Diclorhidrato de zuclopentixol para la esquizofrenia
Referencias
Referencias de los estudios incluidos en esta revisión
Referencias de los estudios excluidos de esta revisión
Referencias de los estudios en espera de evaluación
Referencias adicionales
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Allocation: randomised. | |
| Participants | Diagnosis: paranoid schizophrenia. | |
| Interventions | 1. Cis(Z)‐clopenthixol hydrochloride: dose range 5‐50 mg/day. N=4. | |
| Outcomes | Leaving the study early. Unable to use ‐ | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Methods | Allocation: randomised. | |
| Participants | Diagnosis: psychosis, schizophrenia (68), schizoaffective (7), hypomania (4)(RDC). | |
| Interventions | 1. Zuclopenthixol: dose range 25‐150 mg/day. N=50. Additional medication: amitriptyline, temazepam, procyclidine. | |
| Outcomes | Leaving the study early. Unable to use ‐ | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Methods | Allocation: randomised. | |
| Participants | Diagnosis: schizophrenia. | |
| Interventions | 1. Clopenthixol: dose range 50‐200 mg/day. N=10. | |
| Outcomes | Global state: CGI. Unable to use ‐ | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Methods | Allocation: randomised. | |
| Participants | Diagnosis: chronic schizophrenia. | |
| Interventions | 1. Clopenthixol: dose 132.5 mgm/day, range 25‐250 mgm/day. N=25. | |
| Outcomes | Leaving the study early. Unable to use ‐ | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Methods | Allocation: randomised not clear. | |
| Participants | Diagnosis: acute schizophrenia. | |
| Interventions | 1. Clopenthixol: dose 100 mg/day. N=36. | |
| Outcomes | Adverse effects: Drowsiness, stimulation, confusion, gastrointestinal side effects, anticholinergic side effects, Dizziness, orthostatic reaction, headache, hypersalivation, hypokinesia, hyperkinesia, dyskinesia, rigor, tremor, akathisia. Unable to use ‐ | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | High risk | C ‐ Inadequate |
| Methods | Allocation: randomisation not clear. | |
| Participants | Diagnosis: schizophrenia (DSM‐III‐R). | |
| Interventions | 1. Clopenthixol: dose 5.5 mg/day, range 3‐7 mg/day. N=10. | |
| Outcomes | Leaving the study early: due to side effects(great number of EEG artifacts). Unable to use ‐ | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | High risk | C ‐ Inadequate |
| Methods | Allocation: randomisation not clear. | |
| Participants | Diagnosis: chronic schizophrenia. | |
| Interventions | 1. Cis(Z) ‐ clopenthixol: dose 62.5 mg/day. N=29. | |
| Outcomes | Global state: CGI. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Methods | Allocation: randomisation not clear. | |
| Participants | Diagnosis: chronic schizophrenia. | |
| Interventions | 1. Cis(Z) ‐ clopenthixol: dose 48 mg/day, range 10‐150 mg/day. N=10. | |
| Outcomes | Leaving the study early. Unable to use‐ | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Methods | Allocation: randomised. | |
| Participants | Diagnosis: chronic schizophrenia. | |
| Interventions | 1. Cis(Z) ‐ clopenthixol: dose 73 mg/day, range 10‐150 mg/day. N=26. | |
| Outcomes | Global state: CGI. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Methods | Allocation: randomised. | |
| Participants | Diagnosis: chronic schizophrenia. | |
| Interventions | 1. Cis(Z)‐zuclopenthixol: dose 40 mg/day. N=30. | |
| Outcomes | Leaving the study early. Unable to use ‐ | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Methods | Allocated: randomised. | |
| Participants | Diagnosis: acute psychosis (ICD‐9). | |
| Interventions | 1. Zuclopenthixol: dose 33.5 mg/day, range 10‐75 mg/day. N=26. Additional medication: biperiden, nitrazepam, chloral hydrate. | |
| Outcomes | Leaving the study early. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Methods | Allocation: randomised. | |
| Participants | Diagnosis: chronic schizophrenia (DSM‐III‐R). | |
| Interventions | 1. Zuclopenthixol: dose 38 mg/day, range 10‐100 mg/day. N=50. | |
| Outcomes | Leaving the study early. Unable to use ‐ | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Methods | Allocation: randomised (each patient was assigned a number belonging to a bottle of medication). | |
| Participants | Diagnosis: acute schizophrenia. | |
| Interventions | 1. Clopenthixol: dose 122 mg/day, range 75‐600 mg/day. N=20. | |
| Outcomes | Leaving the study early. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
| Methods | Allocation: randomised. | |
| Participants | Diagnosis: chronic schizophrenia. | |
| Interventions | 1. Clopenthixol: dose 150 mg/day. N=18. | |
| Outcomes | Adverse effects: number of patients experiencing side effects. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Methods | Allocation: randomised. | |
| Participants | Diagnosis: acute schizophrenia (RDC). | |
| Interventions | 1. Zuclopenthixol dihydrochloride: dose range 25‐150 mg/day. N=30. Additional medication: temazepam, procyclidine, amitriptyline. | |
| Outcomes | Global state: CGI. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Methods | Allocation: randomised. | |
| Participants | Diagnosis: acute psychosis or exacerbation of chronic psychosis. | |
| Interventions | 1. Zuclopenthixol: dose 37 mg/day, range 10‐120 mg/day. N=27. Additional medication: benzodiazepine, methotrimeprazine, antiparkinsonian drugs. | |
| Outcomes | Leaving the study early. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Methods | Allocation: randomised. | |
| Participants | Diagnosis: chronic schizophrenia. | |
| Interventions | 1. Clopenthixol: dose 205 mg/day. N=15. Additional medication: antiparkinsonian medication, sedative. | |
| Outcomes | Leaving the study early. Unable to use ‐ | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Methods | Allocation: randomised. | |
| Participants | Diagnosis: schizophrenia. | |
| Interventions | 1. Clopenthixol: dose 150 mg/day. N=10. | |
| Outcomes | Leaving the study early. Unable to use ‐ | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
BPRS ‐ Brief Psychiatric Rating Scale
CGA ‐ Clinical Global Assessment
CGI ‐ Clinical Global Impression
CPRS ‐ Comprehensive Psychopathological Rating Scale
NOSIE ‐ Nourses' Observation Scale for Inpatient Evaluation
PANSS ‐ Positive and Negative Syndrome Scale
PIP ‐ Psychotic Inpatient Profile
RDC ‐ Research Diagnostic Criteria
SHBS ‐ Schedule for Handicaps, Behaviour, and Skills
TESS ‐ Treatment Emergent Symptom Scale
