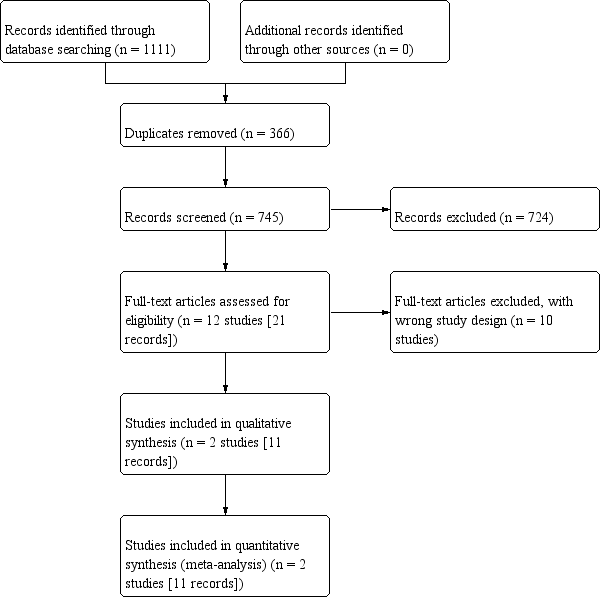
Crioterapia primaria para el cáncer de próstata localizado o localmente avanzado
Referencias
Referencias de los estudios incluidos en esta revisión
Referencias de los estudios excluidos de esta revisión
Referencias adicionales
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | Study design: parallel randomised controlled trial Study conducted: 1999 to 2002 Setting: single institution Geographic location: Canada | |
| Participants | Inclusion criteria: men with histologically proven prostate cancer, clinically staged as T2c, T3a or T3b based on digital rectal examination or transrectal ultrasound findings, or both, negative computerised tomography of the abdomen and pelvis, negative bone scan and serum PSA < 25 ng/mL Exclusion criteria: men with node‐positive disease and distant metastases, prior pelvic radiotherapy or hormone therapy, prostate volume > 75 mL or American Society of Anesthesiology Risk Class > 3 Total number randomly assigned: 63 Group A (whole gland cryotherapy)
Group B (EBRT)
| |
| Interventions | Group A (whole gland cryotherapy): Cryocare System (Endocare Inc, Irvine, CA, USA) was used under general or spinal anaesthesia using transrectal ultrasound‐guided probe placement. In most cases, 5 cryoprobes (range 2–8) were used and 2 freeze–thaw cycles were administered with the urethra protected by a urethra‐warming device (Cook Urologic Inc, Spencer, IN, USA). 3 thermocouple probes at the respective neurovascular bundles and in the midline apex were placed for monitoring purposes and to ensure that the required temperature of < −40 °C was reached. A trocar suprapubic catheter was inserted intraoperatively and kept open for 3 weeks. Group B (EBRT): 66 Gy in 33 fractions, administered at 2 Gy per day, 5 days a week for 6.5 weeks, directed at the prostate, seminal vesicles, and peri‐prostatic region. Co‐interventions: 6 months of hormonal therapy with LHRH agonists (goserelin) was administered starting 3 months before the date of cryosurgery or start of the radiotherapy sessions Follow‐up period (median): 105 months | |
| Outcomes | Primary outcomes: overall survival or disease specific survival
Secondary outcomes: biochemical disease‐free survival or clinical progression
Adverse events
| |
| Funding sources | Research grant from Astra‐Zeneca, Canada | |
| Declarations of interest | Primary author: financial interest or relationship with US HIFU, or both | |
| Notes | Publication status: full text publication Language of publication: English | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comments: not described |
| Allocation concealment (selection bias) | Unclear risk | Comments: not described |
| Blinding of participants and personnel (performance bias) | Unclear risk | Comments: not described |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comments: not described |
| Blinding of outcome assessment (detection bias) | Low risk | Comments: objective outcomes were probably not affected by lack of blinding |
| Incomplete outcome data (attrition bias) | Low risk | Comments: 31/32 (96.8%) and 31/31 (100.0%) men randomised to cryotherapy and EBRT were included in analysis |
| Incomplete outcome data (attrition bias) | Unclear risk | Comments: the outcome was not measured. |
| Incomplete outcome data (attrition bias) | Low risk | Comments: 31/32 (96.8%) and 31/31 (100.0%) of men randomised to cryotherapy and EBRT were included in analysis |
| Incomplete outcome data (attrition bias) | Unclear risk | Comments: the outcome was not measured. |
| Selective reporting (reporting bias) | High risk | Comments: protocol was not published and treatment failure (secondary outcome of study) data were not reported |
| Other bias | Unclear risk | Comments: only 64 out of the planned 150 participants who planned to be randomised were accrued. Lower average prostate volumes likely favor the cryotherapy group. |
| Methods | Study design: parallel randomised controlled trial Study conducted: December 1997 to February 2003 Setting: single institution Geographic location: Canada | |
| Participants | Inclusion criteria: men with histologically proven adenocarcinoma of the prostate, a biopsy tumour classification of T2 or T3, no evidence of lymph node or distant metastases, a pretreatment PSA level < 20 ng/mL, and a gland volume < 60 cm3 Exclusion criteria: men were ineligible if they had: clinically bulky T3 tumour; received prior pelvic radiation; received previous androgen‐deprivation therapy (ADT) at any time; or had undergone transurethral resection of prostate within the previous 3 months Total number randomly assigned: 244 Group A (whole gland cryotherapy)
Group B (EBRT)
| |
| Interventions | Group A (whole gland cryotherapy): transrectal ultrasound guidance with argon/helium third‐generation equipment was used in all participants. The investigators routinely applied thermosensor monitoring, urethral warming, and saline injections to separate the anterior rectal wall from the posterior prostate. 2 freeze‐thaw cycles were used in all men. Group B (EBRT): 4‐field box technique, received 2 Gy daily (5 days per week) using high‐energy mega‐voltage X‐rays (> 10 MV), prescribed dose was 68 Gy initially then increased to 70 Gy in 2000 and 73.5 Gy in 2002 based on standard practice. Clinical target volume included prostate gland and seminal vesicle base (if biopsy tumour classification < T3c) or entirety (if biopsy tumour classification T3c). Co‐interventions: all participants received ADT using LHRH agonist therapy. A single 3‐month depot of LHRH agonist was given followed by local treatment between 90 days and 120 days after the injection. In 2001, the local standard care changed to include 6 months of ADT before EBRT using 2 consecutive 3‐month depots of LHRH, and the protocol was amended accordingly to reflect this change with local treatment commencing between 180 days and 210 days after the first injection. Follow‐up period (median, range): 100 months (53‐128) | |
| Outcomes | Primary outcomes: treatment failure
Secondary outcomes: overall survival or disease‐specific survival or prevalence of positive biopsies
QoL
Adverse events
| |
| Funding sources | Grant from the National Cancer Institute of Canada and the Calgary Health Region | |
| Declarations of interest | Supported by the National Cancer Institute of Canada, and the Alberta Cancer Board. One of coauthor (Dr Rewcastle) was research director for Endocare, Inc | |
| Notes | Publication status: full text publication Language of publication: English | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The study biostatistician (P.M.A.B.) randomly assigned eligible patients to receive 1 of 2 treatments, with stratification according to biopsy tumor classification (bT2 vs bT3), Gleason score (2‐4 vs 5‐7 vs 8‐10), and PSA (7.5 ng/mL vs >7.5 ng/mL) with use of dynamic randomization with a biased coin". |
| Allocation concealment (selection bias) | Unclear risk | Comments: not described |
| Blinding of participants and personnel (performance bias) | High risk | Quote: Study reported as "Open Label" in protocol |
| Blinding of outcome assessment (detection bias) | High risk | Quote: Study reported as "Open Label" in protocol; outcome assessment not explicitly reported but judged to have been unlikely |
| Blinding of outcome assessment (detection bias) | Low risk | Comments: objective outcomes were not likely to be affected by lack of blinding |
| Incomplete outcome data (attrition bias) | Low risk | Comments: 117/122 (95.9%) and 114/122 (93.4%) men randomised to cryotherapy and EBRT were included in analysis |
| Incomplete outcome data (attrition bias) | High risk | Comments: Short term: 106/122 (86.8%) and 88/122 (72.1%) men randomised to cryotherapy and EBRT were included in analysis Long term: 98/122 (80.3%) and 97/122 (79.5%) men randomised to cryotherapy and EBRT were included in analysis |
| Incomplete outcome data (attrition bias) | Low risk | Comments: 117/122 (95.9%) and 114/122 (93.4%) men randomised to cryotherapy and EBRT were included in analysis |
| Incomplete outcome data (attrition bias) | Unclear risk | Comments: the outcome was not measured. |
| Selective reporting (reporting bias) | Low risk | Protocol (NCT00489060) was published and all review outcomes were well described |
| Other bias | Unclear risk | Quote: "Trial was closed prematurely because of diminishing patient accrual, and not because of adverse events", "One interim analysis was planned"; some participants with PSA > 20 ng/mL were included in EBRT group. |
