Adenosina versus antagonistas de los canales del calcio intravenosos para el tratamiento de la taquicardia supraventricular en adultos
Información
- DOI:
- https://doi.org/10.1002/14651858.CD005154.pub4Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 12 octubre 2017see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Corazón
- Copyright:
-
- Copyright © 2017 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
S Alabed: selection of studies, data extraction and analysis, and review writing and editing.
A Sabouni: selection of studies and data extraction.
R Providencia: review editing and clinical expertise.
E Atallah: co‐writing of review and data extraction.
M Qintar: review editing, selection of studies, data extraction, and clinical expertise.
T JA Chicho: review editing, data extraction, and clinical expertise.
Sources of support
Internal sources
-
None, Not specified.
External sources
-
National Institute for Health Research (NIHR), UK.
S Alabed currently holds an NIHR Academic Clinical Fellowship (ACF)
-
National Institutes of Health (NIH), USA.
M Qintar is supported by The National Heart, Lung, and Blood Institute of the NIH under Award Number T32HL110837
Declarations of interest
SA: none known.
AS: none known.
RP: has received a research grant from Medtronic for a clinical epidemiology study on sudden cardiac death, and proctored and lectured for Medtronic and Pfizer, respectively, on topics related to atrial fibrillation. However, these topics are not directly related to treatment of supraventricular arrhythmias (which do not include atrial fibrillation) in A&E.
EA: none known.
MQ: none known.
T JA C: none known.
Acknowledgements
The authors of this updated review thank the authors of the original version of this review, A Holdgate and A Foo.
The authors of this review update would like to thank Charlene Bridge, Nicole Martin and all the peer reviewers, editors and copy‐editors from the Cochrane Heart Group for providing precious help.
Version history
| Published | Title | Stage | Authors | Version |
| 2017 Oct 12 | Adenosine versus intravenous calcium channel antagonists for supraventricular tachycardia | Review | Samer Alabed, Ammar Sabouni, Rui Providencia, Edmond Atallah, Mohammed Qintar, Timothy JA Chico | |
| 2012 Feb 15 | Adenosine versus intravenous calcium channel antagonists for the treatment of supraventricular tachycardia in adults | Review | Anna Holdgate, Angeline Foo | |
| 2006 Oct 18 | Adenosine versus intravenous calcium channel antagonists for the treatment of supraventricular tachycardia in adults | Review | Anna Holdgate, Angeline Foo | |
| 2005 Jan 24 | Adenosine versus intravenous calcium channel antagonists for the treatment of supraventricular tachycardia in adults | Protocol | Anna Holdgate, Angeline Foo | |
Differences between protocol and review
Differences between original review in 2006 and update in 2017
The main changes in review methods compared with those used in the original review include the following.
-
Excluding studies of induced SVT: We excluded studies involving induced SVTs as they are not relevant to patients presenting acutely to the emergency department. Patients with inducible SVT may not necessarily be affected by SVT in their daily life. Induced SVTs can be terminated with pacing manoeuvres, whereas spontaneous SVTs treated in emergency rooms/A&E may last for hours and may require IV treatment for control.
-
Excluding quasi‐randomised trials: Although the review protocol mentioned inclusion of quasi‐RCTs, we decided to exclude trials with major violations in randomisation methods or treatment allocation. We also excluded studies reported to be randomised but showing no data on baseline differences between treatment interventions, and those in which major differences occurred at a rate of > 1 per 20 comparisons (which makes them unlikely to have occurred by chance) (Carlisle 2015; Carlisle 2017). When we had concerns about study methods, we excluded the study if study authors did not respond to our requests for clarification.
-
Using odds ratio instead of Peto odds ratio: The Cochrane Handbook for Systematic Reviews of Interventions discourages use of the Peto odds ratio and recommends use of the odds ratio instead (Higgins 2011).
-
Summary of findings tables: We prepared these in accordance with new requirements provided in the Cochrane Handbook for Systematic Reviews of Interventions.
-
Search for ongoing trials: The protocol and the original review did not plan or perform this.
-
Remove "in adults" from title: The protocol did not attempt to include adults only, and the original review included only one study in children (Greco 1982).
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
- Adenosine [adverse effects, *therapeutic use];
- Anti‐Arrhythmia Agents [adverse effects, *therapeutic use];
- Calcium Channel Blockers [adverse effects, *therapeutic use];
- Emergency Service, Hospital [statistics & numerical data];
- Hypotension [chemically induced];
- Randomized Controlled Trials as Topic;
- Tachycardia, Supraventricular [*drug therapy];
- Verapamil [adverse effects, therapeutic use];
Medical Subject Headings Check Words
Adult; Humans;
PICO

Study flow diagram.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
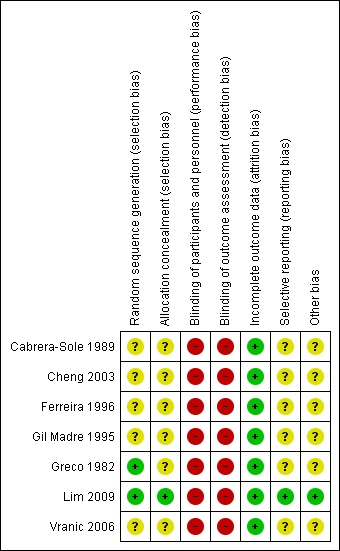
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
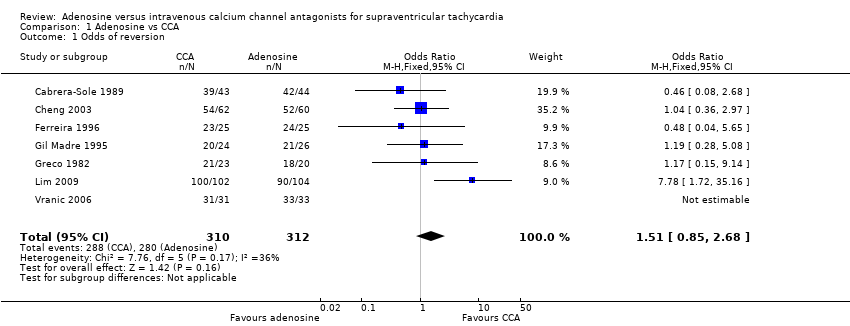
Comparison 1 Adenosine vs CCA, Outcome 1 Odds of reversion.
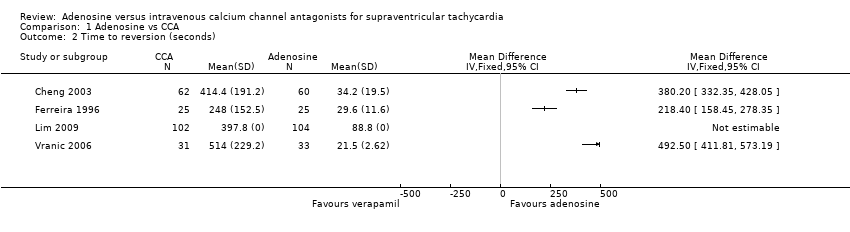
Comparison 1 Adenosine vs CCA, Outcome 2 Time to reversion (seconds).

Comparison 1 Adenosine vs CCA, Outcome 3 Relapse to SVT post reversion.

Comparison 1 Adenosine vs CCA, Outcome 4 Minor adverse events.

Comparison 1 Adenosine vs CCA, Outcome 5 Hypotension.
| Adenosine compared with calcium channel antagonists for supraventricular tachycardia | |||||||||
| Patient or population: patients with supraventricular tachycardia | |||||||||
| Outcomes | Number of participants | Number of studies | Odds ratio | Absolute effects (95% CI) | Follow‐up | Quality of the evidence | What happens | ||
| With adenosine | With CCA | Difference | |||||||
| Odds of reversion | 622 | 7 RCTs | OR 1.51 (0.85 to 2.68) | 89.7% | 92.9% | 3.2% lower odds of reversion with adenosine | Until reversion occurred | ⊕⊕⊕⊝ | Higher odds of reversion indicate better effect. |
| Major adverse event: | 306 | 3 RCTs | OR 3.09 | 0.0% | 0.0% (0.0 to 0.0) | 0.0% fewer (0 fewer to 0 fewer) | Up to 2 hours after infusion | ⊕⊕⊝⊝ | Lower hypotension rate indicates fewer adverse events. |
| Length of stay in hospital | Not reported | 0 | |||||||
| Patient satisfaction | Not reported | 0 | |||||||
| CI: confidence interval; OR: odds ratio. | |||||||||
| GRADE Working Group grades of evidence | |||||||||
| aQuality of the evidence downgraded by one level for imprecision. Moderate to wide confidence intervals. bQuality of the evidence downgraded by one level for study limitations. Judgements of high risk of bias in all studies, as none of the studies were blinded. | |||||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Odds of reversion Show forest plot | 7 | 622 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.51 [0.85, 2.68] |
| 2 Time to reversion (seconds) Show forest plot | 4 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3 Relapse to SVT post reversion Show forest plot | 4 | 358 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.09, 1.69] |
| 4 Minor adverse events Show forest plot | 3 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Chest tightness | 3 | 222 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.09 [0.02, 0.50] |
| 4.2 Shortness of breath | 2 | 171 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.23 [0.04, 1.37] |
| 4.3 Flushing | 1 | 50 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.01 [0.00, 0.24] |
| 5 Hypotension Show forest plot | 3 | 306 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.09 [0.12, 76.71] |

