避免早產兒於母乳哺育建立期使用瓶餵牛奶
Abstract
Background
Preterm infants start milk feeds by gavage tube. As they mature, sucking feeds are gradually introduced. Women who choose to breast feed their preterm infant are not always able to be in hospital with their baby and need an alternative approach to feeding. Most commonly, milk (expressed breast milk or formula) is given by bottle. Whether using bottles during establishment of breast feeds is detrimental to breast feeding success is a topic of ongoing debate.
Objectives
To identify the effects of avoidance of bottle feeds during establishment of breast feeding on the likelihood of successful breast feeding, and to assess the safety of alternatives to bottle feeds.
Search methods
We used the standard search strategy of the Cochrane Neonatal Review Group to search the Cochrane Central Register of Controlled Trials (CENTRAL; 2016, Issue 2), MEDLINE via PubMed (1966 to July 2016), Embase (1980 to July 2016) and CINAHL (1982 to July 2016). We also searched databases of clinical trials and the reference lists of retrieved articles for randomised controlled trials and quasi‐randomised trials.
Selection criteria
Randomised and quasi‐randomised controlled trials comparing avoidance of bottles with use of bottles in women who have chosen to breast feed their preterm infant.
Data collection and analysis
Two review authors independently assessed trial quality and extracted data. When appropriate, we contacted study authors for additional information. Review authors used standard methods of The Cochrane Collaboration and the Cochrane Neonatal Review Group.
Main results
We included seven trials with 1152 preterm infants. Five studies used a cup feeding strategy, one used a tube feeding strategy and one used a novel teat when supplements to breast feeds were needed. We included the novel teat study in this review, as the teat was designed to more closely mimic the sucking action of breast feeding. The trials were of small to moderate size, and two had high risk of attrition bias. Adherence with cup feeding was poor in one of the studies, indicating dissatisfaction with this method by staff and/or parents; the remaining four cup feeding studies provided no such reports of dissatisfaction or low adherence. Meta‐analyses provided evidence of low to moderate quality indicating that avoiding bottles increases the extent of breast feeding on discharge home (full breast feeding typical risk ratio (RR) 1.47, 95% confidence interval (CI) 1.19 to 1.80; any breast feeding RR 1.11, 95% CI 1.06 to 1.16). Limited available evidence for three months and six months post discharge shows that avoiding bottles increases the occurrence of full breast feeding and any breast feeding at discharge and at six months post discharge, and of full (but not any) breast feeding at three months post discharge. This effect was evident at all time points for the tube alone strategy and for all except any breast feeding at three months post discharge for cup feeding. Investigators reported no clear benefit when the novel teat was used. No other benefits or harms were evident, including, in contrast to the previous (2008) review, length of hospital stay.
Authors' conclusions
Evidence of low to moderate quality suggests that supplementing breast feeds by cup increases the extent and duration of breast feeding. Current insufficient evidence provides no basis for recommendations for a tube alone approach to supplementing breast feeds.
PICO
Plain language summary
避免早產兒於母乳哺育建立期使用瓶餵牛奶。
文獻回顧問題:欲哺育母乳之早產兒母親使用瓶餵牛奶是否會影響母乳哺餵的成功率?背景:早產兒出生後初始可藉由管路途徑服用牛奶,隨著生理機能的成熟,他們可駕馭利用吸吮的方式進食。因著早產兒生長逐漸成熟,每一次吸吮牛奶的量也會顯著增加。然而當選擇母乳哺育的婦女無法在早產兒需要母乳提供親餵時,按照慣例會使用瓶裝母乳或是配方奶取代親餵。然而此方法被認為可以會影響母乳哺育的成功率。研究特徵:作者搜尋了截至2016年7月的文獻,最終找到7個符合的文獻(共含1152名早產兒),皆為小型至中型的研究,多數在研究設計及執行上有缺失。主要結果:其中5個研究(包含2個最大的研究)使用杯子哺餵,其中1個使用管路餵食牛奶。1個研究利用特別設計的奶嘴來哺餵並建議此方法比瓶餵更趨近於親餵母乳。大多的研究執行於高收入的國家中,僅有2個研究在中等收入的國家內,此研究並無於低收入國家中進行。總體來說,若無使用瓶餵牛奶(搭配傳統奶嘴),早產兒較可能於出院後完全或至少出院第三及第六個月後部分接受母乳親餵。在哺乳結果上,使用特殊設計奶嘴的研究並無顯示出差異,單獨利用杯子或是管路進食才能改善母乳哺餵的成功率。然而,由於單獨使用管路方式哺餵之研究品質不佳,在有更高證據等級的研究發表前,我們無法推薦使用管路進食策略。我們並沒有於任何報告結果中發現和益處、壞處相關的證據,包括住院天數的長短或是體重增加的影響。
結論:使用杯子取代瓶餵牛奶可增加早產兒母乳哺育的程度以及持續時間。需要進行更多研究,才能確認能否推薦單獨使用管道餵養的方式。
Authors' conclusions
Summary of findings
| Breast feeding with supplemental feeds by other than bottle compared with breast feeding with supplemental feeds by bottle (all trials) in preterm infants | ||||||
| Patient or population: preterm infants | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | |
| Risk with breast feeding with supplemental feeds by bottle (all trials) | Risk with breast feeding with supplemental feeds by other than bottle | |||||
| Full breast feeding at discharge | Study population | RR 1.47 | 1074 | ⊕⊕⊝⊝ | ||
| 44 per 100 | 64 per 100 | |||||
| Full breast feeding at 3 months post discharge | Study population | RR 1.56 | 986 | ⊕⊕⊕⊝ | ||
| 36 per 100 | 57 per 100 | |||||
| Full breast feeding at 6 months post discharge | Study population | RR 1.64 | 887 | ⊕⊕⊝⊝ | ||
| 31 per 100 | 51 per 100 | |||||
| Any breast feeding at discharge | Study population | RR 1.11 | 1138 | ⊕⊕⊕⊝ | ||
| 79 per 100 | 88 per 100 | |||||
| Any breast feeding at 3 months post discharge | Study population | RR 1.31 | 1063 | ⊕⊝⊝⊝ | ||
| 60 per 100 | 78 per 100 | |||||
| Any breast feeding at 6 months post discharge | Study population | RR 1.25 | 886 | ⊕⊝⊝⊝ | ||
| 45 per 100 | 56 per 100 | |||||
| Length of hospital stay | MD 2.25 higher | ‐ | 1004 | ⊕⊝⊝⊝ | ||
| Episodes of infection | Study population | RR 0.70 | 500 | ⊕⊕⊕⊝ | ||
| 7 per 100 | 5 per 100 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| aAttrition bias (14% and 15% attrition in two included studies). bModerate heterogeneity (I2 = 52%). cModerate heterogeneity (I2 = 73%). dModerate heterogeneity (I2 = 71%). | ||||||
Background
Description of the condition
Preterm infants begin sucking feeds when they are mature enough to co‐ordinate sucking and swallowing; this occurs at around 32 to 34 weeks' gestation (Lemons 1996). Milk feeds are given through a gavage tube until infants are able to receive all their intake by sucking feeds. Once sucking feeds begin, they are increased gradually, usually beginning with once a day and increasing as the infant demands or is assessed as ready to progress. As the number of sucking feeds increases, the number of tube feeds decreases until sucking feeds alone provide sufficient intake for growth and development. It is not always possible for a mother to be available to breast feed during this transition time. Also at times after a breast feed, the infant is assessed as having received insufficient milk, and a 'top up' with expressed breast milk or formula is required. In these instances, it is common clinical practice for milk (breast or formula) to be given by bottle in addition to any breast feeds.
Description of the intervention
Alternatives to bottles during this transition time have been reported and include feeding the infant by cup (Lang 1994a), gavage tube (Stine 1990), finger feeding (Healow 1995; Kurokawa 1994), spoon (Aytekin 2014) and paladai ‐ a traditional feeding device used in India (Malhotra 1999). Increased breast feeding prevalence has been reported when bottle feeds were replaced by cup feeds (Abouelfettoh 2008; Gupta 1999; Lang 1994a) or tube feeds (Stine 1990), and infants have been reported to achieve all breast feeds sooner with spoon feeding (Aytekin 2014). However, these studies were small and did not include a control.
How the intervention might work
It has been suggested that using bottles may interfere with establishing successful breast feeding, possibly because of a difference in the sucking action required for the breast versus an artificial nipple (Bu'Lock 1990; Neifert 1995).
Why it is important to do this review
Alternatives to breast feeds are not necessarily benign. With both bottle feeds (Bier 1993; Blaymore Bier 1997; Chen 2000; Young 1995) and cup feeds (Dowling 2002; Freer 1999), mean oxygen saturation was lower and the frequency of oxygen desaturation was greater than with breast feeding, highlighting the importance of considering safety aspects of any alternatives to bottle feeds. Use of both cup and paladai has been associated with a tendency for infants to 'spill' a large proportion of the feed (Aloysius 2007; Dowling 2002). However, other studies have not reported problems associated with cup feeding (Gupta 1999; Lang 1994a).
