Gonadotropinas para la subfertilidad idiopática de factor masculino
Appendices
Appendix 1. MDSG
Keywords CONTAINS "male factor subfertility" or "male factor" or "male fertility" or "male immune subfertility" or "male infertility" or "male subfertility" or "Sperm" or "oligo‐spermatozoa" or "oligoasthenoteratozoospermia"or "oligospermia"or "oligozoospermia"or "asthenospermia"or "asthenozoospermia"or "azoospermia"or "subfertility‐male " or Title CONTAINS "male factor subfertility" or "male factor" or "male fertility" or "male immune subfertility" or "male infertility" or "male subfertility" or "Sperm" or "oligo‐spermatazoa" or "oligoasthenoteratozoospermia"or "oligospermia"or "oligozoospermia"or "asthenospermia"or "asthenozoospermia"or "azoospermia"or "subfertility‐male "
AND
Keywords CONTAINS "gonadotrophins"or"gonadotropin"or"FSH"or"FSH HMG"or"lh"or"follitropin"or"Follitropin A"or"follitropin alfa"or"follicle stimulating hormone"or"urinary FSH"or"u‐FSH "or"u‐hMG"or"u‐LH "or"uFSH"or"uHCG"or"luteinizing hormone"or"Luteinising hormone releasing hormone"or"recombinant FSH"or"recombinant hFSH"or"recombinant HCG"or"recombinant LH"or"human recombinant follitropin‐alpha"or"human menopausal gonadotrophin"or"HMG"or "human menopausal gonadotrophin"or"human chorionic gonadotrophin"or"human chorionic gonadotropin"or"HCG"or"menotropin"or"menotrophin" or Title CONTAINS"gonadotrophins"or"gonadotropin"or"FSH"or"FSH HMG"or"lh"or"follitropin"or"Follitropin A"or"follitropin alfa"or"follicle stimulating hormone"or"urinary FSH"or"u‐FSH "or"u‐hMG"or"u‐LH "or"uFSH"or"uHCG"or"luteinizing hormone"or"Luteinising hormone releasing hormone"or"recombinant FSH"or"recombinant hFSH"or"recombinant HCG"or"recombinant LH"or"human recombinant follitropin‐alpha"
Appendix 2. CENTRAL
The Cochrane Central Register of Controlled Trials (CENTRAL) on Issue 12, 2012 of The Cochrane Library was searched in all fields using the following words:
1 gonadotropins/ or exp gonadotropins, pituitary/ or exp follicle stimulating hormone/ or exp luteinizing hormone/ (3536)
2 gonadotrop$.tw. (2412)
3 follicle stimulating hormone$.tw. (1045)
4 (luteinizing hormone$ or luteinising hormone$).tw. (1193)
5 (LH or FSH).tw. (2778)
6 (ufsh or ulh).tw. (21)
7 (rfsh or rlh).tw. (168)
8 or/1‐7 (6449)
9 exp infertility, male/ or exp aspermia/ or exp asthenozoospermia/ or exp azoospermia/ or exp oligospermia/ (473)
10 (male$ adj2 infertil$).tw. (293)
11 (male$ adj2 subfertil$).tw. (54)
12 (men adj2 infertil$).tw. (103)
13 (men adj2 subfertil$).tw. (19)
