Quinina para los calambres musculares
Referencias
Referencias de los estudios incluidos en esta revisión
Referencias de los estudios excluidos de esta revisión
Referencias adicionales
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | Double‐blind RCT of cross‐over design | |
| Participants | 24 participants (aged 51 to 64 years) who experienced at least 3 nocturnal leg muscle cramps per week | |
| Interventions | A quinine‐vitamin E combination (4 tablets taken daily, each containing 64.8 mg quinine sulphate and 400 IU vitamin E) or quinine sulphate 64.8 mg (4 tablets taken daily) alone taken for 1 week each. 7‐day placebo washout periods before, between and after treatments | |
| Outcomes | Cramp number, cramp duration, cramp intensity after treatment (graded 0 = better to 3 = much worse), adverse events | |
| Notes | Unpublished study conducted by BioDesign (Germany) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Quote: "After randomization..." Comment: patients were allocated a number from 1 to14 and "randomized" to a specific group but no details of the randomization process are provided |
| Allocation concealment? | Unclear risk | No details were given on how the allocation may have been concealed |
| Blinding? | Low risk | Quote: "The study was designed as a double blind..."; "...5 containers with 30 capsules labelled with the number of the treatment week were provided." Comment: probably done as quinine‐vitamin E combination and quinine capsules are similar by description |
| Incomplete outcome data addressed? | Low risk | No drop‐outs from the trial |
| Free of selective reporting? | Low risk | All intended outcome measures were addressed in the results and analysis |
| Free of other bias? | Unclear risk | Conducted by manufacturer of quinine tablets |
| Methods | Double‐blind RCT of cross‐over design | |
| Participants | 69 participants (mean age 51 years) who experienced at least 2 leg cramps per week | |
| Interventions | 2‐week baseline then 2 weeks of quinine sulphate (260 mg) or placebo, then 2‐week washout, then 2 weeks of cross‐over treatment, then 2‐week washout | |
| Outcomes | Cramp number, cramp intensity, cramp duration sleep disturbance, adverse events | |
| Notes | Out of the 69 participants, only 3 were male. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Quote: "Subjects were assigned a study number based upon sequential entry...The study numbers had been previously randomly assigned to either Group 1 or Group 2..." Comment: probably done |
| Allocation concealment? | High risk | Quote: "The identity of the medication will be unknown to the patient and the Investigator but will be identifiable to the Clinical Monitor based on the randomization schedule."; "All patients which the Investigator judges eligible for admission into the study, must be approved by one of the Clinical Monitors either in person or by phone..." Comment: the clinical monitor overseeing the trial was responsible for vetting all candidates before entry into the study and also had access to the randomization schedule |
| Blinding? | Low risk | Quote: "Placebo capsules will be of identical composition to the active capsule, except for the Quinine Sulfate content, and will be identical in appearance."; "The identity of the medications was unknown to the Investigator and the subjects" Comment: probably done |
| Incomplete outcome data addressed? | High risk | 69 out of 84 participants completed the study but no mention is made of the dropouts or the underlying reasons |
| Free of selective reporting? | Low risk | All outcome measures mentioned in protocol addressed in analysis |
| Free of other bias? | Unclear risk | The study was sponsored by Scholl who were marketing quinine as a treatment for cramps. Also 66 of the 69 participants were female though the significance of gender to outcome is not known |
| Methods | Double‐blind RCT of parallel design | |
| Participants | 556 participants (aged 18 to 84 years) who experienced at least 3 nights of nocturnal leg muscle cramps per week | |
| Interventions | 7‐day placebo washout period followed by 2 weeks of a quinine‐vitamin E combination (259.2 mg quinine sulphate and 1600 IU vitamin E daily) or quinine sulphate 259.2 mg alone or vitamin E (1600 IU) alone, or placebo | |
| Outcomes | Cramp number, cramp days, cramp intensity, cramp duration, sleep disturbance, adverse events | |
| Notes | Large multicentre trial. Approximately double number of females than males across all treatment groups. Unpublished | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Quote: "...according to a predetermined randomized schedule" |
| Allocation concealment? | Unclear risk | No details are given regarding how allocation may have been concealed |
| Blinding? | Low risk | Quote: "All capsules will be identical in appearance", "...weeks 2 and 3 will be double‐blind treatment periods" Comment: probably adequate |
| Incomplete outcome data addressed? | Low risk | All withdrawals and those lost to follow up accounted for, and intention‐to‐treat analysis performed |
| Free of selective reporting? | Low risk | All outcomes reported in detail |
| Free of other bias? | Unclear risk | Conducted by manufacturer of quinine tablets |
| Methods | Double‐blind RCT of cross‐over design | |
| Participants | 30 male participants (aged 38 to 73 years) who experienced at least 6 leg cramps per month | |
| Interventions | Quinine sulphate 500 mg daily (200 mg at supper, 300 mg at bedtime) or vitamin E 800 U daily or placebo for a 4‐week treatment period, followed by a 4‐week washout period before cross‐over to a second 4‐ week treatment period | |
| Outcomes | Cramp number, cramp nights, cramp intensity (graded 1 = no pain to 4 = severe), sleep disturbance (graded 1 = none to 4 = severe), adverse events | |
| Notes | Conducted at a Veterans Affairs Medical Center in USA. All subjects were male | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Quote: "Drug‐on periods were assigned in randomly permuted order..." |
| Allocation concealment? | Unclear risk | No details provided on how allocation may have been concealed |
| Blinding? | Low risk | Quote: "All medications were packaged in unit doses and dispensed by the same company." Comment: probably adequate blinding |
| Incomplete outcome data addressed? | Low risk | All 3 patients who failed to complete the study were accounted for |
| Free of selective reporting? | Low risk | All outcome measures mentioned in the protocol were addressed in the analysis |
| Free of other bias? | Unclear risk | All subjects were male as all recruited from Veterans Affairs Medical Center |
| Methods | Double‐blind, parallel group RCT | |
| Participants | 94 participants (aged 18 to 70 years) who experienced at least 6 muscle cramps in 2 weeks | |
| Interventions | Quinine sulphate 400 mg daily or placebo for 2 weeks | |
| Outcomes | Cramp number, cramp days, cramp intensity (scale not stated), sleep disturbance (scale not stated), global efficacy rating by patient and doctor (scale not stated), adverse events | |
| Notes | Multicentre trial in Germany; participants taken from 17 general practices | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Quote: "Randomisation used permuted blocks of four patients stratified by the centre...... when the sealed envelopes were collected and the blind review written, the code was revealed." Comment: probably done |
| Allocation concealment? | Low risk | Quotes: "All investigators enrolled in the study and all participants were unaware of the treatment allocation, because tablets were identical..." & "A sealed envelope assigning either verum or placebo was available in each centre for each patient..." Comment: probably done |
| Blinding? | Low risk | Quote: "...because the quinine and placebo tablets were identical in appearance." |
| Incomplete outcome data addressed? | High risk | According to the table of results ("Table 2" in the study), there were 6 drop outs; none of these are mentioned in the text |
| Free of selective reporting? | Low risk | All outcome measures mentioned in the protocol were addressed in the analysis |
| Free of other bias? | Low risk | Trial completed at designated time period. Well matched patient characteristics at baseline |
| Methods | Double‐blind RCT of cross‐over design | |
| Participants | 28 participants (aged 51 to 82 years) selected from 2 general practices on the basis that they received regular repeat prescriptions for quinine | |
| Interventions | Quinine sulphate 300 mg daily or placebo for a 30‐day treatment period, followed by a 3‐day washout period before cross‐over to a second 30‐day treatment period | |
| Outcomes | Number of cramp nights, adverse events | |
| Notes | Bicentre study in UK. Results were invalidated by a significant carry‐over effect due to a short washout period | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Quote: "...a randomised double blind cross‐over trial..." Comment: details of randomisation not provided |
| Allocation concealment? | Unclear risk | No details regarding allocation concealment are provided |
| Blinding? | Unclear risk | No details regarding methods of blinding are provided |
| Incomplete outcome data addressed? | Unclear risk | Quote: "Of the 28 recruited, 25 completed the two parts of the trial and filled in diary cards successfully. Two of the three drop‐outs did so because of severe cramps during placebo period" Comment: 1 drop‐out not accounted for |
| Free of selective reporting? | Low risk | Both the intended outcome measures were addressed in the results and analysis |
| Free of other bias? | High risk | Cross‐over trial with only 3 days allocated to washout period rendered a significant carry‐over effect of treatment |
| Methods | Double‐blind RCT of cross‐over design | |
| Participants | 9 elderly out‐patients with a history of night cramps of at least 1 year, with at least 2 cramps per week | |
| Interventions | Quinine sulphate 200 mg or placebo at bedtime for a 4‐week treatment period, followed by a 1‐week washout period before cross‐over to a second 4‐week treatment period | |
| Outcomes | Cramp number, cumulative duration of attacks (in minutes), cumulative score of cramp severity (graded 1 = mild to 3 = severe), adverse events | |
| Notes | The cumulative duration of cramps was calculated as was the score for intensity. Duration or intensity per cramp was not calculated. However, these were calculated from individual patient data | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Quote: "...and patients were randomly assigned.." Comment: no details of the randomisation process are provided |
| Allocation concealment? | Unclear risk | No details provided on how allocation was concealed |
| Blinding? | Low risk | Quote: "in a double‐blind manner to begin receiving either quinine or a placebo." Comment: probably done |
| Incomplete outcome data addressed? | Unclear risk | Explanation given for the one dropout, but was not included in analysis on an intention‐to‐treat basis |
| Free of selective reporting? | Low risk | All intended outcome measures are addressed in the results |
| Free of other bias? | Unclear risk | 7 of the eight 8 volunteers who completed the trial were female |
| Methods | Double‐blind, parallel group RCT | |
| Participants | 164 participants (mean age 56 years) suffering from at least 3 nights of leg cramps per week | |
| Interventions | Combination therapy of quinine sulphate plus theophylline ethylene diamine, or quinine alone, or placebo daily for 2 weeks. Before this treatment period, participants were put on placebo as a run‐in phase | |
| Outcomes | Cramp number, cramp nights, cramp intensity (graded 1 mild to 3 = severe), cramp duration, adverse events | |
| Notes | Multicentre study in Germany conducted by Merrell Dow Pharma (now Sanofi‐Aventis) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Quote: "für jedes Zentrum wurde eine Blockrandomisierung vorgenommen....."; [for each centre a blockwise randomization sequence was generated]......" Comment: probably adequate |
| Allocation concealment? | Low risk | Quote: "Die Prüfärzte hatten für jeden Patienten einen verschlossenen Umschlag erhalten, in dem aussen die Randomnummer und innen das Prüfmedikament verzeichnet war" [Each prinicipal investigator was given a sealed envelope for each respective patient with the random number marked on the outside and the medication on the inside] Comment: probably done |
| Blinding? | Low risk | Quote: " 3‐fache blinde Studienanlage" [Triple blind study setting]:; die äusserlich indentischen und nicht voneinander zu unterscheidenen Tabletten..." [from the outside identical tablets indistinguishable with respect to form, taste, colour...] Comment: patients, principal investigators and statistician were all blinded |
| Incomplete outcome data addressed? | Low risk | "Tab.5: Gründe für die fehlende Aufnahme in die inferenzstatistischen Zeitreihenanalysen.." [Tab.5: Reasons for exclusion from statistical analysis] Comment: reasons are given for all patients not included in the statistical analysis |
| Free of selective reporting? | Low risk | Results of all outcome measures are reported |
| Free of other bias? | Unclear risk | Conducted by manufacturer of quinine and also the quinine‐theophylline combination |
| Methods | Double‐blind RCT of cross‐over design | |
| Participants | 62 participants (mean age 47 years) who experienced at least 2 leg cramps per week | |
| Interventions | 2‐week baseline then 2 weeks of quinine sulphate (325 mg) or placebo, then 2‐week washout, then 2 weeks of cross‐over treatment, then 2‐week washout | |
| Outcomes | Cramp number, cramp intensity, cramp days, adverse events | |
| Notes | Second trial by Scholl pharmaceuticals submitted to FDA, but with higher quinine dose of 325 mg. Unpublished. Based in Florida | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Quote: "...medications will be assigned according to a predetermined randomization schedule" Comment: no details of the "randomization schedule" are provided |
| Allocation concealment? | Low risk | Quote: "Each subject's medications will be provided by the Sponsor and distributed by the Invesitgator" Comment: appears that allocation was concealed |
| Blinding? | Low risk | Quote: "Placebo capsules...will be identical in appearance" |
| Incomplete outcome data addressed? | Low risk | All withdrawals were accounted for |
| Free of selective reporting? | Low risk | All outcomes reported on |
| Free of other bias? | Unclear risk | Conducted by manufacturer of quinine tablets |
| Methods | Double‐blind, parallel group RCT | |
| Participants | 20 adult volunteers (median age 55 years) from general population who suffered at least three muscle cramps per week | |
| Interventions | Hydroquinine hydrobromide 300 mg daily (200 mg at supper, 100 mg at bedtime) or placebo for 2 weeks. This was followed by a 2‐week intervention‐free period whereby persistence of drug effect was monitored | |
| Outcomes | Reduction in cramp number from baseline for each treatment group. Cramp severity (scale not stated), cramp duration, cramp location and adverse events were also outcomes | |
