Restricción de líquidos para el tratamiento de lactantes prematuros con enfermedad pulmonar crónica
Información
- DOI:
- https://doi.org/10.1002/14651858.CD005389.pub2Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 08 febrero 2017see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Neonatología
- Copyright:
-
- Copyright © 2017 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
KB designed the protocol and wrote the first draft.
All three authors reviewed the articles found by the literature search.
All three authors edited the review.
Sources of support
Internal sources
-
No sources of support supplied
External sources
-
Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Department of Health and Human Services, USA.
Editorial support of the Cochrane Neonatal Review Group has been funded with Federal funds from the Eunice Kennedy Shriver National Institute of Child Health and Human Development National Institutes of Health, Department of Health and Human Services, USA, under Contract No. HHSN275201600005C
Declarations of interest
None.
Version history
| Published | Title | Stage | Authors | Version |
| 2017 Feb 08 | Fluid restriction for treatment of preterm infants with chronic lung disease | Review | Keith J Barrington, Etienne Fortin‐Pellerin, Thomas Pennaforte | |
| 2005 Jul 20 | Fluid restriction for treatment of preterm babies with chronic lung disease | Protocol | Keith J Barrington, Fahad N Al‐Hazzani | |
Differences between protocol and review
The search strategy was updated to include a search of CINAHL and clinical trial registries.
The methods for the assessment of risk of bias were altered to match current Cochrane standards.
We added the methodology and plan for 'Summary of findings' tables and GRADE recommendations, which were not included in the original protocol.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
- Apnea [epidemiology];
- Bronchopulmonary Dysplasia [*therapy];
- Chronic Disease;
- Fluid Therapy [*methods];
- Infant, Premature;
- Length of Stay [statistics & numerical data];
- Lung Diseases [therapy];
- Oxygen Inhalation Therapy;
- Randomized Controlled Trials as Topic;
- Respiration, Artificial [statistics & numerical data];
- Weight Gain;
Medical Subject Headings Check Words
Humans; Infant, Newborn;
PICO

Study flow diagram.

Comparison 1 Fluid restricted compared to liberal fluids, Outcome 1 Duration of hospitalisation.
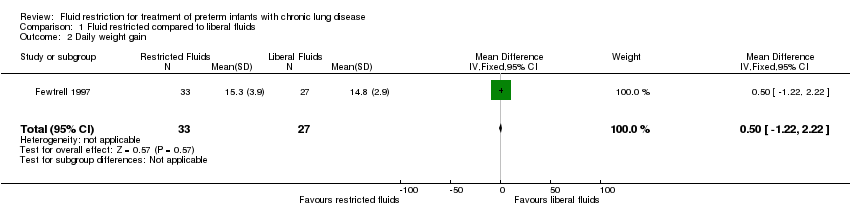
Comparison 1 Fluid restricted compared to liberal fluids, Outcome 2 Daily weight gain.
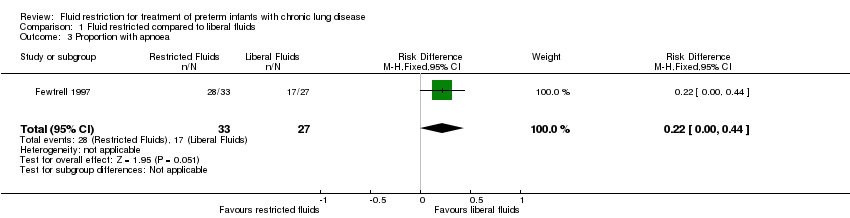
Comparison 1 Fluid restricted compared to liberal fluids, Outcome 3 Proportion with apnoea.
| Fluid restricted compared to liberal fluids compared to placebo for treatment of preterm babies with chronic lung disease | ||||||
| Participant or population: treatment of preterm babies with chronic lung disease | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with placebo | Risk with fluid restricted compared to liberal fluids | |||||
| Duration of oxygen therapy | The mean duration of oxygen therapy was 27.5 days | median 0.5 days higher | ‐ | 60 | ⊕⊕⊝⊝ | |
| Duration of hospitalisation | The mean duration of hospitalisation was 85 days | MD 3 Days higher | ‐ | 60 | ⊕⊕⊝⊝ | |
| Daily weight gain | The mean daily weight gain was 14.8 g/kg/d | MD 0.5 g/kg/d higher | ‐ | 60 | ⊕⊕⊝⊝ | |
| Proportion with apnoea | Study population | RR 1.35 | 60 | ⊕⊕⊝⊝ | ||
| 630 per 1000 | 850 per 1000 | |||||
| Moderate | ||||||
| 630 per 1000 | 850 per 1000 | |||||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Unmasked study 2 Wide confidence limits | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Duration of hospitalisation Show forest plot | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 3.0 [‐7.64, 13.64] |
| 2 Daily weight gain Show forest plot | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.5 [‐1.22, 2.22] |
| 3 Proportion with apnoea Show forest plot | 1 | 60 | Risk Difference (M‐H, Fixed, 95% CI) | 0.22 [‐0.00, 0.44] |