ADT: androgen deprivation therapy; EBRT: external beam radiotherapy; LHRH: luteinising hormone‐releasing hormone; PSA: prostate specific antigen; QoL: quality of life
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| Ineligible study design (single arm) | |
| Ineligible study design (single arm) | |
| Ineligible study design (single arm) | |
| Ineligible study design (single arm) | |
| Ineligible study design (single arm) | |
| Ineligible study design (retrospective study) | |
| Ineligible study design (non‐randomized study) | |
| Ineligible study design (single arm) | |
| Ineligible study design (single arm) | |
| Ineligible study design (single arm) |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Time to death from prostate cancer Show forest plot | 2 | Hazard Ratio (Random, 95% CI) | 1.00 [0.11, 9.45] | |
| Analysis 1.1  Comparison 1 Cryotherapy versus EBRT, Outcome 1 Time to death from prostate cancer. | ||||
| 2 Quality of life (at 3 months follow‐up) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 1.2  Comparison 1 Cryotherapy versus EBRT, Outcome 2 Quality of life (at 3 months follow‐up). | ||||
| 2.1 Urinary function | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Bowel function | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Sexual function | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Quality of life (at 36 months follow‐up) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 1.3  Comparison 1 Cryotherapy versus EBRT, Outcome 3 Quality of life (at 36 months follow‐up). | ||||
| 3.1 Urinary function | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Bowel function | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 Sexual function | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Major adverse events Show forest plot | 2 | 293 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.47, 1.78] |
| Analysis 1.4  Comparison 1 Cryotherapy versus EBRT, Outcome 4 Major adverse events. | ||||
| 5 Time to death from any cause Show forest plot | 2 | Hazard Ratio (Random, 95% CI) | 0.99 [0.05, 18.79] | |
| Analysis 1.5  Comparison 1 Cryotherapy versus EBRT, Outcome 5 Time to death from any cause. | ||||
| 6 Time to biochemical failure Show forest plot | 2 | Hazard Ratio (Random, 95% CI) | 2.15 [0.07, 62.12] | |
| Analysis 1.6  Comparison 1 Cryotherapy versus EBRT, Outcome 6 Time to biochemical failure. | ||||
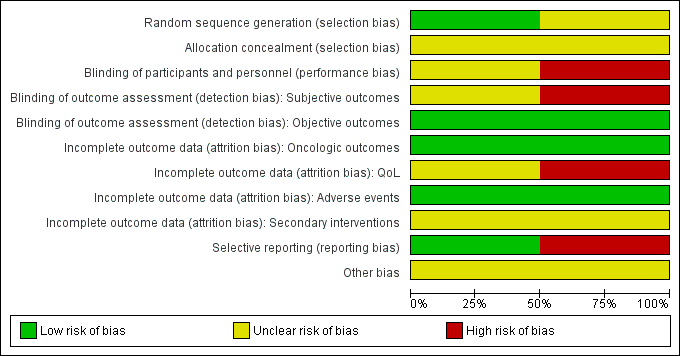
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
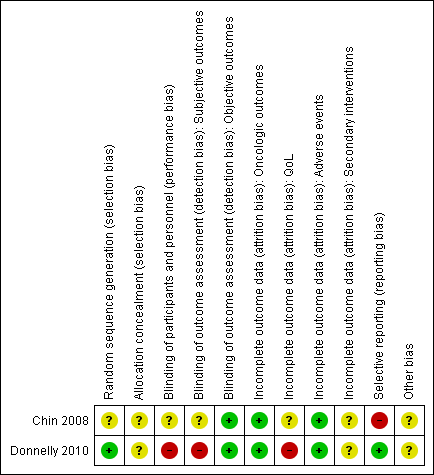
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
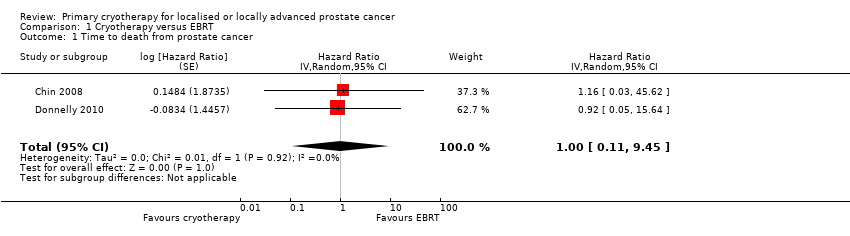
Comparison 1 Cryotherapy versus EBRT, Outcome 1 Time to death from prostate cancer.
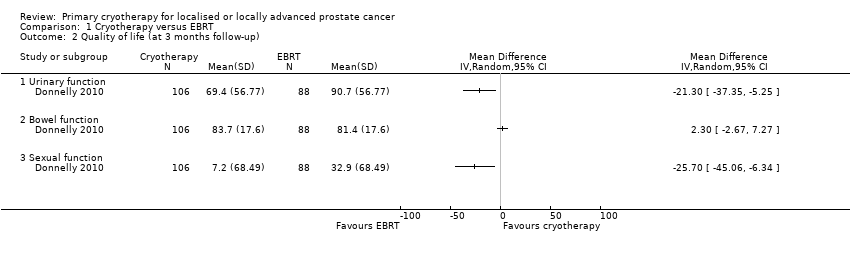
Comparison 1 Cryotherapy versus EBRT, Outcome 2 Quality of life (at 3 months follow‐up).
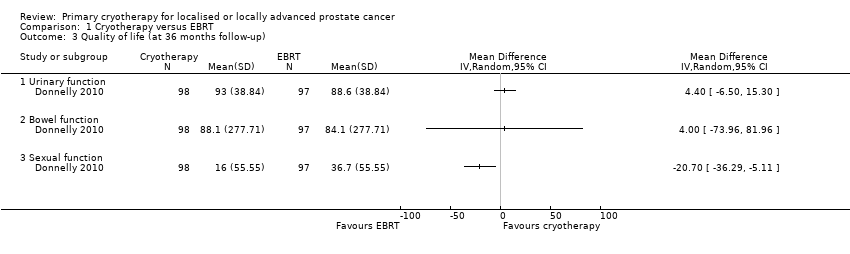
Comparison 1 Cryotherapy versus EBRT, Outcome 3 Quality of life (at 36 months follow‐up).
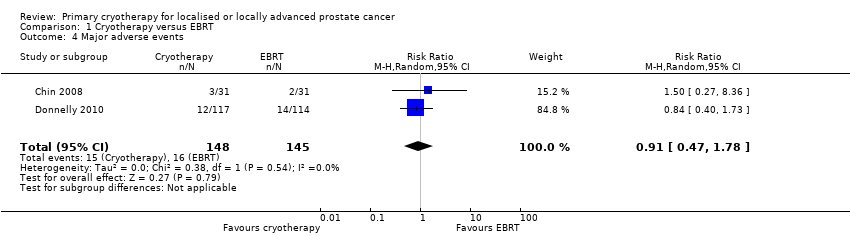
Comparison 1 Cryotherapy versus EBRT, Outcome 4 Major adverse events.
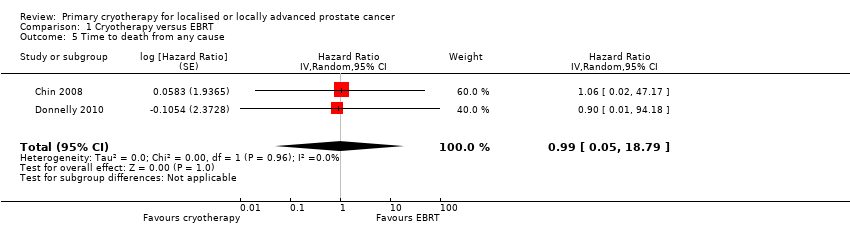
Comparison 1 Cryotherapy versus EBRT, Outcome 5 Time to death from any cause.
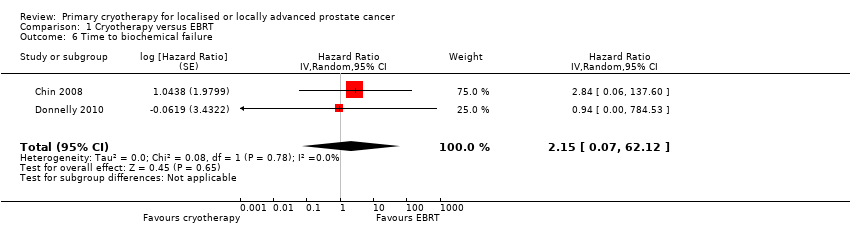
Comparison 1 Cryotherapy versus EBRT, Outcome 6 Time to biochemical failure.
| Participants: men with localised or locally advanced prostate cancer Setting: single institution in Canada Intervention: whole gland cryotherapy Comparator: external beam radiotherapy (EBRT) | |||||
| Outcomes | № of participants | Quality of the evidence | Relative effect | Anticipated absolute effects* (95% CI) | |
| Risk with EBRT | Risk difference with cryotherapy | ||||
| Time to death from prostate cancer (absolute effects: disease‐specific mortality)1 | 293 | ⊕⊝⊝⊝ | HR 1.00 | Study population | |
| 97 per 1000 | 0 fewer per 1000 | ||||
| Quality of life ‐ urinary function Follow‐up: mean 36 months | 195 | ⊕⊝⊝⊝ | ‐ | The mean quality of life ‐ urinary function was 88.6 | MD 4.4 higher |
| Quality of life ‐ bowel function Follow‐up: mean 36 months | 195 | ⊕⊝⊝⊝ | ‐ | The mean quality of life ‐ bowel function was 84.1 | MD 4 higher |
| Quality of life ‐ sexual function Follow‐up: mean 36 months | 195 | ⊕⊝⊝⊝ | ‐ | The mean quality of life ‐ sexual function was 36.7 | MD 20.7 lower |
| Major adverse events | 293 | ⊕⊝⊝⊝ | RR 0.91 | Study population | |
| 110 per 1000 | 10 fewer per 1000 | ||||
| Time to death from any cause (absolute effects: overall mortality)1 | 293 | ⊕⊝⊝⊝ | HR 0.99 | Study population | |
| 166 per 1000 | 2 fewer per 1000 | ||||
| Secondary interventions for treatment failure ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| 1 Mortality instead of survival to estimate anticipated absolute effect is reported for methodological reason. 2 Downgraded by one level for study limitations: unclear or high risk of bias in half of domains in included studies. 3 Downgraded by one level for indirectness (differences in intervention): prescribed dose of radiotherapy in the included studies was lower than 74 Gy as recommended by current guidelines (EAU 2017). Also, radiotherapy should be given in combination with long‐term androgen deprivation therapy (two to three years) in patients with high risk prostate cancer. 4 Downgraded by two levels for imprecision: wide confidence interval cross assumed threshold of clinically important differences. 5 UCLA‐Prostate Cancer Index contains six domains (urinary function (4 items), urinary bother (1 item), sexual function (5 items), sexual bother (1 item), bowel function (3 items), bowel bother (1 item)) which are scored separately (low score = worst, high score = better) (Litwin 1998). 6 Downgraded by one level for imprecision: confidence interval crosses assumed threshold of clinically important difference. | |||||
| Study name | Trial period | Setting/ country | Participants | Intervention(s) and comparator(s) | Age (median, years) | No of men with clinical tumour stage (T2a; 2b; 2c; 3a; 3b) | Biopsy Gleason score (<7; 7; >7) | PSA (median, ng/mL) | Median follow‐up (months) |
| Chin 2008 | 1999 to 2002 | Single institution in Canada | Histologically proven prostate cancer, clinically staged as T2c, T3a or T3b with no evidence of lymph node or distant metastasis and serum PSA < 25 ng/mL | Whole gland cryotherapy | 70.4 | ‐; ‐; 12; 17; 2 | 2; 24; 5 | 11.1 | 105 |
| EBRT | 70.5 | ‐; ‐; 8; 15; 8 | 1; 24; 6 | 8.6 | |||||
| Donnelly 2010 | 1997 to 2003 | Single institution in Canada | Histologically proven adenocarcinoma of the prostate, a biopsy tumour classification of T2 or T3, no evidence of lymph node or distant metastases, a pretreatment PSA level < 20 ng/mL, and a gland volume < 60 cm3 | Whole gland cryotherapy | 69.4 | 22; 28; 49; 17; 6 | 42; 69; 11 | 8.1 | 100 |
| EBRT | 68.6 | 20; 23; 57; 18; 4 | 44; 65; 13 | 9.0 | |||||
| EBRT: external beam radiotherapy; PSA: prostate specific antigen | |||||||||
| Study name | Intervention(s) and comparator(s) | Screened/ eligible | Randomised | Treatment completed | Treatment analysed |
| Chin 2008 | Whole gland cryotherapy | NR/140 | 32 | NR | 31 (96.8%) |
| EBRT | 31 | 31 (100.0%) | |||
| Total | 63 | NR | 62 (98.4%) | ||
| Donnelly 2010 | Whole gland cryotherapy | NR/764 | 122 | NR | 117 (95.9%) |
| EBRT | 122 | 114 (93.4%) | |||
| Total | 244 | NR | 231 (94.6%) | ||
| Grand total | 307 | NR | 293 (95.4%) | ||
| EBRT: external beam radiotherapy; NR: not reported | |||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Time to death from prostate cancer Show forest plot | 2 | Hazard Ratio (Random, 95% CI) | 1.00 [0.11, 9.45] | |
| 2 Quality of life (at 3 months follow‐up) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.1 Urinary function | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Bowel function | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Sexual function | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Quality of life (at 36 months follow‐up) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3.1 Urinary function | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Bowel function | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 Sexual function | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Major adverse events Show forest plot | 2 | 293 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.47, 1.78] |
| 5 Time to death from any cause Show forest plot | 2 | Hazard Ratio (Random, 95% CI) | 0.99 [0.05, 18.79] | |
| 6 Time to biochemical failure Show forest plot | 2 | Hazard Ratio (Random, 95% CI) | 2.15 [0.07, 62.12] | |