| Notes | Adult volunteers were recruited via a notice in a regional newspaper, with a "small financial reward". Randomisation led to quinine group being solely female whilst all males were randomised into placebo group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Quote: "Twenty participants were randomly allocated to receive either active drug or placebo." Comment: no details of the randomisation process are provided |
| Allocation concealment? | Low risk | Quote: "During the trial only the manufacturer knew the codes disclosing drug and placebo." |
| Blinding? | High risk | Quote: "...three quinine users who complained of a bitter taste possibly were not blind to the type of medication..." Comment: inadequate blinding with high risk of bias |
| Incomplete outcome data addressed? | Unclear risk | One participant dropped out of the placebo group but it is unclear if an intention‐to‐treat analysis was performed |
| Free of selective reporting? | High risk | Quote: "Differences in severity, duration and location ... between placebo...and drug treatment were small." Comment: emphasis placed on cramp frequency, with no mention of the results for the 3 other outcomes |
| Free of other bias? | Unclear risk | Volunteers were recruited via notice in a regional newspaper, for a "small financial reward". The quinine group was solely female, whilst all males were randomised into placebo group |
| Methods | Double‐blind, parallel group RCT | |
| Participants | 106 adult participants from general population who suffered at least 3 muscle cramps per week | |
| Interventions | Hydroquinine hydrobromide dihydrate 300 mg daily (200 mg at supper, 100 mg at bedtime) or placebo for 2 weeks | |
| Outcomes | Cramp number, cramp days, cramp intensity (graded 1 mild to 10 = severe), cramp duration, cramp location, adverse events | |
| Notes | Adult volunteers were recruited via a notice in a regional newspaper, and posters in pharmacies and libraries | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Quote: "An independent investigator used the random‐number generator of the SAS program to create the randomisation schedule." |
| Allocation concealment? | Low risk | Quote: "All investigators involved in the study and all participants were unaware of the treatment allocation." |
| Blinding? | High risk | Quote: "The only side‐effect definitely related to hydroquinine was a bitter taste or dry mouth (ten participants)..." Comment: inadequate blinding with high risk of bias |
| Incomplete outcome data addressed? | Low risk | All patients who failed to complete the trial were accounted for |
| Free of selective reporting? | Unclear risk | Data was collected with respect to cramp duration, severity & location, in addition to the primary outcome of frequency. However little actual data is presented to justify the "insignificant differences between drug and placebo" reported, and no mention of results for cramp location is made |
| Free of other bias? | Unclear risk | Quote: "We recruited volunteers through notices in regional newspapers and posters in libraries and pharmacies." |
| Methods | Double‐blind RCT of cross‐over design | |
| Participants | 9 elderly participants seeking treatment from GP for at least 2 cramp nights per week | |
| Interventions | Quinine sulphate 300 mg or placebo daily for a 2‐week treatment period, followed by a 2‐week washout period before cross‐over to a second 2‐week treatment period. A 2‐week run‐in period (of placebo) preceded the first phase of treatment | |
| Outcomes | Improvement in sleep induction (graded 1 = difficult to 10 = easy), sleep quality (graded 1 = poor to 10 = good), cramp severity (graded 1 = mild to 10 =severe), cramp timing (before or after 2 am), cramp duration and adverse events | |
| Notes | Table of results for cramp duration contradicts commentary in results section | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Quote: "The study was double‐blind and crossed over within patients, and randomised..." Comment: details of randomisation not provided |
| Allocation concealment? | Low risk | Quote: "...the study was... randomised and balanced by an independent observer." |
| Blinding? | Unclear risk | Quote: "The study was double‐blind.... the two weeks between treatments were single‐blind with patients taking placebo." Comment: no explicit mention of how blinding was achieved |
| Incomplete outcome data addressed? | Low risk | All 9 patients completed the trial |
| Free of selective reporting? | Low risk | All outcome measures commented upon in analysis, including adverse events |
| Free of other bias? | Low risk | Adequate washout periods. Trial ended at designated time period |
| Methods | Double‐blind RCT of cross‐over design | |
| Participants | 9 participants with chronic renal failure on maintenance haemodialysis 3 times per week, and with frequent muscle cramps | |
| Interventions | Participants given quinine sulphate 320 mg or placebo at the beginning of each dialysis treatment, for a period of 12 weeks | |
| Outcomes | Cramp frequency, cramp intensity (graded mild = cramp lasting < 5 minutes and disappeared spontaneously, moderate = cramp lasting between 5 and 10 minutes and ceased after reduction of dialysis pump‐rate and severe = cramp lasting >15 minutes and unrelieved despite reduction in pump rate), cramp duration, adverse events | |
| Notes | Study conducted in New York. Frequency of muscle cramps expressed as number of dialyses affected by cramps, rather than number or cramps during a fixed period | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Quote: "...a randomised double blind cross‐over trial..." Comment: details of randomisation not provided |
| Allocation concealment? | Unclear risk | No details regarding methods of concealment are provided |
| Blinding? | Low risk | Quote: "Quinine sulphate and placebo were placed in identical gelatin capsules and delivered from the hospital pharmacy...The pharmacy kept a record of the content of the capsule...but this information was withheld from the dialysis staff..." Comment: adequately blinded |
| Incomplete outcome data addressed? | Low risk | All patients completed the trial |
| Free of selective reporting? | High risk | The distribution and timing of cramps, and the blood pressure and dialysis pump rate during an episode were said to be outcomes have but these are not mentioned in the results/discussion sections |
| Free of other bias? | High risk | Only cramps during dialysis sessions were assessed; effect of treatment on cramps outside of dialysis sessions was not measured. Also, there was no washout period between cross‐over treatments |
| Methods | Single‐blind, parallel group RCT | |
| Participants | 31 cirrhotic patients with an average of over 3 muscle cramps per week | |
| Interventions | 4‐week run‐in period, followed by a 4‐week treatment period of either quinidine sulphate 200 mg twice‐daily or placebo twice‐daily | |
| Outcomes | Cramp number, adverse events | |
| Notes | Study conducted on an outpatient basis in Taiwan. 31 participants (mean age 62 years) completed the study. 84% participants were male | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Quote: "...were allocated, using a table of random numbers." Comment: probably done |
| Allocation concealment? | Unclear risk | No mention of how allocation was conveyed to the investigators, though as this was a single‐blinded study, concealment may not have been attempted at all |
| Blinding? | High risk | Quote: "Patients were not aware of which drug was being prescribed, but physicians were." Comment: single blinded study |
| Incomplete outcome data addressed? | Low risk | 31 out of 43 participants completed the study and withdrawals are accounted for (excluded due to low cramp frequency or poor record keeping) and were excluded before randomisation |
| Free of selective reporting? | Low risk | All intended outcome measures are addressed in the results |
| Free of other bias? | Low risk | Except for the lack of double‐blinding counted above, nil else significant |
| Methods | Double‐blind RCT of cross‐over design | |
| Participants | 205 participants (mean age 44 years) who experienced at least 2 leg cramps per week | |
| Interventions | 1‐week washout then four blocks of 5‐day treatment periods separated by 2‐day washouts. Treatments consisted of 129.6 mg quinine sulphate twice daily, or a quinine‐vitamin E combination (129.6 mg quinine sulphate plus 800 Units vitamin E) twice daily, or 800 units vit E twice daily | |
| Outcomes | Cramp number, cramp intensity, cramp days, sleep disturbance, adverse events. | |
| Notes | The second largest trial. 2‐centre trial (New York & California). NB short treatment periods. Unpublished | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Quote: "...patients were assigned at random to 24 treatment sequences according to a randomisation schedule" Comment: no details of how the "randomisation schedule" was generated |
| Allocation concealment? | Low risk | Quote: "...according to a randomisation schedule prepared by an independent person who did not participate in the study" Comment: appears adequate |
| Blinding? | Low risk | Quote: "...under the double‐blind condition for identically appearing study medications" |
| Incomplete outcome data addressed? | Low risk | All withdrawals and those lost to follow were fully accounted for |
| Free of selective reporting? | Low risk | All outcomes are reported in the results |
| Free of other bias? | High risk | High risk of bias caused by very short washout periods between treatments. Also, trial conducted by manufacturer of quinine tablets |
| Methods | Double‐blind RCT of parallel design | |
| Participants | 25 participants on a general medical ward, experiencing at least 2 leg cramps per week | |
| Interventions | Nightly quinine sulphate (300 mg) or placebo for 2 weeks (or less if discharged earlier) | |
| Outcomes | Cramp days, cramp intensity, adverse events | |
| Notes | Poorly designed study with no mention of number of patients in each group. Scanty data also | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Quote: "Each volunteer was randomly allocated to receive either 300 mg quinine or a placebo..." Comment: no description of randomisation protocol |
| Allocation concealment? | Unclear risk | No mention of how allocation may have been concealed |
| Blinding? | Unclear risk | No details regarding methods of blinding are provided |
| Incomplete outcome data addressed? | High risk | Impossible to assess as no mention of number of patients in each group nor of how many actually completed the trial |
| Free of selective reporting? | Low risk | Both the intended outcome measures were addressed in the results and analysis |
| Free of other bias? | High risk | Patients were recruited from a general medical ward as inpatients. Some patients were discharged before the 2‐week follow‐up |
| Methods | RCT of cross‐over design | |
| Participants | 16 participants from general practice (mean age 76 years) who experienced at least 2 leg cramps per week | |
| Interventions | 2‐week washout then 4 blocks of 3‐week treatment periods consisting of quinine bisulphate (300 mg) or placebo or cork or wood in woollen bags | |
| Outcomes | Cramp number, adverse events | |
| Notes | Quinine compared against placebo and folklore. Only data provided is that for adverse events, but how many patients suffered these is not clear | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Quote: "were allocated to receive the three treatments and placebo in random order." Comment: details of randomisation not provided |
| Allocation concealment? | Unclear risk | No mention of how allocation was concealed |
| Blinding? | High risk | Quote: "the two tablets (quinine & placebo) should have been physically identical, but owing to lack of funds this criterion was not met." Comment: treatments were clearly distinguishable |
| Incomplete outcome data addressed? | High risk | There were 6 withdrawals from the trial; it is not clear from which treatment group they withdrew from, and the precise causes of the withdrawals are not given |
| Free of selective reporting? | Low risk | Quote: "During analysis of the data only average cramp number was considered because the duration section of the form was inadequately filled in by the majority of patients." Commment: suggests authors would have, as planned, analysed such data if it was available |
| Free of other bias? | High risk | Treatments were sequential with no dedicated washout period between each phase raising the possibility of significant carry‐over / withdrawal effects |
| Methods | Single‐blind, parallel group RCT | |
| Participants | 24 adult outpatients (mean age 64 years) with nocturnal calf cramps associated with myofascial pain syndrome and gastrocnemius trigger points with at least 4 cramps per month | |
| Interventions | Quinine sulphate 300 mg orally daily at bedtime or 1 to 2 ml 1% xylocaine injection at the gastrocnemius trigger point at the start of the trial. Treatment period for 4 weeks, followed by follow‐up 4 weeks later. All subjects assigned to perform calf stretches daily | |
| Outcomes | Cramp number, cramp intensity (graded 0 = no pain to 10 = severe), cramp duration (minutes), adverse events | |
| Notes | Participants recruited from several outpatient clinics in Thailand. 2 participants withdrew from the study due to cinchonism during the treatment period. 20 of the 22 participants who completed the study were female | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Quote: "They were randomly divided into two groups..." Comment: no description of randomisation protocol |
| Allocation concealment? | Unclear risk | No details regarding how allocation may have been concealed is given |
| Blinding? | High risk | Quote: "...a single‐blinded comparative clinical study." Comment: no control group was used for the injection treatment. The reviewing physician was blinded to the treatment received by the participants |
| Incomplete outcome data addressed? | Unclear risk | Reasons for the 2 participant withdrawals are given but these were not counted in an intention‐to‐treat analysis |
| Free of selective reporting? | Low risk | All intended outcome measures were addressed |
| Free of other bias? | High risk | The number of treatments received by participants in the injection group varied depending on individual cramp frequencies during the follow‐up period; there was therefore no uniform dose/regime for the injections. Significant confounder in the fact that all subjects were to perform calf stretches daily |
| Methods | Double‐blind, parallel group RCT | |
| Participants | 30 participants on dialysis, with a history of leg cramps | |
| Interventions | 2 month placebo run‐in period, then active phase of either daily quinine 325 mg at bedtime with a vitamin E placebo, or vitamin E 400 IU at bedtime with a quinine placebo, for 2 months | |
| Outcomes | Cramp number, cramp intensity (graded 1 = no pain to 6 = excruciating), adverse events | |
| Notes | 29 participants (aged 21 to 73 years) from a community‐based academic hospital in Ohio, USA, completed the study. Study compares quinine to vitamin E as well as vitamin E and quinine to placebo. Although researchers state adverse effects of interventions will be investigated, no mention is made of these in the results or discussion | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Quote: "Patients were then randomized into two groups..." Comments: no description of randomisation protocol |
| Allocation concealment? | Unclear risk | No details regarding how allocation may have been concealed is given |
| Blinding? | Low risk | Quote: "quinine 325 mg at bedtime with a vitamin E placebo or 2) vitamin E 400 IU at bedtime with a quinine placebo." Comment: probably done |
| Incomplete outcome data addressed? | Unclear risk | 11 of 40 participants did not complete the trial, all of whom were accounted for. However, 1 participant died after randomisation but no details were given about which treatment was received or whether the death was related to the medication given. |
| Free of selective reporting? | High risk | Adverse events was an outcome but no results given. Also results are given only for first month of treatment, despite treatment duration being 60 days |
| Free of other bias? | Low risk | |
| Methods | Double‐blind RCT of cross‐over design | |
| Participants | 19 adult participants from general medicine clinic who experienced at least 2 leg cramps per week | |
| Interventions | 2‐week run‐in period, followed by either quinine bisulphate 200 mg at night or placebo daily for 3 weeks before cross‐over to a second 3‐week treatment period | |
| Outcomes | Cramp number, cramp intensity (graded 1 = mild to 10 = severe), cramp duration (seconds), adverse events | |
| Notes | Single centre. Conducted in USA | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Quote: "Randomisation was accomplished using a simple random numbers table..." Comment: probably adequate |
| Allocation concealment? | High risk | No evidence that allocations were concealed |
| Blinding? | Unclear risk | Quote: "Patients were blinded to all study periods. However, study personnel were aware that periods one and three used placebo." Comment: details of how investigators and patients were blinded not provided |
| Incomplete outcome data addressed? | Low risk | All 6 participants who left the study were accounted for |
| Free of selective reporting? | Low risk | All outcome measures mentioned in the methods were addressed in the analysis |
| Free of other bias? | Unclear risk | The study group who successfully completed the study consisted of 14 women and only 2 men |
| Methods | Double‐blind RCT of cross‐over design | |
| Participants | 21 elderly participants who experienced at least 2 cramps per week | |
| Interventions | 2‐week run‐in period, followed by either quinine bisulphate 300 mg at night or placebo daily for 3 weeks before cross‐over to a second 3‐week treatment period | |
| Outcomes | Cramp number, cramp index (incorporating cramp duration and intensity) | |
| Notes | Only 18 participants (mean age 73 years) completed the study ‐ full reasons for withdrawal given | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Quote: " Patients were randomly allocated..." Comment: no description of randomisation protocol |
| Allocation concealment? | Unclear risk | No details given regarding allocation concealment |
| Blinding? | Low risk | Quote: "Treatments were quinine bisulphate in a dose of one tablet (300 mg) at night and an identical sugar‐coated placebo..." Comment: probably done |
| Incomplete outcome data addressed? | Low risk | Only 18 participants completed the study, but reasons for withdrawal were given |
| Free of selective reporting? | High risk | Adverse events was an outcome but no results given |
| Free of other bias? | High risk | There was no washout period between the treatment phases thus leaving open the possibility of a 'carry‐over' effect |
| Methods | Double‐blind RCT of cross‐over design | |
| Participants | 22 elderly out‐patients (mean age 74 years), seeking treatment for leg cramps | |
| Interventions | Quinine bisulphate 300 mg or placebo daily for a 3‐week treatment period, followed by immediate cross‐over onto another 3‐week treatment period (i.e. no washout period in between). A 2‐week run‐in period before the trial involved quinine abstention | |
| Outcomes | Cramp number, "cramp index" (the product of intensity score 1 = mild to 3 = severe and duration < 1 min = 1, 1 to 10 min = 2, 11 to 20 min = 3, 21 to 60 min = 4, or > 60 min = 5), adverse events | |
| Notes | Cramp duration and intensity could not be separated from the "cramp index". Individual patient data were available | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Quote: "the remainder were allocated, using a table of random numbers..." Comment: probably adequate |
| Allocation concealment? | Unclear risk | No details given regarding allocation concealment |
| Blinding? | Low risk | Quote: "Treatments were quinine, 300 mg, at night, or an identical, sugar coated placebo tablet..." Comment: satisfactory blinding |
| Incomplete outcome data addressed? | Unclear risk | 1 patient dropped out during the placebo stage for an unspecified reason and was not included in the final analysis |
| Free of selective reporting? | Low risk | All outcome measures mentioned in the protocol were addressed in the analysis |
| Free of other bias? | High risk | Quote: "followed by two, sequential, 3‐week treatment periods." Comment: no washout period between each treatment phase raises the possibility of significant carry‐over effect |
| Methods | Double‐blind, randomised controlled 'N‐of‐1' trial | |
| Participants | 13 elderly participants (median age 75 years), suffering at least 2 cramps per week | |
| Interventions | 2‐week washout period followed by 3 4‐week treatment blocks in which patients are randomised to either placebo or quinine sulphate (200 to 300 mg) for 2 weeks and then the other treatment for 2 weeks | |
| Outcomes | Cramp number, cramp days | |
| Notes | General practices in New Zealand | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Quote: "Patients were randomly assigned to one of eight possible treatment sequences..."; "...copy of the randomisations code..." Comment: probably done as description suggests centrally‐organised randomisation codes |
| Allocation concealment? | Low risk | Quote: " A sealed copy of the randomisation code..."; " A master copy of the randomisation codes was also held by the research supervisor..." Comment: probably done |
| Blinding? | Low risk | Quote: "Both the patients and the researcher interacting with them and conducting the analyses were blinded..." Comment: adequate double‐blinding |
| Incomplete outcome data addressed? | Low risk | Full explanation provided for the 3 dropouts |
| Free of selective reporting? | Low risk | All outcomes measured that were described in the initial protocol were addressed in the analysis |
| Free of other bias? | Unclear risk | Participants continued with their most recent dose of quinine thus this varied between participants |
RCT: randomised controlled trial
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| This was an observational study where patients already on quinine were started on verapamil instead, and seen to improve | |
| The focus of this RCT was on the cessation of quinine and effect of exercise | |
| The active treatment in this RCT comprised quinine with aminophylline. The effect of quinine alone could therefore not be ascertained | |
| This Spanish paper was translated into English. There was no evidence of randomisation and all cramps were treated with hypertonic saline, meaning that quinine was not given alone. Also the outcome measured was the number of dialysis sessions affected by cramp rather than the cramp number itself | |
| The active treatment in this RCT of haemodialysis patients comprised quinine with aminophylline. The effect of quinine alone could therefore not be ascertained |
RCT: randomised controlled trial
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Difference in number of cramps over 2 weeks (GIV) ‐ fixed‐effect Show forest plot | 14 | Cramp number (Fixed, 95% CI) | ‐1.81 [‐2.20, ‐1.42] | |
| Analysis 1.1  Comparison 1 Quinine versus placebo, Outcome 1 Difference in number of cramps over 2 weeks (GIV) ‐ fixed‐effect. | ||||
| 2 Difference in number of cramps over 2 weeks ‐ random‐effects (minus Connolly 1992) Show forest plot | 13 | Cramp Number (Random, 95% CI) | ‐2.45 [‐3.54, ‐1.36] | |
| Analysis 1.2  Comparison 1 Quinine versus placebo, Outcome 2 Difference in number of cramps over 2 weeks ‐ random‐effects (minus Connolly 1992). | ||||
| 3 Difference in number of cramps according to quinine dose (GIV) ‐ fixed‐effect Show forest plot | 14 | Cramp Number (Fixed, 95% CI) | ‐1.81 [‐2.20, ‐1.42] | |
| Analysis 1.3  Comparison 1 Quinine versus placebo, Outcome 3 Difference in number of cramps according to quinine dose (GIV) ‐ fixed‐effect. | ||||
| 3.1 500 mg quinine | 1 | Cramp Number (Fixed, 95% CI) | ‐8.7 [‐10.30, ‐7.10] | |
| 3.2 400 mg quinine | 2 | Cramp Number (Fixed, 95% CI) | ‐3.36 [‐4.83, ‐1.89] | |
| 3.3 300 to 325 mg quinine | 5 | Cramp Number (Fixed, 95% CI) | ‐0.79 [‐1.31, ‐0.26] | |
| 3.4 260 mg quinine | 3 | Cramp Number (Fixed, 95% CI) | ‐1.29 [‐2.15, ‐0.42] | |
| 3.5 200 mg quinine | 3 | Cramp Number (Fixed, 95% CI) | ‐3.22 [‐4.40, ‐2.04] | |
| 4 Difference in cramp intensity (GIV) ‐ fixed‐effect Show forest plot | 7 | Cramp intensity (Fixed, 95% CI) | ‐0.12 [‐0.20, ‐0.05] | |
| Analysis 1.4  Comparison 1 Quinine versus placebo, Outcome 4 Difference in cramp intensity (GIV) ‐ fixed‐effect. | ||||
| 5 Change in cramp duration (mins) ‐ random‐effects Show forest plot | 2 | Change in duration (Random, 95% CI) | ‐1.35 [‐4.00, 1.30] | |
| Analysis 1.5  Comparison 1 Quinine versus placebo, Outcome 5 Change in cramp duration (mins) ‐ random‐effects. | ||||
| 6 Difference in number of cramp days over 2 weeks (GIV) ‐ random‐effects (minus Connolly 1992 Show forest plot | 7 | Cramp days (Random, 95% CI) | ‐1.15 [‐1.93, ‐0.38] | |
| Analysis 1.6  Comparison 1 Quinine versus placebo, Outcome 6 Difference in number of cramp days over 2 weeks (GIV) ‐ random‐effects (minus Connolly 1992. | ||||
| 7 Participants suffering minor adverse events ‐ fixed‐effect Show forest plot | 16 | 1447 | Risk Difference (M‐H, Fixed, 95% CI) | 0.03 [0.00, 0.06] |
| Analysis 1.7  Comparison 1 Quinine versus placebo, Outcome 7 Participants suffering minor adverse events ‐ fixed‐effect. | ||||
| 8 Participants suffering specific minor adverse events Show forest plot | 18 | Risk Difference (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.8  Comparison 1 Quinine versus placebo, Outcome 8 Participants suffering specific minor adverse events. | ||||
| 8.1 Gastrointestinal | 18 | 1581 | Risk Difference (M‐H, Fixed, 95% CI) | 0.03 [0.01, 0.05] |
| 8.2 Headache | 18 | 1581 | Risk Difference (M‐H, Fixed, 95% CI) | 0.00 [‐0.02, 0.02] |
| 8.3 Tinnitus | 18 | 1581 | Risk Difference (M‐H, Fixed, 95% CI) | 0.01 [‐0.00, 0.02] |
| 9 Participants suffering major adverse events Show forest plot | 18 | 1613 | Risk Difference (M‐H, Fixed, 95% CI) | 0.00 [‐0.01, 0.02] |
| Analysis 1.9  Comparison 1 Quinine versus placebo, Outcome 9 Participants suffering major adverse events. | ||||
| 10 Participants suffering specific major adverse events (gastrointestinal) Show forest plot | 18 | 1613 | Risk Difference (M‐H, Fixed, 95% CI) | 0.01 [‐0.00, 0.02] |
| Analysis 1.10  Comparison 1 Quinine versus placebo, Outcome 10 Participants suffering specific major adverse events (gastrointestinal). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Difference in number of cramps in 2 weeks ‐ random‐effects (minus Connolly 1992) Show forest plot | 3 | No. cramps (Random, 95% CI) | ‐0.24 [‐1.29, 0.81] | |
| Analysis 2.1  Comparison 2 Quinine versus vitamin E, Outcome 1 Difference in number of cramps in 2 weeks ‐ random‐effects (minus Connolly 1992). | ||||
| 2 Difference in cramp intensity ‐ fixed‐effect Show forest plot | 3 | Cramp intensity (Fixed, 95% CI) | ‐0.06 [‐0.17, 0.04] | |
| Analysis 2.2  Comparison 2 Quinine versus vitamin E, Outcome 2 Difference in cramp intensity ‐ fixed‐effect. | ||||
| 3 Difference in number of cramp days over 2 weeks ‐ random‐effects (minus Connolly 1992) Show forest plot | 2 | Cramp days (Random, 95% CI) | ‐0.28 [‐0.98, 0.43] | |
| Analysis 2.3  Comparison 2 Quinine versus vitamin E, Outcome 3 Difference in number of cramp days over 2 weeks ‐ random‐effects (minus Connolly 1992). | ||||
| 4 Participants suffering minor adverse events ‐ random‐effects Show forest plot | 2 | 688 | Risk Difference (M‐H, Random, 95% CI) | 0.02 [‐0.04, 0.09] |
| Analysis 2.4  Comparison 2 Quinine versus vitamin E, Outcome 4 Participants suffering minor adverse events ‐ random‐effects. | ||||
| 5 Participants suffering major adverse events ‐ random‐effects Show forest plot | 3 | 748 | Risk Difference (M‐H, Random, 95% CI) | 0.01 [‐0.01, 0.02] |
| Analysis 2.5  Comparison 2 Quinine versus vitamin E, Outcome 5 Participants suffering major adverse events ‐ random‐effects. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Difference in number of cramps in 2 weeks ‐ random‐effects Show forest plot | 2 | No. cramps (Random, 95% CI) | 1.07 [‐1.08, 3.23] | |
| Analysis 3.1  Comparison 3 Quinine versus a quinine‐vitamin E combination (Q‐Vel), Outcome 1 Difference in number of cramps in 2 weeks ‐ random‐effects. | ||||
| 2 Difference in cramp intensity ‐ random‐effects Show forest plot | 3 | Cramp intensity (Random, 95% CI) | 0.10 [‐0.06, 0.26] | |
| Analysis 3.2  Comparison 3 Quinine versus a quinine‐vitamin E combination (Q‐Vel), Outcome 2 Difference in cramp intensity ‐ random‐effects. | ||||
| 3 Difference in number of cramp days over 2 weeks ‐ random‐effects Show forest plot | 2 | Cramp days (Random, 95% CI) | 0.18 [‐1.13, 1.49] | |
| Analysis 3.3  Comparison 3 Quinine versus a quinine‐vitamin E combination (Q‐Vel), Outcome 3 Difference in number of cramp days over 2 weeks ‐ random‐effects. | ||||
| 4 Participants suffering minor adverse events ‐ random‐effects Show forest plot | 3 | 739 | Risk Difference (M‐H, Random, 95% CI) | 0.03 [‐0.04, 0.10] |
| Analysis 3.4  Comparison 3 Quinine versus a quinine‐vitamin E combination (Q‐Vel), Outcome 4 Participants suffering minor adverse events ‐ random‐effects. | ||||
| 5 Participants suffering major adverse events ‐ fixed‐effect Show forest plot | 3 | 739 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.00 [‐0.01, 0.01] |
| Analysis 3.5  Comparison 3 Quinine versus a quinine‐vitamin E combination (Q‐Vel), Outcome 5 Participants suffering major adverse events ‐ fixed‐effect. | ||||
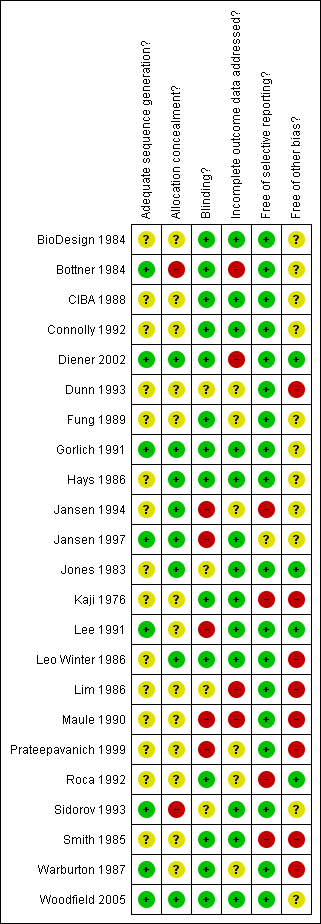
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
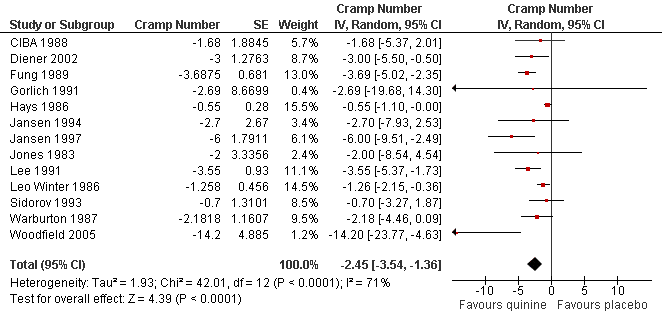
Forest plot of comparison: 1 Quinine versus placebo, outcome: 1.2 Difference in number of cramps over 2 weeks ‐ random‐effects (minus Connolly 1992).

Forest plot of comparison: 2 Quinine versus vitamin E, outcome: 2.1 Difference in number of cramps in 2 weeks ‐ random‐effects (minus Connolly 1992).

Forest plot of comparison: 3 Quinine versus a quinine‐vitamin E combination (Q‐Vel), outcome: 3.1 Difference in number of cramps in 2 weeks ‐ random‐effects.

Forest plot of comparison: 1 Quinine versus placebo, outcome: 1.7 Participants suffering minor adverse events ‐ fixed‐effect.
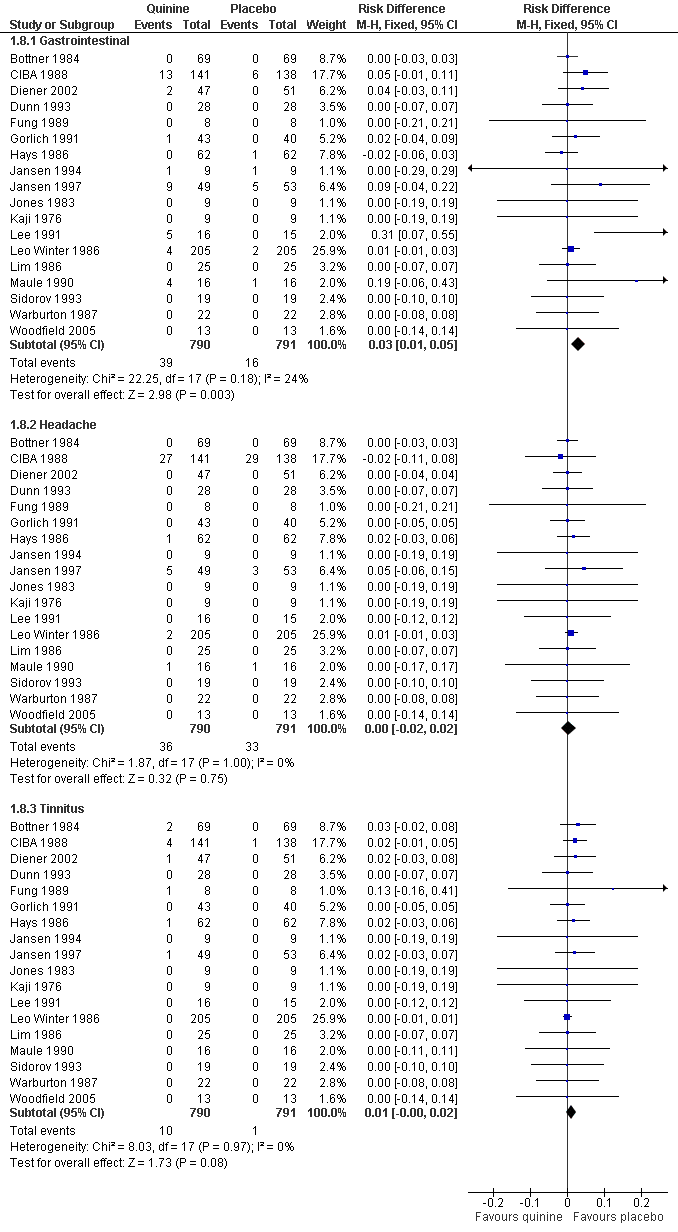
Forest plot of comparison: 1 Quinine versus placebo, outcome: 1.8 Participants suffering specific minor adverse events.
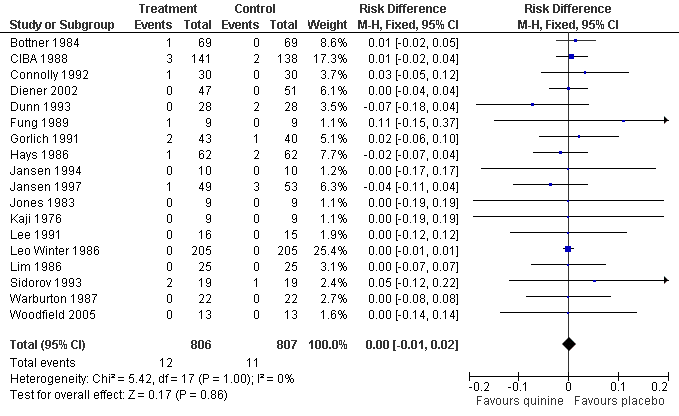
Forest plot of comparison: 1 Quinine versus placebo, outcome: 1.9 Participants suffering major adverse events.

Forest plot of comparison: 1 Quinine versus placebo, outcome: 1.10 Participants suffering specific major adverse events (gastrointestinal).
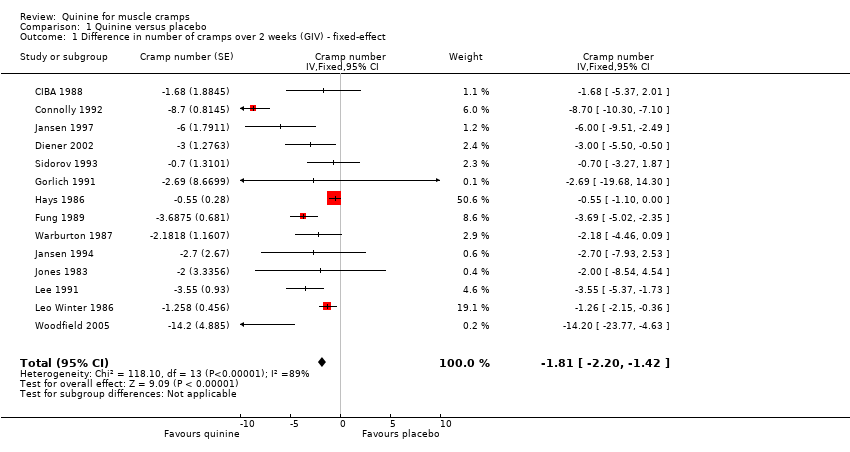
Comparison 1 Quinine versus placebo, Outcome 1 Difference in number of cramps over 2 weeks (GIV) ‐ fixed‐effect.

Comparison 1 Quinine versus placebo, Outcome 2 Difference in number of cramps over 2 weeks ‐ random‐effects (minus Connolly 1992).

Comparison 1 Quinine versus placebo, Outcome 3 Difference in number of cramps according to quinine dose (GIV) ‐ fixed‐effect.

Comparison 1 Quinine versus placebo, Outcome 4 Difference in cramp intensity (GIV) ‐ fixed‐effect.

Comparison 1 Quinine versus placebo, Outcome 5 Change in cramp duration (mins) ‐ random‐effects.

Comparison 1 Quinine versus placebo, Outcome 6 Difference in number of cramp days over 2 weeks (GIV) ‐ random‐effects (minus Connolly 1992.
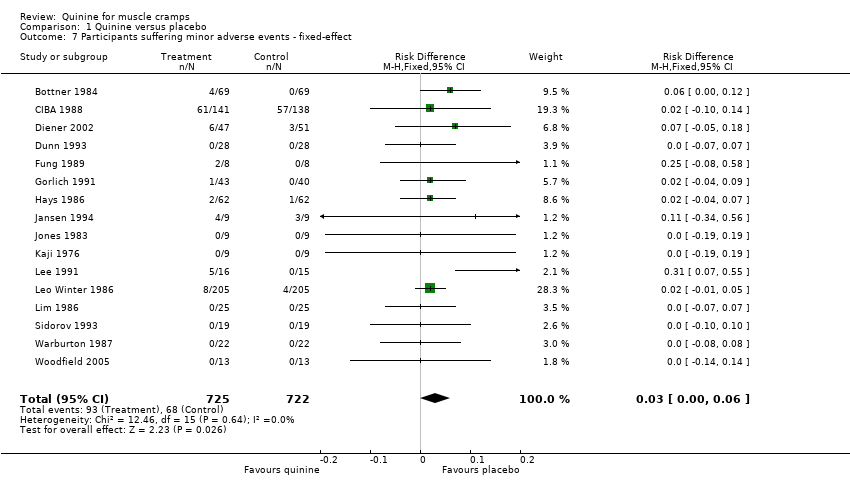
Comparison 1 Quinine versus placebo, Outcome 7 Participants suffering minor adverse events ‐ fixed‐effect.
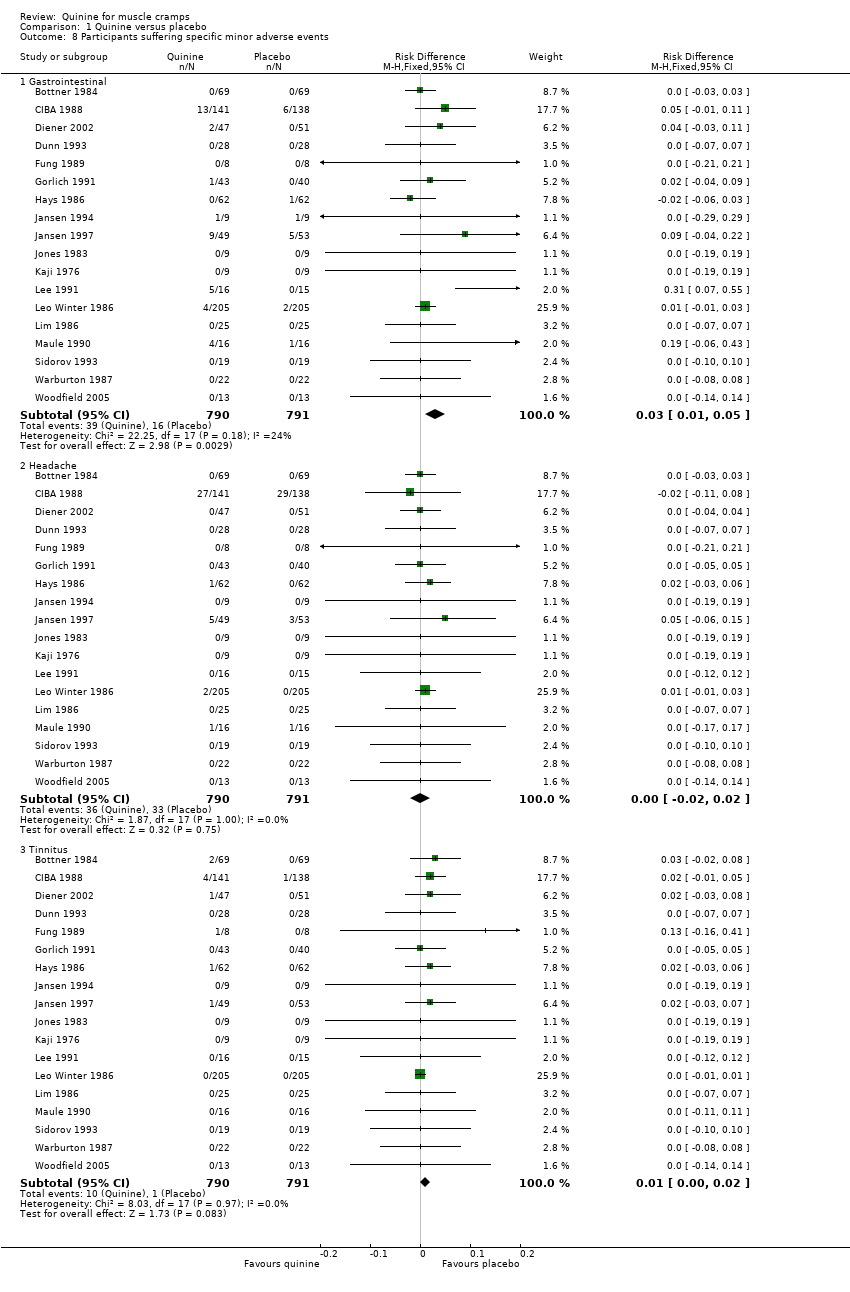
Comparison 1 Quinine versus placebo, Outcome 8 Participants suffering specific minor adverse events.

Comparison 1 Quinine versus placebo, Outcome 9 Participants suffering major adverse events.

Comparison 1 Quinine versus placebo, Outcome 10 Participants suffering specific major adverse events (gastrointestinal).
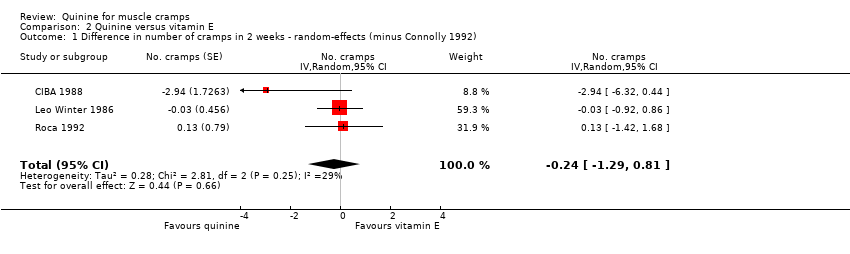
Comparison 2 Quinine versus vitamin E, Outcome 1 Difference in number of cramps in 2 weeks ‐ random‐effects (minus Connolly 1992).

Comparison 2 Quinine versus vitamin E, Outcome 2 Difference in cramp intensity ‐ fixed‐effect.
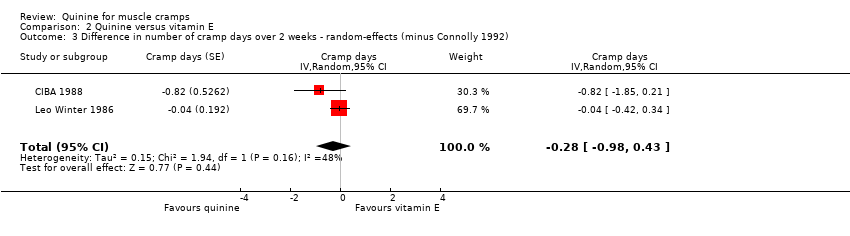
Comparison 2 Quinine versus vitamin E, Outcome 3 Difference in number of cramp days over 2 weeks ‐ random‐effects (minus Connolly 1992).

Comparison 2 Quinine versus vitamin E, Outcome 4 Participants suffering minor adverse events ‐ random‐effects.

Comparison 2 Quinine versus vitamin E, Outcome 5 Participants suffering major adverse events ‐ random‐effects.

Comparison 3 Quinine versus a quinine‐vitamin E combination (Q‐Vel), Outcome 1 Difference in number of cramps in 2 weeks ‐ random‐effects.

Comparison 3 Quinine versus a quinine‐vitamin E combination (Q‐Vel), Outcome 2 Difference in cramp intensity ‐ random‐effects.

Comparison 3 Quinine versus a quinine‐vitamin E combination (Q‐Vel), Outcome 3 Difference in number of cramp days over 2 weeks ‐ random‐effects.

Comparison 3 Quinine versus a quinine‐vitamin E combination (Q‐Vel), Outcome 4 Participants suffering minor adverse events ‐ random‐effects.

Comparison 3 Quinine versus a quinine‐vitamin E combination (Q‐Vel), Outcome 5 Participants suffering major adverse events ‐ fixed‐effect.
| Quinine for muscle cramps | ||||||
| Patient or population: patients with muscle cramps | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Quinine versus placebo | |||||
| Number of cramps over 2 weeks | The mean number of cramps over 2 weeks in the control groups was | The mean Number of cramps over 2 weeks in the intervention groups was | 952 | ⊕⊕⊕⊝ | The difference was statistically significant. | |
| Cramp intensity | The mean cramp intensity in the control groups was | The mean Cramp intensity in the intervention groups was | 666 | ⊕⊕⊕⊝ | The difference was statistically significant. | |
| Participants suffering major adverse events | 14 per 1000 | 15 per 1000 | See comment | 1103 | ⊕⊕⊕⊝ | Risks were calculated from pooled risk differences. The difference was not statistically significant. |
| Participants suffering minor adverse events | 94 per 1000 | 127 per 1000 | See comment | 969 | ⊕⊕⊕⊝ | Risks were calculated from pooled risk differences. The difference was statistically significant. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 There were significant shortcomings in study design in some trials, but the majority of those included in this meta‐analysis were of moderate to high quality | ||||||
| Quinine versus vitamin E for muscle cramps | ||||||
| Patient or population: patients with muscle cramps | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Quinine versus vitamin E | |||||
| Number of cramps over 2 weeks | The mean number of cramps over 2 weeks in the control groups was | The mean Number of cramps over 2 weeks in the intervention groups was | 513 | ⊕⊕⊝⊝ | The difference was not statistically significant. | |
| Cramp intensity | The mean cramp intensity in the control groups was | The mean Cramp intensity in the intervention groups was | 513 | ⊕⊕⊕⊝ | The difference was not statistically significant. | |
| Participants suffering major adverse events | 3 per 1000 | 9 per 1000 | See comment | 513 | ⊕⊕⊕⊝ | Risks were calculated from pooled risk differences. The difference between the two groups was not statistically significant. |
| Participants suffering minor adverse events | 167 per 1000 | 189 per 1000 | See comment | 483 | ⊕⊕⊕⊝ | Risks were calculated from pooled risk differences. The difference between the two groups was not statistically significant. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Only three trials were available for this comparison, two of which were conducted by pharmaceutical investigators on behalf of manufacturers of quinine. A deficiency in the design of one of these trials meant that there was only a two‐day washout between crossover treatments. | ||||||
| Quinine versus a quinine‐vitamin E combination (Q‐Vel) for muscle cramps | ||||||
| Patient or population: patients with muscle cramps | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Quinine versus a quinine‐vitamin E combination (Q‐Vel) | |||||
| Number of cramps over 2 weeks | The mean number of cramps over 2 weeks in the control groups was | The mean Number of cramps over 2 weeks in the intervention groups was | 486 | ⊕⊕⊝⊝ | The difference was not statistically significant. | |
| Cramp intensity | The mean cramp intensity in the control groups was | The mean Cramp intensity in the intervention groups was | 510 | ⊕⊕⊝⊝ | The difference was not statistically significant. | |
| Participants suffering major adverse events | 8 per 1000 | 8 per 1000 | See comment | 510 | ⊕⊕⊕⊝ | Risks were calculated from pooled risk differences. |
| Participants suffering minor adverse events | 173 per 1000 | 202 per 1000 | See comment | 510 | ⊕⊕⊕⊝ | Risks were calculated from pooled risk differences. The difference was not statistically significant. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 The results for cramp number in these two trials were not consistent, each suggesting opposite effects. | ||||||
| Study | Number of participants Study design Patient focus | Mean age (yrs) | Female | Male | F: M ratio | Quinine dose (mg) | Treatment period (d) | Washout period (d) | Treatment comparisons |
| n = 556 Study design = Pb Patient focus = Ib | 45 | 393 | 163 | 2.4 | 260 | 14 | n/ac | Placebo ✓ Vitamin E ✓ Quinine‐vitamin E combination (Q‐Vel®d) ✓ | |
| n = 205 Study design = Cb Patient focus = Ib | 44 | 173 | 32 | 5.4 | 260 | 5 | 2 | Placebo ✓ Vitamin E ✓ Quinine‐vitamin E combination (Q‐Vel®) ✓ | |
| n = 164 Study design = P Patient focus = I | 56 | 119 | 45 | 2.6 | 260 | 14 | n/a | Placebo ✓ Quinine‐theophylline combination (Limptar®d)✓ | |
| n = 106 Study design = P Patient focus = I | 51 | 68 | 44 | 1.5 | 300 | 14 | n/a | Placebo ✓ | |
| n = 94 Study design = P Patient focus = I | 49 | 66 | 32 | 2.1 | 400 | 14 | n/a | Placebo ✓ | |
| n = 69 Study design = C Patient focus = I | 51 | 66 | 3 | 22.0 | 260 | 14 | 14 | Placebo ✓ | |
| n = 62 Study design = C Patient focus = I | 47 | 49 | 13 | 3.8 | 325 | 14 | 14 | Placebo ✓ | |
| n = 31 Study design = P Patient focus = Lb | 62 | 5 | 26 | 0.2 | 400 | 28 | n/a | Placebo ✓ | |
| n = 30 Study design = C Patient focus = I | 59 | 0 | 30 | 0.0 | 500 | 28 | 28 | Placebo ✓ Vitamin E ✓e | |
| n = 30 Study design = P Patient focus = Hb | 48 | 10 | 19 | 0.5 | 325 | 60f | n/a | Vitamin E ✓ | |
| n = 28 Study design = C Patient focus = I | 67 | 17 | 11 | 1.5 | 300 | 30 | 3 | Placebo ✓ | |
| n = 25 Study design = P Patient focus = I | _ | _ | _ | _ | 300 | ≤ 14 | n/a | Placebo ✓ | |
| n = 24 Study design = C Patient focus = I | 57 | 11 | 13 | 0.8 | 260 | 7 | 7 | Quinine‐vitamin E combination (Q‐Vel®) ✓ | |
| n = 24 Study design = P Patient focus = Ig | 64 | 21 | 3 | 7.0 | 300 | 28 | n/a | Xylocaine injection ✓ | |
| n = 22 Study design = C Patient focus = I | 74 | 16 | 6 | 2.7 | 300 | 21 | 0 | Placebo ✓ | |
| n = 20 Study design = P | 55 | 14 | 6 | 2.3 | 300 | 14 | n/a | Placebo ✓ | |
| n = 19 Study design = C Patient focus = I | 58 | 14 | 2 | 7.0 | 200 | 14 | 14 | Placebo ✓ | |
| n = 21 Study design = C Patient focus = I | 73 | _ | _ | _ | 300 | 21 | 0 | Placebo ✓ | |
| n = 16 Study design = C Patient focus = I | 76 | 10 | 6 | 1.7 | 300 | 21 | 0 | Placebo ✓ | |
| n = 13 Study design = N‐of‐1 Patient focus = I | 75 | 7 | 6 | 1.2 | 200 ‐300 | 42 | 0 | Placebo ✓ | |
| n = 9 Study design = C Patient focus = H | _ | _ | _ | _ | 320h | 42h | 0 | Placebo ✓ | |
| n = 9 Study design = C Patient focus = I | _ | _ | _ | _ | 300 | 14 | 14 | Placebo ✓ | |
| n = 9 Study design = C Patient focus = I | 63 | 7 | 1 | 7.0 | 200 | 28 | 7 | Placebo ✓ | |
| aUnpublished. | |||||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Difference in number of cramps over 2 weeks (GIV) ‐ fixed‐effect Show forest plot | 14 | Cramp number (Fixed, 95% CI) | ‐1.81 [‐2.20, ‐1.42] | |
| 2 Difference in number of cramps over 2 weeks ‐ random‐effects (minus Connolly 1992) Show forest plot | 13 | Cramp Number (Random, 95% CI) | ‐2.45 [‐3.54, ‐1.36] | |
| 3 Difference in number of cramps according to quinine dose (GIV) ‐ fixed‐effect Show forest plot | 14 | Cramp Number (Fixed, 95% CI) | ‐1.81 [‐2.20, ‐1.42] | |
| 3.1 500 mg quinine | 1 | Cramp Number (Fixed, 95% CI) | ‐8.7 [‐10.30, ‐7.10] | |
| 3.2 400 mg quinine | 2 | Cramp Number (Fixed, 95% CI) | ‐3.36 [‐4.83, ‐1.89] | |
| 3.3 300 to 325 mg quinine | 5 | Cramp Number (Fixed, 95% CI) | ‐0.79 [‐1.31, ‐0.26] | |
| 3.4 260 mg quinine | 3 | Cramp Number (Fixed, 95% CI) | ‐1.29 [‐2.15, ‐0.42] | |
| 3.5 200 mg quinine | 3 | Cramp Number (Fixed, 95% CI) | ‐3.22 [‐4.40, ‐2.04] | |
| 4 Difference in cramp intensity (GIV) ‐ fixed‐effect Show forest plot | 7 | Cramp intensity (Fixed, 95% CI) | ‐0.12 [‐0.20, ‐0.05] | |
| 5 Change in cramp duration (mins) ‐ random‐effects Show forest plot | 2 | Change in duration (Random, 95% CI) | ‐1.35 [‐4.00, 1.30] | |
| 6 Difference in number of cramp days over 2 weeks (GIV) ‐ random‐effects (minus Connolly 1992 Show forest plot | 7 | Cramp days (Random, 95% CI) | ‐1.15 [‐1.93, ‐0.38] | |
| 7 Participants suffering minor adverse events ‐ fixed‐effect Show forest plot | 16 | 1447 | Risk Difference (M‐H, Fixed, 95% CI) | 0.03 [0.00, 0.06] |
| 8 Participants suffering specific minor adverse events Show forest plot | 18 | Risk Difference (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.1 Gastrointestinal | 18 | 1581 | Risk Difference (M‐H, Fixed, 95% CI) | 0.03 [0.01, 0.05] |
| 8.2 Headache | 18 | 1581 | Risk Difference (M‐H, Fixed, 95% CI) | 0.00 [‐0.02, 0.02] |
| 8.3 Tinnitus | 18 | 1581 | Risk Difference (M‐H, Fixed, 95% CI) | 0.01 [‐0.00, 0.02] |
| 9 Participants suffering major adverse events Show forest plot | 18 | 1613 | Risk Difference (M‐H, Fixed, 95% CI) | 0.00 [‐0.01, 0.02] |
| 10 Participants suffering specific major adverse events (gastrointestinal) Show forest plot | 18 | 1613 | Risk Difference (M‐H, Fixed, 95% CI) | 0.01 [‐0.00, 0.02] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Difference in number of cramps in 2 weeks ‐ random‐effects (minus Connolly 1992) Show forest plot | 3 | No. cramps (Random, 95% CI) | ‐0.24 [‐1.29, 0.81] | |
| 2 Difference in cramp intensity ‐ fixed‐effect Show forest plot | 3 | Cramp intensity (Fixed, 95% CI) | ‐0.06 [‐0.17, 0.04] | |
| 3 Difference in number of cramp days over 2 weeks ‐ random‐effects (minus Connolly 1992) Show forest plot | 2 | Cramp days (Random, 95% CI) | ‐0.28 [‐0.98, 0.43] | |
| 4 Participants suffering minor adverse events ‐ random‐effects Show forest plot | 2 | 688 | Risk Difference (M‐H, Random, 95% CI) | 0.02 [‐0.04, 0.09] |
| 5 Participants suffering major adverse events ‐ random‐effects Show forest plot | 3 | 748 | Risk Difference (M‐H, Random, 95% CI) | 0.01 [‐0.01, 0.02] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Difference in number of cramps in 2 weeks ‐ random‐effects Show forest plot | 2 | No. cramps (Random, 95% CI) | 1.07 [‐1.08, 3.23] | |
| 2 Difference in cramp intensity ‐ random‐effects Show forest plot | 3 | Cramp intensity (Random, 95% CI) | 0.10 [‐0.06, 0.26] | |
| 3 Difference in number of cramp days over 2 weeks ‐ random‐effects Show forest plot | 2 | Cramp days (Random, 95% CI) | 0.18 [‐1.13, 1.49] | |
| 4 Participants suffering minor adverse events ‐ random‐effects Show forest plot | 3 | 739 | Risk Difference (M‐H, Random, 95% CI) | 0.03 [‐0.04, 0.10] |
| 5 Participants suffering major adverse events ‐ fixed‐effect Show forest plot | 3 | 739 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.00 [‐0.01, 0.01] |