Cups and similar feeding vessels are easier to clean than bottles and artificial teats; this fact may be of particular relevance for infection control in low‐ and middle‐income countries.
For women who wish to breast feed their preterm infant, it is important to establish the most efficacious and least harmful method of supplementing breast feeds.
Objectives
To identify the effects of avoidance of bottle feeds during establishment of breast feeding on the likelihood of successful breast feeding, and to assess the safety of alternatives to bottle feeds.
Methods
Criteria for considering studies for this review
Types of studies
All trials using random or quasi‐random participant allocation.
Types of participants
Infants born at less than 37 weeks' gestation whose mothers had chosen to breast feed, and who had not received 'sucking' feeds by bottle or any alternative feeding device at study entry. At enrolment, infants may have been receiving enteral feeds only, parenteral feeds only or a combination of parenteral and enteral feeds. Their enteral milk intake may have been provided via tube (using expressed breast milk and/or formula) or breast feeds. Tube feeds could be continuous or intermittent, and tube placement could be gastric or duodenal.
Types of interventions
-
Experimental intervention: complete avoidance of bottles during the transition to breast feeds. Instead of bottles, alternative feeding devices were used for complementing or supplementing breast feeds, including gavage tube, cup, spoon, dropper, finger feeding, paladai and other.
-
Control intervention: breast feeds complemented or supplemented with bottles during the transition to breast feeds.
Types of outcome measures
Primary outcomes
-
Full breast feeding compared with not breast feeding or partial breast feeding on discharge home and at three months and six months post discharge
-
Any breast feeding (full and partial combined) compared with not breast feeding on discharge home and at three months and six months post discharge
Secondary outcomes
-
Time (days) to reach full sucking feeds
-
Average rate of weight gain (g/d or g/kg/d) to discharge home
-
Length of hospital stay (days)
-
Duration (minutes) of supplementary or complementary feed
-
Volume of supplementary feed taken compared with volume prescribed (mL)
-
Cardiorespiratory stability during and after intervention (mean heart and respiratory rates; proportions of bradycardic and apnoeic events during feed; mean oxygenation measured by oximetry or transcutaneous monitor; proportion of hypoxic events during feed)
-
Episodes of choking/gagging per feed
-
Milk aspiration on radiological assessment
-
Parent/health professional satisfaction with feeding method as measured by self report
-
Episodes of infection per infant
Search methods for identification of studies
Electronic searches
We used the criteria and standard methods of The Cochrane Collaboration and the Cochrane Neonatal Review Group (see the Cochrane Neonatal Group search strategy for specialized register).
For the review update in 2016, we conducted a comprehensive search in July 2016 that included the Cochrane Central Register of Controlled Trials (CENTRAL; 2016, Issue 2) in The Cochrane Library; MEDLINE via PubMed; Embase; and the Cumulative Index to Nursing and Allied Health Literature (CINAHL), using the following search terms: (cup feed* OR (cup AND feed) OR cupfeed* OR gavage OR (tube AND feed*) OR spoon OR dropper OR (finger AND feed*) OR paladai), plus database‐specific limiters for randomised controlled trials (RCTs) and neonates (see Appendix 1 for the full search strategies for each database). We applied no language restrictions.
We searched clinical trials registries for ongoing and recently completed trials (clinicaltrials.gov; the World Health Organization International Trials Registry and Platform www.whoint/ictrp/search/en/ and the ISRCTN Registry).
For the 2008 review, we conducted computerised searches of the Cochrane Central Register of Controlled Trials (CENTRAL; 2007, Issue 4) in The Cochrane Library; MEDLINE (1950 to July week 1 2008); the Cumulative Index to Nursing and Allied Health Literature (CINAHL; 1982 to July week 1 2008); and Embase (1980 to 2008 week 28), using medical subject headings (MeSH): breastfeeding; Milk, human; Lactation; Bottle Feeding; Intubation, Gastrointestinal. We used the following text words: Neonat$, Cup, Cup Feed*, Cupfeed*, Gavage, Gavage feed*, Tube feed*, Spoon, Dropper, Finger Feed*, Palada*. We did not restrict the search by language.
Searching other resources
We checked the bibliographies of published trials.
Data collection and analysis
We used standard methods of the Cochrane Neonatal Review Group.
Selection of studies
We merged search results from different databases, using reference management software, and we removed duplicates. One review author (CC) screened titles and abstracts and removed obviously irrelevant reports. Three review authors (CC, HS, JG) independently reviewed the abstracts of potentially relevant reports. When uncertainty about inclusion of the study arose, we retrieved the full text. Review authors (CC, HS, JG) resolved disagreements on inclusion of studies.
Data extraction and management
Once inclusion of trials was established, two review authors (CC, HS) independently assessed trial methods; extracted data onto paper forms; assessed trial quality; and discussed and resolved disagreements (CC, HS).
For the review updated in 2016, we requested additional information from Garpiel 2012 (only abstract available) and from Yilmaz 2014 (gestational age category used in stratification) but received no response. For the 2008 version of this review, we requested additional information from Gilks 2004, Kliethermes 1999 and Rocha 2002. We received additional information from Kliethermes 1999 (on breast feeding prevalence, apnoeic/bradycardic episodes and blinding of assessment outcome) and from Gilks 2004 (on exclusions post randomisation, years study was conducted, type of cup used, days to reach full sucking feeds and milk aspiration).
Assessment of risk of bias in included studies
We used standard methods of The Cochrane Collaboration and the Cochrane Neonatal Review Group to assess the methodological quality of trials (to meet validity criteria). For each trial, we sought information regarding methods of randomisation and blinding and reporting of all outcomes for all infants enrolled. We assessed each criterion as presenting low, high or unclear risk. Two review authors (CC, HS) separately assessed each study and resolved disagreements by discussion. We made explicit judgements about whether studies were at high risk of bias across six domains according to the criteria suggested in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
We included these items for appraisal.
-
Random sequence generation and allocation concealment, i.e.
-
random sequence generation: selection bias (biased allocation to interventions) due to inadequate generation of a randomised sequence; and
-
allocation concealment: selection bias (biased allocation to interventions) due to inadequate concealment of allocations before assignment.
-
-
Blinding of participants and personnel: performance bias due to knowledge of allocated interventions by participants and personnel during the study.
-
Blinding of outcome assessment: detection bias due to knowledge of allocated interventions by outcome assessors.
-
Incomplete outcome data: attrition bias due to quantity, nature or handling of incomplete outcome data.
-
Selective reporting: reporting bias due to selective outcome reporting.
-
Other bias: bias due to problems not covered elsewhere in the table.
See Appendix 2 for a more detailed description of risk of bias for each domain.
We used 'Risk of bias' tables to illustrate risk across trials. We resolved disagreements by consensus and, if necessary, by adjudication with a third review author.
Measures of treatment effect
We analysed treatment effects in individual trials by using Review Manager 5.3. We analysed dichotomous data using risk ratios (RRs), risk difference (RDs) and numbers needed to treat for an additional beneficial outcome (NNTBs) or numbers needed to treat for an additional harmful outcome (NNTHs). We reported 95% confidence intervals (CIs) for all estimates and used mean differences (MDs) with 95% confidence intervals for outcomes measured on a continuous scale. We analysed differences in the number of events for outcomes measured as count data (e.g. episodes of choking/gagging) by comparing rates of events in the two groups.
Unit of analysis issues
The unit of analysis was the participating infant in individually randomised trials.
Dealing with missing data
We requested additional data from trial investigators when data on important outcomes were missing or were reported unclearly. For included studies, we noted levels of attrition. If we had concerns regarding the impact of including studies with high levels of missing data in the overall assessment of treatment effect, we explored this through sensitivity analysis.
We analysed all outcomes on an intention‐to‐treat basis (i.e. we included in the analyses all participants randomly assigned to each group). The denominator for each outcome in each trial was the number randomly assigned minus any participants whose outcomes were known to be missing.
Assessment of reporting biases
For included trials that were recently performed (and therefore prospectively registered), we explored possible selective reporting of study outcomes by comparing primary and secondary outcomes in the reports versus primary and secondary outcomes proposed at trial registration, using the websites www.clinicaltrials.gov and www.controlled‐trials.com.
Data synthesis
We conducted meta‐analyses using Review Manager software (RevMan 2014), as supplied by The Cochrane Collaboration. We used the Mantel‐Haenszel method to obtain estimates of typical risk ratio and risk difference. For analysis of continuous measures, we used the inverse variance method. For all meta‐analyses, we used a fixed‐effect model.
Quality of evidence
We used the Grading of Recommendations, Assessment, Development and Evaluation (GRADE) approach, as outlined in the GRADE Handbook (Schünemann 2013), to assess the quality of evidence for the following (clinically relevant) outcomes: breast feeding extent and duration, length of hospital stay, growth and episodes of infection.
Two review authors (CC, HS) independently assessed the quality of the evidence for each of the outcomes above. We considered evidence from RCTs as high quality but downgraded the evidence one level for serious (and two levels for very serious) limitations on the basis of the following: design (risk of bias), consistency across studies, directness of the evidence, precision of estimates and presence of publication bias. We used the GRADEpro 2008 Guideline Development Tool to create a ‘Summary of findings’ table to report the quality of the evidence.
The GRADE approach provides an assessment of the quality of a body of evidence based on four grades.
-
High: We are very confident that the true effect lies close to that of the estimate of effect.
-
Moderate: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of effect but may be substantially different.
-
Low: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of effect.
-
Very low: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect.
Subgroup analysis and investigation of heterogeneity
We planned subgroup analyses to determine whether safety and efficacy outcomes were altered by the type of intervention used and the country in which the study took place (low‐ and middle‐income countries vs high‐income countries; classified according to http://data.worldbank.org/about/country‐classifications). When we found moderate to high heterogeneity (I2 > 50%), we used a random‐effects model and investigated potential sources of the heterogeneity (differences in study quality, participants or treatment regimens). When heterogeneity was explained by subgroup analysis, we presented results in this way.
Results
Description of studies
See also Characteristics of included studies, Characteristics of excluded studies and Characteristics of studies awaiting classification.
Results of the search
We identified and included two new trials, resulting in a total of seven trials included in the review; we excluded 10 studies and found one that awaits classification. We identified no ongoing trials (Figure 1).

Study flow diagram: review update.
Included studies
We included seven studies and have provided details of each of these studies (Collins 2004; Gilks 2004; Kliethermes 1999; Mosley 2001; Rocha 2002; Simmer 2016; Yilmaz 2014) in the Characteristics of included studies table. Collins 2004 is a primary study report; a PhD thesis presents additional data related to this study (i.e. extent of breast feeding, any and full, at three months and six months post discharge, time to full sucking feeds, weight gain, milk aspiration and reasons for non‐compliance). Simmer 2016 is a primary study report that was first published in abstract form. Studies were undertaken in neonatal units in Australia (Collins 2004; Simmer 2016), Brazil (Rocha 2002), England (Gilks 2004; Mosley 2001), Turkey (Yilmaz 2014) and the United States of America (Kliethermes 1999). Five trials were single‐centre studies (Gilks 2004; Kliethermes 1999; Mosley 2001; Rocha 2002; Simmer 2016), and two were multi‐centre studies (Collins 2004 ‐ two centres; Yilmaz 2014 ‐ three centres).
Participants
This review included a total of 1152 infants; sample sizes ranged from 14 to 522 participants. All studies included preterm infants, although limits for gestational age and birth weight differed. Four studies included extremely preterm and very preterm infants (Collins 2004 < 34 weeks; Rocha 2002 32 to 34 weeks; Gilks 2004 and Simmer 2016 < 35 weeks), and two included moderate to late preterm infants (Mosley 2001 32 to 37 weeks; Yilmaz 2014 32 to 35 weeks); Kliethermes 1999 used a birth weight criterion of 1000 to 2500 grams.
Five studies stratified infants at randomisation ‐ one by birth weight (Rocha 2002) and four by gestational age (Collins 2004; Gilks 2004; Simmer 2016; Yilmaz 2014).
The average gestational age of included infants across all trials was 32 weeks.
Interventions
Those using alternative feeding devices (cup, gavage tube, paladai, finger feeding, dropper, spoon or other) were classified as the experimental group, and infants who received bottle feeding were classified as the control group.
Five studies compared breast feeding with supplementary feeds given by cup versus breast feeding with supplementary feeds given by bottle (Collins 2004; Gilks 2004; Mosley 2001; Rocha 2002; Yilmaz 2014). One trial compared breast feeding with supplementary feeds by bottle versus breast feeding with supplementary feeds by gavage tube alone (Kliethermes 1999). The Simmer 2016 trial used a specially developed feeding system that incorporated a shut‐off valve in the teat, so that milk flowed only when the infant created a vacuum; collapse of the teat was prevented by a venting system. Infants controlled the flow of milk by raising the tongue when sucking stopped; study authors (Simmer 2016) showed that this action was similar in breast fed term infants (Geddes 2012). Although this intervention uses a bottle and a teat, the review authors agreed to include this study in the review, given that the 'novel teat' causes action that is purportedly similar to the breast feeding action compared with conventional teats used in all other studies.
In all studies, neither bottle feeds nor alternative feeding devices (cup/tube alone/novel teat) were used to replace a breast feed and were given only when the mother was not available to breast feed, or if extra milk was thought necessary after a breast feed and investigators determined that the infant was able to take this orally.
Among the cup feeding studies, four (Collins 2004; Gilks 2004; Rocha 2002; Yilmaz 2014) followed the cup feeding recommendations of Lang (Lang 1994a; Lang 1994b). Rocha 2002 used the protective cap from a bottle, Collins 2004 and Yilmaz 2014 a 60 mL medicine cup and Gilks 2004 an Ameda baby cup. Mosley 2001 did not state the type of cup used and did not describe the cup feeding procedure. An indwelling nasogastric tube remained in situ for both experimental and control groups in two studies in which feeds were given by tube if insufficient milk was taken during cup or breast feeding, or if the infant was not scheduled for a sucking feed (Collins 2004; Gilks 2004). It is not stated whether this occurred for cup feeds in the other studies (Mosley 2001; Rocha 2002; Yilmaz 2014).
For breast feeding with supplementary feeds by bottle compared with breast feeding with supplementary feeds by gavage tube (Kliethermes 1999), all infants received standard care (including non‐nutritive breast feeding) until written orders for oral feedings were given. For the control group, all supplementary feeds were given by bottle, and the indwelling nasogastric tube was removed as directed by the clinical care team. For the experimental group (gavage tube), feeds were given by an indwelling 3.5 gauge French nasogastric tube. The tube was removed during the last 24 to 48 hours of parent 'rooming‐in', at which time a cup or syringe was used if needed.
Skin‐to‐skin contact and non‐nutritive sucking at the breast were encouraged for all infants in three studies (Collins 2004; Kliethermes 1999; Simmer 2016). This was not reported in the remaining studies (Gilks 2004; Mosley 2001; Rocha 2002; Yilmaz 2014).
Sucking feeds for experimental and control groups were commenced and advanced according to individual hospital policy. In one trial (Rocha 2002), this decision was based on weight (1600 grams). In Collins 2004, sucking feeds began when infants were assessed as mature enough to co‐ordinate a suck‐swallow‐breathe reflex. In three studies, sucking feeds occurred at the discretion of the nurse or midwife (Collins 2004), the neonatologist (Collins 2004; Kliethermes 1999; Mosley 2001; Yilmaz 2014) or the neonatal nurse practitioner (Kliethermes 1999; Mosley 2001). This information was not reported in Gilks 2004 and Simmer 2016.
Non‐nutritive sucking with use of a dummy (also known as a pacifier) varied among the included studies. Collins 2004 randomised infants to cup/no dummy, cup/dummy, bottle/no dummy and bottle/dummy, reporting no statistically significant interaction between infants randomised to no dummy or cup; therefore, results from marginal groups (cup vs bottle and dummy vs no dummy) could be analysed independently. In Kliethermes 1999, a dummy was available during tube feedings for the experimental group, and study authors did not report whether a dummy was available outside feeding times in either group. In Rocha 2002, a dummy was not used for the experimental (cup) group, and Mosley 2001 reported that six infants were given a dummy. In Simmer 2016, non‐nutritive sucking was encouraged in both groups, and Gilks 2004 and Yilmaz 2014 did not report dummy use.
Outcomes
All outcomes were not reported in each study.
All studies measured breast feeding outcomes (Collins 2004; Gilks 2004; Kliethermes 1999; Mosley 2001; Rocha 2002; Simmer 2016; Yilmaz 2014). Six studies (Collins 2004; Gilks 2004; Kliethermes 1999; Mosley 2001; Simmer 2016; Yilmaz 2014) measured full breast feeding at discharge home from hospital, four studies (Collins 2004; Kliethermes 1999; Simmer 2016; Yilmaz 2014) at three months post discharge and three studies (Collins 2004; Kliethermes 1999; Yilmaz 2014) at six months post discharge.
Six studies (Collins 2004; Gilks 2004; Kliethermes 1999; Rocha 2002; Simmer 2016; Yilmaz 2014) measured any breast feeding at discharge home from hospital, five studies (Collins 2004; Kliethermes 1999; Rocha 2002; Simmer 2016; Yilmaz 2014) at three months post discharge and three studies (Collins 2004; Kliethermes 1999; Yilmaz 2014) at six months post discharge.
Three studies (Collins 2004; Kliethermes 1999; Yilmaz 2014) used the following definition of full breast feeding: No other solids or liquids were given apart from vitamins, minerals, juice or ritualistic feedings, given infrequently. Mosley 2001 used the term 'exclusive' and Simmer 2016 'fully' breast feeding but did not define the terms; however, these investigators reported both breast feeding and breast milk feeds. Rocha 2002 defined breast feeding as feeding exclusively or partially directly at the breast. Kliethermes 1999 and Gilks 2004 considered infants who were receiving supplementary feeds of expressed breast milk on discharge as partially breast fed, and Collins 2004 considered them fully breast fed. Two per cent of women (n = 6) with 2% of infants (n = 7) in Collins 2004 had chosen to feed their infants expressed breast milk by bottle; researchers randomised three to cup feeds and four to bottle feeds.
At three months and six months post discharge, Collins 2004 used the term 'all breast feeds' to indicate that an infant's milk feeds were breast feeds only when no other types of milk were given, and 'partial breast feeds' to mean that an infant's milk feeds were a combination of breast feeds and other types of milk. The intent was to determine the types of milk feeds infants were receiving (breast or formula), irrespective of whether they were receiving solids. This does not fit with the conventional definition of full breast feeding (Labbok 1990), that is, if an infant is on solids and all milk feeds are breast feeds, the infant is usually classified as 'partially' breast feeding. The Collins 2008 review did not include data for 'all breast feeds' in the meta‐analyses. Given the small number of studies reporting this outcome, review authors reconsidered and have included the data for 'all breast feeds' in the meta‐analysis in this 2016 update.
Three studies (Collins 2004; Gilks 2004; Simmer 2016) measured the time taken to reach full sucking feeds. Three studies (Collins 2004; Rocha 2002; Yilmaz 2014) reported rate of weight gain, four (Collins 2004; Kliethermes 1999; Simmer 2016; Yilmaz 2014) length of hospitalisation and two (Rocha 2002; Yilmaz 2014) supplementary feeding time. No studies reported the volume of supplementary feed taken compared with the volume prescribed.
Two studies reported cardiorespiratory stability. Kliethermes 1999 reported apnoeic or bradycardic episodes, and Rocha 2002 oxygen saturation associated with mode of feeding. No studies reported episodes of choking/gagging, and two trials reported milk aspiration (Collins 2004; Gilks 2004). Collins 2004 reported parental satisfaction, and three studies (Collins 2004; Kliethermes 1999; Simmer 2016) reported episodes of infection.
Excluded studies
See Characteristics of excluded studies for details. We excluded:
-
Abouelfettoh 2008, Aytekin 2014, De Aquino 2009, Harding 2014, Lau 2012 and Ronan 2013 because they were not RCTs;
-
Aloysius 2007 and Lopez 2014 because they were randomised cross‐over trials; and
-
Kumar 2010 and Marofi 2016 because they did not include a bottle control group.
One study (Garpiel 2012) is awaiting classification.
Risk of bias in included studies
We have provided details of the methodological quality of each study in the Characteristics of included studies table (Figure 2).
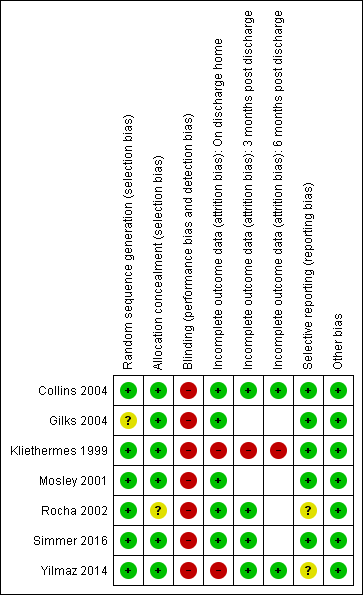
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Risk of selection bias was low with adequate methods of random sequence generation described in six studies (Collins 2004; Kliethermes 1999; Mosley 2001; Rocha 2002; Simmer 2016; Yilmaz 2014) and not described in Gilks 2004. Allocation concealment was adequate in six studies (Collins 2004; Gilks 2004; Kliethermes 1999; Mosley 2001; Simmer 2016; Yilmaz 2014) and was unclear in Rocha 2002.
Blinding
Risk of performance and detection bias was high, as blinding of treatment was not possible in any study. Five studies (Kliethermes 1999; Mosley 2001; Rocha 2002; Simmer 2016; Yilmaz 2014) did not clearly state whether outcome assessment was blinded. Two studies (Collins 2004; Gilks 2004) stated that data for outcomes were collected unblinded. Simmer 2016 was the only study that described blinding of analyses.
Incomplete outcome data
We judged risk of bias due to incomplete outcome data as low in six studies (Collins 2004; Gilks 2004; Mosley 2001; Rocha 2002; Simmer 2016; Yilmaz 2014) and high in Kliethermes 1999. Studies handled protocol violations differently; five studies excluded the infants from analyses (Gilks 2004; Kliethermes 1999; Mosley 2001; Rocha 2002; Yilmaz 2014). Proportions of incomplete outcome data for the primary outcome were as follows: Collins 2004 5%, Gilks 2004 0%. Kliethermes 1999 15%, Mosley 2001 13%, Rocha 2002 6%, Simmer 2016 3% and Yilmaz 2014 14%.
Collins 2004 reported a high proportion of non‐compliance. In the experimental (cup) group, 85 of 151 (56%) had a bottle introduced, and in the control group, 1 of 152 (0.7%) had a cup introduced. Infants were analysed in the group to which they were randomised (Collins 2004).
Selective reporting
We found no evidence of reporting bias.
Other potential sources of bias
We found no evidence of other potential sources of bias.
Effects of interventions
See summary of findings Table for the main comparison. Breast feeding with supplemental feeds by other than bottle versus breast feeding with supplemental feeds by bottle.
This review includes seven studies with 1152 infants.
We conducted subgroup analyses to determine whether outcomes were altered by type of intervention. We incorporated the subgroups into the main structure of each figure.
Full breast feeding (Outcomes 1.1 to 1.3)
At discharge home (Analysis 1.1)
Six studies reported this outcome in 1074 infants (Collins 2004; Gilks 2004; Kliethermes 1999; Mosley 2001; Simmer 2016; Yilmaz 2014). Three trials (Collins 2004; Kliethermes 1999; Yilmaz 2014) as well as the meta‐analysis of data from all trials showed a statistically significantly higher rate of full breast feeding in the experimental (avoid bottle) group (typical risk ratio (RR) 1.47, 95% confidence interval (CI) 1.19 to 1.80; risk difference (RD) 0.21, 95% CI 0.09 to 0.32; number needed to treat for an additional beneficial outcome (NNTB) 5, 95% CI 3 to 11). Heterogeneity between studies was moderate (I2 = 52%).
Subgroup analyses by intervention type: full breast feeding at discharge home (Outcomes 1.1.1 to 1.1.3)
The subgroup interaction test was not statistically significantly different, although the P value = 0.08 indicates that the effect on breast feeding of a tube alone approach may have a more significant impact on breast feeding success than a cup feeding approach. However, only one small study with high risk of bias (Kliethermes 1999) used a tube alone feeding approach.
Four studies with 893 infants (Collins 2004; Gilks 2004; Mosley 2001; Yilmaz 2014) compared cup feeds with bottle feeds. The statistically significant increase in full breast feeding remained (typical RR 1.41, 95% CI 1.14 to 1.75; RD 0.20, 95% CI 0.10 to 0.308; NNTB 5, 95% CI 3 to 10) with low heterogeneity (I2 = 45%). Kliethermes 1999 showed a significant increase in full breast feeding (tube alone vs bottle), and Simmer 2016 when comparing different teats showed no difference in full breast feeding.
Three months post discharge (Analysis 1.2)
Four studies (Collins 2004; Kliethermes 1999; Simmer 2016; Yilmaz 2014) with 986 infants reported this outcome. Two studies (Kliethermes 1999; Yilmaz 2014) and the meta‐analysis showed a statistically significantly higher rate of full breast feeding in the experimental (avoid bottle) group (typical RR 1.56, 95% CI 1.37 to 1.78; RD 0.20, 95% CI 0.15 to 0.26; NNTB 5, 95% CI 4 to 7). We detected low heterogeneity in this analysis (I2 = 37%).
Subgroup analyses by intervention type: full breast feeding at three months post discharge (Outcomes 1.2.1 to 1.2.3)
Cup feeding compared with bottle feeding (Collins 2004; Yilmaz 2014) showed a significant increase in full breast feeding (typical RR 1.54, 95% CI 1.34 to 1.77; RD 0.21, 95% CI 0.15 to 0.27; NNTB 5, 95% CI 4 to 7) but with moderate heterogeneity (I2 = 61%). Setting, participants and risk of bias differed in the two cup feeding studies. Collins 2004 was conducted in a high‐income country, included more immature infants (mean gestational age, 30 weeks) and reported low adherence with the intervention and overall low risk of bias, whereas Yilmaz 2014 included more mature infants (mean gestational age, 33 weeks) and high adherence with the intervention, was conducted in a high‐middle‐income country and had high risk of attrition bias. Kliethermes 1999 reported that tube alone versus bottle showed increased full breast feeding, and Simmer 2016 described no differences when different teats were compared.
Six months post discharge (Analysis 1.3)
Full breast feeding was significantly increased at six months post discharge in the experimental (avoid bottle) group in both individual trials and the meta‐analysis (887 infants, three studies: Collins 2004; Kliethermes 1999; Yilmaz 2014) (typical RR 1.64, 95% CI 1.14 to 2.36; RD 0.15, 95% CI 0.07 to 0.24; NNTB 7, 95% CI 4 to 14). Moderate heterogeneity was detected (I2 = 52%).
Subgroup analyses by intervention type: full breast feeding at six months home (Outcomes 1.3.1 to 1.3.3)
Tube alone versus bottle statistically significantly increased full breast feeding (Kliethermes 1999). The subgroup interaction test (P = 0.06) indicated that the effect on breast feeding of the tube alone approach (Kliethermes 1999) may have a more significant impact on breast feeding success than the cup feeding approach, as described above. The two cup feeding trials (Collins 2004; Yilmaz 2014) noted an increase in full breast feeding in the cup group (typical RR 1.54, 95% CI 1.34 to 1.77; RD 0.13, 95% CI 0.07 to 0.19, NNTB 8, 95% CI 5 to 14) with no heterogeneity (I2= 0%).
Any breast feeding (Outcomes 1.4 to 1.6)
At discharge home (Analysis 1.4)
Data were obtained from six trials including 1138 infants (Collins 2004; Gilks 2004; Kliethermes 1999; Rocha 2002; Simmer 2016; Yilmaz 2014). Two studies (Kliethermes 1999; Yilmaz 2014) as well as the meta‐analysis showed a statistically significantly higher rate of any breast feeding on discharge home in the experimental (avoid bottle) group (typical RR 1.11, 95% CI 1.06 to 1.16; RD 0.09, 95% CI 0.05 to 0.13; NNTB 11, 95% CI 8 to 20) with no heterogeneity detected.
Subgroup analyses by intervention type: any breast feeding at discharge home (Outcomes 1.4.1 to 1.4.3)
One of the cup feeding studies (Yilmaz 2014) and the meta‐analysis revealed a significant increase in breast feeding (Collins 2004; Gilks 2004; Rocha 2002; Yilmaz 2014; 957 infants; typical RR 1.09, 95% CI 1.03 to 1.15; RD .07, 95% CI .03 to .11; NNTB 14. 95% CI 9 to 33, no heterogeneity), as did the tube alone trial (Kliethermes 1999), but Simmer 2016 noted no such increase upon comparing two different types of teats.
Three months post discharge (Analysis 1.5)
Five studies with 1063 infants (Collins 2004; Kliethermes 1999; Rocha 2002; Simmer 2016; Yilmaz 2014) contributed data to this outcome. Two studies (Kliethermes 1999; Rocha 2002) and a meta‐analysis of data showed a statistically significant increase in the rate of any breast feeding in the experimental (avoid bottle) group (typical RR 1.31, 95% CI 1.01 to 1.71; RD 0.14, 95% CI 0.04 to 0.24; NNTB 7, 95% CI 4 to 25), with moderate heterogeneity detected (I2 = 73%).
Subgroup analyses by intervention type: any breast feeding at three months post discharge (Outcomes 1.5.1 to 1.5.3)
Researchers found no clear benefit of cup feeding for any breast feeding at three months post discharge (883 infants, three trials; Collins 2004; Rocha 2002; Yilmaz 2014). Tube alone showed a statistically significant increase in any breast feeding (Kliethermes 1999) but with no statistically significant differences between novel and conventional teats (Simmer 2016).
Six months post discharge (Analysis 1.6)
Three studies with 886 infants (Collins 2004; Kliethermes 1999; Yilmaz 2014) provided data on this outcome. Two studies (Kliethermes 1999; Yilmaz 2014) and a meta‐analysis showed a statistically significant increase in the rate of any breast feeding at six months post discharge in experimental (avoid bottle) groups (typical RR 1.25, 95% CI 1.10 to 1.41; RD 0.11, 95% CI 0.05 to 0.17; NNTB 9, 95% CI 6 to 20), with moderate heterogeneity detected (I2 = 50%).
Subgroup analyses by intervention type: any breast feeding at three months post discharge (Outcomes 1.6.1 to 1.6.3)
Cup feeding in Collins 2004 and Yilmaz 2014 (803 infants) resulted in a statistically significant increase in any breast feeding at six months post discharge (typical RR 1.20, 95% CI 1.06 to 1.36; RD 0.09, 95% CI 0.03 to 0.16; NNTB 11, 95% CI 6 to 22) with no heterogeneity. Tube alone also showed a statistically significant increase in any breast feeding (Kliethermes 1999). The subgroup interaction test P value = 0.06 in Kliethermes 1999 indicated that the effect on breast feeding of a tube alone approach may have a more significant impact on breast feeding success than a cup feeding approach. However, only one small study with high risk of bias used a tube alone approach.
Time (days) to reach full sucking feeds (Outcome 1.7)
Four studies measured this outcome (Analysis 1.7) in 513 infants (Collins 2004; Gilks 2004; Kliethermes 1999; Simmer 2016). Two studies (Collins 2004; Kliethermes 1999) showed a significant increase in days to reach full sucking feeds in the experimental (avoid bottle) group. Kliethermes 1999 did not report standard deviations, so their data could not be included in the meta‐analysis; however, the increase (7.5 days) was of the same magnitude as reported in Collins 2004 (10.5 days). Meta‐analysis (Collins 2004; Gilks 2004; Simmer 2016) revealed no clear effect on days to taken to reach full sucking feeds in the experimental (avoid bottle) group.
Subgroup analyses by intervention type: time (days) to reach full sucking feeds (Outcomes 1.7.1, 1.7.2)
Neither the two cup feeding trials (Collins 2004; Gilks 2004; 332 infants) nor the novel teat feeding trial (Simmer 2016) showed a clear increase or reduction in days to reach full sucking feeds. The tube alone study (Kliethermes 1999) reported a significant increase in days to reach full sucking feeds.
Weight gain (g/kg/d or g/d) (Outcome 1.8)
Three studies with 893 infants (all cup) reported no statistically significant differences in weight gain (g/kg/d; Analysis 1.8) when measured from birth to discharge home (Collins 2004), or one week after oral feeds were commenced (Rocha 2002; Yilmaz 2014). A meta‐analysis was not possible because different units of measurement were used. Simmer 2016 reported that infants in the experimental (novel teat) group were statistically significantly lighter on discharge home (mean difference (MD) ‐186 grams, 95% CI ‐317 to ‐56).
Length of hospital stay, days (Outcome 1.9)
We obtained data from four studies with 1004 infants (Analysis 1.9) (Collins 2004; Kliethermes 1999; Simmer 2016; Yilmaz 2014). Collins 2004 showed a statistically significant increase in length of hospital stay of 10 days with the experimental (cup) group, but meta‐analysis revealed no statistically significant difference (MD 2.25, 95% CI ‐3.36 to 7.86 days). Moderate heterogeneity was present in this analysis (I2 = 73%).
Subgroup analyses by intervention type: length of hospital stay (days) (Outcomes 1.9.1 to 1.9.3)
The two cup feeding trials with 823 infants (Collins 2004; Yilmaz 2014) showed no clear difference in length of hospital stay (MD 4.45, 95% CI ‐5.57 to 14.48 days). High heterogeneity was present (I2 = 90%). The overall length of stay differed between these studies owing to differences in the maturity of included infants. Collins 2004 suggested that increased length of stay may have been related to problems with staff and acceptance by parents of cup feeding, with some infants less satisfied and more difficult to feed by cup as they matured, resulting in feeding by tube and delayed onset of all sucking feeds ‐ a requirement for discharge home. Kliethermes 1999 (tube alone) and Simmer 2016 (novel teat) also showed no statistically significant differences in length of hospital stay.
Duration (minutes) of supplementary feed (Outcome 1.10)
Two studies with 600 infants (Rocha 2002; Yilmaz 2014; both cup intervention) measured duration of supplementary feeds and showed no significant differences in time taken to cup feed versus time taken to bottle feed (MD ‐0.42, 95% CI ‐1.96 to 1.12 minutes). Moderate heterogeneity was present in this analysis (I2 = 60%) (Analysis 1.10) and is not explained by the maturity of included infants, as infants in both studies were at a mean of 33 weeks' gestation.
Episodes of infection (Outcome 1.11)
Three studies with 500 infants reported infection (Analysis 1.11): Collins 2004 reported necrotising enterocolitis; Kliethermes 1999 reported infection not defined; and Simmer 2016 reported late‐onset sepsis. All participants were from high‐income countries, and neither trials nor the meta‐analysis showed a statistically significant difference in episodes of infection (typical RR 0.70, 95% CI 0.35 to 1.42). No heterogeneity was detected.
Cardiorespiratory stability
One trial (Kliethermes 1999) reported the total number of episodes of apnoea and bradycardia per infant. Researchers described significantly fewer apnoeic and bradycardic incidents for the experimental (tube alone) group (mean 127, SD not reported) compared with the control (bottle) group (mean 136, SD not reported; P = 0.0006). However, the breast feeding plus bottle group had significantly more episodes requiring stimulation (mean 32.7 episodes, SD not reported vs mean 23.3 episodes, SD not reported; P = 0.0001). Investigators measured apnoeic and bradycardic episodes over the entire hospital stay ‐ not just episodes associated with feeding. Rocha 2002 reported mean oxygen saturation during feeds and showed no statistically significant difference in the mean of the lowest oxygen saturation during feeds (cup mean 90.8, SD 4.8, range 75 to 99; bottle mean 87.7, SD 7.6, range 68 to 97). Rocha 2002 also reported oxygen desaturation during feeds and showed no difference in desaturation episodes of less than 90% with cup feeds (18/44, 40.9%) compared with the bottle group (19/34, 55.9%). Researchers reported a statistically significant difference in the proportion of desaturation episodes less than 85%, with fewer occurring in cup groups (6/44, 13.6%) than in bottle groups (12/34, 35.3%; P = 0.02).
Milk aspiration on radiological assessment
The three studies that reported this outcome (Collins 2004; Gilks 2004; Yilmaz 2014) described no episodes of milk aspiration.
Satisfaction with feeding method
One study included views of parents on the method of feeding (Collins 2004) and noted a high rate of non‐compliance, with 56% of infants in the intervention (breast feeding with supplemental feeds by cup) group (n = 85/151) having a bottle introduced. Compliance differed between recruiting hospitals; the hospital at which cup feeding was introduced specifically for this study had a higher rate of compliance than the other recruiting hospital, where cup feeding had been practised for three years before the study began. Researchers collected data on reasons for the introduction of a bottle from the medical records or after discussion with attending nurses or midwives. Reasons for introducing a bottle were available for 74% (n = 63) of the 85 infants randomised to cup feeds who had a bottle introduced. In 65% (n = 41) of cases, the reason given for introduction of a bottle was that it was introduced at the request of the mother, and the staff initiated the bottle in 29% (n = 18) of cases. In 10% (n = 6) of cases, researchers introduced a bottle because the baby was not satisfied with cup feeds or would not settle down. One infant randomised to the bottle group had a cup introduced because of transfer to a peripheral hospital, where cup feeding was routinely done.
The three month post discharge questionnaire included a question to the mother on reasons for introduction of a bottle. Reasons for introducing a bottle were available for 91% (n = 77) of the 85 infants randomised to cup feeds who had a bottle introduced. Women could select from a list of options, and additional space was provided for any other comments. A total of 44% (n = 34) indicated that the decision to introduce a bottle was theirs, and 33% (n = 25) were advised by the nurse or midwife (some responded yes to both of these statements). In all, 26% (n = 20) had problems with cup feeding including inability of the infant to do it, frequent spills, dissatisfaction with cup feeds and unacceptably long feeding times. Ten (13%) of the respondents did not like cup feeds and changed feeding method because of this. Nine (12%) respondents said that the staff refused to cup feed their infant. Collins 2004 reported that some infants became less satisfied with cup feeds and more difficult to feed by this method as they neared discharge, generally during the last week of their hospital stay. Because of this, if the mother could not be present to breast feed, the infant would be tube fed. The criterion for discharge home was that the infant had to be on full sucking feeds. This may have contributed to increased length of stay in this study. However, the study author cautions that reliable data on this point were not collected (Collins 2004).
Outcomes not reported
No studies reported the volume of supplementary feed taken compared with the volume prescribed, nor did they describe episodes of choking or gagging.
Subgroup analysis: trials conducted in low‐ and middle‐income countries
Two trials were conducted in upper‐middle‐income countries: Rocha 2002 in Brazil and Yilmaz 2014 in Turkey. Meta‐analyses were limited and showed no substantial differences from the meta‐analysis of all trials together (Analysis 1.1; Analysis 1.2; Analysis 1.3; Analysis 1.4; Analysis 1.5; Analysis 1.6; Analysis 1.8; Analysis 1.9; Analysis 1.10). These studies did not report infection rates.
Discussion
Summary of main results
The strategy of avoiding bottles while an infant breast feeds is being established in preterm infants, resulting in statistically significant increases in the extent and duration of breast feeding. Studies included in this review compared cup feeding with a tube alone approach or a novel teat versus bottle feeding with a conventional teat. Across all time points (discharge home, three months and six months post discharge), both a cup feeding strategy and a tube alone strategy increased breast feeding success. We included the novel teat study in this review, as the design of the teat, in which a vacuum is created, attempted to mimic the sucking action required for breast feeding, thereby potentially supporting breast feeding success when compared with a conventional teat. Investigators found no differences in breast feeding outcomes with the novel teat, rather the cup and tube alone studies reported an increase in breast feeding. Meta‐analysis of data from trials that included length of stay, weight gain and infection showed no clear evidence of benefit or harm. Limited evidence from the two trials that assessed cardiorespiratory stability suggests improved stability with avoidance of bottles.
Overall completeness and applicability of evidence
The trials reviewed provide no information on the volume of feed consumed compared with the volume prescribed nor on episodes of choking/gagging per feed. We found limited information on cardiorespiratory stability and parent and health professional satisfaction with the feeding method. No studies were conducted in low‐income countries, and two were completed in middle‐income countries. No reports describe infants dissatisfied with tube or cup, except Collins 2004, in which adherence with cup feeding was poor. In contrast, cup feeding had not previously been used in Yilmaz 2014, and staff acceptance was high, with high adherence to the intervention. Both of the largest studies were cup feeding studies, but they were conducted in different populations and settings. Collins 2004 was conducted in a high‐income country in very and extremely preterm infants, whereas Yilmaz 2014 included moderate to late preterm infants in a high‐middle‐income country. Lang 1997 suggested that as preterm infants mature, they may be able to bottle feed with no interference with breast feeds, but she cautions that the introduction of a bottle should occur only when breast feeding is well established. Such a strategy might be more acceptable to staff and parents, but no randomised controlled trials have investigated this approach.
Quality of the evidence
We included in this review seven studies with 1152 infants. Blinding was not possible in any of the included studies and therefore was subject to caregiver influence. We graded the level of evidence for full breast feeding as low or moderate, and for any breast feeding as moderate or very low (summary of findings Table for the main comparison). We graded the level of evidence for episodes of infection and length of hospital stay as moderate and very low (summary of findings Table for the main comparison). We downgraded outcomes because of attrition and moderate to high heterogeneity. The direction of effects of all included trials was consistent (favouring avoiding bottles) for breast feeding outcomes, but the magnitude of effects in Kliethermes 1999 was inconsistent with that in the other studies. The most likely reason for this heterogeneity was the difference in the intervention provided or the poorer quality of the study. Kliethermes 1999 used supplemental feeding by tube, and Simmer 2016 a novel teat, whereas the remaining trials used supplemental feeds by cup. Heterogeneity was considerable between cup feeding studies that reported length of stay (Collins 2004; Yilmaz 2014), with length of stay increased by a mean of 10 days in Collins 2004, and no difference was reported in Yilmaz 2014.
Potential biases in the review process
Assessment of risk of bias involves subjective judgements. Review authors therefore independently assessed studies and resolved disagreements through discussion (Higgins 2011). We attempted to identify all relevant studies by screening the reference lists of included trials and related reviews.

Study flow diagram: review update.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
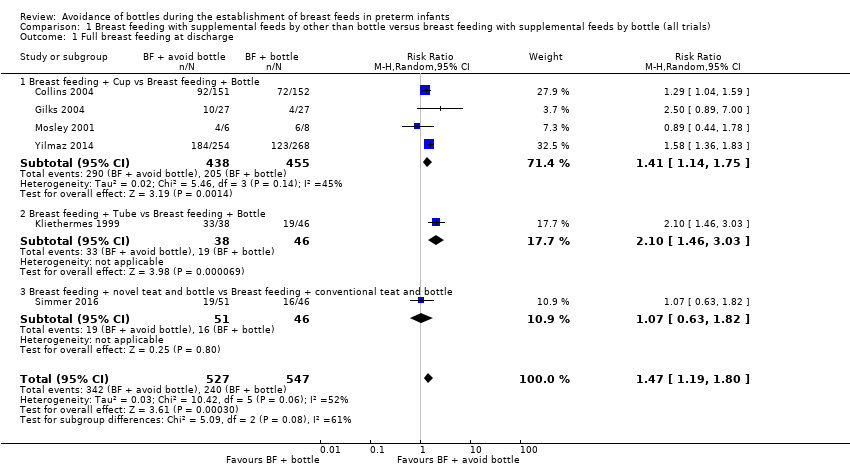
Comparison 1 Breast feeding with supplemental feeds by other than bottle versus breast feeding with supplemental feeds by bottle (all trials), Outcome 1 Full breast feeding at discharge.
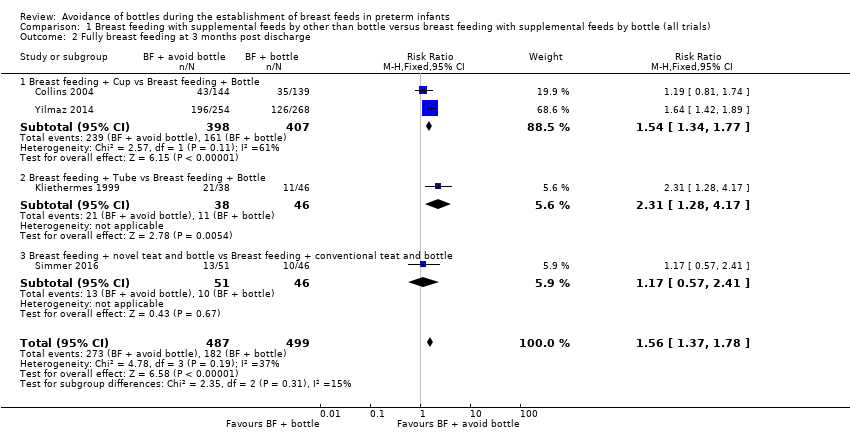
Comparison 1 Breast feeding with supplemental feeds by other than bottle versus breast feeding with supplemental feeds by bottle (all trials), Outcome 2 Fully breast feeding at 3 months post discharge.
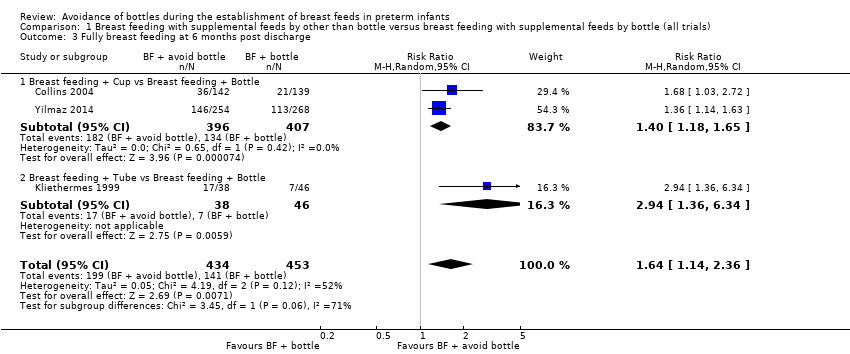
Comparison 1 Breast feeding with supplemental feeds by other than bottle versus breast feeding with supplemental feeds by bottle (all trials), Outcome 3 Fully breast feeding at 6 months post discharge.
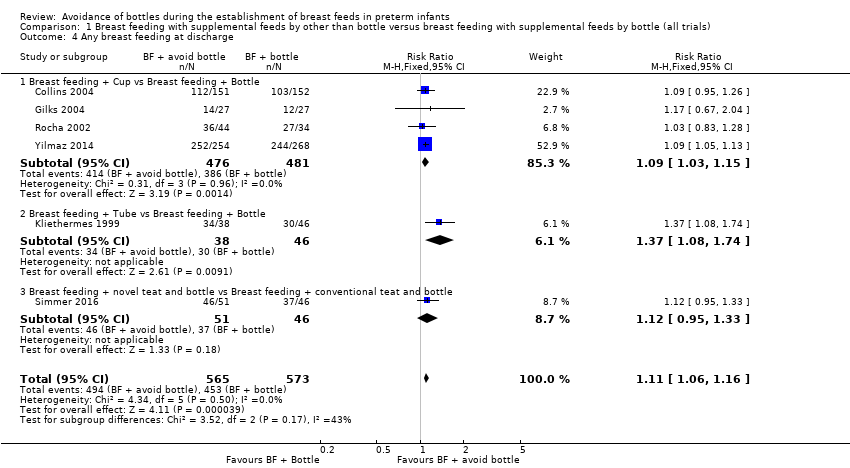
Comparison 1 Breast feeding with supplemental feeds by other than bottle versus breast feeding with supplemental feeds by bottle (all trials), Outcome 4 Any breast feeding at discharge.

Comparison 1 Breast feeding with supplemental feeds by other than bottle versus breast feeding with supplemental feeds by bottle (all trials), Outcome 5 Any breast feeding at 3 months post discharge.

Comparison 1 Breast feeding with supplemental feeds by other than bottle versus breast feeding with supplemental feeds by bottle (all trials), Outcome 6 Any breast feeding at 6 months post discharge.

Comparison 1 Breast feeding with supplemental feeds by other than bottle versus breast feeding with supplemental feeds by bottle (all trials), Outcome 7 Days to reach full sucking feeds.
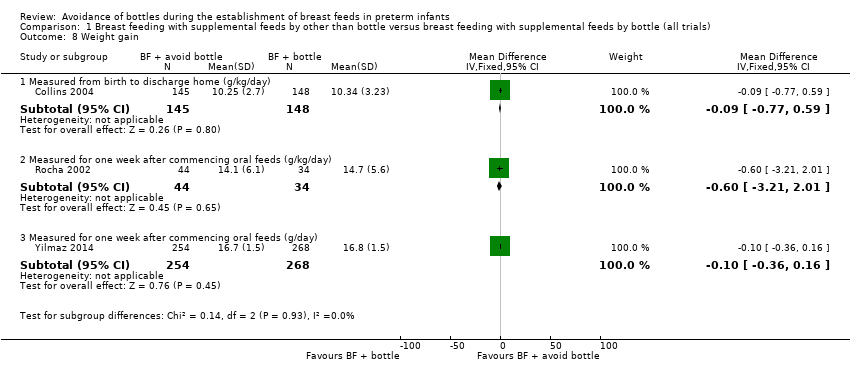
Comparison 1 Breast feeding with supplemental feeds by other than bottle versus breast feeding with supplemental feeds by bottle (all trials), Outcome 8 Weight gain.
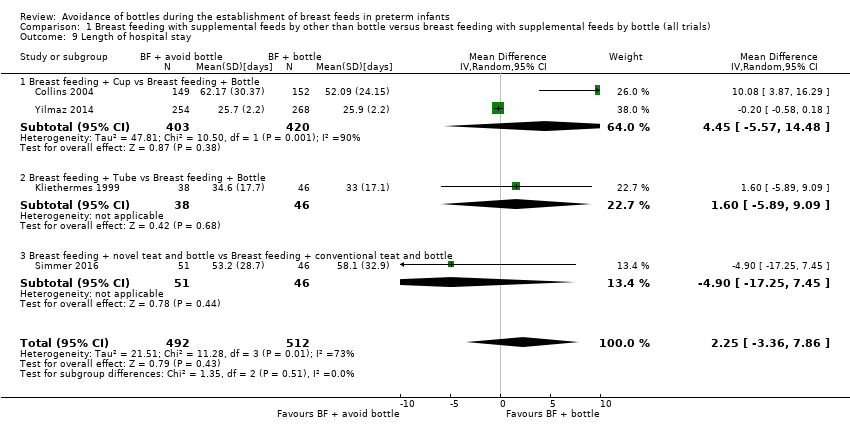
Comparison 1 Breast feeding with supplemental feeds by other than bottle versus breast feeding with supplemental feeds by bottle (all trials), Outcome 9 Length of hospital stay.
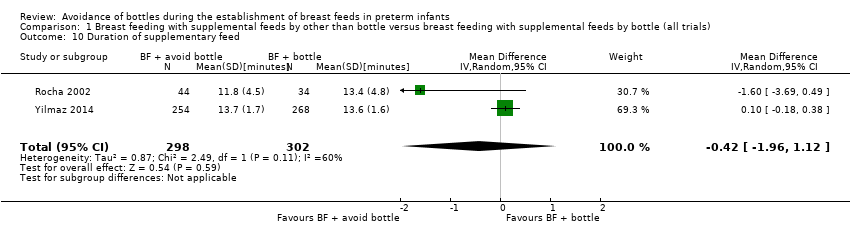
Comparison 1 Breast feeding with supplemental feeds by other than bottle versus breast feeding with supplemental feeds by bottle (all trials), Outcome 10 Duration of supplementary feed.
