Profilaxis con antibióticos para los pacientes sometidos a colecistectomía laparoscópica electiva
Referencias
Referencias de los estudios incluidos en esta revisión
Referencias de los estudios excluidos de esta revisión
Referencias adicionales
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | Randomised clinical trial with a parallel design. Generation of the allocation sequence: unclear. Allocation concealment: unclear. Blinding: not performed. Outcome assessment was made by surgeons of the treatment group, but it was not stated if they were blinded. Follow‐up: adequate; without lost to follow‐up at 15 days. Intention‐to‐treat analysis: no. Sample size calculations: no. | |
| Participants | 105 patients randomised (50 for antibiotic group and 55 for no‐antibiotic group). Sex: 32 men/73 women. Mean age 48.1 to 49.3. Age of inclusion not reported. Inclusion criteria: clinical diagnosis of chronic cholecystitis undergoing elective cholecystectomy. Exclusions criteria: conversion to open surgery and acute cholecystitis. Trial duration: 14 months. | |
| Interventions | Intravenous single cefazolin dose, 1 g given at the time of induction compared with no antibiotic in the control group. | |
| Outcomes | Surgical site infection determined by pus drainage and extra‐abdominal infections. | |
| Notes | Contacted authors April 9/2007. It was impossible to get more information about excluded patients because data were not available. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear. "Randomly distributed into two groups", "Se dividieron en dos grupos al azar". |
| Allocation concealment? | Unclear risk | Unclear. "Randomly distributed into two groups", "Se dividieron en dos grupos al azar". |
| Blinding? | High risk | "Patients were controlled during hospital stay and then, 15 days after discharge in an out‐patient setting, recording data about infection", "Los pacientes fueron controlados en su estadia intrahospitalaria y luego hasta 15 dias en forma ambulatoria consignando la presencia de cualquier infeccion" |
| Incomplete outcome data addressed? | High risk | Per protocol analysis. |
| Vested interest bias | Unclear risk | It is not possible to say if there were or there were not any financial interests. |
| Methods | Randomised clinical trial with a parallel design. Generation of the allocation sequence: unclear. Allocation concealment: adequate; sealed envelopes. Blinding: single‐blind. Outcome assessment was made by surgeons of the treatment group blind to intervention. Follow‐up: adequate: without lost to follow‐up (time not stated). Intention‐to‐treat analysis: no. Sample size calculations: no. | |
| Participants | 76 patients (39 for antibiotic group and 37 for no‐antibiotic group). Sex distribution not reported. Mean age not reported. Age of inclusion not reported. Inclusion criteria: patients undergoing elective cholecystectomy. Exclusions criteria: need to carry out cholangiography, concomitant usage of antibiotics, spillage of gallbladder content, and conversion to open surgery. Trial duration: 14 months. | |
| Interventions | Intravenous single cefuroxime dose, 750 mg given at the time of induction compared with removing of the gallbladder with a plastic bag. | |
| Outcomes | Surgical site infection determined by pus drainage. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear."Patients ... were randomised to receive...". |
| Allocation concealment? | Low risk | "Randomisation ... was done using a sealed envelope technique". |
| Blinding? | Low risk | "An infection control sister followed up the patients". |
| Incomplete outcome data addressed? | High risk | Per protocol analysis. |
| Vested interest bias | Unclear risk | It is not possible to say if there were or there were not financial interests. |
| Methods | Randomised clinical trial with a parallel design. Generation of the allocation sequence: unclear. Allocation concealment: adequate; sealed envelopes. Blinding: double‐blind. Use of placebo. Outcome assessment was made by surgeons of the treatment group blind to intervention. Follow‐up: adequate: without lost to follow‐up at 30 days. Intention‐to‐treat analysis: no. Sample size calculations: yes. Power of 80%, alfa error of 5%. | |
| Participants | 412 patients (277 for antibiotics groups and 135 for placebo). Sex distribution not reported. Mean age: 47.1 to 48.2. Age of inclusion 18 to 80. Inclusion criteria: patients scheduled to elective cholecystectomy. Exclusions criteria: pregnant or lactating women, antibiotic allergy, previous antibiotic therapy, acute cholecystitis or cholangitis, jaundice, previous biliary surgery, choledocholithiasis, prosthetic valves, contraindication to laparoscopy. Trial duration: 25 months. | |
| Interventions | Intravenous single cefotetan and cefazolin dose, 1 g each one given at the time of induction compared with placebo. | |
| Outcomes | Surgical site infection determined by pus drainage and extra‐abdominal infections "any infection remote to the surgical site". | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear. |
| Allocation concealment? | Low risk | Sealed envelopes. |
| Blinding? | Low risk | Outcome assessment was made by surgeons of the treatment group blind to intervention. |
| Incomplete outcome data addressed? | Low risk | Per protocol analysis. |
| Vested interest bias | Unclear risk | It is not possible to say if there were or there were not financial interests. |
| Methods | Randomised clinical trial with a parallel design. Generation of the allocation sequence: unclear. Allocation concealment: unclear. Blinding: not performed. Outcome assessment was made by surgeons of the treatment group, but it was not stated whether they were blinded. Follow‐up: adequate. Lost to follow‐up of 2.4% at 30 days. Intention‐to‐treat analysis: yes. Sample size calculations: yes. Power of 80%, alfa error of 5%, absolute difference to identify of 4%. | |
| Participants | 250 patients (128 for antibiotic group and 122 for no‐antibiotic group). Sex: 47 men/203 women. Mean age: 46.5 to 47.3. Age of inclusion not reported. Inclusion criteria: patients undergoing elective cholecystectomy. Exclusions criteria: acute cholecystitis, obstructive jaundice, immunosuppression, pregnancy, artificial device or graft in place, use of antibiotics 7 days prior to surgery. Trial duration: 25 months. | |
| Interventions | Intravenous preoperative dose of cefazolin, 1 g given at the time of induction and followed by two doses at 8 and 16 hours postoperatively compared with no antibiotic in the control group. | |
| Outcomes | Surgical site infection determined by pus drainage and extra‐abdominal infections "Lower urinary tract infection (UTIs, with symptomatic bacteriuria)...and any other problem with local effects only ...". | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Patients were randomised to receive prophylactic antibiotics. |
| Allocation concealment? | Unclear risk | Patients were randomised to receive prophylactic antibiotics. |
| Blinding? | High risk | Complications were reported to the investigators as they occurred. |
| Incomplete outcome data addressed? | Low risk | Intention to treat analysis. |
| Vested interest bias | Unclear risk | It is not possible to say if there were or there were not financial interests. |
| Methods | Randomised clinical trial with a parallel design. Generation of the allocation sequence: unclear. Allocation concealment: unclear. Blinding: yes. Use of placebo. Outcome assessment was made by surgeons of the treatment group, blinded to the interventions. Follow‐up: adequate. No losses to follow up. Intention‐to‐treat analysis: no. Sample size calculations: no. | |
| Participants | 92 patients (49 for antibiotic group and 43 for no‐antibiotic group). Sex: 33 men/59 women. Mean age: 47.5 to 50.1. Age of inclusion reported as adults. Inclusion criteria: patients undergoing elective cholecystectomy. Exclusions criteria: antibiotic allergy, acute cholecystitis, previous biliary surgery, obstructive jaundice, immunosuppression, artificial device or graft in place, use of antibiotics 7 days prior to surgery. Trial duration: 12 months. | |
| Interventions | Intravenous preoperative dose of cefotaxime, 2 g given at the time of induction and followed by a dose at 24 hours postoperatively compared with placebo in the control group. | |
| Outcomes | Surgical site infection determined by pus drainage. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | After the patients were confirmed for the study, they were randomised.... |
| Allocation concealment? | Unclear risk | After the patients were confirmed for the study, they were randomised... . |
| Blinding? | Low risk | Both the surgical team and the patients were blinded to the groups. |
| Incomplete outcome data addressed? | High risk | Per protocol analysis. |
| Vested interest bias | Unclear risk | It is not possible to say if there were or there were not financial interests. |
| Methods | Randomised clinical trial with a parallel design. Generation of the allocation sequence: Random number table. Allocation concealment: unclear. Blinding: yes. Use of placebo. Outcome assessment was made by residents of the treatment group, blinded to the interventions. Follow‐up: adequate. No losses to follow up. Intention‐to‐treat analysis: No. Sample size calculations: reported, but not clear. | |
| Participants | 93 patients (40 for antibiotic group and 53 for no‐antibiotic group). Sex: 25 men/68 women. Mean age: 42.04 to 42.38. Age of inclusion reported as adults. Inclusion criteria: patients undergoing elective cholecystectomy. Exclusions criteria: antibiotic allergy, acute cholecystitis, previous biliary surgery, obstructive jaundice, immunosuppression, artificial device or graft in place, use of antibiotics 7 days prior to surgery, conversion to open surgery. Trial duration: 12 months. | |
| Interventions | Intravenous preoperative dose of cefotaxime, 1.5 g given at the time of induction compared with placebo in the control group. | |
| Outcomes | Surgical site infection determined by pus drainage and extra‐abdominal infections (pyrexia of more than 38 C (excluding the first postoperative day), positive bacteriological culture from possible infection sites such as wounds, the urinary or respiratory tract..." | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Random number table. |
| Allocation concealment? | Unclear risk | Unclear. |
| Blinding? | Low risk | Outcome assessment was made by residents of the treatment group, blinded to the interventions. |
| Incomplete outcome data addressed? | High risk | Per protocol analysis. |
| Vested interest bias | Unclear risk | It is not possible to say that there were or there were not financial interests. |
| Methods | Randomised clinical trial with a parallel design. Generation of the allocation sequence: adequate. Allocation concealment: unclear. Blinding: no. Outcome assessment was made by surgeons of the treatment group, but it was not stated if they were blinded. Follow‐up: adequate. Lost to follow‐up of 0.9%. Intention‐to‐treat analysis: no. One patient who was lost to follow‐up was excluded from the analysis. Sample size calculations: no. | |
| Participants | 100 patients (50 for antibiotic group and 50 for no‐antibiotic group). Sex: 23 men/77 women. Mean age: 51 to 52.2. Age of inclusion: 15 to 80 years old. Inclusion criteria: patients undergoing elective cholecystectomy. Exclusions criteria: antibiotic allergy, acute cholecystitis, obstructive jaundice, immunosuppression, artificial device or graft in place, use of antibiotics 48 hours prior to surgery. Duration of treatment: 7 months. | |
| Interventions | Intravenous preoperative dose of cefazolin, 1 g given at the time of induction compared with no antibiotic in the control group. | |
| Outcomes | Surgical site infection determined by pus drainage. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | By block randomisation. |
| Allocation concealment? | Unclear risk | By block randomisation. |
| Blinding? | High risk | All patients were followed‐up for 30 days after the procedure at the out‐patient clinic or by telephone contact. |
| Incomplete outcome data addressed? | High risk | Per protocol analysis. |
| Vested interest bias | Unclear risk | It is not possible to say if there were or there were not financial interests. |
| Methods | Randomised clinical trial with a parallel design. Generation of the allocation sequence: adequate. Allocation concealment: adequate. Blinding: yes. Use of placebo. Outcome assessment was made by residents of the treatment group, blinded to the interventions. Follow‐up: adequate. Lost to follow‐up of 0%. Intention‐to‐treat analysis: no. Sample size calculations: no. | |
| Participants | 100 patients (50 for antibiotic group and 50 for no‐antibiotic group). Sex: 10 men/90 women. Mean age: 39 to 39. Age of inclusion: Older than 18 years old. Inclusion criteria: patients undergoing elective cholecystectomy for symptomatic gallstone disease. Exclusions criteria: jaundice, acute cholecystitis, cholangitis, acute pancreatitis or other acute inflammation, conversion to open cholecystectomy, immunosuppressive therapy, cardiac disorders mandating prophylactic use of antibiotics, or antibiotic use in the preceding seven days. Duration of treatment: not reported. | |
| Interventions | Intravenous preoperative dose of ceftriaxone, 1 g given at the time of induction compared with physiologic saline as placebo in the control group. | |
| Outcomes | "Superficial SSI was defined as erythema or purulent discharge at the surgical site above the deep fascia. A deep infection was defined as purulent material deep to the fascia or near the gallbladder fossa. A distant infection was defined as any infection remote from the surgical site." | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | "Anesthetist randomly opened one of a collection of sealed envelopes" |
| Allocation concealment? | Low risk | "Anesthetist randomly opened one of a collection of sealed envelopes", "The surgeon and medical staff were unaware of which treatment the patient received." |
| Blinding? | Low risk | "The surgeon and medical staff were unaware of which treatment the patient received." |
| Incomplete outcome data addressed? | High risk | Per protocol analysis. |
| Vested interest bias | Unclear risk | It is not possible to say if there were or there were not financial interests. |
| Methods | Randomised clinical trial with a parallel design. Generation of the allocation sequence: adequate. Computer randomisation. Allocation concealment: unclear. Blinding: yes. Use of placebo. Outcome assessment was made by surgeons of the treatment group who were blinded to the intervention. Follow‐up: adequate. No losses to follow up. Intention‐to‐treat analysis: no. One patient who was lost to follow‐up was excluded from the analysis. Sample size calculations: no. | |
| Participants | 84 patients (44 for antibiotic group and 40 for no‐antibiotic group). Sex: 33 men/51 women. Mean age: 49.5 to 53.6. Age of inclusion not stated. Inclusion criteria: patients undergoing elective cholecystectomy. Exclusions criteria: antibiotic allergy, acute cholecystitis, obstructive jaundice or choledocholithiasis, previous ERCP, corticosteroid therapy, use of antibiotics 7 days prior to surgery, conversion to open surgery. Trial duration: 2 years. | |
| Interventions | Intravenous preoperative dose of cefotaxime, 2 g given at the time of induction and followed by a dose at 24 hours postoperatively compared with placebo in the control group. | |
| Outcomes | Surgical site infection determined by pus drainage and extra‐abdominal infections "infectious complications were defined as pyrexia with a body temperature higher than 380 C twice a day (excluding the first postoperative day) and culture findings positive for pathogens from infectious sites such as wounds, the urinary or respiratory tract...". | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Compute‐matrix randomisation. |
| Allocation concealment? | Unclear risk | Compute‐matrix randomisation. |
| Blinding? | Low risk | Patients were followed postoperatively. |
| Incomplete outcome data addressed? | High risk | Per protocol analysis. |
| Vested interest bias | Unclear risk | It is not possible to say if there were or there were not financial interests. |
| Methods | Randomised clinical trial with a parallel design. Generation of the allocation sequence: adequate. Random selection. Allocation concealment: Closed envelopes. Blinding: No. Use of placebo. Outcome assessment was made by surgeons of the treatment group who were not blinded to the intervention. Follow‐up: adequate. No losses to follow up. Intention‐to‐treat analysis: no. Patients converted to open cholecystectomy were excluded Sample size calculations: no. | |
| Participants | 144 patients (68 for antibiotic group and 76 for no‐antibiotic group). Sex: 22 men/122 women. Mean age: 42.5 to 44.6. Age of inclusion not stated. Excluded patients older than 60 years Inclusion criteria: patients undergoing elective cholecystectomy. Exclusions criteria: patients older than 60 years; antibiotic intake in the 7 days prior to surgery; acute cholecystitis in the 6 months prior to the procedure; evidence of cholangitis and/or obstructive jaundice and biliary pancreatitis; regular corticosteroid therapy; pregnancy or lactation; previous biliary tract surgery or previous endoscopic retrograde cholangiopancreatography; presence of American Society of Anesthesiologists classification (ASA) higher than score II; evidence of diabetes mellitus; body mass index higher than 30; and conversion to open cholecystectomy. Trial duration: 3 years. | |
| Interventions | Intravenous preoperative dose of cefazolin, 1 g given at the time of induction compared with placebo in the control group. | |
| Outcomes | Surgical site infection determined by pus drainage and extra‐abdominal infections "number of septic complications". | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Random selection "One of 2 packages containing either cefazolin or placebo was chosen randomly by the anaesthesiologist for each patient." |
| Allocation concealment? | Low risk | Closed envelope method "The package was opened by the anaesthesiologist, and the type of solution administered (cefazolin or placebo) was recorded. The medical staff and the patient were unaware of the content of the solution." |
| Blinding? | Unclear risk | Patients were followed postoperatively by the same surgical group. |
| Incomplete outcome data addressed? | High risk | Per protocol analysis. |
| Vested interest bias | Unclear risk | It is not possible to say if there were or there were not financial interests. |
| Methods | Randomised clinical trial with a parallel design. Generation of the allocation sequence: adequate. Unclear. Allocation concealment: unclear. Blinding: yes. Use of placebo. Outcome assessment was made by surgeons of the treatment group who were blinded to the intervention. Follow‐up: adequate. No losses to follow up. Intention‐to‐treat analysis: no. One patient who was lost to follow‐up was excluded from the analysis. Sample size calculations: no. | |
| Participants | 208 patients (105 for antibiotic group and 103 for no‐antibiotic group). Sex: 58 men/150 women. Mean age: 49.9 to 51.3. Age of inclusion not stated. Inclusion criteria: patients undergoing elective cholecystectomy. Exclusions criteria: conversion to laparotomy, acute cholecystitis, evidence of obstructive jaundice, history of biliary tract surgery, prosthetic valves, chronic hepatic diseases, acute pancreatitis, immunosuppression, steroid therapy, chronic systemic infections, allergy to β‐lactam antibiotics. Trial duration: 3 years. | |
| Interventions | Intravenous preoperative dose of cefazolin, 1 g given at the time of induction compared with placebo in the control group. | |
| Outcomes | Surgical site infection determined by body temperature higher than 38°C, purulent discharge from the incisions, and any abdominal signs of infection. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear. |
| Allocation concealment? | Unclear risk | Unclear. |
| Blinding? | Low risk | "The patients were followed up regularly until the day of discharge and on 7th and 30th days postoperatively by the same surgical team (Dr. OA and Dr. EH) who were blind to AP." |
| Incomplete outcome data addressed? | High risk | Per protocol analysis. |
| Vested interest bias | Unclear risk | It is not possible to say if there were or there were not financial interests. |
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| Quasi‐randomised trial. Randomisation was performed after date of birth. | |
| Included patients with acute cholecystitis. | |
| Published as a theses. We could not obtain copy of the thesis. | |
| Comparative, not randomised trial. |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Surgical site infection Show forest plot | 11 | 1664 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.49, 1.54] |
| Analysis 1.1  Comparison 1 Antibiotic prophylaxis versus placebo or no‐prophylaxis, Outcome 1 Surgical site infection. | ||||
| 1.1 Per protocol analysis (Intention‐to‐treat analysis not performed) | 11 | 1664 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.49, 1.54] |
| 2 Extra‐abdominal infections Show forest plot | 7 | 1188 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.25, 1.74] |
| Analysis 1.2  Comparison 1 Antibiotic prophylaxis versus placebo or no‐prophylaxis, Outcome 2 Extra‐abdominal infections. | ||||
| 3 Surgical site infection (Intention‐to‐treat analysis worst‐best case scenario) Show forest plot | 11 | 1756 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.80 [1.75, 4.46] |
| Analysis 1.3  Comparison 1 Antibiotic prophylaxis versus placebo or no‐prophylaxis, Outcome 3 Surgical site infection (Intention‐to‐treat analysis worst‐best case scenario). | ||||
| 4 Surgical site infection (Intention‐to‐treat analysis best‐worst case scenario) Show forest plot | 11 | 1756 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.21, 0.54] |
| Analysis 1.4  Comparison 1 Antibiotic prophylaxis versus placebo or no‐prophylaxis, Outcome 4 Surgical site infection (Intention‐to‐treat analysis best‐worst case scenario). | ||||
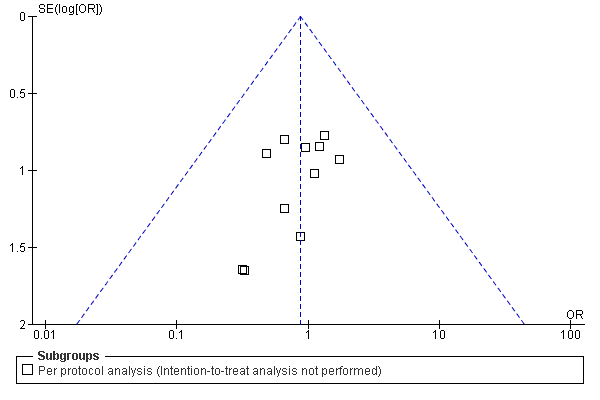
Funnel plot of comparison: 1 Antibiotic prophylaxis versus no‐prophylaxis, outcome: 1.1 Surgical site infection.

Funnel plot of comparison: 1 Antibiotic prophylaxis versus no‐prophylaxis, outcome: 1.2 Global infections.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Antibiotic prophylaxis versus placebo or no‐prophylaxis, Outcome 1 Surgical site infection.

Comparison 1 Antibiotic prophylaxis versus placebo or no‐prophylaxis, Outcome 2 Extra‐abdominal infections.

Comparison 1 Antibiotic prophylaxis versus placebo or no‐prophylaxis, Outcome 3 Surgical site infection (Intention‐to‐treat analysis worst‐best case scenario).

Comparison 1 Antibiotic prophylaxis versus placebo or no‐prophylaxis, Outcome 4 Surgical site infection (Intention‐to‐treat analysis best‐worst case scenario).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Surgical site infection Show forest plot | 11 | 1664 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.49, 1.54] |
| 1.1 Per protocol analysis (Intention‐to‐treat analysis not performed) | 11 | 1664 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.49, 1.54] |
| 2 Extra‐abdominal infections Show forest plot | 7 | 1188 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.25, 1.74] |
| 3 Surgical site infection (Intention‐to‐treat analysis worst‐best case scenario) Show forest plot | 11 | 1756 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.80 [1.75, 4.46] |
| 4 Surgical site infection (Intention‐to‐treat analysis best‐worst case scenario) Show forest plot | 11 | 1756 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.21, 0.54] |