14 oligospermia.tw. (43)
15 asthenospermia.tw. (26)
16 azoospermia.tw. (122)
17 asthenozoospermia.tw. (35)
18 oligoasthenoteratozoospermia.tw. (7)
19 (sperm or semen).tw. (1668)
20 or/9‐19 (1906)
21 8 and 20 (492)
22 limit 21 to yr="2007 ‐Current" (86)
Appendix 3. MEDLINE
The MEDLINE search was combined with the Cochrane highly sensitive search strategy for identifying randomized trials which appears in the Cochrane Handbook of Systematic Reviews of Interventions (Version 5.0.2 chapter 6, 6.4.11)
MEDLINE database was searched using the following subject headings and keywords:
1 gonadotropins/ or exp gonadotropins, pituitary/ or exp follicle stimulating hormone/ or exp luteinizing hormone/ (92423)
2 gonadotrop$.tw. (54032)
3 follicle stimulating hormone$.tw. (14034)
4 (luteinizing hormone$ or luteinising hormone$).tw. (24704)
5 (LH or FSH).tw. (52061)
6 (ufsh or ulh).tw. (75)
7 (rfsh or rlh).tw. (533)
8 or/1‐7 (139500)
9 exp infertility, male/ or exp aspermia/ or exp asthenozoospermia/ or exp azoospermia/ or exp oligospermia/ (20736)
10 (male$ adj2 infertil$).tw. (6771)
11 (male$ adj2 subfertil$).tw. (538)
12 (men adj2 infertil$).tw. (2860)
13 (men adj2 subfertil$).tw. (387)
14 oligospermia.tw. (1043)
15 asthenospermia.tw. (240)
16 azoospermia.tw. (4131)
17 asthenozoospermia.tw. (439)
18 oligoasthenoteratozoospermia.tw. (192)
19 (sperm or semen).tw. (61703)
20 or/9‐19 (74403)
21 8 and 20 (6570)
22 randomized controlled trial.pt. (318727)
23 controlled clinical trial.pt. (83412)
24 randomized.ab. (234709)
25 placebo.tw. (136295)
26 clinical trials as topic.sh. (157393)
27 randomly.ab. (172722)
28 trial.ti. (100443)
29 (crossover or cross‐over or cross over).tw. (52145)
30 or/22‐29 (781229)
31 (animals not (humans and animals)).sh. (3557624)
32 30 not 31 (721295)
33 32 and 21 (580)
34 (2007$ or 2008$ or 2009$ or 2010$ or 2011$ or 2012$).ed. (4353905)
35 33 and 34 (134)
Appendix 4. EMBASE
The EMBASE search is combined with trial filters developed by the Scottish Intercollegiate Guidelines Network (SIGN) http://www.sign.ac.uk/mehodology/filters.html#random
The EMBASE database was searched using the following subject headings and keywords:
1 exp gonadotropin/ (19928)
2 exp follitropin/ (38779)
3 exp luteinizing hormone/ (44768)
4 gonadotrop$.tw. (54764)
5 follitropin.tw. (578)
6 luteinizing hormone$.tw. (22001)
7 follicle stimulating hormone$.tw. (13564)
8 luteinising hormone$.tw. (1169)
9 (LH or FSH).tw. (54401)
10 (ufsh or ulh).tw. (93)
11 (rfsh or rlh).tw. (776)
12 or/1‐11 (120267)
13 exp male infertility/ or exp asthenospermia/ or exp azoospermia/ or exp male sterility/ or exp oligospermia/ (27195)
14 (male$ adj2 infertil$).tw. (8279)
15 (male$ adj2 subfertil$).tw. (641)
16 (men adj2 infertil$).tw. (3292)
17 (men adj2 subfertil$).tw. (412)
18 oligospermia.tw. (1072)
19 asthenospermia.tw. (286)
20 azoospermia.tw. (4550)
21 asthenozoospermia.tw. (488)
22 oligoasthenoteratozoospermia.tw. (239)
23 (sperm or semen).tw. (65277)
24 or/13‐23 (82036)
25 24 and 12 (7630)
26 Clinical Trial/ (823603)
27 Randomized Controlled Trial/ (296357)
28 exp randomization/ (55579)
29 Single Blind Procedure/ (14735)
30 Double Blind Procedure/ (102763)
31 Crossover Procedure/ (31733)
32 Placebo/ (191694)
33 Randomi?ed controlled trial$.tw. (68283)
34 Rct.tw. (8403)
35 random allocation.tw. (1087)
36 randomly allocated.tw. (16142)
37 allocated randomly.tw. (1728)
38 (allocated adj2 random).tw. (691)
39 Single blind$.tw. (11480)
40 Double blind$.tw. (120977)
41 ((treble or triple) adj blind$).tw. (256)
42 placebo$.tw. (164540)
43 prospective study/ (181244)
44 or/26‐43 (1171442)
45 case study/ (14547)
46 case report.tw. (213228)
47 abstract report/ or letter/ (806112)
48 or/45‐47 (1029684)
49 44 not 48 (1137623)
50 25 and 49 (1089)
51 (2010$ or 2011$ or 2012$).em. (2585757)
52 50 and 51 (211)
Appendix 5. PSYCINFO
PSYCINFO was searched using the following words:
1 exp Gonadotropic Hormones/ (3427)
2 exp Luteinizing Hormone/ or exp Follicle Stimulating Hormone/ (665)
3 gonadotrop$.tw. (1195)
4 follicle stimulating hormone$.tw. (414)
5 (luteinizing hormone$ or luteinising hormone$).tw. (1128)
6 (LH or FSH).tw. (2253)
7 (ufsh or ulh).tw. (1)
8 (rfsh or rlh).tw. (9)
9 or/1‐8 (5830)
10 exp Infertility/ (1489)
11 (male$ adj2 infertil$).tw. (135)
12 (male$ adj2 subfertil$).tw. (6)
13 (men adj2 infertil$).tw. (57)
14 (men adj2 subfertil$).tw. (0)
15 oligospermia.tw. (15)
16 asthenospermia.tw. (2)
17 azoospermia.tw. (15)
18 asthenozoospermia.tw. (0)
19 oligoasthenoteratozoospermia.tw. (1)
20 (sperm or semen).tw. (1703)
21 or/10‐20 (3130)
22 9 and 21 (64)
23 random.tw. (34342)
24 control.tw. (267676)
25 double‐blind.tw. (15544)
26 clinical trials/ (5727)
27 placebo/ (3095)
28 exp Treatment/ (506101)
29 or/23‐28 (765513)
30 22 and 29 (28)
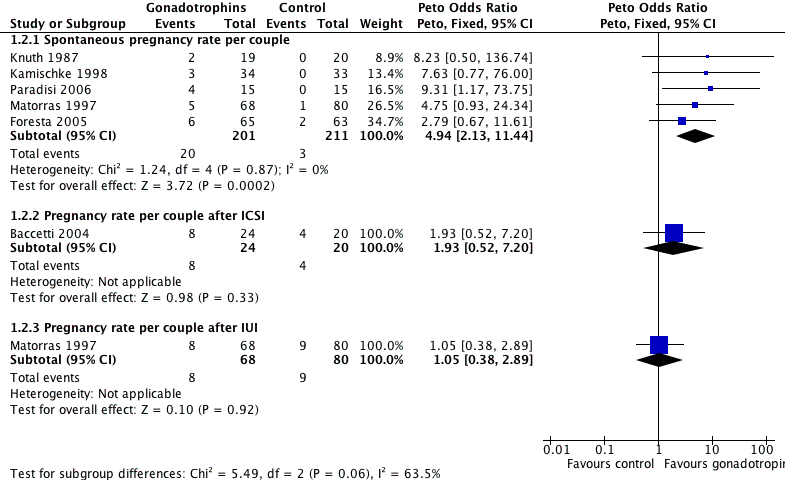
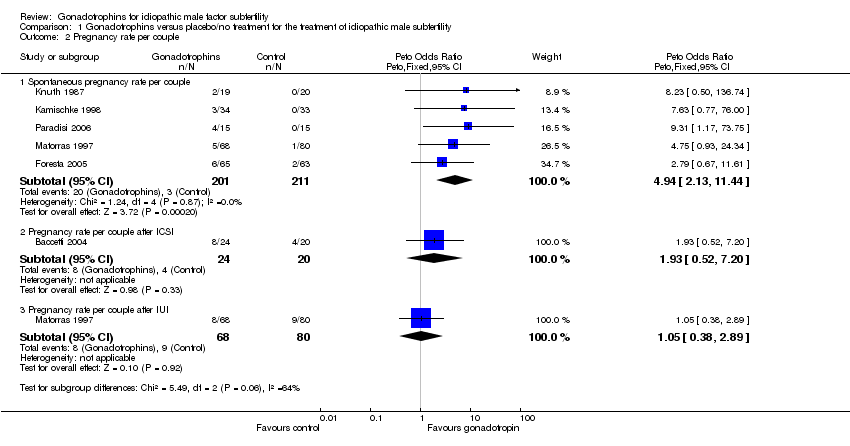
Forest plot of comparison: 1 Gonadotrophins versus placebo/no treatment for the treatment of idiopathic male subfertility, outcome: 1.2 Pregnancy rate per couple randomly assigned.

Forest plot of comparison: 1 Gonadotrophins versus placebo/no treatment for the treatment of idiopathic male subfertility, outcome: 1.3 Subgroup analysis: pregnancy rate per couple randomly assigned with no female factor.

Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
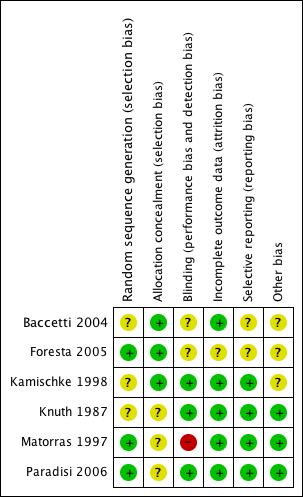
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.

Forest plot of comparison: 1 Gonadotrophins versus placebo/no treatment for the treatment of idiopathic male subfertility, outcome: 1.1 live‐birth rate per couple randomly assigned.
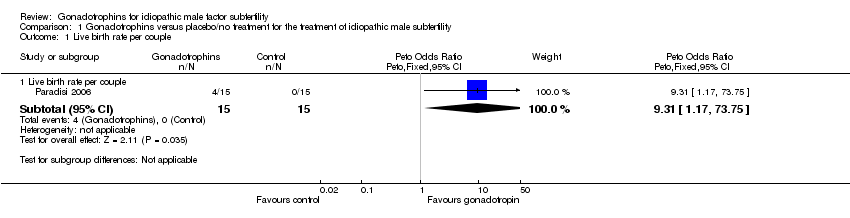
Comparison 1 Gonadotrophins versus placebo/no treatment for the treatment of idiopathic male subfertility, Outcome 1 Live birth rate per couple.
Comparison 1 Gonadotrophins versus placebo/no treatment for the treatment of idiopathic male subfertility, Outcome 2 Pregnancy rate per couple.

Comparison 1 Gonadotrophins versus placebo/no treatment for the treatment of idiopathic male subfertility, Outcome 3 Subgroup analysis: Pregnancy rate per couple with no female factor.
| Gonadotrophins versus placebo/no treatment for the treatment of idiopathic male subfertility | ||||||
| Population: Men with idiopathic male factor subfertility Setting: Assisted reproduction | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo/no treatment for the treatment of idiopathic male subfertility | Gonadotrophins | |||||
| Live birth rate per couple randomly assigned | 0 per 1000 | 0 per 1000 | OR 9.31 | 30 | ⊕⊝⊝⊝ | |
| Spontaneous pregnancy rate per couple randomly assigned | 14 per 1000 | 67 per 1000 | OR 4.94 | 412 | ⊕⊕⊕⊝ | |
| *The basis for the assumed risk is the median control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence: | ||||||
| 1Authors did not report on allocation concealment. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Live birth rate per couple Show forest plot | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 1.1 Live birth rate per couple | 1 | 30 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 9.31 [1.17, 73.75] |
| 2 Pregnancy rate per couple Show forest plot | 6 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 2.1 Spontaneous pregnancy rate per couple | 5 | 412 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 4.94 [2.13, 11.44] |
| 2.2 Pregnancy rate per couple after ICSI | 1 | 44 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.93 [0.52, 7.20] |
| 2.3 Pregnancy rate per couple after IUI | 1 | 148 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.05 [0.38, 2.89] |
| 3 Subgroup analysis: Pregnancy rate per couple with no female factor Show forest plot | 5 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 3.1 Spontaneous pregnancy rate per couple with no female factor | 4 | 264 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 5.00 [1.88, 13.34] |
| 3.2 Pregnancy rate per couple after ICSI with no female factor | 1 | 44 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.93 [0.52, 7.20] |