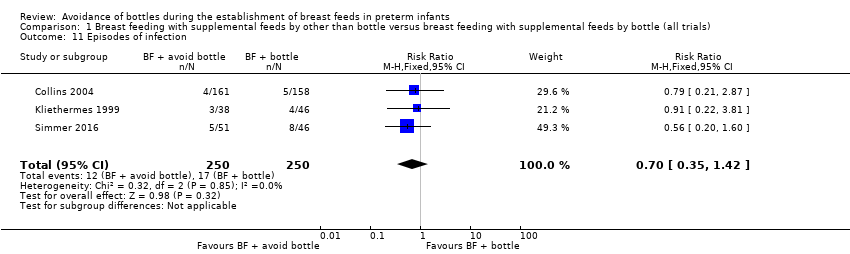
Comparison 1 Breast feeding with supplemental feeds by other than bottle versus breast feeding with supplemental feeds by bottle (all trials), Outcome 11 Episodes of infection.
| Breast feeding with supplemental feeds by other than bottle compared with breast feeding with supplemental feeds by bottle (all trials) in preterm infants | ||||||
| Patient or population: preterm infants | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | |
| Risk with breast feeding with supplemental feeds by bottle (all trials) | Risk with breast feeding with supplemental feeds by other than bottle | |||||
| Full breast feeding at discharge | Study population | RR 1.47 | 1074 | ⊕⊕⊝⊝ | ||
| 44 per 100 | 64 per 100 | |||||
| Full breast feeding at 3 months post discharge | Study population | RR 1.56 | 986 | ⊕⊕⊕⊝ | ||
| 36 per 100 | 57 per 100 | |||||
| Full breast feeding at 6 months post discharge | Study population | RR 1.64 | 887 | ⊕⊕⊝⊝ | ||
| 31 per 100 | 51 per 100 | |||||
| Any breast feeding at discharge | Study population | RR 1.11 | 1138 | ⊕⊕⊕⊝ | ||
| 79 per 100 | 88 per 100 | |||||
| Any breast feeding at 3 months post discharge | Study population | RR 1.31 | 1063 | ⊕⊝⊝⊝ | ||
| 60 per 100 | 78 per 100 | |||||
| Any breast feeding at 6 months post discharge | Study population | RR 1.25 | 886 | ⊕⊝⊝⊝ | ||
| 45 per 100 | 56 per 100 | |||||
| Length of hospital stay | MD 2.25 higher | ‐ | 1004 | ⊕⊝⊝⊝ | ||
| Episodes of infection | Study population | RR 0.70 | 500 | ⊕⊕⊕⊝ | ||
| 7 per 100 | 5 per 100 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| aAttrition bias (14% and 15% attrition in two included studies). bModerate heterogeneity (I2 = 52%). cModerate heterogeneity (I2 = 73%). dModerate heterogeneity (I2 = 71%). | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Full breast feeding at discharge Show forest plot | 6 | 1074 | Risk Ratio (M‐H, Random, 95% CI) | 1.47 [1.19, 1.80] |
| 1.1 Breast feeding + Cup vs Breast feeding + Bottle | 4 | 893 | Risk Ratio (M‐H, Random, 95% CI) | 1.41 [1.14, 1.75] |
| 1.2 Breast feeding + Tube vs Breast feeding + Bottle | 1 | 84 | Risk Ratio (M‐H, Random, 95% CI) | 2.10 [1.46, 3.03] |
| 1.3 Breast feeding + novel teat and bottle vs Breast feeding + conventional teat and bottle | 1 | 97 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.63, 1.82] |
| 2 Fully breast feeding at 3 months post discharge Show forest plot | 4 | 986 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.56 [1.37, 1.78] |
| 2.1 Breast feeding + Cup vs Breast feeding + Bottle | 2 | 805 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.54 [1.34, 1.77] |
| 2.2 Breast feeding + Tube vs Breast feeding + Bottle | 1 | 84 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.31 [1.28, 4.17] |
| 2.3 Breast feeding + novel teat and bottle vs Breast feeding + conventional teat and bottle | 1 | 97 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.57, 2.41] |
| 3 Fully breast feeding at 6 months post discharge Show forest plot | 3 | 887 | Risk Ratio (M‐H, Random, 95% CI) | 1.64 [1.14, 2.36] |
| 3.1 Breast feeding + Cup vs Breast feeding + Bottle | 2 | 803 | Risk Ratio (M‐H, Random, 95% CI) | 1.40 [1.18, 1.65] |
| 3.2 Breast feeding + Tube vs Breast feeding + Bottle | 1 | 84 | Risk Ratio (M‐H, Random, 95% CI) | 2.94 [1.36, 6.34] |
| 4 Any breast feeding at discharge Show forest plot | 6 | 1138 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [1.06, 1.16] |
| 4.1 Breast feeding + Cup vs Breast feeding + Bottle | 4 | 957 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [1.03, 1.15] |
| 4.2 Breast feeding + Tube vs Breast feeding + Bottle | 1 | 84 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.37 [1.08, 1.74] |
| 4.3 Breast feeding + novel teat and bottle vs Breast feeding + conventional teat and bottle | 1 | 97 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.95, 1.33] |
| 5 Any breast feeding at 3 months post discharge Show forest plot | 5 | 1063 | Risk Ratio (M‐H, Random, 95% CI) | 1.31 [1.01, 1.71] |
| 5.1 Breast feeding + Cup vs Breast feeding + Bottle | 3 | 883 | Risk Ratio (M‐H, Random, 95% CI) | 1.24 [0.89, 1.71] |
| 5.2 Breast feeding + Tube vs Breast feeding + Bottle | 1 | 83 | Risk Ratio (M‐H, Random, 95% CI) | 1.69 [1.19, 2.41] |
| 5.3 Breast feeding + novel teat and bottle vs Breast feeding + conventional teat and bottle | 1 | 97 | Risk Ratio (M‐H, Random, 95% CI) | 1.20 [0.80, 1.80] |
| 6 Any breast feeding at 6 months post discharge Show forest plot | 3 | 886 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [1.10, 1.41] |
| 6.1 Breast feeding + Cup vs Breast feeding + Bottle | 2 | 803 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.20 [1.06, 1.36] |
| 6.2 Breast feeding + Tube vs Breast feeding + Bottle | 1 | 83 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.07 [1.18, 3.64] |
| 7 Days to reach full sucking feeds Show forest plot | 3 | 429 | Mean Difference (IV, Random, 95% CI) | 2.56 [‐7.17, 12.28] |
| 7.1 Breast feeding + Cup vs Breast feeding + Bottle | 2 | 332 | Mean Difference (IV, Random, 95% CI) | 5.08 [‐6.43, 16.59] |
| 7.2 Breast feeding + novel teat and bottle vs Breast feeding + conventional teat and bottle | 1 | 97 | Mean Difference (IV, Random, 95% CI) | ‐4.0 [‐15.63, 7.63] |
| 8 Weight gain Show forest plot | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 8.1 Measured from birth to discharge home (g/kg/day) | 1 | 293 | Mean Difference (IV, Fixed, 95% CI) | ‐0.09 [‐0.77, 0.59] |
| 8.2 Measured for one week after commencing oral feeds (g/kg/day) | 1 | 78 | Mean Difference (IV, Fixed, 95% CI) | ‐0.60 [‐3.21, 2.01] |
| 8.3 Measured for one week after commencing oral feeds (g/day) | 1 | 522 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.36, 0.16] |
| 9 Length of hospital stay Show forest plot | 4 | 1004 | Mean Difference (IV, Random, 95% CI) | 2.25 [‐3.36, 7.86] |
| 9.1 Breast feeding + Cup vs Breast feeding + Bottle | 2 | 823 | Mean Difference (IV, Random, 95% CI) | 4.45 [‐5.57, 14.48] |
| 9.2 Breast feeding + Tube vs Breast feeding + Bottle | 1 | 84 | Mean Difference (IV, Random, 95% CI) | 1.60 [‐5.89, 9.09] |
| 9.3 Breast feeding + novel teat and bottle vs Breast feeding + conventional teat and bottle | 1 | 97 | Mean Difference (IV, Random, 95% CI) | ‐4.90 [‐17.25, 7.45] |
| 10 Duration of supplementary feed Show forest plot | 2 | 600 | Mean Difference (IV, Random, 95% CI) | ‐0.42 [‐1.96, 1.12] |
| 11 Episodes of infection Show forest plot | 3 | 500 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.35, 1.42] |