VTSRS ‐ Verdun Target Symptom Rating Scale
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Allocation: randomised. | |
| Allocation: randomised. | |
| Allocation: randomised. | |
| Allocation: randomised. | |
| Allocation: randomised. | |
| Allocation: not randomised, case‐series. | |
| Allocation: not randomised, case series. | |
| Allocation: randomised. | |
| Allocation: randomised. | |
| Allocation: randomised. | |
| Allocation: randomised. | |
| Allocation: randomised. | |
| Allocation: randomised. | |
| Allocation: not clear, double‐blind. | |
| allocation: not clear, double‐blind. | |
| Allocation: randomised. | |
| Allocation: randomised. | |
| Allocation: randomised, blindness not clear. | |
| Allocation: randomised, double‐blind. | |
| Allocation: randomised. | |
| Allocation: randomised. | |
| Allocation: not randomised. | |
| Allocation: randomised. | |
| Allocation: randomised. | |
| Allocation: randomised. | |
| Allocation: randomised. | |
| Allocation: randomised. | |
| Allocation: randomised. | |
| Allocation: randomised. | |
| Allocation: randomised. | |
| Allocation: randomised. | |
| Allocation: randomised. | |
| Allocation: not randomised, case series. | |
| Allocation: randomised. | |
| Allocation: randomised. | |
| Allocation: not randomised. | |
| Allocation: randomised. | |
| Allocation: randomised. | |
| Allocation: not clear, double ‐blind. | |
| Allocation: randomised. | |
| Allocation: randomisation not clear. | |
| Allocation: randomised. | |
| Allocation: randomised. | |
| Allocation: randomised. | |
| Allocation: randomised. | |
| Allocation: randomised. | |
| Allocation: randomised. | |
| Allocation: not randomised, case series. | |
| Allocation: randomised. | |
| Allocation: randomised. | |
| Allocation: randomised. | |
| Allocation: randomised. | |
| Allocation: randomised. | |
| Allocation: not clear. |
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Adverse effects: 1. Autonomic ‐ specific ‐ orthostatic Show forest plot | 1 | 28 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.01, 6.60] |
| Analysis 1.1  Comparison 1 ZUCLOPENTHIXOL vs PLACEBO (only short term), Outcome 1 Adverse effects: 1. Autonomic ‐ specific ‐ orthostatic. | ||||
| 2 Adverse effects: 2. Extrapyramidal effects Show forest plot | 2 | 64 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.73 [1.12, 29.34] |
| Analysis 1.2  Comparison 1 ZUCLOPENTHIXOL vs PLACEBO (only short term), Outcome 2 Adverse effects: 2. Extrapyramidal effects. | ||||
| 2.1 general ‐ extrapyramidal effects ‐ UKU side effect rating scale | 1 | 28 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.07 [0.86, 43.04] |
| 2.2 specific ‐ tremor | 1 | 36 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.0 [0.26, 97.37] |
| 3 Adverse effects: 3. 'Psychic' side effects ‐ related to level of arousal Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.3  Comparison 1 ZUCLOPENTHIXOL vs PLACEBO (only short term), Outcome 3 Adverse effects: 3. 'Psychic' side effects ‐ related to level of arousal. | ||||
| 3.1 specific ‐ excitation | 1 | 28 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.63 [0.12, 59.40] |
| 3.2 specific ‐ sleepiness / sedation | 1 | 28 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.89 [1.01, 8.30] |
| 4 Adverse effects: 5. Weight ‐ change (kg) Show forest plot | 1 | 28 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.17, 1.11] |
| Analysis 1.4  Comparison 1 ZUCLOPENTHIXOL vs PLACEBO (only short term), Outcome 4 Adverse effects: 5. Weight ‐ change (kg). | ||||
| 4.1 loss or gain of weight of 10 pounds (high change=poor) | 1 | 28 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.17, 1.11] |
| 5 Leaving the study early Show forest plot | 1 | 28 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.01, 6.60] |
| Analysis 1.5  Comparison 1 ZUCLOPENTHIXOL vs PLACEBO (only short term), Outcome 5 Leaving the study early. | ||||
| 5.1 due to adverse effects | 1 | 28 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.01, 6.60] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size | ||||||||||||||||||||||||||||||||||||||||||||||||||
| 1 Global state: 1. Not improved (CGI) Show forest plot | 7 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 2.1  Comparison 2 ZUCLOPENTHIXOL vs OTHER TYPICAL ANTIPSYCHOTICS (only short term), Outcome 1 Global state: 1. Not improved (CGI). | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 1.1 not 'mildly ill or normal' | 2 | 114 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.55, 1.66] | ||||||||||||||||||||||||||||||||||||||||||||||||||
| 1.2 unchanged or worse | 7 | 357 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.53, 0.98] | ||||||||||||||||||||||||||||||||||||||||||||||||||
| 2 Global state: 2. Average score (CGI, high score = poor) Show forest plot | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐1.30, 0.90] | ||||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 2.2  Comparison 2 ZUCLOPENTHIXOL vs OTHER TYPICAL ANTIPSYCHOTICS (only short term), Outcome 2 Global state: 2. Average score (CGI, high score = poor). | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3 Mental state: 1. Average scores (BPRS, high score=poor) Show forest plot | 1 | 41 | Mean Difference (IV, Fixed, 95% CI) | ‐2.66 [‐9.09, 3.77] | ||||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 2.3  Comparison 2 ZUCLOPENTHIXOL vs OTHER TYPICAL ANTIPSYCHOTICS (only short term), Outcome 3 Mental state: 1. Average scores (BPRS, high score=poor). | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 4 Mental state: 2. Average scores (data skewed, high score=poor) Show forest plot | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 2.4
Comparison 2 ZUCLOPENTHIXOL vs OTHER TYPICAL ANTIPSYCHOTICS (only short term), Outcome 4 Mental state: 2. Average scores (data skewed, high score=poor). | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 4.1 BPRS | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| 4.2 CPRS | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| 5 Adverse effects: 1. General non‐specific Show forest plot | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 2.5  Comparison 2 ZUCLOPENTHIXOL vs OTHER TYPICAL ANTIPSYCHOTICS (only short term), Outcome 5 Adverse effects: 1. General non‐specific. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 5.1 rated on TESS ‐ unspecified | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.5 [0.99, 2.27] | ||||||||||||||||||||||||||||||||||||||||||||||||||
