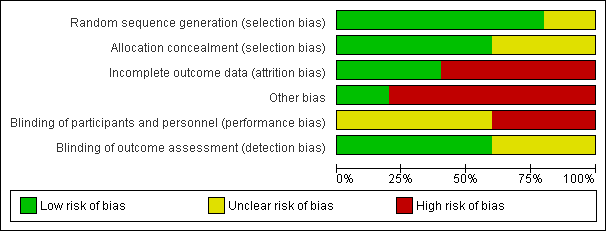
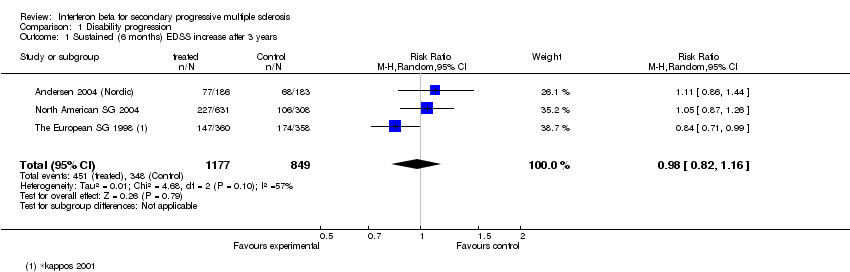
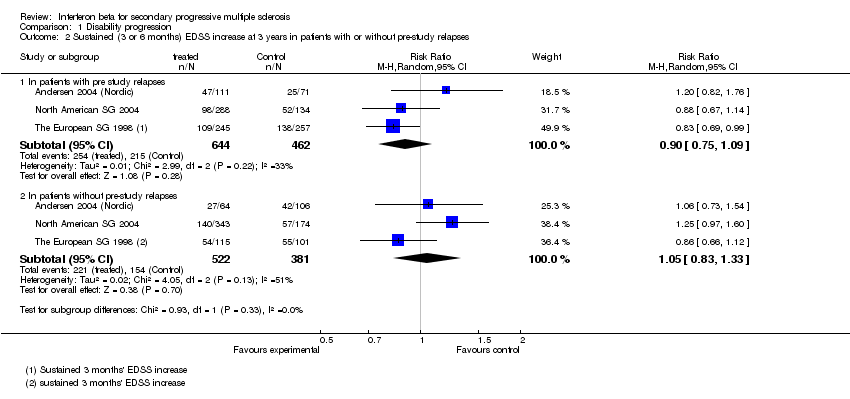
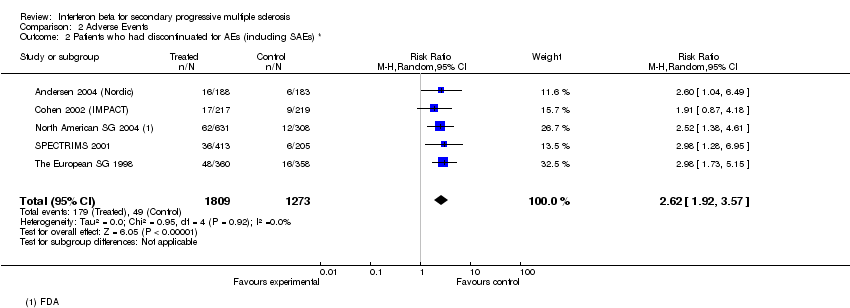
Contenido relacionado
Revisiones y protocolos relacionados
Yousheng Xiao, Jianyi Huang, Hongye Luo, Jin Wang | 7 febrero 2014
Eugenio Pucci, Giorgio Giuliani, Alessandra Solari, Silvana Simi, Silvia Minozzi, Carlo Di Pietrantonj, Ian Galea | 5 octubre 2011
Jian Zhang, Shengliang Shi, Yueling Zhang, Jiefeng Luo, Yousheng Xiao, Lian Meng, Xiaobo Yang | 27 noviembre 2017
Loredana La Mantia, Carlo Di Pietrantonj, Marco Rovaris, Giulio Rigon, Serena Frau, Francesco Berardo, Anna Gandini, Anna Longobardi, Bianca Weinstock‐Guttman, Alberto Vaona | 24 noviembre 2016
Dian He, Chao Zhang, Xia Zhao, Yifan Zhang, Qingqing Dai, Yuan Li, Lan Chu | 22 marzo 2016
Graziella Filippini, Cinzia Del Giovane, Laura Vacchi, Roberto D'Amico, Carlo Di Pietrantonj, Deirdre Beecher, Georgia Salanti | 6 junio 2013
Juan Ignacio Rojas, Marina Romano, Agustín Ciapponi, Liliana Patrucco, Edgardo Cristiano | 20 enero 2010
Jia Liu, Lu‐Ning Wang, Siyan Zhan, Yinyin Xia | 23 diciembre 2013
Loredana La Mantia, Irene Tramacere, Belal Firwana, Ilaria Pacchetti, Roberto Palumbo, Graziella Filippini | 19 abril 2016
Jin Wang, Yousheng Xiao, Man Luo, Hongye Luo | 7 diciembre 2011
Respuestas clínicas Cochrane
Jane Burch, Sera Tort | 26 marzo 2021