为疑似脑膜炎球菌病患者在入院前使用抗生素
摘要
研究背景
脑膜炎球菌病可在发病后数小时内导致死亡或残疾。入院前使用抗生素旨在通过防止在确诊前延迟开始治疗来降低患严重疾病和死亡的风险。
研究目的
研究入院前使用抗生素与不使用抗生素或安慰剂的疗效和安全性,以及不同的入院前抗生素方案在降低疑似脑膜炎球菌病患者的死亡率、临床失败和发病率方面的有效性和安全性。
检索策略
截止至2017年1月,我们检索了Cochrane对照试验中心注册库(Cochrane Central Register of Controlled Trials, CENTRAL)(2017年1月6日)、MEDLINE(1966年至2017年1月6日)、Embase(1980年至2017年1月6日)、Web of Science(1985年至2017年1月6日)、LILACS(1982年至2017年1月6日)和前瞻性试验注册库。我们之前检索了1985年至2015年6月的CAB摘要,但在2017年1月没有更新此检索。
纳入排除标准
随机对照试验(randomised controlled trials, RCTs)或半随机对照试验,针对疑似脑膜炎球菌感染的患者比较了抗生素与安慰剂或无干预情况,或在入院或确诊前使用不同抗生素的情况。
资料收集与分析
两位系统综述作者独立评价了试验质量,并从检索结果中提取了资料。对于二分类资料,我们计算了的风险比(risk ration, RR)和95%置信区间(confidence interval, CI)。我们只纳入了一项试验,因此没有进行资料综合。我们使用GRADE方法评价了证据的总体质量。
主要结果
我们没有发现比较入院前使用抗生素与没有使用抗生素或安慰剂的情况的随机对照试验。我们纳入了一项开放标签、非劣效性随机对照试验,共有510名受试者,在尼日尔疫情期间进行,评价单剂量肌肉注射头孢曲松与单剂量肌注长效(油性)氯霉素的疗效。头孢曲松在降低死亡率(RR=1.21,95% CI [0.57, 2.56];N=503;308例确诊脑膜炎球菌性脑膜炎;26例死亡;中等质量证据)、临床失败(RR=0.83,95% CI [0.32, 2.15];N=477;18例临床失败;中等质量证据)、或神经系统后遗症(RR=1.29,95% CI [0.63, 2.62];N=477;29例有后遗症;低质量证据)。未报告治疗的不良反应。估计的治疗费用相似。没有关于后遗症导致的疾病负担的现有资料。
作者结论
我们没有发现可靠的证据支持对非严重脑膜炎球菌病疑似病例在入院前使用抗生素。来自一项随机对照试验的中等质量证据表明,单次肌肉注射头孢曲松和长效氯霉素在减少严重不良结局方面同样有效、安全和经济。这些抗生素之间的选择应该基于负担能力、可用性和抗生素耐药性模式。
进一步的随机对照试验比较不同的入院前的抗生素治疗,并辅以强化支持措施,这在病情较轻的人群中是合理的,但需要在不同的临床环境中提供可靠的证据。
PICO
简语概要
确诊前使用抗生素治疗疑似脑膜炎球菌感染的脑膜炎病例
系统综述问题
我们想知道,因感染脑膜炎球菌而怀疑使大脑和脊髓膜发炎(脑膜炎)的患者是否应该在确诊前服用抗生素,以防止死亡或残疾。我们发现了一项相关研究。
研究背景
脑膜炎球菌病是一种进展迅速的传染性细菌感染,可引起严重的脑部和血液疾病的流行。如果不及早治疗,许多人将会死亡或永久残疾。如果及早使用抗生素,则会非常有效。但等待实验室检查确诊可能会导致延迟开始使用抗生素的时间。基于临床猜测(经验性治疗)尽早给予抗生素可以防止治疗延误以及随之而来的死亡和残疾。然而,这样做也可能导致不必要的治疗。
研究特征
我们检索了比较给予与不给予经验性抗生素的研究,或者比较了疑似脑膜炎球菌病患者使用不同抗生素的研究。我们发现了一项随机试验比较了两种不同长效抗生素的单次肌肉注射剂量。证据检索日期截至到2017年1月。
这项纳入的研究是2003年脑膜炎球菌病爆发期间在尼日尔的九个初级保健机构进行的。在510名接受研究的成人和儿童中,251人接受了头孢曲松治疗,259人接受了氯霉素治疗。该项研究由无国界医生组织资助。
主要研究结果
根据经验,使用两种抗生素导致死亡、对治疗无反应或神经功能障碍的人数没有差异。随后确诊的患者结果相似。两种抗生素都没有明显的不良反应。
证据质量
尽管这项研究进行得很好,但由于该研究排除了两个月以下的儿童、孕妇和重病患者,因此死亡和治疗失败的总体证据质量仅为中等。由于随访时间短,神经功能障碍的证据质量低。
由于脑膜炎球菌病会造成严重的后果,因此不凭经验使用抗生素是不道德的。然而,未来的研究需要对不同年龄和疾病严重程度的人群比较不同的抗生素,以便在不同的临床环境中提供可靠的证据。
Authors' conclusions
Summary of findings
| Ceftriaxone versus long‐acting (oily) chloramphenicol in people suspected to have meningococcal disease | ||||||
| Patient or population: people suspected to have meningococcal disease (adults and children) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Long‐acting (oily) chloramphenicol | Intramuscular ceftriaxone | |||||
| Death ‐ in all participants ‐ short term | 47 per 10001 | 57 per 1000 | RR 1.21 | 503 | ⊕⊕⊕⊝ | All outcomes in this table are from 1 trial that randomised 510 participants to either intervention (Nathan 2005) |
| Death ‐ in confirmed cases of meningococcal meningitis (subgroup) | 34 per 10001 | 38 per 1000 | RR 1.11 | 308 | ⊕⊕⊕⊝ | |
| Clinical failure ‐ in all participants ‐ short term | 41 per 10001 | 34 per 1000 | OR 0.83 | 477 | ⊕⊕⊕⊝ | |
| Clinical failure ‐ in confirmed cases of meningococcal meningitis (subgroup) | 14 per 10001 | 19 per 1000 | OR 1.39 | 308 | ⊕⊕⊕⊝ | |
| Neurological sequelae ‐ in all participants‐ short term | 53 per 10001 | 68 per 1000 | RR 1.29 | 477 | ⊕⊕⊝⊝ | |
| Neurological sequelae ‐ in confirmed cases of meningococcal meningitis (subgroup) | 63 per 10001 | 91 per 1000 | RR 1.44 | 297 | ⊕⊝⊝⊝ | |
| Adverse events ‐ short term | See comment | See comment | Not estimable | 0 | See comment | No adverse events were detected with either intervention |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Basis of the assumed risk: control group risk. | ||||||
Background
This is an update of a Cochrane review first published in the Cochrane Database of Systematic Reviews 2008, Issue 1 and updated in 2011 and 2013 (Sudarsanam 2008; Sudarsanam 2011; Sudarsanam 2013).
Description of the condition
Meningococcal disease is a contagious bacterial disease caused by Neisseria meningitidis. N meningitidis strains are classified primarily into serogroups based on the type of polysaccharide capsule expressed. While 13 serogroups have been described (A, B, C, D, 29E, H, I, K, L, Y, W‐135, X, and Z), most of the disease is caused by strains belonging to serogroups A, B, C, X, Y, and W‐135 (Hill 2010). Serogroups B and C account for most of the cases in Europe and the Americas (Al‐Tawfiq 2010). Serogroups A and C are responsible for most of the cases in Asia and Africa (Schwartz 1989; Sinclair 2010). In the African 'meningitis belt' that extends from Ethiopia in the east to Senegal in the west, newer strains of serogroup A meningococcal disease have occurred in epidemic form that have posed a recurrent threat to public health, and rates of meningococcal disease in this region are several times higher than in high‐income countries (WHO 2007).
In a systematic review of 132 studies published between 1980 to 2008 that reported on the incidence of disabling sequelae of bacterial meningitis in 18,183 adult and child survivors of bacterial meningitis, Haemophilus influenzae type b (Hib) was the most common cause of bacterial meningitis (35.5%), with pneumococcus accounting for 19.6%, meningococcus for 16.4% and other pathogens for 12% (Edmond 2010). However, the relative frequency of disease caused by N meningitidis has increased in recent years due to the widespread and successful use of an effective vaccine for H influenzae B and a conjugate vaccine for Streptococcus pneumoniae, leaving N meningitidis as the most common cause of bacterial meningitis, particularly in some parts of Africa where surveys indicate that N meningitidis was responsible for 60% to 70% of cases of meningitis (Hsu 2009; WHO 2010). Meningococcal disease is most common in children (due to waning maternal antibody levels), adolescents, and young adults (WHO 2010).
Up to 5% to 10% of a population may be asymptomatic carriers of N meningitidis, in whom the bacteria are harmless commensals of the nasopharyngeal mucosa (WHO 2010). Meningococcal disease is caused by a combination of bacterial virulence factors (particularly the ability to express capsules) and host susceptibility, including age, prior viral infection, overcrowding, smoking, co‐infections, and genetic polymorphisms (Hill 2010; Stephens 2007). Meningococcal disease is spread by person‐to‐person contact through respiratory droplets from infected people. Meningococcal disease attack rates can be as high as 100 to 800 cases per 100,000 but individual communities have on occasion reported rates as high as 1000 per 100,000 (WHO 2004). In 1996, the largest outbreak ever reported occurred in the meningitis belt, in which the total number of cases was over 250,000 with 25,000 reported deaths (Rosenstein 2001).
Mortality
The onset of symptoms of meningococcal disease is sudden, and death can follow within hours (Borg 2009; Hackett 2002; Perea‐Milla 2009). It is estimated that around 50,000 people, mostly children and young adults, die every year from among the approximately 500,000 cases of meningococcal disease reported annually (Granoff 2009). Case fatality rates from invasive meningococcal disease are usually in the range of 10% to 15%, but mortality rates depend on the type and severity of invasive disease, and are greatest in people with meningococcaemia, fulminant septicaemia and shock (50% to 60%), followed by those with meningitis and associated septicaemia (up to 25%), and are lowest for meningitis without sepsis (less than 5%) (Borg 2009; Ferguson 2002; Hill 2010).
Meningococcal disease differs from other gram‐negative bacterial infections by the release of lipopolysaccharide endotoxins and vesicles that cause rapidly progressing skin haemorrhage and necrosis, disseminated intravascular coagulation, and shock (Brandtzaeg 2005). Other meningococcal virulence factors have been identified such as capsular polysaccharides (serogroups A, B, C, W‐135, Y, and X) and a number of surface‐expressed adhesive proteins, including factor H‐binding protein (fHbp), Opa, and Opc, that contribute to the ability to avoid innate immune responses and which aid adherence to mucosal surfaces (Hill 2010; Rouphael 2012; Schneider 2006; Stephens 2007). Capsules of N meningitidis help with transmission and colonisation, and the ability to express and modify capsule is associated with its epidemic potential (Stephens 2007).
Sequelae
There is significant resultant morbidity in 10% to 15% of survivors (Baraff 1993; Borg 2009); from reports published between 1988 to 2008, the pooled median risk (and interquartile range) of developing at least one major sequela after hospital discharge in people with invasive meningococcal disease was 7.2% (4.3% to 11.2%) (Edmond 2010). The risk of major sequelae is greatest in the African and Southeast Asian regions and in low‐income countries (Edmond 2010; Ramakrishnan 2009). Major sequelae are permanent neurological defects caused by pathophysiological inflammatory responses during infections. These involve increased blood‐brain barrier permeability; a large compartmentalised inflammatory response in the subarachnoid space, with pronounced increase in concentrations of tumour necrosis factor α (TNF‐α), interleukins, chemokines and other mediators; and increased resistance to the outflow of cerebrospinal fluid and oedema of the brain leading to elevated intracranial pressure and alterations in cerebral blood flow (Hill 2010; Stephens 2007; Tunkel 1993). The alteration in the permeability of the blood‐brain barrier is caused in part by inflammatory mediators such as matrix metalloproteinases (particularly MMP‐8), leading to disassembly of brain microvascular endothelial cell junction components and cell adhesion during meningococcal infection (Schubert‐Unkmeir 2010).
The resultant neurological deficits include hearing loss or deafness (most common), vision defects, speech disorders, amputation of limbs or digits and scarring of skin (due to extensive necrosis), hydrocephalus, mental retardation, spasticity, paralysis, and seizures, and present a long‐term and serious challenge for families with limited means to care for a disabled child, especially in resource‐poor settings (Borg 2009; Edmond 2010; Ramakrishnan 2009; WHO 2004). Prevalence estimates of these sequelae do not account for the increased mortality or social drift further down the socio‐economic ladder in those with such disabilities, particularly in low‐income families and in resource‐poor settings, leading to underestimates of prevalence in surveys of postmeningitis sequelae (Edmond 2010). Even in high‐income countries, impaired cognitive functions and behavioural sequelae result in impairments in many areas of social, educational, occupational functioning and quality of life after bacterial meningitis (Borg 2009).
These estimates of the sequelae of invasive meningococcal disease also do not account for deaths that might have occurred before admission to hospital due to difficulties in establishing a clinical diagnosis, lack of clinical suspicion in areas not prone to epidemics, and delays in instituting effective treatment in areas with health systems unable to respond to these needs. Meningococcal disease has many clinical manifestations and is often difficult to differentiate from common, less serious illnesses. The infectious syndromes associated with meningococcaemia include meningitis, bacteraemia, pneumonia, epiglottitis, otitis, and focal diseases such as urethritis, conjunctivitis, arthritis, and pericarditis (Stephens 2007). Clinical suspicion of meningococcal infection may vary with the geographic locale of the study, the age group of the patients being studied (children or adults), and the criteria used for clinical diagnosis of meningococcal disease. Meningococcal disease is suspected when there is a characteristic skin rash, headache, weakness, fever, vomiting, and depressed sensorium, with or without evidence of sepsis (Hahné 2006; Harnden 2006).
Diagnosis
The definitive diagnosis of meningococcal infection requires isolation of N meningitidis (a gram‐negative intracellular diplococcus that ferments glucose and maltose) from a sterile body fluid such as blood, cerebrospinal fluid (CSF), or synovial, pleural, or pericardial fluids. In meningococcal meningitis, sterilisation of the CSF occurs rapidly (within two hours) after the instigation of antibiotics (Kanegaye 2001). Culture confirmation occurs in only a third of clinically diagnosed cases, yet meningococcal DNA can be detected in 88% of admission blood samples from the same patients, and molecular techniques using polymerase chain reaction (PCR) on the CSF are informative, even after starting antibiotics (Hackett 2002). Polymerase chain reaction of the blood is highly specific for N meningitidis, can be used for subgroup and serotyping, and may even be of prognostic significance; quantitative PCR reveals higher bacterial DNA load to be associated with greater severity of illness and greater risk of mortality (El Bashir 2003; Hackett 2002). Polymerase chain reaction is more sensitive than culture, particularly in the context of pre‐admission treatment. The UK National Institute for Health and Care Excellence (NICE) guideline recommends that blood real‐time PCR should be done to confirm a diagnosis of meningococcal disease, and that CSF should also be submitted for PCR if the CSF culture is negative (NICE 2010). However, these techniques may not be readily available in resource‐poor settings.
Description of the intervention
The use of antibiotics has dramatically reduced mortality due to meningococcal disease. Once diagnosis is confirmed, crystalline penicillin is commonly given intravenously every 4 to 6 hours for 7 to 10 days in individuals not at risk of anaphylaxis. Rates of bacterial penicillin resistance vary. In areas where penicillin resistance predominates, third‐generation cephalosporins such as cefotaxime and ceftriaxone are recommended by the Scottish Intercollegiate Guidelines Network (SIGN) guidelines, SIGN 2008, and the NICE guidelines, NICE 2010, respectively. However, quinolones are not approved for routine paediatric usage and ceftriaxone requires parenteral administration (Girgis 1998). Sulphonamides are now rarely used, as intermediate resistance to these drugs is reported in some areas (Eickhoff 1965). Oral macrolide and beta‐lactam antibiotics are also effective in treating invasive meningococcal disease and averting mortality, though their routine use in suspected cases of invasive meningococcal disease may depend on 'indication bias' (bias in initiating oral versus parenteral or no antibiotics due to perceptions of mild to moderate, as opposed to severe, illness severity) (Perea‐Milla 2009). A Cochrane review studied osmotic agents for bacterial meningitis and found no benefit (Wall 2013).
The efficacy of short courses of ceftriaxone and oily chloramphenicol has been demonstrated in the treatment of meningococcal meningitis in adults (El Filali 1993). In one epidemic, a single intramuscular injection of an oily suspension of long‐acting chloramphenicol proved as effective as a five‐day course of crystalline penicillin (WHO 1995). Chloramphenicol is bactericidal for N meningitidis and penetrates the blood‐brain barrier more effectively than beta‐lactam antibiotics (Pecoul 1991). Intravenous cefotaxime plus either ampicillin or amoxacillin is recommended in children under three years of age (NICE 2010). Cefotaxime or ceftriaxone, often combined with vancomycin, are also used in high‐income countries until the causative agent has been identified (Stephens 2007). A systematic review did not reveal important differences between third‐generation cephalosporins or conventional antibiotics in averting death or deafness in people with bacterial meningitis (Prasad 2011)
A recent Cochrane review found that rifampicin, ciprofloxacin, ceftriaxone, or penicillin are effective for prophylaxis, with rifampicin resistance being an issue, as mentioned above (Zalmanovici 2013).
How the intervention might work
The aim of pre‐admission antibiotic therapy is to reduce the risk of serious disease by preventing delays in starting therapy. This delay may occur if confirmation of meningococcus is sought before initiation of therapy. It is believed that if treatment is begun early, the associated severe complications and mortality may be avoided or minimised by preventing or reducing the effects of the systemic inflammatory response of the body, including reduced inflammatory cytokines and chemokines, and endotoxin production; and also lead to a reduction in bacterial proliferation (Brandtzaeg 1989; Kanegaye 2001; Wang 2000). Preventing meningococcal shock is dependent on reducing endotoxin levels and meningococcal bacterial load (Hackett 2002), and given that meningococcaemia is a rapidly progressive disease, with an estimated doubling time of meningococci of 30 to 40 minutes (Stephens 2007), the time available for early administration of antibiotics is limited.
With this in mind, the concept of empiric antibiotic use based on clinical suspicion, before a confirmed bacteriological diagnosis is made, or the 'pre‐admission' use of antibiotics prescribed or administered by the doctor in first contact with the patient, has been found to be effective in reducing mortality and complications due to meningococcal disease in observational studies (Hahné 2006; Perea‐Milla 2009; Strang 1992; Wang 2000).
Why it is important to do this review
Standard policy in many countries (Hahné 2006), backed by recommendations from professional associations, mandates the initiation of antibiotics, particularly penicillin, once criteria for bacterial meningitis are met. Some policies contend that initiating antibiotic therapy requires the prior collection of CSF for bacterial confirmation or the use of PCR of the CSF, though there is a lack of consensus on the need for confirmation of the diagnosis before starting antibiotics (Hahné 2006). The NICE guideline states that children with suspected meningitis and meningococcal disease should have a lumbar puncture unless specifically contraindicated (NICE 2010).
However, it is not clear whether treating all suspected cases is associated with improved outcomes, since the effects of confounding are difficult to interpret in the observational studies supporting this view (Hahné 2006; Harnden 2006; Keeley 2006; Sorensen 1998).
In previous versions of this review we identified no trials comparing pre‐admission antibiotics to no antibiotic prior to confirmation of meningococcal meningitis (Sudarsanam 2008; Sudarsanam 2011; Sudarsanam 2013). We identified one trial that demonstrated the non‐inferiority of a single dose of intramuscular ceftriaxone versus a single dose of intramuscular long‐acting (oily) chloramphenicol in reducing serious outcomes.
This review update sought additional trials assessing the use of antibiotic therapy in suspected cases of meningococcal disease, and those that compared different classes of antibiotics used for this infection before confirmation of the diagnosis.
Objectives
To study the effectiveness and safety of pre‐admission antibiotics versus no pre‐admission antibiotics or placebo, and different pre‐admission antibiotic regimens in decreasing mortality, clinical failure and morbidity in people suspected of meningococcal disease.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) or quasi‐RCTs.
Types of participants
People of all ages who were suspected to have meningococcal infection in whom antibiotics were started presumptively before confirmation of the diagnosis, or transfer to hospital.
Participants of trials that looked at treatment of 'meningitis' or 'bacterial meningitis', where it may not have been possible to distinguish the results that applied to meningococcal disease, were treated as having 'suspected meningococcal infection'.
Types of interventions
-
Antibiotic treatment versus placebo or no intervention.
-
Any antibiotic versus another antibiotic from a different class.
-
Combinations of antibiotics versus another antibiotic or combinations of other antibiotics.
Pre‐admission antibiotic treatment refers to the use of antibiotic treatment for an initial dose, or doses, by any route before the diagnosis is confirmed.
Types of outcome measures
Primary outcomes
-
Mortality: death before reaching hospital, in hospital, or within a month of discharge or leaving the hospital.
-
Lack of clinical improvement: as defined by individual trials.
-
Morbidity: persistent neurological defects in the form of vision and hearing loss, speech disorders, persistent cognitive or intellectual impairment, and paralysis, or any other recorded persistent neurological defects.
Secondary outcomes
-
Burden of disease: on the family, individual, or caregiver; as reported using a validated scale or measure.
-
Adverse events: antibiotic‐related clinical adverse effects.
-
Economic costs of the intervention: if reported as done alongside the conduct of included RCTs.
We grouped the primary outcome of morbidity and all secondary outcomes by time. We defined 'short term' as less than six weeks; 'medium term' as six weeks to six months; and 'long term' as more than six months after the onset of symptoms subsequently confirmed to be due to meningococcal meningitis.
Search methods for identification of studies
Electronic searches
For this update we searched the Cochrane Central Register of Controlled Trials (CENTRAL; 2016, Issue 12), part of the Cochrane Library, www.cochranelibrary.com/ (accessed 6 January 2017), which includes the Cochrane Acute Respiratory Infections Group's Specialised Register, MEDLINE Ovid (1966 to 6 January 2017), Embase.com (1980 to 6 January 2017), Web of Science (1985 to 6 January 2017), and LILACS (Latin American and Caribbean Health Sciences Literature) (1982 to 6 January 2017). We did not update the previous CAB Abstracts search (1985 to June 2015) in January 2017 due to lack of institutional access to that database. See Appendix 1 for details of previous searches.