| 5.2 needing treatment with drugs not used for psychiatric disorders | 1 | 63 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.47, 2.10] | ||||||||||||||||||||||||||||||||||||||||||||||||||
| 5.3 needing treatment with hypnotics/sedative drugs | 3 | 162 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.76, 1.56] | ||||||||||||||||||||||||||||||||||||||||||||||||||
| 5.4 needing treatment with additional neuroleptic drugs | 1 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.35, 1.12] | ||||||||||||||||||||||||||||||||||||||||||||||||||
| 5.5 adverse effects interfering with patient's functioning | 1 | 63 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.64, 1.29] | ||||||||||||||||||||||||||||||||||||||||||||||||||
| 6 Adverse effects: 2. Autonomic side effects Show forest plot | 2 | 92 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.35, 2.24] | ||||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 2.6  Comparison 2 ZUCLOPENTHIXOL vs OTHER TYPICAL ANTIPSYCHOTICS (only short term), Outcome 6 Adverse effects: 2. Autonomic side effects. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 6.1 general ‐ autonomic side effects(UKU side effect rating scale) | 1 | 49 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.36 [0.71, 7.85] | ||||||||||||||||||||||||||||||||||||||||||||||||||
| 6.2 specific ‐ orthostatic side effects | 1 | 43 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.11 [0.01, 1.73] | ||||||||||||||||||||||||||||||||||||||||||||||||||
| 7 Adverse effects: 3. Extrapyramidal effects Show forest plot | 8 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 2.7  Comparison 2 ZUCLOPENTHIXOL vs OTHER TYPICAL ANTIPSYCHOTICS (only short term), Outcome 7 Adverse effects: 3. Extrapyramidal effects. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 7.1 general ‐ needing antiparkinsonian medication | 6 | 280 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.86, 1.33] | ||||||||||||||||||||||||||||||||||||||||||||||||||
| 7.2 general ‐ extrapyramidal effects ‐ UKU side effect rating scale | 2 | 92 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.56, 1.09] | ||||||||||||||||||||||||||||||||||||||||||||||||||
| 7.3 specific ‐ akathisia | 1 | 49 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.06 [0.60, 7.07] | ||||||||||||||||||||||||||||||||||||||||||||||||||
| 7.4 specific ‐ hypokinesia / akinesia | 1 | 49 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.30, 1.93] | ||||||||||||||||||||||||||||||||||||||||||||||||||
| 7.5 specific ‐ dystonia | 1 | 49 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.30 [0.01, 6.94] | ||||||||||||||||||||||||||||||||||||||||||||||||||
| 7.6 specific ‐ tremor | 2 | 85 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.29, 1.73] | ||||||||||||||||||||||||||||||||||||||||||||||||||
| 8 Adverse effects: 4. 'Psychic' side effects ‐ related to level of arousal Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 2.8  Comparison 2 ZUCLOPENTHIXOL vs OTHER TYPICAL ANTIPSYCHOTICS (only short term), Outcome 8 Adverse effects: 4. 'Psychic' side effects ‐ related to level of arousal. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 8.1 general ‐ 'psychic' side effects(UKU side effect rating scale) | 1 | 49 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.77 [0.79, 3.95] | ||||||||||||||||||||||||||||||||||||||||||||||||||
| 8.2 specific ‐ asthenia | 1 | 49 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.77 [0.50, 6.28] | ||||||||||||||||||||||||||||||||||||||||||||||||||
| 8.3 specific ‐ excitation | 1 | 43 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.07, 5.47] | ||||||||||||||||||||||||||||||||||||||||||||||||||
| 8.4 specific ‐ sleepiness / sedation | 2 | 92 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.57 [0.93, 2.65] | ||||||||||||||||||||||||||||||||||||||||||||||||||
| 9 Adverse effects: 5a. Weight ‐ change (kg) Show forest plot | 1 | 43 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.20, 1.23] | ||||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 2.9  Comparison 2 ZUCLOPENTHIXOL vs OTHER TYPICAL ANTIPSYCHOTICS (only short term), Outcome 9 Adverse effects: 5a. Weight ‐ change (kg). | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 9.1 loss or gain of weight of 10 pounds (high change=poor) | 1 | 43 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.20, 1.23] | ||||||||||||||||||||||||||||||||||||||||||||||||||
| 10 Adverse effects: 5b. Weight ‐ total weight increase (kg) Show forest plot | 1 | 83 | Mean Difference (IV, Fixed, 95% CI) | ‐1.89 [‐7.89, 4.11] | ||||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 2.10  Comparison 2 ZUCLOPENTHIXOL vs OTHER TYPICAL ANTIPSYCHOTICS (only short term), Outcome 10 Adverse effects: 5b. Weight ‐ total weight increase (kg). | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 11 Leaving the study early Show forest plot | 10 | 488 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.52, 0.97] | ||||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 2.11  Comparison 2 ZUCLOPENTHIXOL vs OTHER TYPICAL ANTIPSYCHOTICS (only short term), Outcome 11 Leaving the study early. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 11.1 any reason | 8 | 424 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.51, 0.95] | ||||||||||||||||||||||||||||||||||||||||||||||||||
| 11.2 due to adverse effects | 2 | 64 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.28 [0.18, 8.82] | ||||||||||||||||||||||||||||||||||||||||||||||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size | ||||||||||||||||||||||||
| 1 Global state: Unchanged or worse(CGI) Show forest plot | 1 | 98 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.30 [0.80, 2.11] | ||||||||||||||||||||||||
| Analysis 3.1  Comparison 3 ZUCLOPENTHIXOL vs ATYPICAL ANTIPSYCHOTICS (only short term), Outcome 1 Global state: Unchanged or worse(CGI). | ||||||||||||||||||||||||||||
| 2 Mental state: 1. No clinical response Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |||||||||||||||||||||||||
| Analysis 3.2  Comparison 3 ZUCLOPENTHIXOL vs ATYPICAL ANTIPSYCHOTICS (only short term), Outcome 2 Mental state: 1. No clinical response. | ||||||||||||||||||||||||||||
| 2.1 not achieving clinical response (at least 20% reduction in the total PANSS score) | 1 | 98 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.39 [0.92, 2.10] | ||||||||||||||||||||||||
| 2.2 not achieving clinical response for the total PANSS ‐ derived BPRS score | 1 | 98 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.82, 1.86] | ||||||||||||||||||||||||
| 3 Mental state: 2. Average scores(BPRS, data skewed, high score=poor) Show forest plot | Other data | No numeric data | ||||||||||||||||||||||||||
| Analysis 3.3
Comparison 3 ZUCLOPENTHIXOL vs ATYPICAL ANTIPSYCHOTICS (only short term), Outcome 3 Mental state: 2. Average scores(BPRS, data skewed, high score=poor). | ||||||||||||||||||||||||||||
| 4 Adverse effects: 1. General ‐ reporting adverse effects (UKU‐side effect rating scale) Show forest plot | 1 | 98 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.87, 1.11] | ||||||||||||||||||||||||
| Analysis 3.4  Comparison 3 ZUCLOPENTHIXOL vs ATYPICAL ANTIPSYCHOTICS (only short term), Outcome 4 Adverse effects: 1. General ‐ reporting adverse effects (UKU‐side effect rating scale). | ||||||||||||||||||||||||||||
| 5 Adverse effects: 2. Autonomic side effects Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |||||||||||||||||||||||||
| Analysis 3.5  Comparison 3 ZUCLOPENTHIXOL vs ATYPICAL ANTIPSYCHOTICS (only short term), Outcome 5 Adverse effects: 2. Autonomic side effects. | ||||||||||||||||||||||||||||
| 5.1 specific ‐ gastrointestinal side effects | 1 | 74 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.15 [0.01, 2.82] | ||||||||||||||||||||||||
| 5.2 specific ‐ anticholinergic side effects | 1 | 74 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.29, 2.63] | ||||||||||||||||||||||||
| 5.3 specific ‐ orthostatic reaction | 1 | 74 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.01, 8.36] | ||||||||||||||||||||||||
| 5.4 specific ‐ headache | 1 | 74 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.07, 16.25] | ||||||||||||||||||||||||
| 5.5 specific ‐ hypersalivation | 1 | 74 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.07, 16.25] | ||||||||||||||||||||||||
| 5.6 specific ‐ dizziness | 1 | 74 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.16, 7.10] | ||||||||||||||||||||||||
| 6 Adverse effects: 3. Extrapyramidal effects Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |||||||||||||||||||||||||
| Analysis 3.6  Comparison 3 ZUCLOPENTHIXOL vs ATYPICAL ANTIPSYCHOTICS (only short term), Outcome 6 Adverse effects: 3. Extrapyramidal effects. | ||||||||||||||||||||||||||||
| 6.1 general ‐ extrapyramidal side effects(ESRS) | 1 | 98 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.5 [0.92, 2.44] | ||||||||||||||||||||||||
| 6.2 general ‐ needing antiparkinsonian medication | 1 | 98 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.92 [1.12, 3.28] | ||||||||||||||||||||||||
| 6.3 specific ‐ hypokinesia | 1 | 74 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.07, 16.25] | ||||||||||||||||||||||||
| 6.4 specific ‐ hyperkinesia | 1 | 74 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.16 [0.13, 75.20] | ||||||||||||||||||||||||
| 6.5 specific ‐ rigor | 1 | 74 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.11 [0.20, 22.29] | ||||||||||||||||||||||||
| 6.6 specific ‐ tremor | 1 | 74 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.11 [0.20, 22.29] | ||||||||||||||||||||||||
| 6.7 specific ‐ akathisia | 1 | 74 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.07, 16.25] | ||||||||||||||||||||||||
| 7 Adverse effects: 4. 'Psychic' side effects ‐ related to level of arousal Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |||||||||||||||||||||||||
| Analysis 3.7  Comparison 3 ZUCLOPENTHIXOL vs ATYPICAL ANTIPSYCHOTICS (only short term), Outcome 7 Adverse effects: 4. 'Psychic' side effects ‐ related to level of arousal. | ||||||||||||||||||||||||||||
| 7.1 specific ‐ drowsiness | 1 | 74 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.29, 3.91] | ||||||||||||||||||||||||
| 7.2 specific ‐ stimulation | 1 | 74 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.24, 1.83] | ||||||||||||||||||||||||
| 7.3 specific ‐ confusion | 1 | 74 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.01, 8.36] | ||||||||||||||||||||||||
| 8 Adverse effects: 5. Weight ‐ change (kg) Show forest plot | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | ‐1.60 [‐10.06, 6.86] | ||||||||||||||||||||||||
| Analysis 3.8  Comparison 3 ZUCLOPENTHIXOL vs ATYPICAL ANTIPSYCHOTICS (only short term), Outcome 8 Adverse effects: 5. Weight ‐ change (kg). | ||||||||||||||||||||||||||||
| 9 Leaving the study early Show forest plot | 2 | 159 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.48 [0.98, 2.22] | ||||||||||||||||||||||||
| Analysis 3.9  Comparison 3 ZUCLOPENTHIXOL vs ATYPICAL ANTIPSYCHOTICS (only short term), Outcome 9 Leaving the study early. | ||||||||||||||||||||||||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Global state: Unchanged or worse (CGI) Show forest plot | 3 | 131 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.76, 1.52] |
| Analysis 4.1  Comparison 4 CIS‐(Z) ZUCLOPENTHIXOL vs CIS(Z)+TRANS(E) FORM OF ZUCLOPENTHIXOL (only short term), Outcome 1 Global state: Unchanged or worse (CGI). | ||||
| 2 Adverse effects: 1. General non‐specific ‐ interfering with functioning/outweighing therapeutic effect Show forest plot | 3 | 131 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.46, 1.21] |
| Analysis 4.2  Comparison 4 CIS‐(Z) ZUCLOPENTHIXOL vs CIS(Z)+TRANS(E) FORM OF ZUCLOPENTHIXOL (only short term), Outcome 2 Adverse effects: 1. General non‐specific ‐ interfering with functioning/outweighing therapeutic effect. | ||||
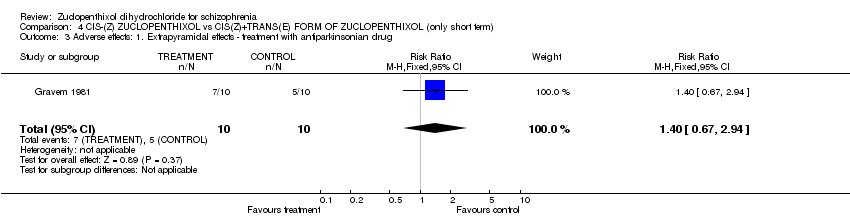
| 3 Adverse effects: 1. Extrapyramidal effects ‐ treatment with antiparkinsonian drug Show forest plot | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.4 [0.67, 2.94] |
| Analysis 4.3  Comparison 4 CIS‐(Z) ZUCLOPENTHIXOL vs CIS(Z)+TRANS(E) FORM OF ZUCLOPENTHIXOL (only short term), Outcome 3 Adverse effects: 1. Extrapyramidal effects ‐ treatment with antiparkinsonian drug. | ||||
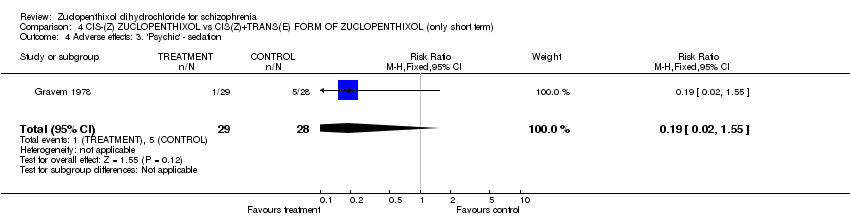
| 4 Adverse effects: 3. 'Psychic' ‐ sedation Show forest plot | 1 | 57 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.19 [0.02, 1.55] |
| Analysis 4.4  Comparison 4 CIS‐(Z) ZUCLOPENTHIXOL vs CIS(Z)+TRANS(E) FORM OF ZUCLOPENTHIXOL (only short term), Outcome 4 Adverse effects: 3. 'Psychic' ‐ sedation. | ||||
| 5 Leaving the study early ‐ by 1 week Show forest plot | 2 | 29 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.0 [0.27, 92.62] |
| Analysis 4.5  Comparison 4 CIS‐(Z) ZUCLOPENTHIXOL vs CIS(Z)+TRANS(E) FORM OF ZUCLOPENTHIXOL (only short term), Outcome 5 Leaving the study early ‐ by 1 week. | ||||

Comparison 1 ZUCLOPENTHIXOL vs PLACEBO (only short term), Outcome 1 Adverse effects: 1. Autonomic ‐ specific ‐ orthostatic.
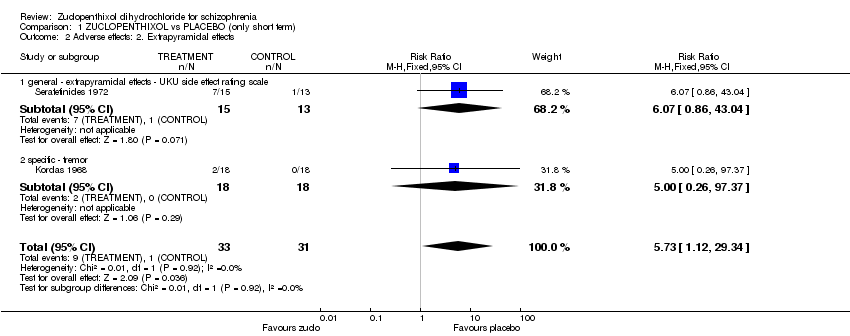
Comparison 1 ZUCLOPENTHIXOL vs PLACEBO (only short term), Outcome 2 Adverse effects: 2. Extrapyramidal effects.

Comparison 1 ZUCLOPENTHIXOL vs PLACEBO (only short term), Outcome 3 Adverse effects: 3. 'Psychic' side effects ‐ related to level of arousal.

Comparison 1 ZUCLOPENTHIXOL vs PLACEBO (only short term), Outcome 4 Adverse effects: 5. Weight ‐ change (kg).

Comparison 1 ZUCLOPENTHIXOL vs PLACEBO (only short term), Outcome 5 Leaving the study early.

Comparison 2 ZUCLOPENTHIXOL vs OTHER TYPICAL ANTIPSYCHOTICS (only short term), Outcome 1 Global state: 1. Not improved (CGI).

Comparison 2 ZUCLOPENTHIXOL vs OTHER TYPICAL ANTIPSYCHOTICS (only short term), Outcome 2 Global state: 2. Average score (CGI, high score = poor).

Comparison 2 ZUCLOPENTHIXOL vs OTHER TYPICAL ANTIPSYCHOTICS (only short term), Outcome 3 Mental state: 1. Average scores (BPRS, high score=poor).
| Study | Intervention | Mean | SD | N |
| BPRS | ||||
| Balasubramanian 1991 | Zuclopenthixol | 13.1 | 11.7 | 50 |
| Balasubramanian 1991 | Chlorpromazine | 12.9 | 9.3 | 44 |
| Heikkila 1992 | Zuclopenthixol | 11.6 | 10.05 | 20 |
| Heikkila 1992 | Haloperidol | 10.3 | 9.5 | 18 |
| CPRS | ||||
| Remvig 1987 | Zuclopenthixol | 6.3 | 5.5 | 22 |
| Remvig 1987 | Perphenazine | 5.9 | 6.5 | 18 |
Comparison 2 ZUCLOPENTHIXOL vs OTHER TYPICAL ANTIPSYCHOTICS (only short term), Outcome 4 Mental state: 2. Average scores (data skewed, high score=poor).
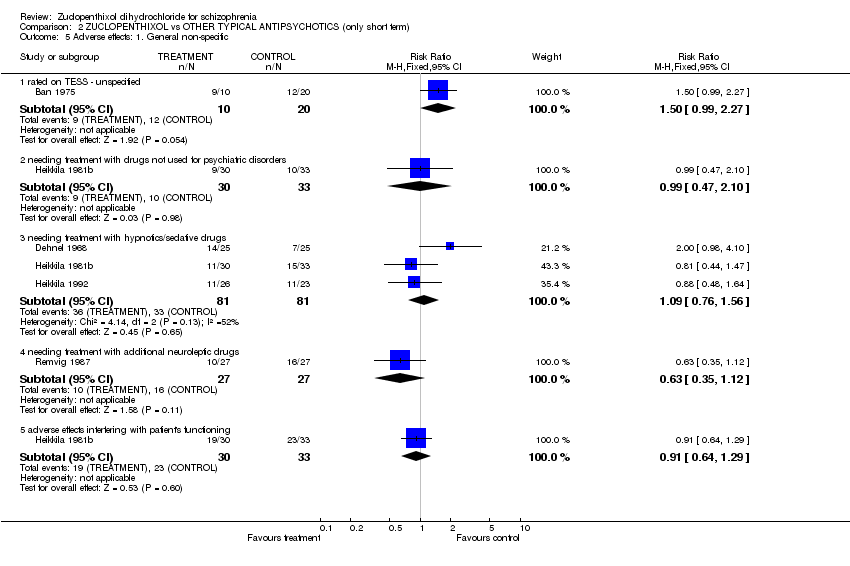
Comparison 2 ZUCLOPENTHIXOL vs OTHER TYPICAL ANTIPSYCHOTICS (only short term), Outcome 5 Adverse effects: 1. General non‐specific.

Comparison 2 ZUCLOPENTHIXOL vs OTHER TYPICAL ANTIPSYCHOTICS (only short term), Outcome 6 Adverse effects: 2. Autonomic side effects.

Comparison 2 ZUCLOPENTHIXOL vs OTHER TYPICAL ANTIPSYCHOTICS (only short term), Outcome 7 Adverse effects: 3. Extrapyramidal effects.
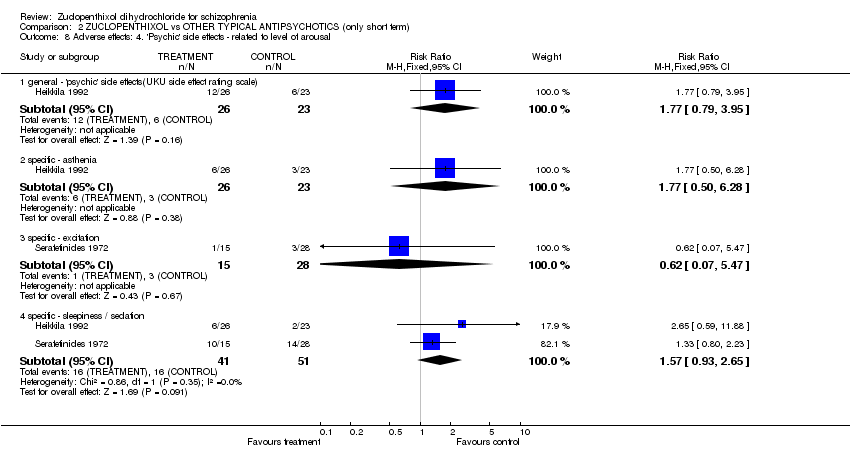
Comparison 2 ZUCLOPENTHIXOL vs OTHER TYPICAL ANTIPSYCHOTICS (only short term), Outcome 8 Adverse effects: 4. 'Psychic' side effects ‐ related to level of arousal.

Comparison 2 ZUCLOPENTHIXOL vs OTHER TYPICAL ANTIPSYCHOTICS (only short term), Outcome 9 Adverse effects: 5a. Weight ‐ change (kg).

Comparison 2 ZUCLOPENTHIXOL vs OTHER TYPICAL ANTIPSYCHOTICS (only short term), Outcome 10 Adverse effects: 5b. Weight ‐ total weight increase (kg).

Comparison 2 ZUCLOPENTHIXOL vs OTHER TYPICAL ANTIPSYCHOTICS (only short term), Outcome 11 Leaving the study early.
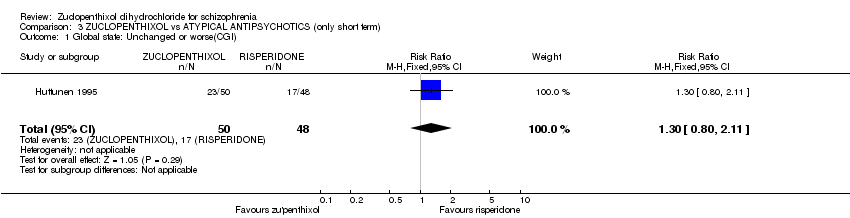
Comparison 3 ZUCLOPENTHIXOL vs ATYPICAL ANTIPSYCHOTICS (only short term), Outcome 1 Global state: Unchanged or worse(CGI).

Comparison 3 ZUCLOPENTHIXOL vs ATYPICAL ANTIPSYCHOTICS (only short term), Outcome 2 Mental state: 1. No clinical response.
| Study | Intervention | Mean | SD | N | Notes |
| Mahadevan 1991 | Zuclopenthixol | 5.7 | 5.3 | 16 | |
| Mahadevan 1991 | Sulpiride | 7.0 | 9.3 | 24 | |
Comparison 3 ZUCLOPENTHIXOL vs ATYPICAL ANTIPSYCHOTICS (only short term), Outcome 3 Mental state: 2. Average scores(BPRS, data skewed, high score=poor).

Comparison 3 ZUCLOPENTHIXOL vs ATYPICAL ANTIPSYCHOTICS (only short term), Outcome 4 Adverse effects: 1. General ‐ reporting adverse effects (UKU‐side effect rating scale).

Comparison 3 ZUCLOPENTHIXOL vs ATYPICAL ANTIPSYCHOTICS (only short term), Outcome 5 Adverse effects: 2. Autonomic side effects.

Comparison 3 ZUCLOPENTHIXOL vs ATYPICAL ANTIPSYCHOTICS (only short term), Outcome 6 Adverse effects: 3. Extrapyramidal effects.

Comparison 3 ZUCLOPENTHIXOL vs ATYPICAL ANTIPSYCHOTICS (only short term), Outcome 7 Adverse effects: 4. 'Psychic' side effects ‐ related to level of arousal.

Comparison 3 ZUCLOPENTHIXOL vs ATYPICAL ANTIPSYCHOTICS (only short term), Outcome 8 Adverse effects: 5. Weight ‐ change (kg).

Comparison 3 ZUCLOPENTHIXOL vs ATYPICAL ANTIPSYCHOTICS (only short term), Outcome 9 Leaving the study early.

Comparison 4 CIS‐(Z) ZUCLOPENTHIXOL vs CIS(Z)+TRANS(E) FORM OF ZUCLOPENTHIXOL (only short term), Outcome 1 Global state: Unchanged or worse (CGI).

Comparison 4 CIS‐(Z) ZUCLOPENTHIXOL vs CIS(Z)+TRANS(E) FORM OF ZUCLOPENTHIXOL (only short term), Outcome 2 Adverse effects: 1. General non‐specific ‐ interfering with functioning/outweighing therapeutic effect.
Comparison 4 CIS‐(Z) ZUCLOPENTHIXOL vs CIS(Z)+TRANS(E) FORM OF ZUCLOPENTHIXOL (only short term), Outcome 3 Adverse effects: 1. Extrapyramidal effects ‐ treatment with antiparkinsonian drug.
Comparison 4 CIS‐(Z) ZUCLOPENTHIXOL vs CIS(Z)+TRANS(E) FORM OF ZUCLOPENTHIXOL (only short term), Outcome 4 Adverse effects: 3. 'Psychic' ‐ sedation.

Comparison 4 CIS‐(Z) ZUCLOPENTHIXOL vs CIS(Z)+TRANS(E) FORM OF ZUCLOPENTHIXOL (only short term), Outcome 5 Leaving the study early ‐ by 1 week.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Adverse effects: 1. Autonomic ‐ specific ‐ orthostatic Show forest plot | 1 | 28 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.01, 6.60] |
| 2 Adverse effects: 2. Extrapyramidal effects Show forest plot | 2 | 64 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.73 [1.12, 29.34] |
| 2.1 general ‐ extrapyramidal effects ‐ UKU side effect rating scale | 1 | 28 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.07 [0.86, 43.04] |
| 2.2 specific ‐ tremor | 1 | 36 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.0 [0.26, 97.37] |
| 3 Adverse effects: 3. 'Psychic' side effects ‐ related to level of arousal Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 specific ‐ excitation | 1 | 28 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.63 [0.12, 59.40] |
| 3.2 specific ‐ sleepiness / sedation | 1 | 28 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.89 [1.01, 8.30] |
| 4 Adverse effects: 5. Weight ‐ change (kg) Show forest plot | 1 | 28 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.17, 1.11] |
| 4.1 loss or gain of weight of 10 pounds (high change=poor) | 1 | 28 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.17, 1.11] |
| 5 Leaving the study early Show forest plot | 1 | 28 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.01, 6.60] |
| 5.1 due to adverse effects | 1 | 28 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.01, 6.60] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Global state: 1. Not improved (CGI) Show forest plot | 7 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 not 'mildly ill or normal' | 2 | 114 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.55, 1.66] |
| 1.2 unchanged or worse | 7 | 357 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.53, 0.98] |
| 2 Global state: 2. Average score (CGI, high score = poor) Show forest plot | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐1.30, 0.90] |
| 3 Mental state: 1. Average scores (BPRS, high score=poor) Show forest plot | 1 | 41 | Mean Difference (IV, Fixed, 95% CI) | ‐2.66 [‐9.09, 3.77] |
| 4 Mental state: 2. Average scores (data skewed, high score=poor) Show forest plot | Other data | No numeric data | ||
| 4.1 BPRS | Other data | No numeric data | ||
| 4.2 CPRS | Other data | No numeric data | ||
| 5 Adverse effects: 1. General non‐specific Show forest plot | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 rated on TESS ‐ unspecified | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.5 [0.99, 2.27] |
| 5.2 needing treatment with drugs not used for psychiatric disorders | 1 | 63 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.47, 2.10] |
| 5.3 needing treatment with hypnotics/sedative drugs | 3 | 162 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.76, 1.56] |
| 5.4 needing treatment with additional neuroleptic drugs | 1 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.35, 1.12] |
| 5.5 adverse effects interfering with patient's functioning | 1 | 63 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.64, 1.29] |
| 6 Adverse effects: 2. Autonomic side effects Show forest plot | 2 | 92 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.35, 2.24] |
| 6.1 general ‐ autonomic side effects(UKU side effect rating scale) | 1 | 49 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.36 [0.71, 7.85] |
| 6.2 specific ‐ orthostatic side effects | 1 | 43 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.11 [0.01, 1.73] |
| 7 Adverse effects: 3. Extrapyramidal effects Show forest plot | 8 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 general ‐ needing antiparkinsonian medication | 6 | 280 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.86, 1.33] |
| 7.2 general ‐ extrapyramidal effects ‐ UKU side effect rating scale | 2 | 92 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.56, 1.09] |
| 7.3 specific ‐ akathisia | 1 | 49 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.06 [0.60, 7.07] |
| 7.4 specific ‐ hypokinesia / akinesia | 1 | 49 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.30, 1.93] |
| 7.5 specific ‐ dystonia | 1 | 49 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.30 [0.01, 6.94] |
| 7.6 specific ‐ tremor | 2 | 85 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.29, 1.73] |
| 8 Adverse effects: 4. 'Psychic' side effects ‐ related to level of arousal Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.1 general ‐ 'psychic' side effects(UKU side effect rating scale) | 1 | 49 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.77 [0.79, 3.95] |
| 8.2 specific ‐ asthenia | 1 | 49 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.77 [0.50, 6.28] |
| 8.3 specific ‐ excitation | 1 | 43 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.07, 5.47] |
| 8.4 specific ‐ sleepiness / sedation | 2 | 92 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.57 [0.93, 2.65] |
| 9 Adverse effects: 5a. Weight ‐ change (kg) Show forest plot | 1 | 43 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.20, 1.23] |
| 9.1 loss or gain of weight of 10 pounds (high change=poor) | 1 | 43 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.20, 1.23] |
| 10 Adverse effects: 5b. Weight ‐ total weight increase (kg) Show forest plot | 1 | 83 | Mean Difference (IV, Fixed, 95% CI) | ‐1.89 [‐7.89, 4.11] |
| 11 Leaving the study early Show forest plot | 10 | 488 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.52, 0.97] |
| 11.1 any reason | 8 | 424 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.51, 0.95] |
| 11.2 due to adverse effects | 2 | 64 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.28 [0.18, 8.82] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Global state: Unchanged or worse(CGI) Show forest plot | 1 | 98 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.30 [0.80, 2.11] |
| 2 Mental state: 1. No clinical response Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 not achieving clinical response (at least 20% reduction in the total PANSS score) | 1 | 98 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.39 [0.92, 2.10] |
| 2.2 not achieving clinical response for the total PANSS ‐ derived BPRS score | 1 | 98 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.82, 1.86] |
| 3 Mental state: 2. Average scores(BPRS, data skewed, high score=poor) Show forest plot | Other data | No numeric data | ||
| 4 Adverse effects: 1. General ‐ reporting adverse effects (UKU‐side effect rating scale) Show forest plot | 1 | 98 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.87, 1.11] |
| 5 Adverse effects: 2. Autonomic side effects Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 specific ‐ gastrointestinal side effects | 1 | 74 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.15 [0.01, 2.82] |
| 5.2 specific ‐ anticholinergic side effects | 1 | 74 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.29, 2.63] |
| 5.3 specific ‐ orthostatic reaction | 1 | 74 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.01, 8.36] |
| 5.4 specific ‐ headache | 1 | 74 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.07, 16.25] |
| 5.5 specific ‐ hypersalivation | 1 | 74 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.07, 16.25] |
| 5.6 specific ‐ dizziness | 1 | 74 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.16, 7.10] |
| 6 Adverse effects: 3. Extrapyramidal effects Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 general ‐ extrapyramidal side effects(ESRS) | 1 | 98 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.5 [0.92, 2.44] |
| 6.2 general ‐ needing antiparkinsonian medication | 1 | 98 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.92 [1.12, 3.28] |
| 6.3 specific ‐ hypokinesia | 1 | 74 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.07, 16.25] |
| 6.4 specific ‐ hyperkinesia | 1 | 74 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.16 [0.13, 75.20] |
| 6.5 specific ‐ rigor | 1 | 74 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.11 [0.20, 22.29] |
| 6.6 specific ‐ tremor | 1 | 74 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.11 [0.20, 22.29] |
| 6.7 specific ‐ akathisia | 1 | 74 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.07, 16.25] |
| 7 Adverse effects: 4. 'Psychic' side effects ‐ related to level of arousal Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 specific ‐ drowsiness | 1 | 74 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.29, 3.91] |
| 7.2 specific ‐ stimulation | 1 | 74 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.24, 1.83] |
| 7.3 specific ‐ confusion | 1 | 74 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.01, 8.36] |
| 8 Adverse effects: 5. Weight ‐ change (kg) Show forest plot | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | ‐1.60 [‐10.06, 6.86] |
| 9 Leaving the study early Show forest plot | 2 | 159 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.48 [0.98, 2.22] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Global state: Unchanged or worse (CGI) Show forest plot | 3 | 131 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.76, 1.52] |
| 2 Adverse effects: 1. General non‐specific ‐ interfering with functioning/outweighing therapeutic effect Show forest plot | 3 | 131 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.46, 1.21] |
| 3 Adverse effects: 1. Extrapyramidal effects ‐ treatment with antiparkinsonian drug Show forest plot | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.4 [0.67, 2.94] |
| 4 Adverse effects: 3. 'Psychic' ‐ sedation Show forest plot | 1 | 57 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.19 [0.02, 1.55] |
| 5 Leaving the study early ‐ by 1 week Show forest plot | 2 | 29 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.0 [0.27, 92.62] |

