为疑似脑膜炎球菌病患者在入院前使用抗生素
Appendices
Appendix 1. Details of previous searches
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) (the Cochrane Library) (2007, Issue 1 and 2013, Issue 5), MEDLINE (1966 to February 2007 and to May 2013) and EMBASE (1980 to February 2007 and to May 2013), and handsearched the references of all identified studies.
The following search terms were run over MEDLINE and CENTRAL. The MEDLINE search was combined with the search strategy designed by The Cochrane Collaboration for identifying randomised controlled trials. See Appendix 3 for the EMBASE search strategy.
MEDLINE (Ovid)
1 exp MENINGOCOCCAL INFECTIONS/
2 exp Neisseria meningitides/
3 (neisseria adj mening$).mp.
4 meningococ$.mp
5 or/1‐4
6 exp Anti‐bacterial Agents/
7 (antibiotic$ or penicillin or cefotaxime or ampicillin$ or sulfa$ or ciprofloxacin$ or norfloxaci$ or ofloxaci$ or quinol$ or fluoroquinol$ or fluoro‐quinolon$ or ceftriaxon$ or rifampi$ or azithromyci$ or minocyclin$ or macrolid$ or cephalospori$.).mp.
8 or/6‐7
9 exp Patient Admission/
10 (preadmission or pre‐admission).mp.
11 empiric.mp.
12 or/9‐11
13 5 and 8 and 12
Embase.com
#16 #6 and #10 and #15
#15 #11 or #12 or #13 or #14
#14 (empiric in ti) or (empiric in ab)
#13 (preadmission or pre‐admission)in ab
#12 (preadmission or pre‐admission)in ti
#11 explode 'hospital‐admission' / all subheadings in DEM,DER,DRM,DRR
#10 #7 or #8 or #9
#9 (antibiotic* or penicillin or cefotaxime or ampicilli* or sulfa* or ciprofloxacin* or norfloxaci* or ofloxaci* or quinol* or fluoroquinol* or fluoro‐quinolon* or ceftriaxon* or rifampi* or azithromyci* or minocyclin* or macrolid* or cephalospori*) in ab
#8 (antibiotic* or penicillin or cefotaxime or ampicilli* or sulfa* or ciprofloxacin* or norfloxaci* or ofloxaci* or quinol* or fluoroquinol* or fluoro‐quinolon* or ceftriaxon* or rifampi* or azithromyci* or minocyclin* or macrolid* or cephalospori*) in ti
#7 'antibiotic‐agent' / all subheadings in DEM,DER,DRM,DRR
#6 #1 or #2 or #3 or #4 or #5
#5 (neisseria adj mening*) in ab
#4 (neisseria adj mening*) in ti
#3 explode 'Neisseria‐meningitidis' / all subheadings in DEM,DER,DRM,DRR
#2 (meningococcal infection* in ti) or (meningococcal infection* in ab)
#1 explode 'meningococcosis‐' / all subheadings in DEM,DER,DRM,DRR
Appendix 2. MEDLINE (Ovid) search strategy
1 exp Meningococcal Infections/
2 exp Neisseria meningitidis/
3 (neisseria adj2 mening*).tw.
4 meningococc*.tw.
5 Meningitis/
6 meningit*.tw.
7 or/1‐6
8 exp Anti‐Bacterial Agents/
9 antibiotic*.tw,nm.
10 (penicillin* or cefotaxim* or ampicillin* or sulfa* or ciprofloxacin* or norfloxacin* or ofloxacin* or quinol* or fluoroquinol* or fluoro‐quinol* or ceftriaxon* or rifampi* or azithromyci* or minocyclin* or macrolid* or cephalosporin*).tw,nm.
11 or/8‐10
12 Patient Admission/
13 (patient* adj2 (admis* or admit*)).tw.
14 ((pre or before or prior or previous) adj2 hospital*).tw.
15 ((previous or prior or before) adj2 (admit* or admiss*)).tw.
16 (preadmit* or pre admit* or pre‐admit* or preadmiss* or pre admiss* or pre‐admiss*).tw.
17 empiric.tw.
18 or/12‐17
19 7 and 11 and 18
Appendix 3. Embase.com search strategy
#31 #22 AND #30
#30 #25 NOT #29
#29 #26 NOT #28
#28 #26 AND #27
#27 'human'/de
#26 'nonhuman'/de OR 'animal'/de OR 'animal experiment'/de
#25 #23 OR #24
#24 random*:ab,ti OR placebo*:ab,ti OR crossover*:ab,ti OR 'cross over':ab,ti OR allocat*:ab,ti OR trial:ti OR (doubl* NEXT/1 blind*):ab,ti
#23 'randomized controlled trial'/exp OR 'single blind procedure'/exp OR 'double blind procedure'/exp OR 'crossover procedure'/exp
#22 #7 AND #21
#21 #11 AND #20
#20 #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19
#19 (emergenc* NEAR/3 treat*):ab,ti OR triage:ab,ti
#18 'emergency health service'/de
#17 empiric*:ab,ti
#16 preadmit*:ab,ti OR preadmis*:ab,ti OR (pre NEXT/1 (admit* OR admis*)):ab,ti
#15 ((previous OR prior OR before) NEAR/3 (admit* OR admis*)):ab,ti
#14 ((pre OR before OR prior OR previous) NEAR/5 hospital*):ab,ti
#13 ((hospital* OR patient*) NEAR/3 (admis* OR admit*)):ab,ti
#12 'hospital admission'/de
#11 #8 OR #9 OR #10
#10 penicillin*:ab,ti OR cefotaxim*:ab,ti OR ampicillin*:ab,ti OR sulfa*:ab,ti OR ciprofloxacin*:ab,ti OR norfloxacin*:ab,ti OR ofloxacin*:ab,ti OR quinol*:ab,ti OR fluoroquinol*:ab,ti OR fluoro‐quinol*:ab,ti OR ceftriaxon*:ab,ti OR rifampi*:ab,ti OR azithromyci*:ab,ti OR minocyclin*:ab,ti OR macrolid*:ab,ti OR cephalosporin*:ab,ti
#9 antibiotic*:ab,ti
#8 'antibiotic agent'/exp
#7 #1 OR #2 OR #3 OR #4 OR #5 OR #6
#6 meningococc*:ab,ti OR meningit*:ab,ti OR (neisseria NEAR/2 mening*):ab,ti OR 'n. meningitidis':ab,ti
#5 'neisseria meningitidis'/de
#4 'meningitis'/de
#3 'bacterial meningitis'/de
#2 'epidemic meningitis'/de
#1 'meningococcosis'/exp
Appendix 4. Web of Science (Thomson Reuters) search strategy
| # 3 | #2 AND #1 Databases=SCI‐EXPANDED, CPCI‐S, CCR‐EXPANDED, IC Timespan=1985‐2012 Lemmatization=Off |
| # 2 | Title=(trial) OR Topic=(random* or placebo* or ((singl* or doubl*) NEAR/1 blind*)) Databases=SCI‐EXPANDED, CPCI‐S, CCR‐EXPANDED, IC Timespan=1985‐2012 Lemmatization=Off |
Appendix 5. LILACS (BIREME) search strategy
Search > (MH:"Meningococcal Infections" OR "Infecciones Meningocócicas" OR "Infecções Meningocócicas" OR MH:C01.252.400.625.549$ OR MH:"Neisseria meningitidis" OR MH:B03.440.400.425.550.550.641$ OR MH:B03.660.075.525.520.500$ OR "Neisseria meningitidis" OR "N. meningitidis" OR meningit$ OR meningococ$ OR MH:Meningitis OR MH:"Meningitis, Bacterial" OR "Meningitis Bacteriana" OR "Meningite Bacteriana" OR "Bacterial Meningitis" OR MH:"Meningitis, Meningococcal" OR "Meningitis Meningocócica" OR "Meningite Meningocócica" OR "Meningitis Meningocóccica" OR "Meningite Meningocóccica") AND (MH:"Anti‐Bacterial Agents" OR antibacter$ OR antibiotic$ OR Antibióticos OR Antibacterianos OR MH:D27.505.954.122.085$ OR penicillin$ OR cefotaxim$ OR ampicillin$ OR sulfa$ OR ciprofloxacin$ OR norfloxacin$ OR ofloxacin$ OR quinol$ OR fluoroquinol$ OR fluoro‐quinol$ OR ceftriaxon$ OR rifampi$ OR azithromycin$ minocyclin$ OR macrolid$ OR cephalosporin$) > clinical_trials
Appendix 6. CAB Abstracts (Thomson Reuters) search strategy
| # 3 | #2 AND #1 Databases=CAB Abstracts Timespan=1985‐2012 Lemmatization=Off |
| # 2 | Title=(trial) AND Topic=((random* or placebo* or ((singl* or doubl*) NEXT/1 blind*))) Databases=CAB Abstracts Timespan=1985‐2012 Lemmatization=Off |
| # 1 | Topic=((meningococcal OR "Neisseria meningitidis" OR "N. meningitidis" OR meningitis)) AND Topic=(antibiotic* OR penicillin* OR cefotaxim* OR ampicillin* OR sulfa* OR ciprofloxacin* OR norfloxacin* OR ofloxacin* OR quinol* OR fluoroquinol* OR fluoro‐quinol* OR ceftriaxon* OR rifampi* OR azithromycin* OR minocyclin* OR macrolid* OR cephalosporin*) Databases=CAB Abstracts Timespan=1985‐2012 Lemmatization=Off |

Study flow diagram

'Risk of bias' summary: review authors' judgements about each risk of bias item for Nathan 2005.

Forest plot of comparison: 1 Ceftriaxone versus long‐acting (oily) chloramphenicol, outcome: 1.1 Death

Forest plot of comparison: 1 Ceftriaxone versus long‐acting (oily) chloramphenicol, outcome: 1.2 Clinical failure

Forest plot of comparison: 1 Ceftriaxone versus long‐acting (oily) chloramphenicol, outcome: 1.3 Neurological sequelae
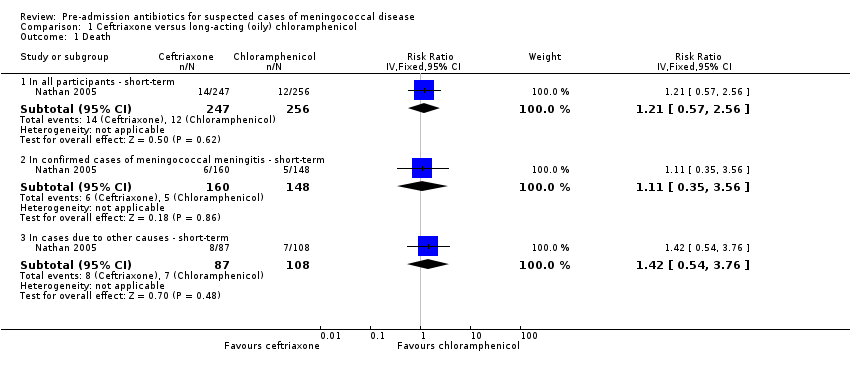
Comparison 1 Ceftriaxone versus long‐acting (oily) chloramphenicol, Outcome 1 Death.

Comparison 1 Ceftriaxone versus long‐acting (oily) chloramphenicol, Outcome 2 Clinical failure.

Comparison 1 Ceftriaxone versus long‐acting (oily) chloramphenicol, Outcome 3 Neurological sequelae.
| Ceftriaxone versus long‐acting (oily) chloramphenicol in people suspected to have meningococcal disease | ||||||
| Patient or population: people suspected to have meningococcal disease (adults and children) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Long‐acting (oily) chloramphenicol | Intramuscular ceftriaxone | |||||
| Death ‐ in all participants ‐ short term | 47 per 10001 | 57 per 1000 | RR 1.21 | 503 | ⊕⊕⊕⊝ | All outcomes in this table are from 1 trial that randomised 510 participants to either intervention (Nathan 2005) |
| Death ‐ in confirmed cases of meningococcal meningitis (subgroup) | 34 per 10001 | 38 per 1000 | RR 1.11 | 308 | ⊕⊕⊕⊝ | |
| Clinical failure ‐ in all participants ‐ short term | 41 per 10001 | 34 per 1000 | OR 0.83 | 477 | ⊕⊕⊕⊝ | |
| Clinical failure ‐ in confirmed cases of meningococcal meningitis (subgroup) | 14 per 10001 | 19 per 1000 | OR 1.39 | 308 | ⊕⊕⊕⊝ | |
| Neurological sequelae ‐ in all participants‐ short term | 53 per 10001 | 68 per 1000 | RR 1.29 | 477 | ⊕⊕⊝⊝ | |
| Neurological sequelae ‐ in confirmed cases of meningococcal meningitis (subgroup) | 63 per 10001 | 91 per 1000 | RR 1.44 | 297 | ⊕⊝⊝⊝ | |
| Adverse events ‐ short term | See comment | See comment | Not estimable | 0 | See comment | No adverse events were detected with either intervention |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Basis of the assumed risk: control group risk. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Death Show forest plot | 1 | Risk Ratio (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 In all participants ‐ short‐term | 1 | 503 | Risk Ratio (IV, Fixed, 95% CI) | 1.21 [0.57, 2.56] |
| 1.2 In confirmed cases of meningococcal meningitis ‐ short‐term | 1 | 308 | Risk Ratio (IV, Fixed, 95% CI) | 1.11 [0.35, 3.56] |
| 1.3 In cases due to other causes ‐ short‐term | 1 | 195 | Risk Ratio (IV, Fixed, 95% CI) | 1.42 [0.54, 3.76] |
| 2 Clinical failure Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 In all participants ‐ short‐term | 1 | 477 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.32, 2.15] |
| 2.2 In confirmed cases of meningococcal meningitis ‐ short‐term | 1 | 308 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.39 [0.23, 8.47] |
| 2.3 In cases due to other causes ‐ short‐term | 1 | 169 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.25, 2.58] |
| 3 Neurological sequelae Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 In all participants ‐ short‐term | 1 | 477 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.63, 2.62] |
| 3.2 In confirmed cases of meningococcal meningitis ‐ short‐term | 1 | 297 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.44 [0.65, 3.23] |
| 3.3 In cases due to other causes ‐ short‐term | 1 | 180 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.12, 3.40] |