We used the search strategy in Appendix 2 to search MEDLINE and CENTRAL. We combined the MEDLINE search with the Cochrane Highly Sensitive Search Strategy for identifying randomised trials in MEDLINE: sensitivity‐maximising version (2008 revision); Ovid format (Lefebvre 2011). We adapted the search strategy for Embase.com (Appendix 3), Web of Science (Appendix 4), LILACS (Appendix 5), and CAB Abstracts (Appendix 6). We applied no language or publication restrictions.
The PRISMA figure summarises this process (Figure 1).

Study flow diagram
Searching other resources
We also searched the World Health Organization International Clinical Trials Registry Platform (WHO ICTRP) (apps.who.int/trialsearch/default.aspx), ClinicalTrials.gov (clinicaltrials.gov), and the ISRCTN register (www.controlled‐trials.com/isrctn/search.html) for ongoing and completed clinical trials, using the search terms 'meningococcal' AND 'meningitis' (searched 13 February 2017). We searched the references of all identified studies, as well as major reviews, for additional studies.
Data collection and analysis
Selection of studies
Two review authors (TS, PR) independently inspected each reference identified by the electronic searches and applied the inclusion criteria. We retrieved the full articles of trials that were deemed potentially relevant and in cases of disagreement. Two review authors (TS, PR) independently inspected the full articles to determine if they met the inclusion criteria, consulting a third review author (PT) when disagreements persisted. We discarded reports that were clearly irrelevant. We recorded studies on pre‐admission antibiotics that did not fulfil the inclusion criteria along with the reasons for their exclusion in the Characteristics of excluded studies table.
Data extraction and management
Two review authors (TS, PR) independently extracted data. We discussed the data extraction, documented decisions and, where necessary, contacted the trial authors for clarification. A third review author (PT) independently checked the extracted data.
We extracted, checked, and recorded the following data.
-
Characteristics of trials: design, date, location, and setting of trial; publication status; sponsor of trial (specified, known or unknown); duration of follow‐up.
-
Characteristics of participants: age; number of participants in each group; gender, setting, location.
-
Characteristics of interventions: type of antibiotics; dose, mode of administration, schedule; length of treatment and follow‐up.
-
Characteristics of outcome measures: we recorded data for events listed in the Types of outcome measures section for each intervention arm.
-
For economic analyses, key items of resource use (costs) and outcomes (beneficial and adverse), cost per unit of effort, quality adjusted life years (QALYs), or cost‐benefit analyses (resource inputs and effects of alternative interventions expressed in monetary units), if detailed in trial reports. If available, we would have recorded the following: analytic perspective adopted (for example, societal; national/subnational; third‐party payer; institution); time horizon for both costs (resource use) and effects (beneficial and adverse effects); and sources of resource use, unit costs, and (if applicable) effects and benefit valuation data (Campbell Collaboration 2008).
Assessment of risk of bias in included studies
Two review authors (TS, PR) independently assessed the included studies for risk of bias using Cochrane's 'Risk of bias' assessment tool on the following six domains: sequence generation, allocation concealment, blinding or masking, incomplete outcome data, selective outcome reporting, and other biases (Higgins 2011a). A third review author (PT) checked this assessment.
We judged each of these six domains as low risk of bias, high risk of bias, or unclear risk of bias, the last when due to lack of information in the report or after contacting the trial authors we were unable to make a reliable assessment. We used the criteria summarised in Table 8.5.c of the Cochrane Handbook for Systematic Reviews of Interventions to make judgements (Higgins 2011a), and recorded these assessments in the standard 'Risk of bias' tables in Review Manager 5 (RevMan 2014). We presented these evaluations in the 'Risk of bias' summary figure (Figure 2) and discussed them further in the Risk of bias in included studies section. We incorporated these judgements in assessing limitations in study design for critical and important outcomes in the summary of findings Table for the main comparison.

'Risk of bias' summary: review authors' judgements about each risk of bias item for Nathan 2005.
Measures of treatment effect
Only one study fulfilled the inclusion criteria, and we analysed dichotomous data from this trial by calculating the risk ratio (RR) and 95% confidence intervals (CI) for each outcome.
Unit of analysis issues
No non‐standard designs were used in the single included trial.
Had more trials been included in this review and had the included trials randomised participants by clusters, such as villages or health centres, and had the results been adjusted for clustering, we would have combined the adjusted measures of effects of these cluster‐randomised trials. If results had not been adjusted for clustering, we would have attempted to adjust the results for clustering, by multiplying the standard errors of the estimates by the square root of the design effect (where the design effect is calculated as DEff = 1 + (M ‐ 1) ICC, where M is the average cluster size and ICC is the intracluster coefficient). If this was not possible, we would not have combined the trials in a meta‐analysis, but would have presented the results in an Additional table.
If time‐to‐event outcomes had been reported, we would have extracted the estimates of the log hazard ratio and its standard error. If standard errors were unavailable, we would have extracted alternative statistics such as confidence intervals or P values.
Dealing with missing data
We contacted trial authors for missing data. We planned to exclude any trial with more than 20% unexplained dropouts in any arm. However, the included trial reported reasons for dropouts, and the trial authors provided supplementary information. The data were adequately presented, and the report provided intention‐to‐treat (ITT) and per‐protocol analyses data, as well as a participant flow diagram in a sufficiently detailed manner as to facilitate data retrieval.
We extracted data to allow an ITT analysis in which all randomised participants were analysed in the groups to which they were originally assigned. If there was a discrepancy in the number randomised and the numbers analysed in each treatment group, we calculated the percentage loss to follow‐up in each group and reported this information. If unexplained dropouts exceeded 10% in either group, we would have assigned the worst outcome to those lost to follow‐up for dichotomous outcomes (except for mortality) and assessed the impact of this in sensitivity analyses with the results of completers.
For continuous outcomes, if provided and where possible, we would have calculated missing standard deviations from other available data such as standard errors (Higgins 2011b). However, we would not have imputed missing values in order to present these in the analyses. We would not have made any assumptions about loss to follow‐up for continuous data and would have analysed results for those participants who completed the trial.
Assessment of heterogeneity
Had we included additional trials, we would have supplemented the inspection of the graphical display of results for non‐overlapping CIs among individual trials with the Mantel‐Haenszel Chi2 test of heterogeneity. Since this test has low power to detect heterogeneity, we would have interpreted a significance level of less than 0.10 as evidence of heterogeneity.
In addition, we would have quantified inconsistency across studies and its impact on the meta‐analysis by examining the value of the I2 statistic to estimate the percentage of variability due to intertrial variability rather than random error. We would have interpreted an I2 statistic of 50% or greater as indicating substantial levels of heterogeneity (Higgins 2003).
Assessment of reporting biases
Had there been sufficient studies (at least 10), we would have used a funnel plot of treatment effect against its standard error (as a measure of study size) to assess possible publication bias or small‐study effects.
Data synthesis
Since only one trial fulfilled the inclusion criteria, we presented the RR and 95% Cl for the prespecified outcomes from this trial.
Had there been more included trials, we would have pooled data for dichotomous outcomes using the inverse variance fixed‐effect model; if heterogeneity was deemed substantial, we would have used the random‐effects model.
For continuous data such as caregiver burden or quality of life assessments measured in similar ways, we had planned to calculate the difference in means weighted by the inverse of the variance. We would have used the standardised mean difference to pool results if continuous outcome data assessing similar outcomes were measured in different ways. If the distribution of the outcome data was significantly skewed and the studies were small, we would have looked for a suitable normalisation method or requested more appropriate summaries of the data from the investigators.
GRADE and 'Summary of findings' table
We created a 'Summary of findings' table using the following outcomes: death in all participants with a short‐term follow‐up of 72 hours; death in confirmed cases of meningococcal meningitis; clinical failure in confirmed cases of meningococcal meningitis; neurological sequelae in all participants with a short‐term follow‐up of 72 hours; neurological sequelae in confirmed cases of meningococcal meningitis; and adverse events with a short‐term follow‐up of 72 hours. We used the five GRADE considerations (study limitations, consistency of effect, imprecision, indirectness, and publication bias) to assess the quality of a body of evidence as it relates to the studies that contribute data to the meta‐analyses for the prespecified outcomes (Atkins 2004). We used methods and recommendations described in Section 8.5 and Chapter 12 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a), employing GRADEpro GDT software (GRADEpro GDT 2014). We justified all decisions to down‐ or upgrade the quality of studies using footnotes, and we made comments to aid the reader's understanding of the review where necessary.
Subgroup analysis and investigation of heterogeneity
We would have evaluated the pooled results from additional studies that used comparable participants, interventions, methods, and outcomes for evidence of significant heterogeneity that would have precluded a reliable interpretation of a common 'average' effect of the combined effects of the interventions.
Had the I2 statistic exceeded 75% with no explanation for this inconsistency (see below) in the effects of interventions across trials, we would have presented the results in a forest plot without pooling data.
We anticipated between‐trial variations in the following situations and therefore intended to perform subgroup analyses when:
-
baseline risk levels (severity of infection) differed between trials;
-
trials presented results for people with suspected versus confirmed meningococcal meningitis; analysing the results separately allows for evaluating the effects of differences in these proportions across trials and the effects of different indices of suspicion for bacterial meningitis during and outside of epidemic settings;
-
trials differed by levels of health service delivery (low‐income versus moderate‐ to high‐income settings);
-
trials differed by duration of follow‐up (short term (< six weeks); medium term (six weeks to six months); long term (> than six months).
If subgroups appeared to differ in the effects of interventions, as evident by non‐overlapping Cls, we would have performed formal tests for subgroup differences using the methods described in Higgins 2011b, which are possible in Review Manager 5 when the inverse variance method is used to pool dichotomous data (RevMan 2014).
Sensitivity analysis
We had also planned to perform sensitivity analyses to assess the robustness of our findings to different aspects of risk of bias among included trials and to evaluate the assumptions made in ITT and completer analyses.
Results
Description of studies
Results of the search
Our original review retrieved 136 reports (Sudarsanam 2008). Of these, we obtained hard copies of 30 potentially eligible trials. Only Nathan 2005 met the inclusion criteria. The search also retrieved a systematic review on the same topic that was published while the first version of this review was underway (Hahné 2006). Hahné 2006 included 14 cohort studies but did not include any RCTs. Another review of observational studies looked at seven trials and also did not include any RCTs (Leclerc 2001).
An updated search in June 2010 retrieved 46 records, only one of which was relevant to this review (Sudarsanam 2011). It was a retrospective analysis that controlled for the effects of indication bias in prescribing antibiotics by using propensity scores to evaluate the effects of pre‐admission antibiotics in preventing deaths due to meningococcal disease during an epidemic in Spain (Perea‐Milla 2009). This is listed in the Characteristics of excluded studies table.
Our 2013 review update retrieved 125 records (Sudarsanam 2013). We identified no relevant trials.
This 2017 update retrieved 77 records. We identified no relevant trials (Figure 1).
Included studies
Our protocol predefined participants as those with suspected cases of meningococcal meningitis awaiting transfer to hospital and randomised to treatment before confirmation of diagnosis. The trial by Nathan 2005, funded by Médecins Sans Frontières, was conducted between March and April 2003 in Niger, during an epidemic of meningococcal infection, and recruited people with suspected meningococcal meningitis from one of eight peripheral health centres, as well from a regional hospital at Zinder, Niger.
Clarifications from the trial authors revealed that anyone presenting with suspected meningitis to any site included in the study, who met the inclusion criteria, was invited to participate. If informed consent was obtained, a lumbar puncture was conducted (along with a rapid diagnostic test for malaria), and the participant was then randomised to the interventions. Participants remained at the site to which they had initially presented (and had received the intervention) for a minimum of 72 hours of follow‐up. If a second treatment dose was required, or if an alternative treatment was necessary, these too were administered at the original site. No transfer of participants between sites occurred. Of the 510 participants originally included in the study, 97 (19%) were recruited at the National Hospital in Zinder and 41 (8%) at a district hospital in Matameye. The trial authors clarified that the hospital in Matameye was a hospital by name, but had no medical facilities beyond those of the other health centres. The remaining 372 (73%) were treated in peripheral health centres.
We felt that as 81% of participants were treated in what were effectively peripheral health centres, and since participants were randomised to the interventions before confirmation of diagnosis, the data from this trial could be used without biasing our review's stated objectives.
The sample size in this trial was chosen to show non‐inferiority between the two groups (less than 10% difference in the failure rate between the two groups at 72 hours) for the primary outcome of treatment failure (death at 72 hours or clinical failure). Most participants (˜55% to 57%) were in the five‐to‐14‐years age group, with 31% under the age of five years but greater than two months old. The trial evaluated the effects of a single intramuscular dose of ceftriaxone (a third‐generation cephalosporin) and a single dose of oily chloramphenicol (long‐acting intramuscular chloramphenicol) in people suspected of having meningococcal disease. This trial is further described in the Characteristics of included studies table.
Excluded studies
Thirty‐one studies are described in the Characteristics of excluded studies table. We excluded nine as they randomised only proven, not suspected, cases of meningitis (Barson 1985; Congeni 1984; del Rio 1983; Kavaliotis 1989; Martin 1990; Molyneux 2011; Pecoul 1991; Rodriguez 1986; Schaad 1990). The other two were RCTs of the effects of dexamethasone in children with proven bacterial meningitis with both groups receiving antibiotics (Girgis 1989; Wald 1995). A report of mortality following pre‐admission antibiotics in suspected meningococcal meningitis was a retrospective case‐control study (Harnden 2006). Perea‐Milla 2009 was also a retrospective case‐control study adjusted for indication bias. Riordan 2001 was a prospective cohort where the intervention being studied was the effect of training on 'door to needle time' for giving antibiotics. Most other studies were observational case‐control, case series, or review articles.
Risk of bias in included studies
We found no RCTs or quasi‐RCTs comparing pre‐admission antibiotics for suspected meningococcal infection versus placebo or no intervention. The trial by Nathan 2005, comparing two antibiotics, had the following characteristics.
Allocation
This trial used off‐site computer‐generated codes in blocks of 20. Sealed, numbered, opaque envelopes containing the description of the allocated intervention were delivered to each intervention site in lots of 50 to be opened sequentially by on‐site study physicians who, after obtaining consent and a sample of CSF for diagnostic confirmation, administered the intervention. The study investigators involved in random sequence generation were not involved in recruitment of the study participants.
Blinding
The study physicians were not blinded to the interventions once participants were allocated. However, the pre‐stated outcomes were objective and unlikely to be influenced by assessor bias.
Incomplete outcome data
A total of 510 participants were randomised, 251 to receive ceftriaxone and 259 to receive chloramphenicol. Three participants left the treatment facility in the ceftriaxone arm and four in the chloramphenicol arm within the 72 hours of follow‐up. Reasons were not provided, but it would be difficult to assume that they had poor outcomes. Data on these participants were not included in the ITT analysis reported by the trial authors, but the numbers were few and similar in both arms, so we used data provided in the ITT analysis in the report for evaluating outcomes. We also used as presented data provided in the report on those participants in whom bacteriologic confirmation of meningococcal meningitis was done.
Selective reporting
The trial protocol was not available, and the report did not have any identification to suggest that it had been prospectively registered in a publicly accessible database. However, the trial reported all pre‐stated and expected outcomes and appeared to be free of selective reporting.
Other potential sources of bias
We identified no other potential sources of bias.
Effects of interventions
We found no trials that evaluated the effects of pre‐admission antibiotics versus placebo for suspected cases of meningococcal disease.
The one included trial screened 557 participants and randomised 510, of whom 251 were given intramuscular ceftriaxone and 259 long‐acting intramuscular (oily) chloramphenicol during an epidemic of meningococcal meningitis (Nathan 2005).
Primary outcomes
1. Mortality
Data were only available for short‐term outcomes, as defined in the Types of outcome measures section. At 72 hours, deaths occurred in 14 out of 247 (6%) participants in the ceftriaxone arm and 12 out of 256 (5%) participants in the long‐acting chloramphenicol arm (ITT values from the report). Mortality did not differ significantly between the two interventions (RR 1.21, 95% CI 0.57 to 2.56; N = 503; moderate‐quality evidence) (Figure 3; Analysis 1.1). A subgroup analysis of the participants who were later confirmed to have meningococcal disease also failed to show any difference in mortality between the two study arms (RR 1.11, 95% CI 0.35 to 3.56; N = 308). Mortality did not differ in the remaining 195 participants in the intervention arms without confirmed meningococcal meningitis (RR 1.42, 95% CI 0.54 to 3.76). We did not undertake formal tests for subgroup differences since the confidence intervals for subgroup effects overlapped considerably.

Forest plot of comparison: 1 Ceftriaxone versus long‐acting (oily) chloramphenicol, outcome: 1.1 Death
There were no data on mortality before presentation to the health centres or after discharge from the facilities, though all participants were discharged only when well.
2. Lack of clinical improvement
This composite outcome was defined as a Glasgow Coma Scale of less than 11 at 24 hours or less than 13 at 48 hours; no improvement or worsening in the state of consciousness or neurological status; persistent convulsions; and axillary temperature above 38.5° C. The ITT analysis reported that clinical failure occurred in 8 out of 233 (3%) participants in the ceftriaxone arm and 8 out of 244 (4%) participants in the long‐acting chloramphenicol arm. Again, this difference was not statistically significant (RR 0.83, 95% CI 0.32 to 2.15; N = 477; moderate‐quality evidence) (Figure 4; Analysis 1.2). The interventions did not differ in the proportions of those with clinical failure in the 308 confirmed cases of meningococcal meningitis (RR 1.39, 95% CI 0.23 to 8.47), nor in the 169 participants in whom meningococcal meningitis was not confirmed (RR 0.81, 95% CI 0.25 to 2.58). The interventions did not differ significantly in the proportions of participants requiring a second injection between 48 to 72 hours (risk difference ‐0.9%, 95% CI ‐4.7% to 3.0%).

Forest plot of comparison: 1 Ceftriaxone versus long‐acting (oily) chloramphenicol, outcome: 1.2 Clinical failure
3. Morbidity
Neurological sequelae were recorded in 16 out of 233 (7%) participants in the ceftriaxone arm and 13 out of 244 (5%) participants in the chloramphenicol arm. Sequelae included hearing impairment in 14 participants and motor dysfunction in 15 participants (ataxia, motor deficit, or both), but data for individual sequelae were not separable by intervention arms.
The incidence of sequelae did not differ significantly between interventions among all participants using the ITT analysis in the report (RR 1.29, 95% CI 0.63 to 2.62; N = 477; low‐quality evidence) (Figure 5; Analysis 1.3). The incidence in those with confirmed meningococcal meningitis was also not significantly different (RR 1.44, 95% CI 0.65 to 3.23; N = 297), nor did it differ significantly in those without confirmed meningococcal meningitis (RR 0.64, 95% CI 0.12 to 3.40; N = 180).

Forest plot of comparison: 1 Ceftriaxone versus long‐acting (oily) chloramphenicol, outcome: 1.3 Neurological sequelae
Secondary outcomes
1. Burden of disease
No data were available on the burden of disease on the family, individual, or caregiver as participants were followed up only until discharge.
2. Adverse effects
Neither intervention was associated with adverse effects.
3. Economic costs of the intervention
The trial reported that the average treatment dose used was 2 g per person for both drugs. The treatment cost per patient was estimated as USD 4 to 6 for chloramphenicol and USD 2 to 3 for ceftriaxone. No details were provided on how these costs were arrived at or of other economic issues such as resource use, etc.
Discussion
Summary of main results
Pre‐admission antibiotics versus no antibiotics
Our primary question regarding the efficacy and safety of pre‐admission antibiotics in decreasing mortality or morbidity in people with suspected meningococcal disease remains unanswered, since we did not find any RCTs comparing antibiotics versus placebo or no antibiotic in suspected cases of meningococcal meningitis and the initiation of antibiotics in the control group after confirmation of the diagnosis.
Comparison of a third‐generation cephalosporin (ceftriaxone) versus long‐acting chloramphenicol
The sole eligible RCT included in this review reported that a single dose of ceftriaxone was not inferior to long‐acting chloramphenicol in preventing mortality, neurological sequelae, the need for a second injection, and clinical non‐response (in the first 48 hours after admission), in those with suspected, and those subsequently confirmed, to have meningococcal meningitis who were given the antibiotic at presentation to the healthcare facility during an epidemic of meningitis (summary of findings Table for the main comparison) (Nathan 2005). No adverse events were reported for either drug, and costs were comparable.
The trial excluded very ill participants (people in an unresponsive coma and in shock), thus leaving unanswered the question of the efficacy of the schedule of treatments in such instances and the role of supportive measures, even if antibiotics were to be given early. Even among those with a less severe illness at the start of the trial, treatment failure at 72 hours and death were significantly more likely in those with impaired consciousness prior to admission (univariate odds ratio 5.51, 95% CI 2.90 to 10.45). This suggests that a single dose of antibiotics prior to admission may be insufficient to prevent negative outcomes in some people with moderately severe illness, perhaps due to suboptimal dosage, the toxic effects of rapid bacteriolysis, or the absence of adequate additional interventions to combat or prevent haemodynamic imbalance, respiratory distress, renal insufficiency, dehydration or overhydration, and electrolyte imbalance (Keeley 2006). These speculations are borne out by experiences with intensive treatment of meningococcal disease elsewhere (Booy 2001), though the speculation on the toxic effects of rapid bacteriolysis due to antibiotic treatment have not been borne out uniformly by empiric enquiry (Stephens 2007).
Overall completeness and applicability of evidence
Completeness
We found no reliable evidence on the relative efficacy of giving antibiotics before confirmation of the diagnosis or withholding them until confirmation of the diagnosis.
We believe that we have identified all trials relevant to this review.
Applicability
The trial by Nathan 2005 provided moderate‐quality evidence to endorse the use of a single dose of either ceftriaxone or oily chloramphenicol in reducing mortality, though the short follow‐up precludes the drawing of valid conclusions regarding neurological sequelae.
The external validity of this trial is less clear. When generalising the results to settings and periods other than during epidemics, as in this trial, it is uncertain whether similar mortality and morbidity estimates would be achieved in the absence of high levels of treatment‐seeking and alertness to the possibility of meningococcal meningitis that epidemics engender.
The oily suspension of chloramphenicol is ideal for use in low‐income countries, due to the comparable efficacy of a single intramuscular dose, repeated if needed after 48 hours, to 10 days of intravenous ampicillin given four times a day (Pecoul 1991), achieved at a 10th of the cost. Widespread resistance to chloramphenicol in high‐income countries limits its usefulness in these settings. However, long‐acting chloramphenicol may be a useful drug of choice in low‐income countries where resistance to chloramphenicol is not a major problem and if supply is not compromised (Pecoul 1999).
Ceftriaxone was initially around six to 10 times more expensive than long‐acting chloramphenicol, but patent rights for ceftriaxone have expired in most countries, and the generic drug costs have also fallen (Nathan 2005). Given the comparative efficacy and costs of both agents evaluated in the included trial, the choice of drug for initial pre‐admission antibiotic therapy would depend on the proportion of people with chloramphenicol resistance among the population in question, and the affordability of ceftriaxone at local costs. When antibiotic susceptibility testing is limited in low‐income countries with emerging chloramphenicol resistance, oily chloramphenicol as the initial drug followed by ceftriaxone, if symptoms do not improve within 48 hours and bacterial meningitis is confirmed, is a possible strategy (Duke 2003). Ceftriaxone could be used as the first‐line drug in the presence of widespread chloramphenicol resistance, though the emergence of resistance to ceftriaxone could also result if this use was indiscriminate. Ceftriaxone would also be preferable to chloramphenicol in situations where the suspected case of meningococcal meningitis is caused by H influenzae or S pneumoniae, where chloramphenicol is not as effective.
The other factor that would influence the choice of antibiotic is safety. Both drugs were considered safe in the trial by Nathan 2005.
Additional caveats must be considered before instituting pre‐admission antibiotics in areas with and without epidemic meningitis. One is the need to rule out other common infections with similar presentations, such as malaria, and meningitis due to other infective pathogens. The alarm engendered by epidemics or local outbreaks may also result in the indiscriminate use of antibiotics. In the included trial malaria was diagnosed in 44 (9%) of participants, and three cases of meningitis were due to H influenzae and three to S pneumoniae; moreover 133 (23%) had sterile lumbar punctures (Nathan 2005).
The other caveat is that unless early initiation of antibiotics in suspected cases of meningococcal disease is accompanied by other measures to improve healthcare delivery (increased recognition and case detection, early transfer to hospital and facilities, and the rapid initiation of supportive measures to manage complications of the more severe forms of the illness), the benefits of antibiotics alone are unlikely to affect mortality rates significantly (Booy 2001; NICE 2010).
Quality of the evidence
Design and quality of reporting
The included trial was designed to demonstrate non‐inferiority of ceftriaxone to chloramphenicol, assuming 15% of those allocated to chloramphenicol would be treatment failures, a difference of less than 10% between the interventions, a one‐sided 5% significance level, 80% power, and 10% loss to follow‐up. The trial authors stated that they calculated the risk difference and 90% CIs of the primary and secondary outcomes and considered the difference as equivalent if the upper limit of its 90% CI was below 10%.
Non‐inferiority trials present particular difficulties in design, conduct, analysis, and interpretation, as do trials assessing equivalence. True equivalence is difficult to prove, but the assumption of equivalence in this instance was based on the demonstration of non‐inferiority initially, using one‐sided 5% significance and a one‐sided 90% CI. Equivalence was then assessed using a pre‐stated one‐sided 90% CI of less than 10% (a two‐sided CI might have been more appropriate) and an ITT analysis. The design, conduct, reporting, and interpretation of this trial conformed to the recommendations in the extension to the CONSORT statement for non‐inferiority and equivalence trials (Piaggio 2006). We considered the study to be adequate in reporting randomisation, allocation concealment, and attrition, and although it was an open‐label trial, we considered the risk of detection bias to be low due to the objective outcomes used.
Overall quality of the evidence
We rated the overall quality of the evidence for primary outcomes as moderate, and although there were no limitations in study design, we downgraded the trial for indirectness for all outcomes due to the exclusion of infants, pregnant women, and those with severe disease (summary of findings Table for the main comparison).
We further downgraded the quality of the evidence to low for neurological outcomes since the duration of follow‐up was only 72 hours and was deemed too short to adequately detect neurological outcomes. Trials that assess deficits in the longer term would enable the detection of neurological sequelae that are detected only after discharge, particularly hearing deficits and spasticity in young children, and milder cognitive deficits and behavioural changes in adolescents (Borg 2009). The methods used to detect these outcomes in Nathan 2005 were based on gross clinical evaluation and may also have been insensitive to detect them accurately.
The trial may also have been underpowered to demonstrate non‐inferiority for neurological outcomes in confirmed cases of meningococcal meningitis, and the follow‐up was too short, therefore we judged the quality of the evidence for this subgroup as very low (summary of findings Table for the main comparison).
Potential biases in the review process
Despite the importance of the issue, the surprising lack of RCTs for our main objective is worrying. While it may suggest that we were unable to locate small trials, particularly those with inconclusive results, and indicate publication or retrieval bias, our search of multiple sources with no language restrictions reassures us that this is unlikely. A more credible explanation is that pre‐admission antibiotics have become the standard of care in many countries and hence an RCT, especially a placebo‐controlled trial, may be deemed unethical.
At first reading, the sole included trial seemed to not fulfil our inclusion criteria of 'pre‐admission' use of antibiotics (Nathan 2005), since participants were those treated in health centres; written communication with trial authors confirmed that the first dose of antibiotic was administered to people suspected as having meningococcal meningitis prior to seeking confirmation of the diagnosis. The trial authors also clarified that the majority of the treatment facilities (8/9) were primary care centres, and the majority of participants were treated at these centres (81%). The results of this trial also showed that 195/503 (39%) randomised participants were not subsequently confirmed to have meningococcal meningitis on culture or by polymerase chain reaction, attesting to the actual use of antibiotics prior to disease confirmation and the suitability of this trial for inclusion in this review. None of the excluded RCTs fulfilled this definition of antibiotic use.
Agreements and disagreements with other studies or reviews
Pre‐admission antibiotics versus no antibiotics
The available evidence for this comparison comes from retrospective case series, case‐control, and cohort studies. Considering the non‐specific nature of symptoms, especially in milder cases and in young children, and the lack of sensitivity of the specific clinical signs of both Kernig and Brudzinski, in adults as well as in children (El Bashir 2003), it is likely that data from these sources are confounded by diagnostic errors and inclusion of the more severely ill (Hahné 2006; Harnden 2006; Keeley 2006; Sorensen 1998). The latter are more likely to seek help in clinic‐based studies, be perceived to have meningitis by clinicians in these and in community‐based studies, and to be treated with parenteral antibiotics early, leading to 'indication bias' (Perea‐Milla 2009). Consequently, the often paradoxical outcomes of these observational studies, both positive and negative, are confounded by disease severity, and the proportions that received pre‐admission antibiotics (Hahné 2006; Harnden 2006; Perera 2006). Similarly, observational studies of oral pre‐admission antibiotics that report lower mortality and morbidity are also confounded by indication bias, in this case the inclusion of people with less severe or non‐meningococcal disease (Harnden 2006; Keeley 2006; Perea‐Milla 2009).
A retrospective analysis of 848 people admitted with invasive meningococcal disease, 49 of whom died (6%), from 1995 to 2000 in 31 hospitals in Spain, used the 'propensity score' to assign patients the probability of receiving pre‐hospital antibiotics prior to admission, based on clinical symptoms, and matched the 228 who had received oral antibiotics in the 48 hours prior to admission with controls who had not received pre‐hospital antibiotics, again based on their propensity scores for not being thus treated (Perea‐Milla 2009). Adjusted multivariate analyses indicated that pre‐hospital antibiotics appeared to protect against death (odds ratio 0.37, 95% CI 0.15 to 0.93). The propensity score technique has been equated with randomisation in situations where randomisation is difficult, and this trial attempted to adjust for the indication bias that has confounded interpretation of previous observational studies. The imprecision of the effect estimate (the confidence intervals suggest a protective effect that could be clinically very important to only marginally important) is in concordance with evidence from case‐control studies that indicate, on balance, that early diagnosis, early admission to hospital (within three hours), and early initiation of supportive measures are as important as the early commencement of antibiotics (Keeley 2006).
Pre‐admission cephalosporins versus chloramphenicol
Previous NICE guidance recommended that children with suspected meningococcal disease be given parenteral antibiotics (benzylpenicillin or a third‐generation cephalosporin) at the earliest opportunity (NICE 2007). The SIGN guideline on the management of invasive meningococcal disease in children and young people recommends that parenteral antibiotics (benzylpenicillin or cefotaxime) should be given as soon as invasive meningococcal disease is suspected and even before confirmation of the diagnosis is sought (SIGN 2008). The latest NICE guidance considers transfer to hospital and fluid management of greater priority than administering pre‐hospital antibiotics, unless immediate transfer is not possible, or if the clinical suspicion of meningococcal meningitis is accompanied by a non‐blanching rash or meningococcal septicaemia (NICE 2010). In contrast to earlier guidance, NICE 2010 recommends using ceftriaxone as first‐line treatment for bacterial meningitis and meningococcal disease in children and young people older than three months of age.
An economic analysis, done from the perspective of the National Health Service (NHS) and a socialised medicine approach, strongly suggests that ceftriaxone is the most cost‐effective antibiotic for the treatment of suspected meningococcal disease or suspected meningitis in a majority of children (NICE 2010). This concurs with the cruder estimates in Nathan 2005. The NICE analyses did not take into account variations in drug prices or any effects on health or costs arising from antibiotic resistance, both of which may vary widely in different settings. These estimates also assumed that survival is the only health‐related quality of life outcome of importance with antibiotic use and did not consider other outcomes such as prevention of neurological disability and its (more difficult to estimate) consequences and associated costs, and quality adjusted life years (QALYs) lost or gained.

Study flow diagram

'Risk of bias' summary: review authors' judgements about each risk of bias item for Nathan 2005.

Forest plot of comparison: 1 Ceftriaxone versus long‐acting (oily) chloramphenicol, outcome: 1.1 Death

Forest plot of comparison: 1 Ceftriaxone versus long‐acting (oily) chloramphenicol, outcome: 1.2 Clinical failure

Forest plot of comparison: 1 Ceftriaxone versus long‐acting (oily) chloramphenicol, outcome: 1.3 Neurological sequelae
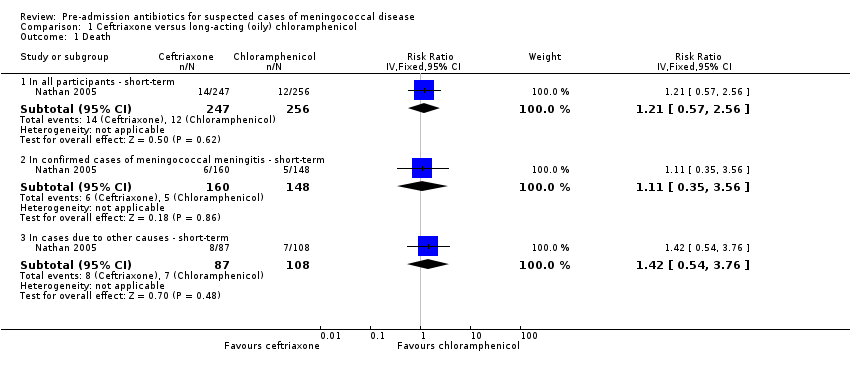
Comparison 1 Ceftriaxone versus long‐acting (oily) chloramphenicol, Outcome 1 Death.

Comparison 1 Ceftriaxone versus long‐acting (oily) chloramphenicol, Outcome 2 Clinical failure.

Comparison 1 Ceftriaxone versus long‐acting (oily) chloramphenicol, Outcome 3 Neurological sequelae.
| Ceftriaxone versus long‐acting (oily) chloramphenicol in people suspected to have meningococcal disease | ||||||
| Patient or population: people suspected to have meningococcal disease (adults and children) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Long‐acting (oily) chloramphenicol | Intramuscular ceftriaxone | |||||
| Death ‐ in all participants ‐ short term | 47 per 10001 | 57 per 1000 | RR 1.21 | 503 | ⊕⊕⊕⊝ | All outcomes in this table are from 1 trial that randomised 510 participants to either intervention (Nathan 2005) |
| Death ‐ in confirmed cases of meningococcal meningitis (subgroup) | 34 per 10001 | 38 per 1000 | RR 1.11 | 308 | ⊕⊕⊕⊝ | |
| Clinical failure ‐ in all participants ‐ short term | 41 per 10001 | 34 per 1000 | OR 0.83 | 477 | ⊕⊕⊕⊝ | |
| Clinical failure ‐ in confirmed cases of meningococcal meningitis (subgroup) | 14 per 10001 | 19 per 1000 | OR 1.39 | 308 | ⊕⊕⊕⊝ | |
| Neurological sequelae ‐ in all participants‐ short term | 53 per 10001 | 68 per 1000 | RR 1.29 | 477 | ⊕⊕⊝⊝ | |
| Neurological sequelae ‐ in confirmed cases of meningococcal meningitis (subgroup) | 63 per 10001 | 91 per 1000 | RR 1.44 | 297 | ⊕⊝⊝⊝ | |
| Adverse events ‐ short term | See comment | See comment | Not estimable | 0 | See comment | No adverse events were detected with either intervention |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Basis of the assumed risk: control group risk. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Death Show forest plot | 1 | Risk Ratio (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 In all participants ‐ short‐term | 1 | 503 | Risk Ratio (IV, Fixed, 95% CI) | 1.21 [0.57, 2.56] |
| 1.2 In confirmed cases of meningococcal meningitis ‐ short‐term | 1 | 308 | Risk Ratio (IV, Fixed, 95% CI) | 1.11 [0.35, 3.56] |
| 1.3 In cases due to other causes ‐ short‐term | 1 | 195 | Risk Ratio (IV, Fixed, 95% CI) | 1.42 [0.54, 3.76] |
| 2 Clinical failure Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 In all participants ‐ short‐term | 1 | 477 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.32, 2.15] |
| 2.2 In confirmed cases of meningococcal meningitis ‐ short‐term | 1 | 308 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.39 [0.23, 8.47] |
| 2.3 In cases due to other causes ‐ short‐term | 1 | 169 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.25, 2.58] |
| 3 Neurological sequelae Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 In all participants ‐ short‐term | 1 | 477 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.63, 2.62] |
| 3.2 In confirmed cases of meningococcal meningitis ‐ short‐term | 1 | 297 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.44 [0.65, 3.23] |
| 3.3 In cases due to other causes ‐ short‐term | 1 | 180 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.12, 3.40] |

