |