نقش مداخلات در پیشگیری و درمان بیماریهای کلیه در پورپورای هنوخ‐شوئنلاین (HSP)
Referencias
منابع مطالعات واردشده در این مرور
منابع مطالعات خارجشده از این مرور
منابع مطالعات در انتظار ارزیابی
منابع اضافی
منابع دیگر نسخههای منتشرشده این مرور
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group
Control group
| |
| Outcomes | Primary outcome
Secondary outcomes at 12 months
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Central randomisation with computer generated random allocation sequence |
| Allocation concealment (selection bias) | Low risk | Central randomisation with computer generated random allocation sequence |
| Blinding of participants and personnel (performance bias) | High risk | No blinding and outcome is likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) | High risk | No blinding and outcome is likely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) | Low risk | All patients accounted for Loss to follow‐up: treatment group (8: death (1), lack of efficacy (1); patient choice (3); adverse event (1); not evaluated (2)); control group (10: deaths (6); lack of efficacy (2); patient choice (1); not evaluated (1)) |
| Selective reporting (reporting bias) | Low risk | Reported all outcomes |
| Other bias | Low risk | Funded by Département a la Recherche Clinique et au Dévelopment |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group
Control group
Co‐interventions
| |
| Outcomes | Primary outcome
Secondary outcomes
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated random number sequence using random number generator |
| Allocation concealment (selection bias) | Low risk | Identical bottles for active and placebo medications coded centrally with trial numbers. |
| Blinding of participants and personnel (performance bias) | Low risk | Trial medications (active and placebo) supplied by same company in identical bottles. Patients, parents, paediatricians and all investigators were blind to treatment management |
| Blinding of outcome assessment (detection bias) | Low risk | Patients, parents, paediatricians and all investigators were blind to outcome assessment |
| Incomplete outcome data (attrition bias) | High risk | Lost to follow‐up: 10 in prednisone group and 6 in placebo group Primary outcome analysed in 71% (123/171) in prednisone group and 75% (124/165) in placebo group Secondary outcome of haematuria or proteinuria analysed in 82% (141/171) in prednisone group and 83% (137/165) in placebo group |
| Selective reporting (reporting bias) | Low risk | Primary outcome pre‐specified as UPC in National Research Register of NHS in UK. Information also provided on dipstick analysis of haematuria and proteinuria. Adverse events reported |
| Other bias | Low risk | Appears to be free of other biases. Funded by Wales Office for Research and Development |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group 1
Treatment group 2
| |
| Outcomes | Primary outcomes
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated random numbers. Information provided by authors |
| Allocation concealment (selection bias) | Low risk | Computer generated. Information provided by authors |
| Blinding of participants and personnel (performance bias) | High risk | No blinding and outcome is likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) | High risk | No blinding and outcome is likely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) | Low risk | All patients accounted for |
| Selective reporting (reporting bias) | Low risk | Data on kidney outcomes and adverse effects provided |
| Other bias | High risk | One author a consultant for Novartis; no full‐text publication after 5 years |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group
Control group
Co‐interventions
| |
| Outcomes |
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) | Unclear risk | Control group given IV vehicle but unclear whether investigators aware of which patients received heparin or vehicle |
| Blinding of outcome assessment (detection bias) | Unclear risk | Control group given IV vehicle but unclear whether investigators aware of which patients received heparin or vehicle |
| Incomplete outcome data (attrition bias) | Unclear risk | No information provided although all patients appeared to have been followed. Minimum follow‐up 6 months. Limited information on adverse effects provided |
| Selective reporting (reporting bias) | Low risk | Information provided on numbers with haematuria alone, haematuria and proteinuria, haematuria with nephrotic syndrome and adverse effects |
| Other bias | Unclear risk | Insufficient information provided. Unclear as to whether same patient could enter the trial twice (at onset or at recurrence) |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group
Control group
Co‐interventions: not reported | |
| Outcomes |
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated sequence of random numbers |
| Allocation concealment (selection bias) | Low risk | Plain sealed numbered envelopes opened at subject randomisation by research pharmacist. Individuals directly involved in the study had no access to these envelopes |
| Blinding of participants and personnel (performance bias) | Low risk | Prednisone and placebo groups received identical number of pills and followed the same schedule. Prednisone and placebo tablets placed in opaque tasteless gelatin capsules The subjects and all individuals involved in the enrolment and assessment of study participants were blinded to the study group |
| Blinding of outcome assessment (detection bias) | Low risk | The subjects and all individuals involved in the enrolment and assessment of study participants were blinded to the study group |
| Incomplete outcome data (attrition bias) | Low risk | One subject enrolled declined randomisation Three children withdrawn from the placebo group due to complications but included in the analysis (intussusception (2); severe rash (1)) |
| Selective reporting (reporting bias) | Low risk | Reported that children with persistent kidney disease had haematuria, proteinuria or both and did not have hypertension or kidney insufficiency Reported adverse effects |
| Other bias | Low risk | The study appears to be free of other sources of bias |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group
Control group
Co‐interventions: not reported | |
| Outcomes |
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) | High risk | No placebo tablets administered in the control group. No information provided on whether outcome assessors were blinded. |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information provided on whether outcome assessors were blinded. Abstract only |
| Incomplete outcome data (attrition bias) | Unclear risk | Unclear whether all eligible patients entered and completed the trial and whether there was any missing data. All reported patients appeared to have completed study. |
| Selective reporting (reporting bias) | Unclear risk | Only outcomes reported were haematuria and proteinuria. No reports separately of more severe kidney disease (acute nephritic syndrome, nephrotic syndrome, hypertension). |
| Other bias | High risk | Insufficient information available to determine however study not published 16 years after abstract first presented |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group
Control group
Co‐interventions
| |
| Outcomes |
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised by a central office |
| Allocation concealment (selection bias) | Low risk | Central randomisation |
| Blinding of participants and personnel (performance bias) | High risk | Open‐label study comparing an orally administered agent with an intravenously administered agent |
| Blinding of outcome assessment (detection bias) | High risk | Open‐label study comparing an orally administered agent with an intravenously administered agent |
| Incomplete outcome data (attrition bias) | High risk | No SD provided with means of urinary protein and SCr at last follow‐up. Duration of study not defined |
| Selective reporting (reporting bias) | Low risk | Data on kidney outcomes and adverse effects provided |
| Other bias | Unclear risk | Insufficient information provided |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group
Control group
Co‐interventions: not reported | |
| Outcomes |
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | High risk | Each patient on entry to the trial was alternatively assigned to one of the two treatment groups |
| Blinding of participants and personnel (performance bias) | High risk | Control group did not receive placebo medications. |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information provided on whether outcome assessors were blinded. Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) | High risk | 19 patients excluded because of insufficient or inadequate follow‐up |
| Selective reporting (reporting bias) | High risk | Information on the numbers with proteinuria, haematuria, hypertension and reduced kidney function provided; no report on adverse effects. |
| Other bias | Unclear risk | Insufficient information provided |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group
Control group
Co‐interventions
| |
| Outcomes |
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) | High risk | No placebo given to control group |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information provided about outcome assessors. Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) | Unclear risk | Unclear whether all entered patients completed trial and were included in the results |
| Selective reporting (reporting bias) | High risk | Information on type of residual kidney disease provided in text. No report of adverse effects |
| Other bias | Unclear risk | Insufficient information provided |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group
Control group
Co‐interventions
| |
| Outcomes |
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation scheme with block size of 6 |
| Allocation concealment (selection bias) | Low risk | Observers and subjects were unaware of randomisation scheme. Pharmia Ltd packed drugs, labelled containers, performed randomisation and retained key to randomisation till end of study |
| Blinding of participants and personnel (performance bias) | Low risk | Prednisone (5 mg tablets) and placebo were similar in size and supplied in lots of 200 tablets in similar containers marked with sequential numbers |
| Blinding of outcome assessment (detection bias) | Low risk | Assessors blinded until end of trial |
| Incomplete outcome data (attrition bias) | Low risk | Three (2 dropped out; 1 protocol violation) excluded from prednisone group. Two (both dropped out) excluded from placebo group. These unlikely to influence final results 138/176 (21%) screened on long‐term follow‐up |
| Selective reporting (reporting bias) | High risk | Data on kidney outcomes extrapolated from graphs. Data on adverse effects included |
| Other bias | Low risk | Appears to be free of other biases |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group
Control group
Co‐interventions
| |
| Outcomes |
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | Low risk | Random allocation at central office |
| Blinding of participants and personnel (performance bias) | High risk | No placebo therapy used. While most outcome measures were laboratory measurements and unlikely to be influenced by lack of blinding, end points in some children were judged on dipstick protein urinalyses, which could have be either doctor or patient reported. |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information provided on outcome assessors. |
| Incomplete outcome data (attrition bias) | Unclear risk | Duration of follow‐up variable and unclear whether all patients completed follow‐up |
| Selective reporting (reporting bias) | Low risk | Data on persistent abnormalities, severe abnormalities and ESKD provided though detailed information on GFR and urinary protein excretion at follow‐up not provided. |
| Other bias | Unclear risk | Insufficient information provided on study design |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group
Control group
Co‐interventions: not reported | |
| Outcomes |
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised but no method mentioned |
| Allocation concealment (selection bias) | Unclear risk | Said to be randomised but no further information provided |
| Blinding of participants and personnel (performance bias) | High risk | No blinding and outcome is likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) | High risk | No blinding and outcome is likely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) | Low risk | All enrolled patients accounted for |
| Selective reporting (reporting bias) | Low risk | Reported all expected outcomes |
| Other bias | Unclear risk | Insufficient information to permit judgement |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group
Control group
Co‐interventions: not reported | |
| Outcomes |
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) | High risk | Control group received vitamin pills. Outcome measures not defined. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) | Unclear risk | Criteria for kidney disease not defined |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information provided |
| Other bias | Unclear risk | Insufficient information available |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group
Control group:
Co‐interventions: not reported | |
| Outcomes |
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not mentioned |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) | High risk | Control group received vitamin pills. Outcome measures not defined |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient data to permit judgement |
| Incomplete outcome data (attrition bias) | Unclear risk | Criteria for kidney disease not defined |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information provided |
| Other bias | Unclear risk | Insufficient information provided |
ACEi ‐ angiotensin converting enzyme inhibitor; ARB ‐ angiotensin receptor blocker; AZA ‐ azathioprine; BP ‐ blood pressure; BUN ‐ blood urea nitrogen; BVAS ‐ Birmingham Vascular Activity Score; CPA ‐ cyclophosphamide; Cr ‐ creatinine; CrCl ‐ creatinine clearance; CSA ‐ cyclosporin; eGFR ‐ estimated glomerular filtration rate; ESKD ‐ end‐stage kidney disease; GFR ‐ glomerular filtration rate; HIV ‐ human immunodeficiency virus; HPF ‐ high power field; HSP ‐ Henoch‐Schönlein Purpura; ISKDC ‐ International Study of Kidney Disease in Children; IV ‐ intravenous; M/F ‐ male/female; MMF ‐ mycophenolate mofetil; RBC ‐ red blood cells; RCT ‐ randomised controlled trial; SCr ‐ serum creatinine; SD ‐ standard deviation; UPC ‐ urinary protein:creatinine ratio
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| Ineligible intervention | |
| Ineligible intervention | |
| Ineligible intervention | |
| Unclear whether study is RCT | |
| Ineligible intervention | |
| Ineligible intervention | |
| Unclear whether study is RCT | |
| Ineligible intervention | |
| Ineligible intervention | |
| Retrospective study | |
| Ineligible intervention | |
| Ineligible population | |
| Ineligible intervention | |
| Ineligible intervention | |
| Ineligible intervention | |
| Ineligible intervention | |
| Ineligible intervention | |
| Ineligible intervention |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Persistent kidney disease at any time after treatment Show forest plot | 5 | 746 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.42, 1.32] |
| Analysis 1.1  Comparison 1 Prednisone versus placebo/supportive treatment for preventing persistent kidney disease, Outcome 1 Persistent kidney disease at any time after treatment. | ||||
| 2 Number of children with any continuing kidney disease at different time points Show forest plot | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.2  Comparison 1 Prednisone versus placebo/supportive treatment for preventing persistent kidney disease, Outcome 2 Number of children with any continuing kidney disease at different time points. | ||||
| 2.1 One month | 4 | 655 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.34, 1.84] |
| 2.2 Three months | 4 | 655 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.46, 1.52] |
| 2.3 Six months | 3 | 379 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.24, 1.11] |
| 2.4 Twelve months | 3 | 455 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.38, 2.91] |
| 3 Any continuing kidney disease at different time points (study with high risk of bias excluded) Show forest plot | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.3  Comparison 1 Prednisone versus placebo/supportive treatment for preventing persistent kidney disease, Outcome 3 Any continuing kidney disease at different time points (study with high risk of bias excluded). | ||||
| 3.1 One month | 3 | 487 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.54, 1.93] |
| 3.2 Three months | 3 | 487 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.70, 1.36] |
| 3.3 Six months | 2 | 211 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.23, 1.50] |
| 3.4 Twelve months | 2 | 287 | Risk Ratio (M‐H, Random, 95% CI) | 1.39 [0.75, 2.59] |
| 4 Number of children with kidney disease in first month/with kidney disease at follow‐up Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 1.4  Comparison 1 Prednisone versus placebo/supportive treatment for preventing persistent kidney disease, Outcome 4 Number of children with kidney disease in first month/with kidney disease at follow‐up. | ||||
| 4.1 One month | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Three months | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.3 Six months | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Number developing severe kidney disease Show forest plot | 2 | 418 | Risk Ratio (M‐H, Random, 95% CI) | 1.58 [0.42, 6.00] |
| Analysis 1.5  Comparison 1 Prednisone versus placebo/supportive treatment for preventing persistent kidney disease, Outcome 5 Number developing severe kidney disease. | ||||
| 6 Duration of kidney disease Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 1.6  Comparison 1 Prednisone versus placebo/supportive treatment for preventing persistent kidney disease, Outcome 6 Duration of kidney disease. | ||||
| 6.1 Haematuria | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 Proteinuria | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Gastrointestinal complications requiring hospital admission Show forest plot | 3 | 517 | Risk Ratio (M‐H, Random, 95% CI) | 0.56 [0.25, 1.23] |
| Analysis 1.7  Comparison 1 Prednisone versus placebo/supportive treatment for preventing persistent kidney disease, Outcome 7 Gastrointestinal complications requiring hospital admission. | ||||
| 8 Eight‐year outcomes Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 1.8 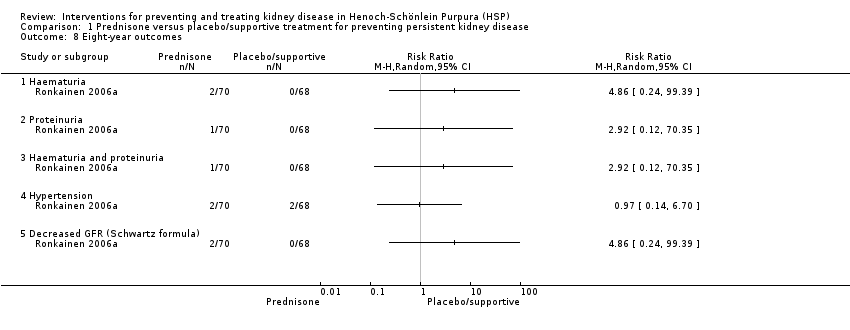 Comparison 1 Prednisone versus placebo/supportive treatment for preventing persistent kidney disease, Outcome 8 Eight‐year outcomes. | ||||
| 8.1 Haematuria | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.2 Proteinuria | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.3 Haematuria and proteinuria | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.4 Hypertension | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.5 Decreased GFR (Schwartz formula) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Kidney disease at any time Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 2.1  Comparison 2 Antiplatelet agents versus supportive treatment for preventing persistent kidney disease, Outcome 1 Kidney disease at any time. | ||||
| 1.1 Dipyridamole ± cyproheptadine in children without kidney disease at entry | 2 | 101 | Risk Ratio (M‐H, Random, 95% CI) | 1.16 [0.46, 2.95] |
| 1.2 Dipyridamole ± cyproheptadine in children with kidney disease at entry | 1 | 19 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.23, 3.72] |
| 2 Kidney disease at any time Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 2.2  Comparison 2 Antiplatelet agents versus supportive treatment for preventing persistent kidney disease, Outcome 2 Kidney disease at any time. | ||||
| 2.1 Aspirin versus supportive treatment | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Any kidney disease at 3 months after onset or relapse Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 3.1  Comparison 3 Heparin versus placebo for preventing persistent kidney disease, Outcome 1 Any kidney disease at 3 months after onset or relapse. | ||||
| 2 Type of kidney disease at 3 months or more after onset or relapse Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 3.2  Comparison 3 Heparin versus placebo for preventing persistent kidney disease, Outcome 2 Type of kidney disease at 3 months or more after onset or relapse. | ||||
| 2.1 Haematuria | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Proteinuria | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Nephrotic syndrome | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Time to development of kidney disease Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 3.3  Comparison 3 Heparin versus placebo for preventing persistent kidney disease, Outcome 3 Time to development of kidney disease. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Persistent kidney disease Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 4.1 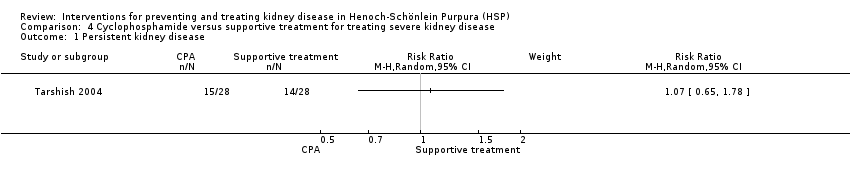 Comparison 4 Cyclophosphamide versus supportive treatment for treating severe kidney disease, Outcome 1 Persistent kidney disease. | ||||
| 2 Persistent severe kidney disease Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 4.2  Comparison 4 Cyclophosphamide versus supportive treatment for treating severe kidney disease, Outcome 2 Persistent severe kidney disease. | ||||
| 3 ESKD Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 4.3  Comparison 4 Cyclophosphamide versus supportive treatment for treating severe kidney disease, Outcome 3 ESKD. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Primary outcome: BVAS at 6 months Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 5.1  Comparison 5 Cyclophosphamide + steroids versus steroids, Outcome 1 Primary outcome: BVAS at 6 months. | ||||
| 1.1 BVAS = 0 at 6 months | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Improvement in BVAS score | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Secondary endpoints at 12 months Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 5.2  Comparison 5 Cyclophosphamide + steroids versus steroids, Outcome 2 Secondary endpoints at 12 months. | ||||
| 2.1 BP > 125/75 mm Hg | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 eGFR < 60 mL/min | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Proteinuria > 1 g/d | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.4 RAS blockers | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.5 Kidney function improvement > 50% | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.6 ESKD | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.7 Mortality | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Adverse effects Show forest plot | 1 | Risk Difference (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 5.3  Comparison 5 Cyclophosphamide + steroids versus steroids, Outcome 3 Adverse effects. | ||||
| 3.1 infection | 1 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Newly diagnosed or deterioration in existing diabetes | 1 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 Depression/anxiety | 1 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.4 Alopecia | 1 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.5 Insomnia | 1 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number with remission at 3 months Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 6.1 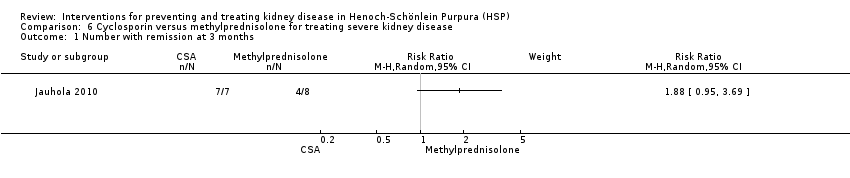 Comparison 6 Cyclosporin versus methylprednisolone for treating severe kidney disease, Outcome 1 Number with remission at 3 months. | ||||
| 2 Number with remission at last follow‐up (mean 6.3 years) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 6.2  Comparison 6 Cyclosporin versus methylprednisolone for treating severe kidney disease, Outcome 2 Number with remission at last follow‐up (mean 6.3 years). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Remission of proteinuria at 1 year Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 7.1  Comparison 7 Mycophenolate mofetil versus azathioprine for treating severe kidney disease, Outcome 1 Remission of proteinuria at 1 year. | ||||
| 2 Regression of histological lesions at 1 year Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 7.2 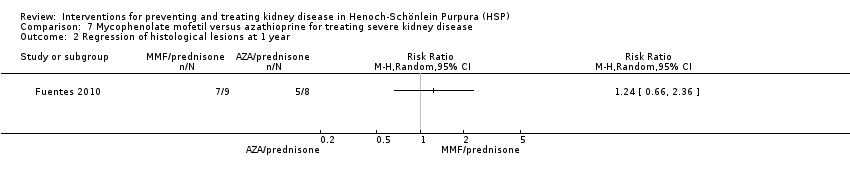 Comparison 7 Mycophenolate mofetil versus azathioprine for treating severe kidney disease, Outcome 2 Regression of histological lesions at 1 year. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Proteinuria Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 8.1  Comparison 8 Fosinopril + supportive treatment versus supportive treatment, Outcome 1 Proteinuria. | ||||
| 1.1 Complete remission of proteinuria < 150 mg/d | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Partial remission | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Minimal response/no response | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
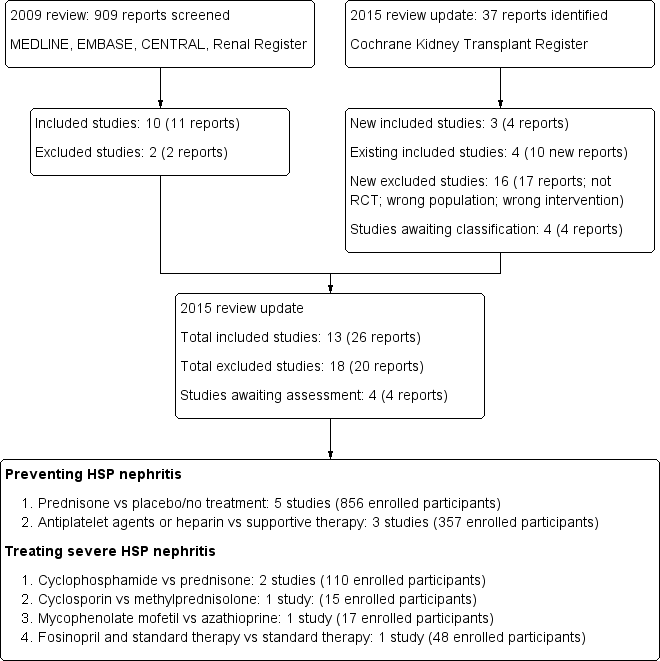
Flow diagram of included and excluded study in Review

Risk of bias: Review authors' judgements about each methodological quality item presented as percentages across all included studies.

Risk of bias: Review authors' judgements about each risk of bias item for each included study

Comparison 1 Prednisone versus placebo/supportive treatment for preventing persistent kidney disease, Outcome 1 Persistent kidney disease at any time after treatment.

Comparison 1 Prednisone versus placebo/supportive treatment for preventing persistent kidney disease, Outcome 2 Number of children with any continuing kidney disease at different time points.

Comparison 1 Prednisone versus placebo/supportive treatment for preventing persistent kidney disease, Outcome 3 Any continuing kidney disease at different time points (study with high risk of bias excluded).
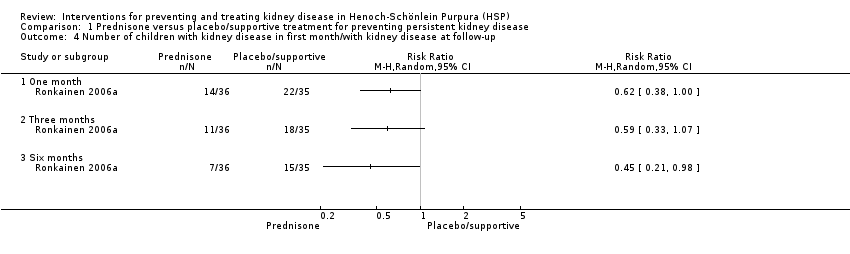
Comparison 1 Prednisone versus placebo/supportive treatment for preventing persistent kidney disease, Outcome 4 Number of children with kidney disease in first month/with kidney disease at follow‐up.

Comparison 1 Prednisone versus placebo/supportive treatment for preventing persistent kidney disease, Outcome 5 Number developing severe kidney disease.

Comparison 1 Prednisone versus placebo/supportive treatment for preventing persistent kidney disease, Outcome 6 Duration of kidney disease.
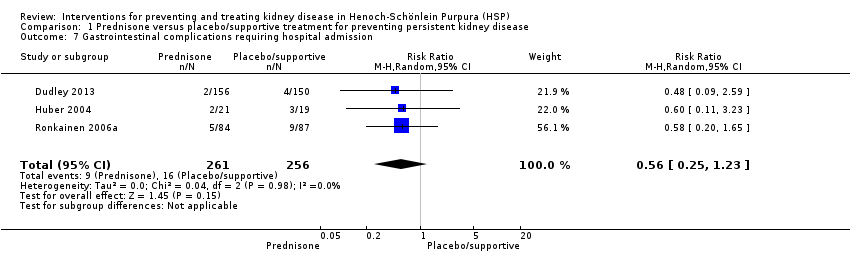
Comparison 1 Prednisone versus placebo/supportive treatment for preventing persistent kidney disease, Outcome 7 Gastrointestinal complications requiring hospital admission.
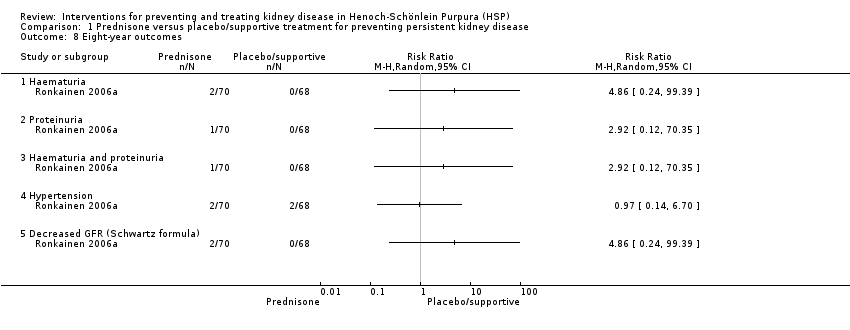
Comparison 1 Prednisone versus placebo/supportive treatment for preventing persistent kidney disease, Outcome 8 Eight‐year outcomes.

Comparison 2 Antiplatelet agents versus supportive treatment for preventing persistent kidney disease, Outcome 1 Kidney disease at any time.

Comparison 2 Antiplatelet agents versus supportive treatment for preventing persistent kidney disease, Outcome 2 Kidney disease at any time.

Comparison 3 Heparin versus placebo for preventing persistent kidney disease, Outcome 1 Any kidney disease at 3 months after onset or relapse.

Comparison 3 Heparin versus placebo for preventing persistent kidney disease, Outcome 2 Type of kidney disease at 3 months or more after onset or relapse.
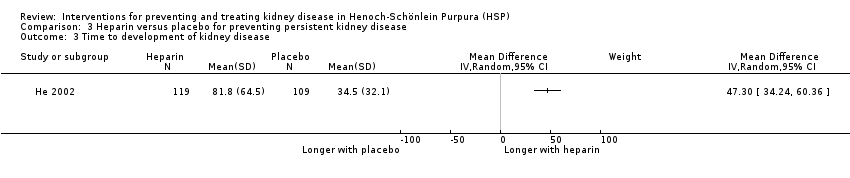
Comparison 3 Heparin versus placebo for preventing persistent kidney disease, Outcome 3 Time to development of kidney disease.

Comparison 4 Cyclophosphamide versus supportive treatment for treating severe kidney disease, Outcome 1 Persistent kidney disease.

Comparison 4 Cyclophosphamide versus supportive treatment for treating severe kidney disease, Outcome 2 Persistent severe kidney disease.

Comparison 4 Cyclophosphamide versus supportive treatment for treating severe kidney disease, Outcome 3 ESKD.
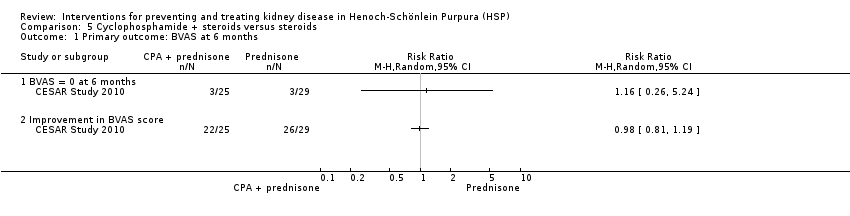
Comparison 5 Cyclophosphamide + steroids versus steroids, Outcome 1 Primary outcome: BVAS at 6 months.
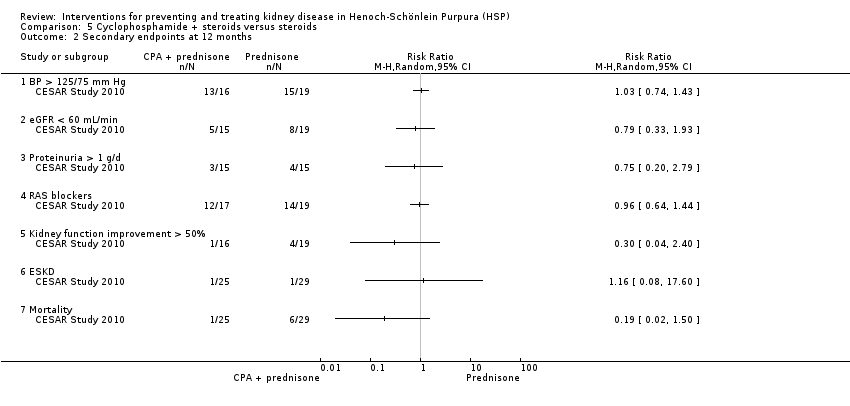
Comparison 5 Cyclophosphamide + steroids versus steroids, Outcome 2 Secondary endpoints at 12 months.

Comparison 5 Cyclophosphamide + steroids versus steroids, Outcome 3 Adverse effects.
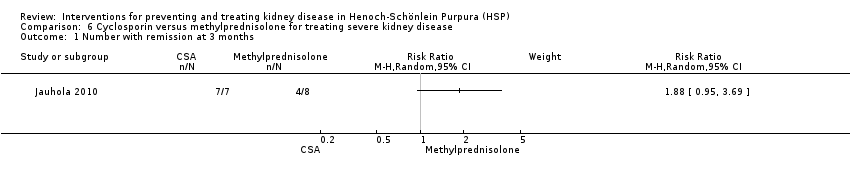
Comparison 6 Cyclosporin versus methylprednisolone for treating severe kidney disease, Outcome 1 Number with remission at 3 months.

Comparison 6 Cyclosporin versus methylprednisolone for treating severe kidney disease, Outcome 2 Number with remission at last follow‐up (mean 6.3 years).

Comparison 7 Mycophenolate mofetil versus azathioprine for treating severe kidney disease, Outcome 1 Remission of proteinuria at 1 year.
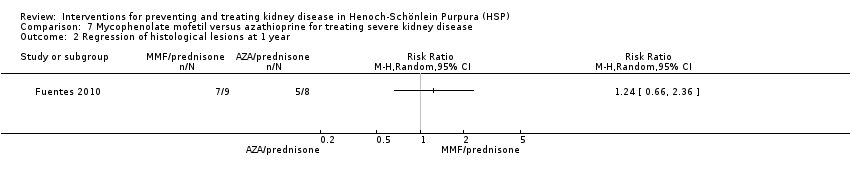
Comparison 7 Mycophenolate mofetil versus azathioprine for treating severe kidney disease, Outcome 2 Regression of histological lesions at 1 year.

Comparison 8 Fosinopril + supportive treatment versus supportive treatment, Outcome 1 Proteinuria.
| Prednisone versus placebo or supportive treatment for preventing persistent kidney disease in patients with Henoch‐Schönlein Purpura (HSP) | ||||||
| Patient or population: patients with HSP | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo or supportive treatment | Prednisone | |||||
| Persistent kidney disease at any time after treatment | Study population | RR 0.74 | 746 (5) | ⊕⊕⊕⊝ | ||
| 143 per 1000 | 106 per 1000 | |||||
| Moderate | ||||||
| 105 per 1000 | 78 per 1000 | |||||
| Number of children with any continuing kidney disease at 3 months | Study population | RR 0.83 | 655 (4) | ⊕⊕⊕⊝ | ||
| 199 per 1000 | 165 per 1000 | |||||
| Moderate | ||||||
| 156 per 1000 | 129 per 1000 | |||||
| Number of children with any continuing kidney disease at 6 months | Study population | RR 0.51 | 379 (3) | ⊕⊕⊕⊝ | ||
| 100 per 1000 | 51 per 1000 | |||||
| Moderate | ||||||
| 53 per 1000 | 27 per 1000 | |||||
| Number of children with any continuing kidney disease at 12 months | Study population | RR 1.06 | 455 (3) | ⊕⊕⊝⊝ | ||
| 84 per 1000 | 89 per 1000 | |||||
| Moderate | ||||||
| 105 per 1000 | 111 per 1000 | |||||
| Any continuing kidney disease at 3 months (study with high risk of bias excluded) | Study population | RR 0.98 | 487 (3) | ⊕⊕⊕⊕ | ||
| 243 per 1000 | 238 per 1000 | |||||
| Moderate | ||||||
| 207 per 1000 | 203 per 1000 | |||||
| Any continuing kidney disease at 12 months (study with high risk of bias excluded) | Study population | RR 1.39 | 287 (2) | ⊕⊕⊕⊝ | ||
| 105 per 1000 | 146 per 1000 | |||||
| Moderate | ||||||
| 105 per 1000 | 146 per 1000 | |||||
| Number developing severe kidney disease | Study population | RR 1.58 | 418 (2) | ⊕⊕⊝⊝ | ||
| 14 per 1000 | 22 per 1000 | |||||
| Moderate | ||||||
| 17 per 1000 | 27 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Two studies had unclear or biased allocation concealment & were not blinded | ||||||
| Study | Timing of outcome | Haematuria | Proteinuria | Blood pressure | Kidney function |
| 1, 3 and 12 months | Any level on dipstick | UPC > 20 mg/mmol Dipstick for protein | Not defined | Not defined | |
| 1, 3, 6 and 12 months | ≥ 5 RBC/HPF or RBC casts | > 300 mg/L on dipstick | > 90th percentile for age and sex | Elevated Cr | |
| Unclear | Not defined | Not defined | Not defined | Not defined | |
| 1, 3, 6 and 12 months | ≥ 10 RBC/HPF | ≥ 4 mg/m²/h | > 2 SD above normal | Cr ≥ 0.8 mg/dL/mm² | |
| During initial 12 months | > 5 RBC/mm² | Not defined | Not defined | Reduced GFR | |
| 1, 3 and 6 months | > 5 RBC/HPF | > 200 mg/L or urinary albumin > 30 mg/L | Not defined | Not defined | |
| 2 years | Not defined | Remission: UPC < 200 mg/mmol or daily urine protein < 40 mg/m²/d | Not defined | Not defined | |
| Mean follow‐up to 7 years | Addis Count > 30,000 RBC/h/m² or ≥ 1+ on dipstick ≥ 3 cells/HPF or > 2 RBC/mm³ | > 4 mg/h/m² or 2+ or more by dipstick Heavy proteinuria > 40 mg/h/m² | Not defined | GFR < 80 mL/min/1.73 m² ESKD | |
| Unclear | Not defined | Not defined | Not defined | Not defined | |
| Unclear | Not defined | Not defined | Not defined | Not defined | |
| Unclear | Not defined | Not defined | Not defined | Not defined | |
| Cr ‐ creatinine; ESKD ‐ end‐stage kidney disease; GFR ‐ glomerular filtration rate; HPF ‐ high power field; UPC ‐ urinary protein:creatinine ratio; RBC ‐ red blood cell | |||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Persistent kidney disease at any time after treatment Show forest plot | 5 | 746 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.42, 1.32] |
| 2 Number of children with any continuing kidney disease at different time points Show forest plot | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 One month | 4 | 655 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.34, 1.84] |
| 2.2 Three months | 4 | 655 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.46, 1.52] |
| 2.3 Six months | 3 | 379 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.24, 1.11] |
| 2.4 Twelve months | 3 | 455 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.38, 2.91] |
| 3 Any continuing kidney disease at different time points (study with high risk of bias excluded) Show forest plot | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 One month | 3 | 487 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.54, 1.93] |
| 3.2 Three months | 3 | 487 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.70, 1.36] |
| 3.3 Six months | 2 | 211 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.23, 1.50] |
| 3.4 Twelve months | 2 | 287 | Risk Ratio (M‐H, Random, 95% CI) | 1.39 [0.75, 2.59] |
| 4 Number of children with kidney disease in first month/with kidney disease at follow‐up Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4.1 One month | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Three months | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.3 Six months | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Number developing severe kidney disease Show forest plot | 2 | 418 | Risk Ratio (M‐H, Random, 95% CI) | 1.58 [0.42, 6.00] |
| 6 Duration of kidney disease Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 6.1 Haematuria | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 Proteinuria | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Gastrointestinal complications requiring hospital admission Show forest plot | 3 | 517 | Risk Ratio (M‐H, Random, 95% CI) | 0.56 [0.25, 1.23] |
| 8 Eight‐year outcomes Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 8.1 Haematuria | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.2 Proteinuria | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.3 Haematuria and proteinuria | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.4 Hypertension | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.5 Decreased GFR (Schwartz formula) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Kidney disease at any time Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Dipyridamole ± cyproheptadine in children without kidney disease at entry | 2 | 101 | Risk Ratio (M‐H, Random, 95% CI) | 1.16 [0.46, 2.95] |
| 1.2 Dipyridamole ± cyproheptadine in children with kidney disease at entry | 1 | 19 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.23, 3.72] |
| 2 Kidney disease at any time Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.1 Aspirin versus supportive treatment | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Any kidney disease at 3 months after onset or relapse Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2 Type of kidney disease at 3 months or more after onset or relapse Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.1 Haematuria | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Proteinuria | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Nephrotic syndrome | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Time to development of kidney disease Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Persistent kidney disease Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2 Persistent severe kidney disease Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3 ESKD Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Primary outcome: BVAS at 6 months Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.1 BVAS = 0 at 6 months | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Improvement in BVAS score | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Secondary endpoints at 12 months Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.1 BP > 125/75 mm Hg | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 eGFR < 60 mL/min | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Proteinuria > 1 g/d | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.4 RAS blockers | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.5 Kidney function improvement > 50% | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.6 ESKD | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.7 Mortality | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Adverse effects Show forest plot | 1 | Risk Difference (M‐H, Random, 95% CI) | Totals not selected | |
| 3.1 infection | 1 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Newly diagnosed or deterioration in existing diabetes | 1 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 Depression/anxiety | 1 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.4 Alopecia | 1 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.5 Insomnia | 1 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number with remission at 3 months Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2 Number with remission at last follow‐up (mean 6.3 years) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Remission of proteinuria at 1 year Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2 Regression of histological lesions at 1 year Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Proteinuria Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.1 Complete remission of proteinuria < 150 mg/d | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Partial remission | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Minimal response/no response | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |

