نقش مداخلات در پیشگیری و درمان بیماریهای کلیه در پورپورای هنوخ‐شوئنلاین (HSP)
چکیده
پیشینه
پورپورای هنوخ‐شوئنلاین (Henoch‐Schönlein Purpura; HSP) شایعترین واسکولیت (vasculitis) دوران کودکی است اما ممکن است در بزرگسالان نیز رخ دهد. این نوع واسکولیت عروق کوچک با پورپورای قابل لمس، درد شکم، آرتریت (arthritis) یا آرترالژی (arthralgia) و درگیری کلیه مشخص میشود. این یک نسخه بهروز شده از مروری است که نخستینبار در سال 2009 منتشر شد.
اهداف
ارزیابی مزایا و آسیبهای عوامل مختلف دارویی (به صورت جداگانه یا ترکیبی) در مقایسه با دارونما (placebo)، عدم‐درمان یا هر عامل دیگری برای: (1) پیشگیری از بروز بیماریهای شدید کلیه در بیماران مبتلا به HSP بدون بیماریهای کلیه در زمان مراجعه؛ (2) پیشگیری از بروز بیماریهای شدید کلیه در بیماران مبتلا به HSP و بیماریهای خفیف کلیه (هماچوری میکروسکوپی (microscopic haematuria)، پروتئینوری (proteinuria) خفیف) در زمان مراجعه؛ (3) درمان بیماریهای شدید کلیه (هماچوری ماکروسکوپی، پروتئینوری، سندرم نفروتیک (nephrotic syndrome)، سندرم نفروتیک با یا بدون نارسایی حاد کلیه) در HSP؛ و (4) پیشگیری از اپیزودهای مکرر بیماریهای کلیه مرتبط با HSP.
روشهای جستوجو
از طریق برقراری ارتباط با هماهنگکننده جستوجوی کارآزماییها (Trials' Search Co‐ordinator) و با جستوجوی واژگان و اصطلاحات مرتبط با این مرور، پایگاه ثبت تخصصی گروه کلیه و پیوند در کاکرین (Cochrane Kidney and Transplant Specialised Register) را تا تاریخ 13 جولای 2015 جستوجو کردیم.
معیارهای انتخاب
کارآزماییهای تصادفیسازی و کنترل شدهای (randomised controlled trials; RCTs) را وارد مرور کردیم که مداخلات مورد استفاده را برای پیشگیری یا درمان بیماریهای کلیه در HSP در مقابل دارونما، عدم‐درمان یا سایر عوامل مقایسه کردند.
گردآوری و تجزیهوتحلیل دادهها
دو نویسنده بهطور مستقل از هم واجد شرایط بودن مطالعه را تعیین کردند، خطر سوگیری (bias) را بررسی کرده و دادهها را از هر مطالعه استخراج کردند. آنالیزهای آماری با استفاده از مدل اثرات‐تصادفی انجام شده و نتایج در قالب خطر نسبی (RR) یا تفاوت خطر (RD) برای پیامدهای دو‐حالتی، و تفاوت میانگین (MD) برای پیامدهای پیوسته با 95% فواصل اطمینان (CI) بیان شدند.
نتایج اصلی
سیزده مطالعه (1403 بیمار ثبتنام شده) شناسایی شدند. مشخصههای خطرات سوگیری (bias) اغلب ضعیف انجام شدند. خطر پائین سوگیری در شش مطالعه (50%) برای تولید توالی (سوگیری انتخاب) و در هفت مطالعه (58%) برای پنهانسازی تخصیص (سوگیری انتخاب) گزارش شد. کورسازی شرکتکنندگان و پرسنل (سوگیری عملکرد) و ارزیابی پیامد (سوگیری تشخیص) در سه مطالعه در معرض خطر پائین سوگیری بود. پنج مطالعه دادههای کامل پیامد (سوگیری ریزش نمونه (attrition bias)) را گزارش کردند در حالی که هشت مطالعه پیامدهای مورد انتظار را گزارش کردند، بنابراین در معرض خطر پائین سوگیری گزارشدهی قرار داشتند.
هشت مطالعه درمان را برای پیشگیری از بیماریهای پایدار کلیه در HSP ارزیابی کردند. هیچ تفاوت معنیداری در خطر ابتلا به بیماریهای پایدار کلیه در هر زمان پس از درمان (5 مطالعه، 746 کودک: RR: 0.74؛ 95% CI؛ 0.42 تا 1.32)، یا در یک، سه، شش و 12 ماه در کودکانی که به دلیل ابتلا به HSP در زمان مراجعه به مدت 14 تا 28 روز به آنها پردنیزون (prednisone) داده شد، در مقایسه با دارونما یا درمان حمایتی، وجود نداشت. تفاوت معنیداری در خطر بیماریهای پایدار کلیه با درمان ضد‐پلاکت در کودکان با یا بدون بیماریهای کلیه در بدو ورود وجود نداشت. هپارین (heparin) در مقایسه با دارونما بهطور قابلتوجهی خطر بیماریهای پایدار کلیه را در مدت سه ماه کاهش داد (1 مطالعه، 228 کودک: RR: 0.27؛ 95% CI؛ 0.14 تا 0.55)؛ خونریزی قابلتوجهی رخ نداد. چهار مطالعه درمان بیماریهای شدید کلیه مرتبط با HSP را بررسی کردند. دو مطالعه (یکی شامل 56 کودک و دیگری شامل 54 بزرگسال) سیکلوفسفامید (cyclophosphamide) را با دارونما یا درمان حمایتی مقایسه کرده و هیچ مزیت قابلتوجهی را از سیکلوفسفامید نیافتند. تفاوت معنیداری در عوارض جانبی وجود نداشت. در یک مطالعه که سیکلوسپورین (cyclosporin) را با متیلپردنیزولون (methylprednisolone) مقایسه کرد (15 کودک) تفاوت معنیداری در بهبودی در پیگیری نهایی با میانگین 6.3 سال وجود نداشت (RR: 1.37؛ 95% CI؛ 0.74 تا 2.54). در یک مطالعه (17 کودک) که مایکوفنولات موفتیل (mycophenolate mofetil) را با آزاتیوپرین (azathioprine) مقایسه کرد، تفاوت معنیداری در بهبودی پروتئینوری در یک سال وجود نداشت (RR: 1.32؛ 95% CI؛ 0.86 تا 2.03). هیچ مطالعهای شناسایی نشد که اثربخشی درمان را بر بیماریهای کلیه در شرکتکنندگان با اپیزودهای مکرر HSP ارزیابی کند.
نتیجهگیریهای نویسندگان
هیچ تغییر اساسی در نتیجهگیریهای حاصل از این نسخه بهروز شده در مقایسه با مرور اولیه دیده نشد. از شواهد کلی با کیفیت پائین، هیچ شواهدی را از RCTها مبنی بر مزیت استفاده از پردنیزون یا عوامل ضد‐پلاکت در پیشگیری از بروز بیماریهای پایدار کلیه در کودکان مبتلا به HSP پیدا نکردیم. اگرچه هپارین موثر به نظر میرسد، استفاده از این درمان بالقوه خطرناک برای پیشگیری از بروز بیماریهای جدی کلیه زمانی که کمتر از 2% از کودکان مبتلا به HSP به بیماریهای شدید کلیه مبتلا میشوند، قابل توجیه نیست. هیچ شواهدی مبنی بر مزیت درمان با سیکلوفسفامید در کودکان یا بزرگسالان مبتلا به HSP و بیماریهای شدید کلیه یافت نشد. به دلیل تعداد کم بیماران و رویدادهایی که منجر به عدم‐دقت در نتایج میشود، هنوز مشخص نیست که سیکلوسپورین و مایکوفنولات موفتیل نقشی در درمان کودکان مبتلا به HSP و بیماریهای شدید کلیه دارند یا خیر.
PICO
خلاصه به زبان ساده
مداخلات برای پیشگیری و درمان بیماریهای کلیه در پورپورای هنوخ‐شوئنلاین
پورپورای هنوخ‐شوئنلاین (Henoch‐Schönlein Purpura; HSP) باعث التهاب عروق خونی کوچک در کودکان شده و سالانه تقریبا 20/100,000 کودک را تحت تاثیر قرار میدهد. نشانهها و علائم آن عبارتند از: بثورات پوستی پورپوریک (شامل لکههای کوچک و کبودیهای بزرگتر)، درد شکم، خونریزی گوارشی، درد و تورم مفاصل، تورم صورت و شواهدی از بیماریهای کلیه همراه با وجود خون و پروتئین در ادرار. بیماریهای کلیه حدود یک‐سوم از کودکان مبتلا به HSP را درگیر میکند. در اکثر آنها این بیماری خفیف است (فقط مقادیر کمی خون در ادرار) و بهطور کامل برطرف میشود، اما تعداد کمی از کودکان دچار بیماریهای پایدار کلیه میشوند که میتواند به نارسایی کلیه تبدیل شود.
این مرور 13 مطالعه (1403 شرکتکننده) را شناسایی کرد که تاثیر مداخلاتی را در پیشگیری از بروز بیماریهای پایدار کلیه مرتبط با HSP یا درمان بیماریهای شدید کلیه بررسی کردند. پنج مطالعه (856 کودک ثبتنام شده) مصرف قرصهای پردنیزون (prednisone) را به مدت 14 تا 28 روز با قرصهای دارونما (placebo) یا عدم‐درمان خاص برای پیشگیری از برزو بیماریهای پایدار کلیه در 6 تا 12 ماه پس از شروع HSP مقایسه کردند. در این مطالعات کاهش قابلتوجهی در فراوانی بیماریهای پایدار کلیه نشان داده نشد. دو مطالعه (129 کودک) هیچ مزیتی را از آسپرین (aspirin) و دیپیریدامول (dipyridamole) (عوامل ضد‐پلاکت) در پیشگیری از بروز بیماریهای پایدار کلیه نشان ندادند. یک مطالعه (228 کودک) نشان داد که هپارین (heparin) تزریقی میتواند خطر ابتلا به بیماریهای پایدار کلیه را کاهش دهد، اما این درمان دارای عوارض جانبی بالقوه از جمله خونریزی شدید است، بنابراین تجویز آن زمانی که فقط یک‐سوم کودکان به هر نوعی از از بیماریهای کلیه مبتلا میشوند، قابل توجیه نیست، این عارضه اغلب جدی نیست و بهطور کامل برطرف میشود. به نظر میرسد که هیچ عارضه جانبی جدی در این مطالعات رخ نداده، اما اطلاعات ارائه شده در مورد عوارض جانبی محدود بود.
در بیماران مبتلا به بیماریهای جدی کلیه، دو مطالعه (یکی در بزرگسالان و دیگری بر کودکان) نشان داد که سیکلوفسفامید (cyclophosphamide) در پیشگیری از آسیب پیشرونده کلیه موثرتر از دارونما یا درمان حمایتی نیست. دو مطالعه کوچک که سیکلوسپورین (cyclosporin) را با متیلپردنیزولون (methylprednisolone)/پردنیزون (15 کودک) و مایکوفنولات موفتیل (mycophenolate mofetil) با آزاتیوپرین (azathioprine) (17 کودک) مقایسه کردند، مزیت قابلتوجهی را از سیکلوسپورین یا مایکوفنولات پیدا نکردند. با این حال، تعداد کودکانی که مورد مطالعه قرار گرفتند بسیار کم بود و نمیتوان وجود مزیت را کاملا رد کرد، بنابراین انجام مطالعات بیشتری لازم است. هیچ عارضه جانبی جدی گزارش نشد.
دادههای کمی از مطالعات تصادفیسازی و کنترل شدهای وجود دارد که مداخلات مورد استفاده را برای پیشگیری یا درمان بیماریهای جدی کلیه در HSP بررسی میکنند، به جز مصرف کوتاه‐مدت پردنیزون برای پیشگیری از بروز بیماریهای کلیه. شواهدی مبنی بر مزیت استفاده از پردنیزون در دورههای کوتاه‐مدت برای پیشگیری از بروز بیماریهای جدی کلیه در HSP وجود نداشت.
Authors' conclusions
Summary of findings
| Prednisone versus placebo or supportive treatment for preventing persistent kidney disease in patients with Henoch‐Schönlein Purpura (HSP) | ||||||
| Patient or population: patients with HSP | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo or supportive treatment | Prednisone | |||||
| Persistent kidney disease at any time after treatment | Study population | RR 0.74 | 746 (5) | ⊕⊕⊕⊝ | ||
| 143 per 1000 | 106 per 1000 | |||||
| Moderate | ||||||
| 105 per 1000 | 78 per 1000 | |||||
| Number of children with any continuing kidney disease at 3 months | Study population | RR 0.83 | 655 (4) | ⊕⊕⊕⊝ | ||
| 199 per 1000 | 165 per 1000 | |||||
| Moderate | ||||||
| 156 per 1000 | 129 per 1000 | |||||
| Number of children with any continuing kidney disease at 6 months | Study population | RR 0.51 | 379 (3) | ⊕⊕⊕⊝ | ||
| 100 per 1000 | 51 per 1000 | |||||
| Moderate | ||||||
| 53 per 1000 | 27 per 1000 | |||||
| Number of children with any continuing kidney disease at 12 months | Study population | RR 1.06 | 455 (3) | ⊕⊕⊝⊝ | ||
| 84 per 1000 | 89 per 1000 | |||||
| Moderate | ||||||
| 105 per 1000 | 111 per 1000 | |||||
| Any continuing kidney disease at 3 months (study with high risk of bias excluded) | Study population | RR 0.98 | 487 (3) | ⊕⊕⊕⊕ | ||
| 243 per 1000 | 238 per 1000 | |||||
| Moderate | ||||||
| 207 per 1000 | 203 per 1000 | |||||
| Any continuing kidney disease at 12 months (study with high risk of bias excluded) | Study population | RR 1.39 | 287 (2) | ⊕⊕⊕⊝ | ||
| 105 per 1000 | 146 per 1000 | |||||
| Moderate | ||||||
| 105 per 1000 | 146 per 1000 | |||||
| Number developing severe kidney disease | Study population | RR 1.58 | 418 (2) | ⊕⊕⊝⊝ | ||
| 14 per 1000 | 22 per 1000 | |||||
| Moderate | ||||||
| 17 per 1000 | 27 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Two studies had unclear or biased allocation concealment & were not blinded | ||||||
Background
Description of the condition
Henoch‐Schönlein Purpura (HSP) is a primary small vessel, non‐granulomatous vasculitis. It is the most common systemic vasculitis in children occurring between the ages of three and 15 years, although adults may also be affected. The annual incidence in a UK population based study was 20/100,000 in children < 17 years of age with a peak incidence of 70/100,000 in children between four and six years of age (Gardner‐Medwin 2002). Clinically the disease is characterised by a tetrad of features including palpable purpura, arthritis or arthralgia, abdominal pain and kidney disease (Saulsbury 1999). According to the 2012 Revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides HSP, (IgA vasculitis) is classified as vasculitis with IgA1‐dominant immune deposits affecting small vessels, predominantly capillaries, venules or arterioles (Jennette 2013). Glomerulonephritis (GN) is the major complication of HSP. Kidney involvement is clinically manifested by microscopic or macroscopic haematuria, proteinuria, nephrotic syndrome and reduced kidney function. In a systematic review of studies (Narchi 2005) of unselected patients, kidney involvement occurred in 34% of children; 80% had isolated haematuria, proteinuria or both while 20% had acute nephritic syndrome or nephrotic syndrome. Kidney disease, if it did occur, developed early ‐ by four weeks in 85% and by six months in nearly all children. Persistent kidney disease (hypertension, reduced function, nephrotic or nephritic syndrome) occurred in 1.8% of children overall but the incidence varied with the severity of the kidney disease at presentation. In general, the prognosis for long‐term kidney function in HSP is excellent in children with microscopic or macroscopic haematuria alone. However patients with nephrotic syndrome and reduced kidney function frequently show a progressive course to end‐stage kidney disease (ESKD). In a study of 78 children with HSP and kidney involvement presenting to two paediatric nephrology services, 44% of children presenting with acute nephritic syndrome, nephrotic syndrome or both compared with 13% presenting with haematuria, proteinuria or both had hypertension or impaired kidney function at a mean follow‐up of 23.4 years (Goldstein 1992).
Description of the intervention
Corticosteroid therapy is commonly used in the acute phase of HSP, particularly for abdominal pain. Controversy has existed as to whether corticosteroids can prevent the development of kidney involvement, reduce its severity or both in HSP. One systematic review concluded that early corticosteroid therapy may reduce the risk of developing persistent kidney disease (Weiss 2007). However two other systematic reviews concluded that the benefit of corticosteroids in preventing persistent kidney disease remained unproven (Wyatt 2001; Zaffanello 2007). There is also considerable uncertainty about the efficacy of therapies to prevent progression to chronic or ESKD in children with HSP‐associated acute nephritis or nephrotic syndrome. Corticosteroid therapy, azathioprine, mycophenolate mofetil, cyclophosphamide, cyclosporin, antiplatelet therapy, anticoagulants and plasmapheresis have been used in such patients (Bergstein 1998; Du 2012; Flynn 2001; Foster 2000; Iijima 1998; Niaudet 1998; Ronkainen 2003; Shenoy 2007) with varying results. However the data come from largely from observational studies rather than from randomised controlled trials (RCTs).
How the intervention might work
HSP nephritis (HSPN) is due to a systemic vasculitis with deposition of immune deposits of IgA1 in the mesangium, activation of the alternative complement pathway and inflammation. Therefore it is has been argued that medications, which treat other immune diseases including kidney diseases, would have a role in preventing or treating HSPN. In particular it was postulated that corticosteroids could prevent the development of significant HSPN in children presenting with HSP. The use of other immunosuppressive agents is based on their efficacy in preventing kidney transplant rejection and in treating other immune complex diseases such as systemic lupus erythematosus. Urokinase, dipyridamole and warfarin have been used because of their roles in inhibiting the mediators of glomerular damage (Kawasaki 2004). Angiotensin‐converting enzyme inhibitors (ACEi) and angiotensin‐receptor blockers (ARB) would be expected to reduce proteinuria via effects on intraglomerular haemodynamics.
Why it is important to do this review
Although multiple treatment modalities have been used to prevent or to treat HSPN, there is no consensus on the efficacy of various therapies. The aims of this systematic review were to determine the benefits and harms of different interventions used to prevent or treat persistent kidney disease in HSP in children and adults. The scope was deliberately broad because RCTs in HSP are few, and variability in the spectrum of kidney disease included in the relevant studies was very likely. This update of this systematic review, originally published in 2009 (Chartapisak 2009), aimed to incorporate any further data from RCTs to provide additional evidence for or against the use of corticosteroids or other therapies to prevent HSPN, for or against the use of immunosuppressive agents to treat established HSPN and to determine the efficacy of ACEi or ARB in reducing proteinuria.
Objectives
To evaluate the benefits and harms of different agents (used singularly or in combination) compared with placebo, no treatment or any other agent for:
-
The prevention of severe kidney disease in patients with HSP without kidney disease at presentation.
-
The prevention of severe kidney disease in patients with HSP and minor kidney disease (microscopic haematuria, mild proteinuria) at presentation.
-
The treatment of established severe kidney disease (macroscopic haematuria, proteinuria, nephritic syndrome, nephrotic syndrome with or without acute kidney failure) in HSP.
-
The prevention of recurrent episodes of HSP‐associated kidney disease.
Methods
Criteria for considering studies for this review
Types of studies
All RCTs and quasi‐RCTS (RCTs in which allocation to treatment was obtained by alternation, use of alternate medical records, date of birth or other predictable methods) looking at the benefits and harms of different therapeutic modalities for the prevention or treatment of kidney disease in HSP. If crossover studies were identified, the first period of randomised crossover studies were to be included.
Types of participants
Inclusion criteria
Patients of any age with HSP with or without kidney disease manifestations (microscopic haematuria, macroscopic haematuria, proteinuria, nephrotic syndrome, acute nephritic syndrome, reduced function, acute kidney failure).
Exclusion criteria
Patients with other forms of primary or secondary GN such as IgA nephropathy, mesangiocapillary GN, membranous GN, systemic lupus erythematosus, rapidly progressive GN not associated with HSP, other systemic vasculitides.
Types of interventions
-
Immunosuppressive agents including corticosteroids, alkylating agents (cyclophosphamide, chlorambucil), azathioprine, mycophenolate, cyclosporin and rituximab
-
Anticoagulants and antiplatelet agents including warfarin, dipyridamole, aspirin, heparin
-
ACEi and ARB
-
Fish oil
-
Immunoglobulin G, plasma exchange, antibody therapy
-
The above agents used individually or in combination were compared with placebo or no specific therapy or compared with other agents
-
Different durations, frequencies or modes of delivery of the same interventions.
Studies of therapies with herbal treatments and non‐pharmacological interventions were excluded.
Types of outcome measures
-
ESKD (including dialysis and transplantation)
-
Significant increase in serum creatinine (SCr) as defined by the investigators
-
Significant reduction in glomerular filtration rate (GFR) as defined by the investigators
-
Hypertension due to HSP‐associated kidney disease
-
Development, persistence or worsening of proteinuria as defined by the investigators
-
The development or persistence of nephrotic syndrome, nephritic syndrome, acute kidney insufficiency
-
Patient mortality
-
Biopsy result including percent of crescent formation, chronicity index, sclerosis, and fibrosis
-
Quality of life
-
Complications of therapy e.g. infection, bleeding, neutropenia, hypertension.
Primary outcomes
-
Reduction in kidney function including ESKD, acute kidney insufficiency or significant increase in SCr, GFR or both as defined by the investigators
-
Development, persistence or worsening of proteinuria, development of nephrotic syndrome or acute nephritic syndrome as defined by the investigators
-
Complications of therapy including infection, bleeding, leucopenia, hypertension
Secondary outcomes
-
Biopsy result including percent of crescent formation, chronicity index, sclerosis, and fibrosis.
-
Quality of life
-
Hypertension due to HSP‐associated kidney disease
-
Patient mortality
Search methods for identification of studies
Electronic searches
We searched the Cochrane Kidney and Transplant Specialised Register up to 13 July 2015 through contact with the Trials Search Co‐ordinator using search terms relevant to this review. The Cochrane Kidney and Transplant's Specialised Register contains studies identified from several sources the following sources.
-
Monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL)
-
Weekly searches of MEDLINE OVID SP
-
Handsearching of kidney‐related journals and the proceedings of major kidney conferences
-
Searching of the current year of EMBASE OVID SP
-
Weekly current awareness alerts for selected kidney and transplant journals
-
Searches of the International Clinical Trials Register (ICTRP) Search Portal and ClinicalTrials.gov.
Studies contained in the Specialised Register are identified through search strategies for CENTRAL, MEDLINE, and EMBASE based on the scope of Cochrane Kidney and Transplant. Details of these strategies, as well as a list of handsearched journals, conference proceedings and current awareness alerts, are available in the Specialised Register section of information about Cochrane Kidney and Transplant.
See Appendix 1 for search terms used in strategies for this review.
Searching other resources
Reference lists of clinical practice guidelines, review articles and relevant studies were also searched.
Data collection and analysis
Selection of studies
The search strategy described was used to obtain titles and abstracts of studies that might be relevant to the review. The titles and abstracts were screened independently by two authors, who discarded studies that were not applicable. However studies and reviews that might have included relevant data or information on studies were retained initially. Three authors independently assessed retrieved abstracts and, if necessary the full text, of these studies to determine which studies satisfied the inclusion criteria.
Data extraction and management
Data extraction was carried out independently by three authors using standard data extraction forms. Studies reported in non‐English language journals were translated before assessment. When more than one publication of one study was identified, reports were grouped together and the publication with the most complete data was used in the analyses. Where relevant outcomes were only published in earlier versions these data were used. Any discrepancy between published versions was highlighted. Where necessary, authors were contacted for additional information about their studies. Disagreements were resolved by discussion.
Assessment of risk of bias in included studies
The following items were used assessed using the risk of bias assessment tool (Higgins 2011) (Appendix 2).
-
Was there adequate sequence generation (selection bias)?
-
Was allocation adequately concealed (selection bias)?
-
Was knowledge of the allocated interventions adequately prevented during the study?
-
Participants and personnel (performance bias)
-
Outcome assessors (detection bias)
-
-
Were incomplete outcome data adequately addressed (attrition bias)?
-
Are reports of the study free of suggestion of selective outcome reporting (reporting bias)?
-
Was the study apparently free of other problems that could put it at a risk of bias?
Measures of treatment effect
For dichotomous outcomes (number with any kidney disease) results were expressed as risk ratio (RR) with 95% confidence intervals (CI). For continuous outcomes (severity or duration of haematuria or proteinuria, creatinine, GFR), the mean difference (MD) with 95% CI were calculated. Where possible the risk difference (RD) with 95% CI was calculated for each adverse effect. Otherwise any adverse effects were listed in the text for each intervention.
Unit of analysis issues
We planned to include data from the first part of any cross‐over study if the data could be separated. However no cross‐over studies were identified.
Dealing with missing data
Any further information required from the original author was requested by written correspondence and any relevant information obtained was included in the review. We aimed to analyse available data in meta‐analyses using the intention‐to‐treat (ITT) data. However where ITT data were only available graphically or were not provided and additional information could not be obtained from the authors, available data were used in analyses. Attrition rates, for example drop‐outs, losses to follow‐up and withdrawals were assessed.
Assessment of heterogeneity
Heterogeneity was analysed using a chi squared test on N‐1 degrees of freedom, with an alpha of 0.05 used for statistical significance and with the I² test (Higgins 2003). I² values of 25%, 50% and 75% correspond to low, medium and high levels of heterogeneity.
Assessment of reporting biases
We planned to assess for reporting bias using funnel plots. However we did not identify sufficient studies on any intervention to allow this assessment.
Data synthesis
We pooled data using the random effects model but we also analysed the fixed effect model to ensure robustness of the model chosen.
Subgroup analysis and investigation of heterogeneity
We planned subgroup analyses to explore possible sources of heterogeneity among participants (severity of kidney disease, kidney pathology, age), interventions (agent, dose and duration of treatment) or associated with risk of bias might explain any observed heterogeneity of treatment effects. Examination of these possible between‐study differences by subgroup analysis was not possible because of insufficient study data.
Sensitivity analysis
Sensitivity analysis was undertaken where significant heterogeneity among studies existed and single studies appeared to be responsible for this heterogeneity. Where required results were reported with and without inclusion of such single studies.
Results
Description of studies
Results of the search
In our 2009 review (Chartapisak 2009) a total of 909 reports were identified (Figure 1). Of these, 13 reports underwent full text review. Ten studies (11 reports) were included (Dudley 2013; He 2002; Huber 2004; Islek 1999; Jauhola 2010; Mollica 1992; Peratoner 1990; Ronkainen 2006a; Tarshish 2004; and one three‐armed study listed as two studies for analysis purposes Yoshimoto 1987a; Yoshimoto 1987b). Two studies were excluded (Hui‐Lan 2001; Jin 2003).

Flow diagram of included and excluded study in Review
In this review update, 37 new reports were identified. There were four reports of three new studies (CESAR Study 2010; Fuentes 2010; Xu 2009) and 10 reports of five already included studies (Dudley 2013; He 2002; Jauhola 2010; Ronkainen 2006a; Tarshish 2004). Sixteen additional studies (17 reports) were excluded.
Further information from the original authors was requested by written correspondence to five authors from whom additional trial information was obtained from three authors (Dudley 2013; Fuentes 2010; Jauhola 2010; Ronkainen 2006a).
Prior to publication a final search of the Specialised Register identified four new potential studies and these will be assessed for inclusion in a future update of this review (Ding 2014; NCT00301613; Wu 2013b; Wu 2014c).
Included studies
In this update 13 studies (26 reports) enrolling 1403 participants were included (CESAR Study 2010; Dudley 2013; Fuentes 2010; He 2002; Huber 2004; Islek 1999; Jauhola 2010; Mollica 1992; Peratoner 1990; Ronkainen 2006a; Tarshish 2004; Xu 2009; Yoshimoto 1987a; Yoshimoto 1987b). One study (Yoshimoto 1987a; Yoshimoto 1987b) compared two different interventions with a single control intervention and was treated as two studies for the analyses. Four studies were available in abstract form only (Fuentes 2010; Islek 1999; He 2002; Yoshimoto 1987a; Yoshimoto 1987b). Twelve studies were published in English; one study published in Chinese was translated before assessment.
Five studies (856 enrolled participants) examined the effects of short‐duration corticosteroids (14 to 28 days) on preventing persistent HSP‐associated kidney disease at six to 12 months after presentation in comparison with placebo (Dudley 2013; Huber 2004; Ronkainen 2006a) or supportive treatment (Islek 1999; Mollica 1992). Three studies included children with kidney disease at randomisation (Dudley 2013; Huber 2004; Ronkainen 2006a). Children considered to have established HSP‐associated kidney disease (proteinuria > 300 mg/L or haematuria > 10 red cells/high power field) were excluded from Ronkainen 2006a while Dudley 2013 and Huber 2004 included children with any degree of kidney disease at randomisation. Islek 1999 and Mollica 1992 only included children with no haematuria or proteinuria at presentation.
Peratoner 1990, Yoshimoto 1987a and Yoshimoto 1987b (129 children) evaluated antiplatelet agents (dipyridamole, cyproheptadine and salicylates) in comparison with supportive treatment and He 2002 (228 children) compared heparin with placebo. Peratoner 1990 provided outcome data separately for children with and without kidney disease at presentation while the other studies only included children without kidney disease at randomisation.
Five studies examined the treatment of severe HSP‐associated kidney disease (nephrotic range proteinuria, International Study of Kidney Disease in Children grade II‐IV changes on biopsy); Tarshish 2004 (56 children) compared cyclophosphamide with no specific treatment and Jauhola 2010 (19 children) compared cyclosporin with methylprednisolone. The CESAR Study 2010 evaluated 54 adults with severe biopsy proven HSP kidney disease including proliferative GN and compared cyclophosphamide and prednisone to prednisone only. Fuentes 2010 (15 children) compared azathioprine with mycophenolate mofetil with both treatment groups receiving prednisone. Xu 2009 evaluated fosinopril compared with supportive treatment in 48 children.
Outcomes were assessed at six to 12 months in eight studies (CESAR Study 2010; Dudley 2013; Fuentes 2010; Huber 2004; Jauhola 2010; Mollica 1992; Peratoner 1990; Ronkainen 2006a) and at two years in one study (Jauhola 2010). Tarshish 2004 reported the outcomes at the end of the study without providing detailed information of the duration of the study. Ronkainen 2006a provided a further long‐term outcome at eight years. The remaining four studies did not specify the timing of the outcome assessment (He 2002; Islek 1999; Xu 2009; Yoshimoto 1987a; Yoshimoto 1987b).
Definitions for significant haematuria were provided in five studies (Huber 2004; Mollica 1992; Peratoner 1990; Ronkainen 2006a; Tarshish 2004). Dudley 2013 regarded any degree of haematuria on dipstick as significant and the other studies did not report a definition of haematuria. Definitions for significant proteinuria using urinary protein:creatinine (UPC) ratio, timed urine specimens or dipstick results were specified in seven studies (CESAR Study 2010, Dudley 2013; Huber 2004; Mollica 1992; Ronkainen 2006a; Jauhola 2010; Tarshish 2004). The remaining studies did not provide a definition of significant proteinuria. See Table 1.
| Study | Timing of outcome | Haematuria | Proteinuria | Blood pressure | Kidney function |
| 1, 3 and 12 months | Any level on dipstick | UPC > 20 mg/mmol Dipstick for protein | Not defined | Not defined | |
| 1, 3, 6 and 12 months | ≥ 5 RBC/HPF or RBC casts | > 300 mg/L on dipstick | > 90th percentile for age and sex | Elevated Cr | |
| Unclear | Not defined | Not defined | Not defined | Not defined | |
| 1, 3, 6 and 12 months | ≥ 10 RBC/HPF | ≥ 4 mg/m²/h | > 2 SD above normal | Cr ≥ 0.8 mg/dL/mm² | |
| During initial 12 months | > 5 RBC/mm² | Not defined | Not defined | Reduced GFR | |
| 1, 3 and 6 months | > 5 RBC/HPF | > 200 mg/L or urinary albumin > 30 mg/L | Not defined | Not defined | |
| 2 years | Not defined | Remission: UPC < 200 mg/mmol or daily urine protein < 40 mg/m²/d | Not defined | Not defined | |
| Mean follow‐up to 7 years | Addis Count > 30,000 RBC/h/m² or ≥ 1+ on dipstick ≥ 3 cells/HPF or > 2 RBC/mm³ | > 4 mg/h/m² or 2+ or more by dipstick Heavy proteinuria > 40 mg/h/m² | Not defined | GFR < 80 mL/min/1.73 m² ESKD | |
| Unclear | Not defined | Not defined | Not defined | Not defined | |
| Unclear | Not defined | Not defined | Not defined | Not defined | |
| Unclear | Not defined | Not defined | Not defined | Not defined |
Cr ‐ creatinine; ESKD ‐ end‐stage kidney disease; GFR ‐ glomerular filtration rate; HPF ‐ high power field; UPC ‐ urinary protein:creatinine ratio; RBC ‐ red blood cell
Among the eight studies evaluating interventions to prevent persistent HSP‐associated kidney disease, Dudley 2013 used UPC ratio as the primary end point while in the remaining studies the primary end point of kidney disease was defined by a composite of haematuria and proteinuria. In the two studies evaluating interventions for severe HSP‐associated kidney disease, the primary end point was defined by a composite of proteinuria and reduced kidney function (Jauhola 2010; Tarshish 2004), while CESAR Study 2010 used a Birmingham Vascular Activity Score (BVAS) of zero at six months as indicating complete disease remission. The primary end point in the study comparing MMF with azathioprine was remission of proteinuria (Fuentes 2010). Jauhola 2010 reported data on included randomised and non‐randomised patients. Using information obtained from the authors, only randomised patients were included in the study analyses.
The primary outcome in Dudley 2013 was UPC ratio at 12 months but of the 296 children who had a 12 month follow‐up visit, results of UPC ratio were only available in 247 children. Data on the number of children with haematuria or proteinuria were used in analyses at one and three months. At these time points, the number of children with available data was less than the number undergoing follow‐up at that point.
No studies examining warfarin, ACEi or ARB, fish oil, immunoglobulin G, plasma exchange, antibody therapy or dapsone were identified.
Excluded studies
Eighteen studies (20 reports) were excluded. Three studies were not randomised, one study was enrolled the wrong population and 14 studies evaluated interventions not relevant to this review.
Risk of bias in included studies
Figure 2 and Figure 3 describe the graphical representation of the risk of bias assessment for all studies.

Risk of bias: Review authors' judgements about each methodological quality item presented as percentages across all included studies.
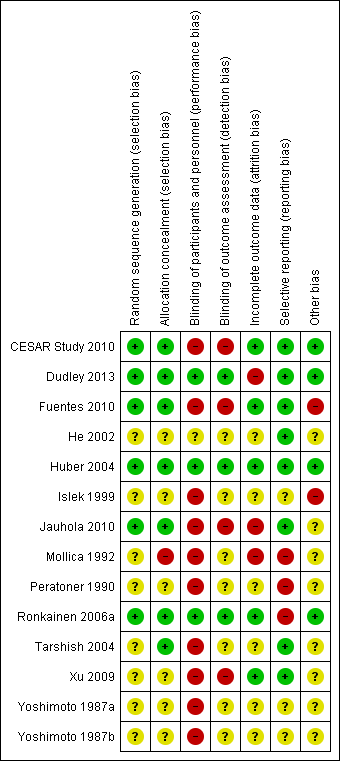
Risk of bias: Review authors' judgements about each risk of bias item for each included study
Allocation
Six studies (CESAR Study 2010; Dudley 2013; Fuentes 2010; Huber 2004; Jauhola 2010; Ronkainen 2006a) were determined to be at low risk of bias for random sequence generation and the risk of bias was unclear in the remaining studies.
Seven studies (CESAR Study 2010; Dudley 2013; Fuentes 2010; Huber 2004; Jauhola 2010Ronkainen 2006a; Tarshish 2004) were determined to be a low risk of bias for allocation concealment, one study was at high risk of bias (Mollica 1992), and the remaining studies had unclear risk of bias.
Blinding
Performance and detection bias was low in three studies (Dudley 2013; Huber 2004; Ronkainen 2006a). In one study participants in both treatment groups received intravenous medications but it was not clear whether the investigators were blinded (He 2002). Blinding of outcome assessors was reported in three studies (Dudley 2013; Huber 2004; Ronkainen 2006a). Fuentes 2010 was an open‐label study and blinding was not reported in the remaining studies. Since the outcome measure of urinalysis reported by participants or investigators is a subjective outcome measure, it could be influenced by lack of blinding so these studies were considered to be at high risk of bias (CESAR Study 2010; Jauhola 2010; Xu 2009).
Incomplete outcome data
Four studies were considered to be at low risk of attrition bias (CESAR Study 2010; Fuentes 2010; Huber 2004; Xu 2009). In two studies (Dudley 2013; Mollica 1992) reporting of outcome data was considered at high risk of bias because of loss to follow‐up or exclusion of data from the analyses. In the remaining studies insufficient information was provided to determine whether all patients entering the study were included in the analysis so the risk of bias was unclear.
Selective reporting
Reporting included all important kidney outcomes and adverse effects of medications in nine studies (CESAR Study 2010, Dudley 2013; Fuentes 2010; He 2002; Huber 2004; Mollica 1992; Peratoner 1990; Jauhola 2010; Tarshish 2004). One study was considered at high risk of reporting bias as data on treatment outcomes was only available in graphical form (Ronkainen 2006a). In the remaining studies it was unclear whether important kidney outcomes including nephrotic syndrome, reduced kidney function and adverse effects of medications had not occurred or had not been reported.
Other potential sources of bias
Four studies appeared free of other potential sources of bias (CESAR Study 2010; Dudley 2013; Huber 2004; Ronkainen 2006a). One author in Fuentes 2010 was a consult for a pharmaceutical company and Islek 1999 was an abstract‐only publication with no full‐text report identified and these two studies were judged to be at high risk of bias. In the remaining studies there was insufficient information provided to determine if there were other potential sources of bias.
Effects of interventions
Preventing persistent kidney disease
Prednisone versus placebo or supportive treatment
In children with newly diagnosed HSP and without significant kidney disease, there was no significant difference in the risk of any kidney disease following prednisone treatment compared with placebo or supportive treatment (Analysis 1.1 (5 studies, 746 children): RR 0.73, 95% CI 0.43 to 1.24; I2 = 44%).
There was no significant difference in the risk of development or persistence of kidney disease with prednisone compared with placebo or supportive treatment at one (Analysis 1.2.1 (4 studies, 655 participants): RR 0.80, 95% CI 0.34 to 1.84; I2 = 72%), three (Analysis 1.2.2 (4 studies, 655 participants): RR 0.83, 95% CI 0.46 to 1.52; I2 = 44%), six (Analysis 1.2.3 (3 studies, 379 participants): RR 0.51, 95% CI 0.24 to 1.11; I2 = 0%) and 12 months (Analysis 1.2.4 (3 studies, 455 participants): RR 1.06, 95% CI 0.38 to 2.91; I2 = 32%). There was substantial heterogeneity in study outcomes at one, three and 12 months, which was largely due to Mollica 1992. This study, which was at high risk of bias due to inadequate allocation concealment, showed a large benefit of prednisone in contrast to the other three studies. Sensitivity analysis with exclusion of this study eliminated the heterogeneity except at one month with no change to significance (Analysis 1.3).
In Ronkainen 2006a post hoc subgroup analysis of 71 children with kidney disease at or within one month of randomisation found that kidney disease was significantly less common at six months after prednisone therapy compared with placebo (Analysis 1.4.3: RR 0.45, 95% CI 0.21 to 0.98).
Two studies (Dudley 2013; Ronkainen 2006a) reported the number of children, who developed severe kidney disease with nephrotic range proteinuria, hypertension or reduced kidney function. Again there was no significant difference in the risk of severe kidney disease between children treated with prednisone or placebo (Analysis 1.5; (2 studies, 418 children): RR 1.58, 95% CI 0.42 to 6.00; I2 = 0%).
Islek 1999 assessed the duration of haematuria and proteinuria and found no significant difference in the duration of haematuria (Analysis 1.6.1 (33 children); MD ‐1.00, 95% CI ‐10.26 to 8.26) or proteinuria (Analysis 1.6.2 (33 children): MD ‐1.60, 95% CI ‐15.62 to 12.42).
The risk of gastrointestinal involvement requiring hospital admission was not significantly different between prednisone and placebo or supportive treatment (Analysis 1.7 (3 studies, 517 participants): RR 0.56, 95% CI 0.25 to 1.23; I2 = 0%). In Huber 2004, two children in the placebo group required surgery for intussusception and were withdrawn from the study. Based on patient diary records in Ronkainen 2006a, children on prednisone had a significantly lower pain severity score for abdominal or joint pain and had significantly shorter durations of abdominal pain but not joint pain compared with placebo.
Ronkainen 2006a completed an eight‐year follow‐up on 138/176 patients originally randomised. They reported minor abnormalities after two clinical screenings in 10 patients; eight who had received prednisone and two who received placebo. There was no significant differences in haematuria Analysis 1.8.1: RR 4.86, 95% CI 0.24 to 99.39), proteinuria (Analysis 1.8.2: RR 2.92, 95% CI 0.12 to 70.35), haematuria and proteinuria (Analysis 1.8.3: RR 2.92, 95% CI 0.12 to 70.35), hypertension (Analysis 1.8.4: RR 0.97, 95% CI 0.14 to 6.70), and decreased GFR by Schwartz formula (Analysis 1.8.5: RR 4.86, 95% CI 0.24 to 99.39).
Huber 2004 and Ronkainen 2006a reported there were no serious adverse effects caused by prednisone or placebo. In Ronkainen 2006a, children receiving prednisone had a 1 kg greater increase in weight and 4 mm Hg increase in diastolic blood pressure during treatment. In Dudley 2013, one child developed behavioural problems and one had an infection, these were considered related to prednisone therapy, while one child developed abdominal pain in the placebo group. Adverse effects were not recorded in Islek 1999 or Mollica 1992.
Antiplatelet agents versus supportive treatment
In children treated with antiplatelet agents compared with support treatment only, there was no significant difference in the risk of kidney disease occurring at any time during follow‐up in children without kidney disease at entry (Analysis 2.1.1 (2 studies, 101 children): RR 1.16, 95% CI 0.46 to 2.95; I2 = 0%). Peratoner 1990 reported no significant difference in kidney disease persisting in children with kidney disease at entry (Analysis 2.1.2 (19 children): RR 0.92, 95% CI 0.23 to 3.72).
Yoshimoto 1987a reported no significant difference in the risk of kidney disease with aspirin compared with supportive treatment only (Analysis 2.2 (18 children): RR 0.14, 95% CI 0.01 to 2.42).
Duration of follow‐up and adverse effects were not recorded in these studies.
Heparin versus placebo
He 2002 reported IV heparin significantly reduced kidney disease (Analysis 3.1 (228 children): RR 0.27, 95% CI 0.14 to 0.55), in haematuria (Analysis 3.2.1 (22 children): RR 0.15, 95% CI 0.03 to 0.67) and in proteinuria (Analysis 3.2.2 (228 children): RR 0.37, 95% CI 0.15 to 0.91) at three months or longer after the onset or relapse of HSP compared to placebo. The risk for nephrotic syndrome did not differ significantly between the groups but event numbers were small resulting in wide confidence intervals (Analysis 3.2.3 (228 children): RR 0.31, 95% CI 0.03 to 2.89). The development of kidney disease was significantly delayed in the heparin group compared with placebo (Analysis 3.3 (228 children): MD 47.3 days, 95% CI 34.24 to 60.36). No child developed severe bleeding.
Treating severe kidney disease
Cyclophosphamide versus supportive treatment
In a single study of children (Tarshish 2004) with significant HSP‐associated kidney disease (proteinuria, reduced kidney function, crescents, segmental lesions or both on kidney biopsy) treated within three months of onset of HSP, there was no significant difference in the risk for persistent kidney disease of any severity (Analysis 4.1 (56 children): RR 1.07, 95% CI 0.65 to 1.78), severe kidney disease (heavy proteinuria, reduced GFR, ESKD) (Analysis 4.2 (56 children): RR 0.88, 95% CI 0.37 to 2.09) or ESKD (Analysis 4.3 (56 children): RR 0.75, 95% CI 0.18 to 3.05) during follow‐up between patients treated with cyclophosphamide and those given supportive treatment. Adverse effects of cyclophosphamide were not reported.
Cyclophosphamide plus steroids versus steroids
CESAR Study 2010 compared cyclophosphamide and methylprednisolone followed by prednisone with methylprednisolone and prednisone in adults. There was no significant difference in the number of patients, who achieved a BVAS of zero by six months (Analysis 5.1.1 (54 participants): RR 1.16, 95% CI, 0.26 to 5.24) or in the number of patients whose BVAS score improved by six months (Analysis 5.1.2 (54 participants): RR 0.98, 95% CI 0.81 to 1.19). No significant differences were reported in the secondary outcomes including hypertension, reduced GFR, proteinuria, improvement in renal function and ESKD at 12 months (Analysis 5.2). Mortality was higher in the prednisone treated group, however this was not significant (Analysis 5.2.7 (54 participants): RR 0.19, 95% CI 0.02 to 1.50). These deaths were not considered to be related to the treatments received and the authors noted that patients in the prednisone group had significantly more severe disease at baseline based on BVAS scores. There were no significant differences in adverse effects (Analysis 5.3).
Cyclosporin versus methylprednisolone
Jauhola 2010 compared cyclosporin with methylprednisolone in children with severe kidney disease. All seven children treated with cyclosporin compared with four of eight treated with methylprednisolone were in remission by three months but the difference was not significant (Analysis 6.1 (15 children): RR 1.88, 95% CI 0.95 to 3.69) due to small patient numbers. At the two‐year follow‐up, 23 patients were assessed including 8 non‐randomised patients; remission rate was 70% in children treated with cyclosporin and 58% in children treated with methylprednisolone. At final follow‐up at a mean of 6.3 years, 6/7 children treated with cyclosporin compared with 5/8 treated with methylprednisolone were in remission, however the difference was not significant (Analysis 6.2 (15 children): RR 1.37, 95% CI 0.74,2.54). Adverse effects related to cyclosporin and methylprednisolone were not reported separately for the randomised patients.
Azathioprine plus prednisone versus mycophenolate mofetil plus prednisone
Fuentes 2010 compared azathioprine plus prednisone versus mycophenolate mofetil plus prednisone in children with biopsy proven HSPN (Class I‐III). There was no significant difference in protein remission between the two groups (Analysis 7.1 (17 children): RR 1.32, 95% CI 0.86 to 2.02). There was no significant difference in the number of children with improvement in kidney histology between treatment groups (Analysis 7.2 (17 children): RR 1.24,95% CI 0.66 to 2.36).
Fosinopril plus supportive treatment versus supportive treatment alone
Xu 2009 reported fosinopril given for two months significantly increased the number of children with complete remission of proteinuria compared with supportive treatment (Analysis 8.1 (48 children): RR 5.83, 95% CI 1.50 to 22.74).
Other outcomes
In most studies the severity of haematuria and proteinuria, the degree of kidney dysfunction and the presence of hypertension were not specified. Dudley 2013 provided information on UPC ratios and Tarshish 2004 provided separate information on ESKD.
Discussion
Summary of main results
We identified 13 studies of which eight examined the efficacy of therapies to prevent persistent kidney disease in HSP and five examined therapies to treat established severe kidney disease.
Prevention of persistent kidney disease ‐ prednisone
A meta‐analysis of five studies showed no evidence of benefit of prednisone over placebo or no treatment in preventing persistent kidney disease in children with none or minor kidney disease at presentation. Thus there is now considerable evidence that the use of steroid therapy at presentation of HSP is not indicated for the prevention of persistent kidney disease as determined by the presence of abnormal UPC ratio or persistent or new abnormalities on urinalysis. Data overall and at specified time points after presentation revealed no significant difference in the number of children with persistent kidney disease. Eight‐year follow‐up in one study found no significant differences in the numbers with urinary abnormalities or hypertension.
Only two studies assessed the use of prednisone in patients with severe kidney disease (nephrotic syndrome, nephritic syndrome, reduced kidney function) and identified no evidence of benefit. Because of small numbers of events resulting in wide confidence intervals and inadequate definition of severe disease, there remains uncertainty as to the efficacy of prednisone in preventing severe HSP‐associated kidney disease
The lack of significant differences between prednisone and placebo or supportive treatment in the numbers with kidney disease at one, three and six months suggests that prednisone did not result in more rapid resolution of kidney disease overall. However Ronkainen 2006a presented a post hoc analysis of 71 children, who had kidney disease at or within one month of presentation. Prednisone therapy for 28 days significantly reduced the number of children with persistent kidney disease at six months. The study was not stratified before randomisation for the presence or absence of kidney disease and the sample size was small so the results can only be considered as hypothesis‐generating. The study only provided outcome data to six months after randomisation so it is unclear whether prednisone treatment reduced the number of patients with persistent HSP‐associated kidney disease overall or promoted more rapid resolution of kidney disease compared with placebo. In addition, children considered to have established kidney disease at randomisation were not included in this study. In the other two well‐designed studies (Dudley 2013; Huber 2004) of prednisone therapy, children with any severity of kidney disease at presentation were potentially included and a meta‐analysis of these studies showed no significant difference in the risk for persistent HSPN at 12 months.
Two studies found no significant difference in the number of children with abdominal complications of HSP (Huber 2004; Ronkainen 2006a). However Ronkainen 2006a) reported that the severity and duration of abdominal pain as well as the duration of joint pain were significantly less severe in children treated with prednisone.
Prevention of persistent kidney disease ‐ antiplatelet agents and heparin
No evidence of benefit of antiplatelet agents (dipyridamole, cyproheptadine, salicylates) was demonstrated in two small studies suggesting that these agents have no role in preventing kidney disease in HSP. One study (He 2002), available in abstract form only, demonstrated that heparin reduced the number of children with kidney disease. Heparin or placebo were administered to children at onset of disease and at relapse and it was not possible to determine how many children in each group received more than one period of treatment. Though bleeding was not reported in this study, the use of such a potentially dangerous therapy is not justified when only about a third of children with HSP develop kidney disease and less than 2% develop severe kidney disease (nephrotic syndrome, nephritic syndrome, kidney failure) (Narchi 2005).
Treatment of severe kidney disease
Two studies evaluated cyclophosphamide in children and adults with HSPN and nephrotic range proteinuria. Tarshish 2004 reported no evidence of benefit of cyclophosphamide alone compared with no specific therapy. CESAR Study 2010 is the first study in adults with severe HSPN; they observed no evidence of benefit with the addition of cyclophosphamide to prednisone. There were no significant differences in the adverse events between the two groups.
While preliminary data in Jauhola 2010 suggested that cyclosporin may be more effective than methylprednisolone and prednisone in inducing remission in children with HSP and nephrotic range proteinuria, long‐term follow‐up at a mean of 6.3 years showed no statistical difference in the remission rates between cyclosporin and methylprednisolone groups. However study numbers were very small limiting the value of this study.
Fuentes 2010 compared azathioprine and mycophenolate mofetil therapy in children with severe kidney disease and reported the number with remission of proteinuria did not differ significantly between treatment groups but there was considerable imprecision because of small patient numbers.
Xu 2009 compared fosinopril with supportive treatment and reported the number achieving complete remission of proteinuria was higher in the fosinopril group.
Overall completeness and applicability of evidence
Thirteen studies enrolling 1403 participants were included in this review update; four studies only available in the abstract form and several studies included small numbers of patients with incomplete outcome data. Incomplete reporting of these studies may result in incomplete information being included in this systematic review.
Three well‐designed, placebo controlled studies (Dudley 2013; Huber 2004; Ronkainen 2006a) have provided data in over 400 children that there is no significant benefit of prednisone therapy at six to 12 months in children with no or minor kidney involvement at presentation. In addition, eight year follow‐up data in Ronkainen 2006a found no longer term benefit. Therefore further RCTs to evaluate prednisone to prevent kidney disease in this group of children presenting with HSP are unlikely to be justified.
Based on observational studies (Niaudet 1998), intravenous methylprednisolone followed by oral prednisone is commonly used in patients with nephrotic range proteinuria and no reduction in kidney function. However there are no RCTs evaluating methylprednisolone and prednisone compared with placebo or no treatment in patients with nephrotic range proteinuria and normal kidney function at or soon after presentation. Because of the known risk of permanent kidney damage and reduced kidney function, it is unlikely that investigators would be prepared to enrol their patients if such a study was proposed.
At present there are few RCTs with small patient numbers evaluating the treatment of HSPN with immunosuppressive medication to determine the benefits and harms of such treatment for patients with nephrotic syndrome with or without reduced kidney function and with or without more than 50% crescents on biopsy. Nevertheless immunosuppressive agents are commonly used in an attempt to treat established severe kidney disease in HSP with observational studies suggesting benefit of methylprednisolone (Niaudet 1998), azathioprine (Bergstein 1998), mycophenolate mofetil (Du 2012), cyclophosphamide (Flynn 2001; Iijima 1998; Tanaka 2003) and cyclosporin (Ronkainen 2003). However in the four RCTs included in this review, no significant benefits of cyclophosphamide, cyclosporin or mycophenolate mofetil were identified (CESAR Study 2010; Fuentes 2010; Jauhola 2010; Tarshish 2004). Patient numbers in the studies of cyclosporin and mycophenolate were very small resulting in considerable imprecision of results so these treatment modalities need further study. Cyclophosphamide is recommended for patients with crescentic GN (KDIGO 2012). The lack of significant benefit of cyclophosphamide may be related to the types of patients included in the two studies. Although all children included in the Tarshish 2004 study had crescentic GN, the delay between onset of disease and treatment (at least three months) may have allowed the development of chronic changes, which were not amenable to therapy. In addition treatment may have been more effective if methylprednisolone and then oral high dose prednisone had been administered also. In the CESAR Study 2010 only 30% of participants had crescentic disease, which may have influenced why there was no significant additional benefit of cyclophosphamide over methylprednisolone and prednisone alone. However while these issues might suggest that further studies of cyclophosphamide in patients with crescentic GN associated with HSP are indicated, the potential long term adverse effects of cyclophosphamide particularly in children (Coutinho 2001) mean that such studies are unlikely to be performed.
Adverse events of therapies were not all well reported. Limited reporting revealed small numbers of adverse events in all studies with no significant difference between interventions. No studies were identified which evaluated therapy to prevent or treat persistent kidney disease in participants with recurrent episodes of HSP.
In the studies evaluating prednisone therapy, the outcomes reported were poorly defined except for one study (Dudley 2013), which used UPC ratio as the primary outcome. The potential significance for long‐term kidney function of any residual urinary abnormalities could not be assessed in the other studies, since they reported the end point as the presence of haematuria or proteinuria or both without measuring the degree of proteinuria.
No RCTs were identified which examined intravenous immunoglobulin, rituximab or plasma exchange. No studies examining fish oil, ACEi or ARB were identified. No studies specifically addressing whether therapy reduced the risk of recurrent episodes of HSP were identified.
Quality of the evidence
Sequence generation and allocation concealment were at low risk of bias in six (50%) and seven (58%) respectively. This may be attributed to poorer reporting of these parameters in the earlier studies. Blinding of participants, investigators and outcome assessors was only reported in three studies, reflecting a high risk of bias in the remaining studies since knowledge of treatment groups could influence patient management and reporting. Only five studies were at low risk of attrition bias. The otherwise robust study of Dudley 2013, was at high risk of attrition bias due to a significant drop out rate with only 72% (123/171) reporting the primary outcome. Eight studies were at low risk of reporting bias. Studies with a high risk of bias are associated with an increased likelihood of results favouring the study intervention (Schulz 1995; Wood 2008). Exclusion of the study with a high risk of bias removed heterogeneity between studies without altering the overall result, reinforcing the strength of the evidence suggesting that short courses of prednisone do not prevent serious kidney disease in children with HSP.
Only the five studies comparing prednisone with placebo or supportive treatment for the prevention of persistent kidney disease in HSP could be assessed in a summary of findings table (summary of findings Table for the main comparison). The overall quality of the studies was considered moderate for persistence of kidney disease at any time after treatment and for the number of children with continuing kidney disease at varying time points. With the removal of one study, which had inadequate allocation concealment and no blinding, the remaining three studies were graded as of high quality at the three month interval but only moderate at 12 months because of the small number of events and high loss to follow‐up in Dudley 2013. The number developing severe kidney disease was graded as low in two studies, as a result of significant loss to follow‐up in the largest included study and small numbers of events.
The remaining eight studies could not be included into summary of findings tables as they were single studies with small numbers or data could not be included in meta‐analyses.
Potential biases in the review process
A thorough search utilising Cochrane Kidney and Transplant's Specialised Register was completed in July 2015.
The Specialised Register includes published studies and conference abstracts with no restriction of language. The omission of eligible studies was therefore minimised, although potentially eligible studies identified prior to publication have yet to be included (Ding 2014; NCT00301613; Wu 2013b; Wu 2014c). However 40% of study reports in the Specialised Register have been identified by handsearching of conference proceedings so it remains possible that further studies of therapy to prevent or treat serious kidney disease in HSP will be identified as conference proceedings from different congresses are searched.
Four (33%) of the included studies were only available in the abstract form, thus limiting information on study methods and outcomes. Four of the studies were published prior to 2000 before the CONSORT checklist (first published in 1996) would influence trial methodology and reporting. Incomplete reporting of these studies may result in incomplete information being included in this systematic review.
Two authors independently undertook all the steps of this review thereby minimising risks of errors in determining study eligibility, data extraction and risk of bias assessment and data synthesis.
Agreements and disagreements with other studies or reviews
Three systematic reviews have previously assessed the effects of corticosteroid therapy to prevent or alter the course of kidney disease in HSP (Weiss 2007; Wyatt 2001; Zaffanello 2007). All three included data from RCTs and observational studies. Conclusions based on non‐randomised study designs are more likely to be biased towards a benefit of treatment (Chalmers 1983). Two reviews determined that it remained unclear whether corticosteroid therapy prevented or altered the course of HSP‐associated kidney disease (Wyatt 2001; Zaffanello 2007). The third review concluded that corticosteroids decreased the likelihood of developing persistent kidney disease but did not prevent kidney disease (Weiss 2007). This conclusion was based on a meta‐analysis of the ad hoc subgroup of children with kidney abnormalities within one month of presentation from Ronkainen 2006a combined with data from two other studies (Huber 2004; Mollica 1992). With the inclusion in this review of another large study (Dudley 2013), which showed no significant benefit of corticosteroid therapy, the evidence base from RCTs supports the conclusion that corticosteroids given at initial presentation of HSP do not prevent the development or persistence of kidney disease in HSP.
One review also evaluated immunosuppressive and other therapies in HSP (Zaffanello 2007). It concluded based on observational studies that cyclophosphamide was of value in treating HSP‐associated kidney disease. The two studies evaluating cyclophosphamide in this review did not show any benefit of cyclophosphamide (CESAR Study 2010, Tarshish 2004).The studies comparing cyclosporin with methylprednisolone and MMF with azathioprine were too small to establish whether or not these treatments were effective (Fuentes 2010; Jauhola 2010).
The KDIGO guidelines (KDIGO 2012) on HSP recommend treatment with ACEi or ARB in patients with HSPN and persistent low grade proteinuria and recommend corticosteroids after a trial of ACEi or ARB in patients with larger amounts of proteinuria as in patients with IgA nephropathy. However these recommendations have been challenged on the basis that the natural history of HSPN is different from IgA nephropathy with the risk of early severe inflammatory episodes with glomerular crescents followed by a rapid evolution to glomerulosclerosis. Thus delayed treatment may increase the risk of CKD progression so early treatment with methylprednisolone pulses with immunosuppressive drugs, plasma exchange or both is commonly commenced based on observational data. However currently there are no data from RCTs to support or refute this alternative management (Davin 2013).
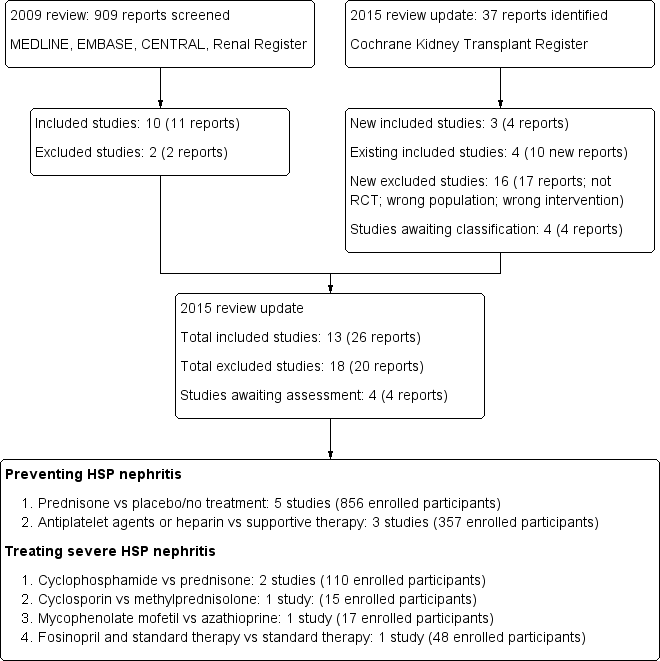
Flow diagram of included and excluded study in Review

Risk of bias: Review authors' judgements about each methodological quality item presented as percentages across all included studies.

Risk of bias: Review authors' judgements about each risk of bias item for each included study

Comparison 1 Prednisone versus placebo/supportive treatment for preventing persistent kidney disease, Outcome 1 Persistent kidney disease at any time after treatment.

Comparison 1 Prednisone versus placebo/supportive treatment for preventing persistent kidney disease, Outcome 2 Number of children with any continuing kidney disease at different time points.

Comparison 1 Prednisone versus placebo/supportive treatment for preventing persistent kidney disease, Outcome 3 Any continuing kidney disease at different time points (study with high risk of bias excluded).
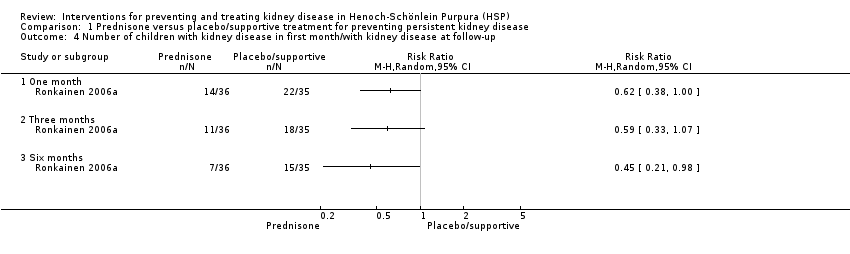
Comparison 1 Prednisone versus placebo/supportive treatment for preventing persistent kidney disease, Outcome 4 Number of children with kidney disease in first month/with kidney disease at follow‐up.

Comparison 1 Prednisone versus placebo/supportive treatment for preventing persistent kidney disease, Outcome 5 Number developing severe kidney disease.

Comparison 1 Prednisone versus placebo/supportive treatment for preventing persistent kidney disease, Outcome 6 Duration of kidney disease.
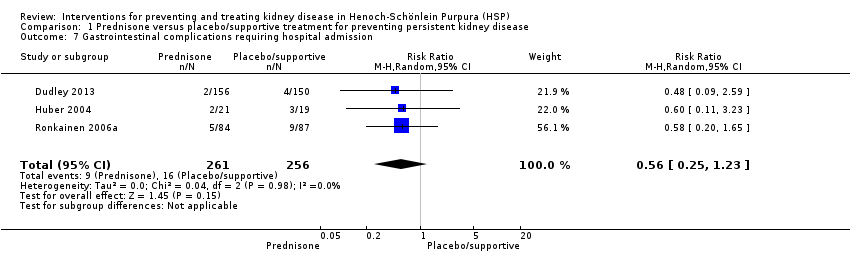
Comparison 1 Prednisone versus placebo/supportive treatment for preventing persistent kidney disease, Outcome 7 Gastrointestinal complications requiring hospital admission.
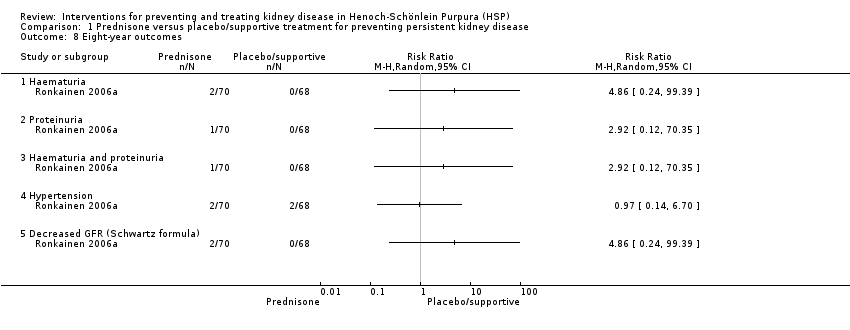
Comparison 1 Prednisone versus placebo/supportive treatment for preventing persistent kidney disease, Outcome 8 Eight‐year outcomes.

Comparison 2 Antiplatelet agents versus supportive treatment for preventing persistent kidney disease, Outcome 1 Kidney disease at any time.

Comparison 2 Antiplatelet agents versus supportive treatment for preventing persistent kidney disease, Outcome 2 Kidney disease at any time.

Comparison 3 Heparin versus placebo for preventing persistent kidney disease, Outcome 1 Any kidney disease at 3 months after onset or relapse.

Comparison 3 Heparin versus placebo for preventing persistent kidney disease, Outcome 2 Type of kidney disease at 3 months or more after onset or relapse.
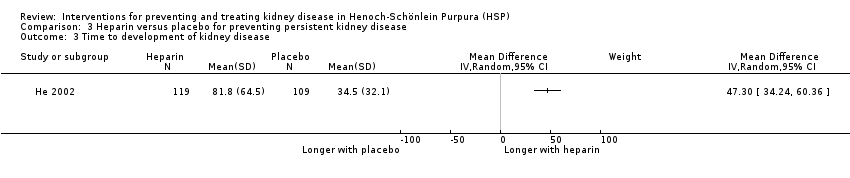
Comparison 3 Heparin versus placebo for preventing persistent kidney disease, Outcome 3 Time to development of kidney disease.

Comparison 4 Cyclophosphamide versus supportive treatment for treating severe kidney disease, Outcome 1 Persistent kidney disease.

Comparison 4 Cyclophosphamide versus supportive treatment for treating severe kidney disease, Outcome 2 Persistent severe kidney disease.

Comparison 4 Cyclophosphamide versus supportive treatment for treating severe kidney disease, Outcome 3 ESKD.
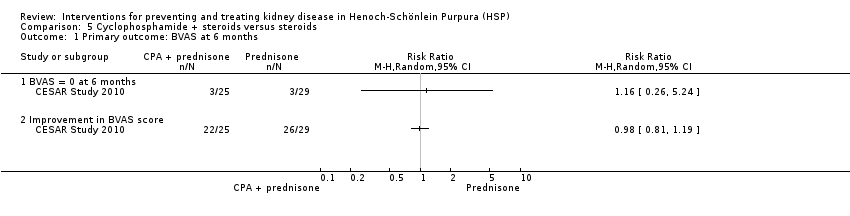
Comparison 5 Cyclophosphamide + steroids versus steroids, Outcome 1 Primary outcome: BVAS at 6 months.
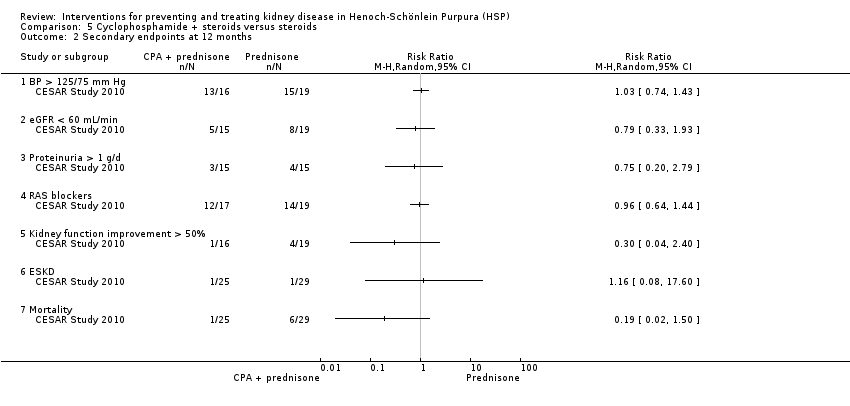
Comparison 5 Cyclophosphamide + steroids versus steroids, Outcome 2 Secondary endpoints at 12 months.

Comparison 5 Cyclophosphamide + steroids versus steroids, Outcome 3 Adverse effects.
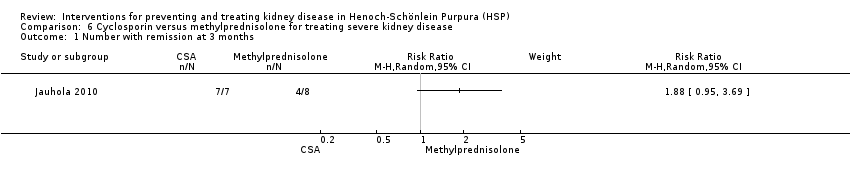
Comparison 6 Cyclosporin versus methylprednisolone for treating severe kidney disease, Outcome 1 Number with remission at 3 months.

Comparison 6 Cyclosporin versus methylprednisolone for treating severe kidney disease, Outcome 2 Number with remission at last follow‐up (mean 6.3 years).

Comparison 7 Mycophenolate mofetil versus azathioprine for treating severe kidney disease, Outcome 1 Remission of proteinuria at 1 year.
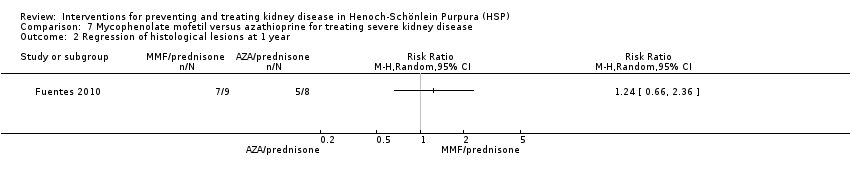
Comparison 7 Mycophenolate mofetil versus azathioprine for treating severe kidney disease, Outcome 2 Regression of histological lesions at 1 year.

Comparison 8 Fosinopril + supportive treatment versus supportive treatment, Outcome 1 Proteinuria.
| Prednisone versus placebo or supportive treatment for preventing persistent kidney disease in patients with Henoch‐Schönlein Purpura (HSP) | ||||||
| Patient or population: patients with HSP | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo or supportive treatment | Prednisone | |||||
| Persistent kidney disease at any time after treatment | Study population | RR 0.74 | 746 (5) | ⊕⊕⊕⊝ | ||
| 143 per 1000 | 106 per 1000 | |||||
| Moderate | ||||||
| 105 per 1000 | 78 per 1000 | |||||
| Number of children with any continuing kidney disease at 3 months | Study population | RR 0.83 | 655 (4) | ⊕⊕⊕⊝ | ||
| 199 per 1000 | 165 per 1000 | |||||
| Moderate | ||||||
| 156 per 1000 | 129 per 1000 | |||||
| Number of children with any continuing kidney disease at 6 months | Study population | RR 0.51 | 379 (3) | ⊕⊕⊕⊝ | ||
| 100 per 1000 | 51 per 1000 | |||||
| Moderate | ||||||
| 53 per 1000 | 27 per 1000 | |||||
| Number of children with any continuing kidney disease at 12 months | Study population | RR 1.06 | 455 (3) | ⊕⊕⊝⊝ | ||
| 84 per 1000 | 89 per 1000 | |||||
| Moderate | ||||||
| 105 per 1000 | 111 per 1000 | |||||
| Any continuing kidney disease at 3 months (study with high risk of bias excluded) | Study population | RR 0.98 | 487 (3) | ⊕⊕⊕⊕ | ||
| 243 per 1000 | 238 per 1000 | |||||
| Moderate | ||||||
| 207 per 1000 | 203 per 1000 | |||||
| Any continuing kidney disease at 12 months (study with high risk of bias excluded) | Study population | RR 1.39 | 287 (2) | ⊕⊕⊕⊝ | ||
| 105 per 1000 | 146 per 1000 | |||||
| Moderate | ||||||
| 105 per 1000 | 146 per 1000 | |||||
| Number developing severe kidney disease | Study population | RR 1.58 | 418 (2) | ⊕⊕⊝⊝ | ||
| 14 per 1000 | 22 per 1000 | |||||
| Moderate | ||||||
| 17 per 1000 | 27 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Two studies had unclear or biased allocation concealment & were not blinded | ||||||
| Study | Timing of outcome | Haematuria | Proteinuria | Blood pressure | Kidney function |
| 1, 3 and 12 months | Any level on dipstick | UPC > 20 mg/mmol Dipstick for protein | Not defined | Not defined | |
| 1, 3, 6 and 12 months | ≥ 5 RBC/HPF or RBC casts | > 300 mg/L on dipstick | > 90th percentile for age and sex | Elevated Cr | |
| Unclear | Not defined | Not defined | Not defined | Not defined | |
| 1, 3, 6 and 12 months | ≥ 10 RBC/HPF | ≥ 4 mg/m²/h | > 2 SD above normal | Cr ≥ 0.8 mg/dL/mm² | |
| During initial 12 months | > 5 RBC/mm² | Not defined | Not defined | Reduced GFR | |
| 1, 3 and 6 months | > 5 RBC/HPF | > 200 mg/L or urinary albumin > 30 mg/L | Not defined | Not defined | |
| 2 years | Not defined | Remission: UPC < 200 mg/mmol or daily urine protein < 40 mg/m²/d | Not defined | Not defined | |
| Mean follow‐up to 7 years | Addis Count > 30,000 RBC/h/m² or ≥ 1+ on dipstick ≥ 3 cells/HPF or > 2 RBC/mm³ | > 4 mg/h/m² or 2+ or more by dipstick Heavy proteinuria > 40 mg/h/m² | Not defined | GFR < 80 mL/min/1.73 m² ESKD | |
| Unclear | Not defined | Not defined | Not defined | Not defined | |
| Unclear | Not defined | Not defined | Not defined | Not defined | |
| Unclear | Not defined | Not defined | Not defined | Not defined | |
| Cr ‐ creatinine; ESKD ‐ end‐stage kidney disease; GFR ‐ glomerular filtration rate; HPF ‐ high power field; UPC ‐ urinary protein:creatinine ratio; RBC ‐ red blood cell | |||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Persistent kidney disease at any time after treatment Show forest plot | 5 | 746 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.42, 1.32] |
| 2 Number of children with any continuing kidney disease at different time points Show forest plot | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 One month | 4 | 655 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.34, 1.84] |
| 2.2 Three months | 4 | 655 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.46, 1.52] |
| 2.3 Six months | 3 | 379 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.24, 1.11] |
| 2.4 Twelve months | 3 | 455 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.38, 2.91] |
| 3 Any continuing kidney disease at different time points (study with high risk of bias excluded) Show forest plot | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 One month | 3 | 487 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.54, 1.93] |
| 3.2 Three months | 3 | 487 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.70, 1.36] |
| 3.3 Six months | 2 | 211 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.23, 1.50] |
| 3.4 Twelve months | 2 | 287 | Risk Ratio (M‐H, Random, 95% CI) | 1.39 [0.75, 2.59] |
| 4 Number of children with kidney disease in first month/with kidney disease at follow‐up Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4.1 One month | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Three months | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.3 Six months | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Number developing severe kidney disease Show forest plot | 2 | 418 | Risk Ratio (M‐H, Random, 95% CI) | 1.58 [0.42, 6.00] |
| 6 Duration of kidney disease Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 6.1 Haematuria | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 Proteinuria | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Gastrointestinal complications requiring hospital admission Show forest plot | 3 | 517 | Risk Ratio (M‐H, Random, 95% CI) | 0.56 [0.25, 1.23] |
| 8 Eight‐year outcomes Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 8.1 Haematuria | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.2 Proteinuria | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.3 Haematuria and proteinuria | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.4 Hypertension | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.5 Decreased GFR (Schwartz formula) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Kidney disease at any time Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Dipyridamole ± cyproheptadine in children without kidney disease at entry | 2 | 101 | Risk Ratio (M‐H, Random, 95% CI) | 1.16 [0.46, 2.95] |
| 1.2 Dipyridamole ± cyproheptadine in children with kidney disease at entry | 1 | 19 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.23, 3.72] |
| 2 Kidney disease at any time Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.1 Aspirin versus supportive treatment | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Any kidney disease at 3 months after onset or relapse Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2 Type of kidney disease at 3 months or more after onset or relapse Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.1 Haematuria | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Proteinuria | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Nephrotic syndrome | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Time to development of kidney disease Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Persistent kidney disease Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2 Persistent severe kidney disease Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3 ESKD Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Primary outcome: BVAS at 6 months Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.1 BVAS = 0 at 6 months | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Improvement in BVAS score | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Secondary endpoints at 12 months Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.1 BP > 125/75 mm Hg | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 eGFR < 60 mL/min | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Proteinuria > 1 g/d | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.4 RAS blockers | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.5 Kidney function improvement > 50% | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.6 ESKD | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.7 Mortality | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Adverse effects Show forest plot | 1 | Risk Difference (M‐H, Random, 95% CI) | Totals not selected | |
| 3.1 infection | 1 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Newly diagnosed or deterioration in existing diabetes | 1 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 Depression/anxiety | 1 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.4 Alopecia | 1 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.5 Insomnia | 1 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number with remission at 3 months Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2 Number with remission at last follow‐up (mean 6.3 years) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Remission of proteinuria at 1 year Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2 Regression of histological lesions at 1 year Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Proteinuria Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.1 Complete remission of proteinuria < 150 mg/d | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Partial remission | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Minimal response/no response | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |

