Ejercicios para mujeres que reciben tratamiento adyuvante para el cáncer de mama
Referencias
References to studies included in this review
References to studies excluded from this review
References to studies awaiting assessment
References to ongoing studies
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | RCT, 2 groups Study start and stop dates: not reported Length of intervention: 15 weeks Length of follow‐up: 5 to 7 weeks after the intervention | |
| Participants | 20 breast cancer patients due to receive adjuvant therapy | |
| Interventions | Intervention (n = 10): Aerobic and resistance training at 40% to 60% maximum exercise capacity and stretching; 2/week, up to 60 minutes per session Control (n = 10): usual care | |
| Outcomes |
Outcomes were measured at baseline and postintervention and at 3 time points during treatment Adverse events: "No cases of injury or any cancer treatment complications impeded subjects in the exercise group from completing the exercise protocol two times a week." | |
| Notes | Funding: Grants obtained through the University of Northern Colorado (Dissertation), University of Northern Colorado, Sponsored Programs and Academic Research Center (2007) Conflicts of interest: The authors declared there was no potential conflict of interest | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Drawing of numbers (1 to 20) by the participants. Participants who drew even numbers were placed into the experimental group, while participants who drew odd numbers were placed into the control group |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants | High risk | Not done |
| Blinding of personnel/care providers | High risk | Not reported. Comment: probably not done |
| Blinding of outcome assessment (detection bias) | High risk | Not reported. Comment: probably not done |
| Blinding of outcome assessment (detection bias) | High risk | Self reported items |
| Incomplete outcome data (attrition bias) | Unclear risk | There was no description of missing outcome data or attrition from the trial |
| Selective reporting (reporting bias) | Low risk | Dissertation with all assessed outcomes available |
| Group similarity at baseline | Low risk | No significant differences for weight, age, body fat, fitness (cardiorespiratory and strength) |
| Adherence | Low risk | "The adherence rate among all the subjects was 100%." All study participants completed the study protocol. One participant missed 1 week of exercise for reasons unrelated to the study |
| Contamination | Unclear risk | Not reported |
| Methods | RCT, 2 groups Study start and stop dates: March 2004 to July 2006 | |
| Participants | 50 newly diagnosed breast cancer patients, aged 35 to 75, who had not yet begun or had only recently begun adjuvant treatment (completed fewer than 2 weeks of radiation or 2 cycles of chemotherapy) | |
| Interventions | Intervention (n = 25): home‐based exercise program, type of exercise up to the women’s choice, weekly telephone calls, information, heart monitor, activity logs, 60% to 80% of predicted maximal heart rate, 5 days per week, 30 minutes, 120 sessions | |
| Outcomes |
Outcomes were measured at baseline and 6 months Adverse events: none reported | |
| Notes | Funding: Lance Armstrong Foundation, American Cancer Society, Susan G. Komen, National Institutes of Health Conflict of interest: None reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A computer program randomly assigned each study participant with equal probability to the exercise group or the usual‐care group |
| Allocation concealment (selection bias) | Low risk | The randomisation code for each participant was obtained by the principal investigator (who was not involved in recruitment or data collection) only after baseline measures for that woman had been completed, and staff conducting clinic visits did not have access to the randomisations program |
| Blinding of participants | High risk | Not done |
| Blinding of personnel/care providers | High risk | Not reported. Comment: probably not done |
| Blinding of outcome assessment (detection bias) | High risk | Self reported items |
| Incomplete outcome data (attrition bias) | Low risk | 5 women (10%) had missing 6‐month data (3 exercisers and 2 usual‐care group participants). All analyses were conducted according to the intention‐to‐treat principle. Baseline QoL values were carried forward for the 5 IMPACT study participants who had missing 6‐month data. Not reported for other outcomes |
| Selective reporting (reporting bias) | Unclear risk | Study protocol not published prospectively |
| Group similarity at baseline | Unclear risk | "No differences between exercise and usual care groups at baseline. Exception: exercisers were more likely to receive lumpectomy than usual care group participants (P < 0.05)." |
| Adherence | High risk | Participants performed 144 (SD = 75) minutes of activity per week throughout the 6 months (range: 0 to 253). 64% met the goal of 150 min per week |
| Contamination | High risk | Women didn't have to be sedentary at baseline. 36% of IMPACT study control group participants reported no sports/recreational physical activity at 6‐month follow‐up; the remaining 64% of controls reported between 35 and 378 min/week of activity, with a median of 181 minutes. Obese cohort (mean BMI > 30 kg/m2) |
| Methods | RCT, 2 groups Study start and stop dates: not reported Study was discontinued due to peripheral neuropathy | |
| Participants | 25 breast cancer patients undergoing neoadjuvant or adjuvant chemotherapy treatment | |
| Interventions | Intervention (n = 13): home‐based, low‐intensity level strength training and functional endurance regimen (strength training combined with walking) Control (n = 12): usual care | |
| Outcomes | Primary outcome:
Secondary outcomes:
Adverse events: Detailed adverse event data were not collected for participants in this study. Outcomes were measured at baseline and 2 weeks after the last chemotherapy treatment | |
| Notes | Funding: None reported Conflict of interest: None reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerised randomisation program |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants | High risk | Not reported |
| Blinding of personnel/care providers | High risk | Not reported. Comment: probably not done |
| Blinding of outcome assessment (detection bias) | High risk | Not reported. Comment: probably not done |
| Blinding of outcome assessment (detection bias) | High risk | Self reported items |
| Incomplete outcome data (attrition bias) | High risk | 3 participants were not able to start the study owing to changes in treatment plan and were not included in any analysis.
> 30% drop‐out Treatment of missing data was not described |
| Selective reporting (reporting bias) | Unclear risk | No study protocol published |
| Group similarity at baseline | Low risk | No significant differences |
| Adherence | High risk | Once peripheral neuropathy developed, these participants (12 of 13) adopted a sporadic pattern of "walking only", which lasted for approximately 4 weeks before coming to an abrupt end. Participants in this study were not able to recall the times they exercised during the telephone contacts. The investigator was not able to measure adherence, which was defined as the number of sessions attended by each participant |
| Contamination | Unclear risk | Participants did not have to be sedentary |
| Methods | RCT, 2 groups Study start and stop dates: not reported | |
| Participants | 22 breast cancer patients, after surgery, receiving chemotherapy or radiotherapy | |
| Interventions | Intervention (n = 12): supervised group exercise: aerobic and resistance training (walking, cycling, low‐level aerobics, muscle‐strengthening exercises, circuits), behaviour change communication, 60% to 75% HRmax, 10 to 20 min per session exercise, plus warm‐up, cool‐down, relaxation, 2 sessions per week Control (n = 10): no intervention | |
| Outcomes |
Outcomes were measured at baseline and 12 weeks. Adverse events: "There were no adverse reactions to taking part in the exercise intervention" | |
| Notes | Funding: Greater Glasgow NHS Trust Conflicts of interest: None reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated numbers, stratification by adjuvant cancer treatment |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants | High risk | Not done |
| Blinding of personnel/care providers | High risk | Not reported. Comment: probably not done |
| Blinding of outcome assessment (detection bias) | High risk | Not reported. Comment: probably not done |
| Blinding of outcome assessment (detection bias) | High risk | Self administered questionnaires were returned to researcher in sealed envelopes. Comment: high risk because items were self reported items |
| Incomplete outcome data (attrition bias) | Low risk | Drop‐out:
All participants: 3/22 (13.7%) No imputation of missing data |
| Selective reporting (reporting bias) | Unclear risk | No study protocol published |
| Group similarity at baseline | Low risk | Similarity for the most important prognostic indicators |
| Adherence | High risk | Adherence: 70% of all sessions |
| Contamination | Low risk | Control group more physically active at baseline than intervention group (421 min vs 330 min). Controls remained at baseline level, whereas intervention group increased level. The self reported levels of physical activity at baseline for both groups and at follow‐up for the control group were similar to those found in sedentary populations |
| Methods | RCT, 2 groups Study start and stop dates: Recruitment between June 2011 and June 2012 | |
| Participants | 44 outpatient breast cancer patients with HER2‐negative status randomised, scheduled for adjuvant or neoadjuvant chemotherapy with 6 cycles (3FEC100+3 taxanes), followed by radiotherapy | |
| Interventions | Intervention (n = 22): aerobic and resistance training, 9 supervised sessions of resistance training and 72 unsupervised home‐based sessions (resistance and aerobic training): bicycle or walking 20 to 40 minutes 2/week, resistance training with resistance bands 1/week. Control (n = 22): adjuvant treatment only | |
| Outcomes | Primary outcome:
Secondary outcomes:
Outcomes were measured at time point: before start of chemotherapy, after 27 weeks of treatment, and after 27 weeks of follow‐up Adverse events: no adverse events were reported | |
| Notes | Funding: Sponsored by Limoges University Hospital. Supported by a grant from the Ligue Contre le Cancer (19‐87) and the ALAIR‐AVD. Conflicts of interest: Not reported. Study registration: NCT01322412 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Block randomisations with variable block size, 1:1, no stratification, further details not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants | High risk | Open study |
| Blinding of personnel/care providers | High risk | Open study |
| Blinding of outcome assessment (detection bias) | High risk | Open study |
| Blinding of outcome assessment (detection bias) | High risk | Self reported |
| Incomplete outcome data (attrition bias) | High risk | Cardiorespiratory fitness and strength: 30/42 participants (68%) took part in all fitness evaluations. QoL, fatigue, psychological distress, physical activity: 19/42 (45.2%) participants in the analysis at the end of the intervention. ITT analysis for cardiorespiratory fitness (VO peak, 6‐MWT) and strength with imputation for missing values. "Several methods were tested and last observation carried forward (LOCF) was used." Otherwise no imputation for missing data |
| Selective reporting (reporting bias) | Low risk | Trial prospectively registered |
| Group similarity at baseline | High risk | Significant baseline differences in cardiorespiratory fitness and BMI |
| Adherence | High risk | 9 of 14 participants took part in more than 70% of the exercise program |
| Contamination | Unclear risk | The control group was not asked to abstain from physical activity |
| Methods | RCT, 3 groups Study start and stop dates: 2003 and 2005 | |
| Participants | 242 breast cancer patients initiating adjuvant chemotherapy | |
| Interventions | Intervention group 1 (n = 78): 'Courneya AET' aerobic ‐ endurance exercise: cycle ergometer, treadmill, elliptical Intervention group 2 (n = 82): 'Courneya RET' muscular endurance exercise: weight machines (set with 9 exercises) Control group (n = 82): Usual care; women were asked not to initiate an exercise programme | |
| Outcomes | Primary outcome:
Secondary outcomes:
Patient‐rated outcomes were assessed at baseline (1 to 2 weeks after starting chemotherapy), midpoint (middle of chemotherapy), after the intervention (3 to 4 weeks after chemotherapy), and at the 6‐month follow‐up. Objectively measured outcomes were assessed at baseline and after intervention. Adverse events: "exercise did not cause adverse events" | |
| Notes | Study description for Courneya AET (aerobic exercise training) and Courneya RET (resistance exercise training) Funding: Canadian Breast Cancer Research Alliance; the Canada Research Chairs Program, Research Team Grant from the National Cancer Institute of Canada with funds from the Canadian Cancer Society (CCS) and the NCIC/CCS Sociobehavioral Cancer Research Network, New Investigator Award from the Heart and Stroke Foundation of Canada; a New Investigator Award from the Canadian Institutes of Health Research and a Health Scholar Award from the Alberta Heritage Foundation for Medical Research; Canada Graduate Scholarship from the Canadian Institutes of Health Research and an Incentive Award from the Alberta Heritage Foundation for Medical Research. Conflict of interest: Authors declared no potential conflict of interest | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated program, stratification by centre and chemotherapy protocol |
| Allocation concealment (selection bias) | Low risk | “The allocation sequence was...concealed from the project directors at each site who assigned participants to groups” |
| Blinding of participants | High risk | Not done |
| Blinding of personnel/care providers | High risk | Not reported. Comment: probably not done |
| Blinding of outcome assessment (detection bias) | High risk | Not reported. Comment: probably not done |
| Blinding of outcome assessment (detection bias) | High risk | Self reported items |
| Incomplete outcome data (attrition bias) | Low risk | Drop‐out:
All participants: 7.9% (19/242) |
| Selective reporting (reporting bias) | Unclear risk | No study protocol published |
| Group similarity at baseline | Low risk | No significant differences |
| Adherence | High risk | Aerobic exercise group: 72% sessions; 95.6% met duration; 87.2% met intensity |
| Contamination | Unclear risk | Women were asked not to initiate an exercise programme. Otherwise not reported |
| Methods | RCT, 3 groups Study start and stop dates: 2003 and 2005 | |
| Participants | 242 breast cancer patients initiating adjuvant chemotherapy | |
| Interventions | Intervention group 1 (n = 78): 'Courneya AET' aerobic ‐ endurance exercise: cycle ergometer, treadmill, elliptical Intervention group 2 (n = 82): 'Courneya RET' muscular endurance exercise: weight machines (set with 9 exercises) Control group (n = 82): Usual care; women were asked not to initiate an exercise programme | |
| Outcomes | Primary outcome:
Secondary outcomes:
Patient‐rated outcomes were assessed at baseline (1 to 2 weeks after starting chemotherapy), midpoint (middle of chemotherapy), after the intervention (3 to 4 weeks after chemotherapy), and at the 6‐month follow‐up. Objectively measured outcomes were assessed at baseline and after intervention. Adverse events: "exercise did not cause adverse events" | |
| Notes | Study description for Courneya AET (aerobic exercise training) and Courneya RET (resistance exercise training) Funding: Canadian Breast Cancer Research Alliance; the Canada Research Chairs Program, Research Team Grant from the National Cancer Institute of Canada with funds from the Canadian Cancer Society (CCS) and the NCIC/CCS Sociobehavioral Cancer Research Network, New Investigator Award from the Heart and Stroke Foundation of Canada; a New Investigator Award from the Canadian Institutes of Health Research and a Health Scholar Award from the Alberta Heritage Foundation for Medical Research; Canada Graduate Scholarship from the Canadian Institutes of Health Research and an Incentive Award from the Alberta Heritage Foundation for Medical Research. Conflict of interest: Authors declared no potential conflict of interest | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated program, stratification by centre and chemotherapy protocol |
| Allocation concealment (selection bias) | Low risk | “The allocation sequence was...concealed from the project directors at each site who assigned participants to groups” |
| Blinding of participants | High risk | Not done |
| Blinding of personnel/care providers | High risk | Not reported. Comment: probably not done |
| Blinding of outcome assessment (detection bias) | High risk | Not reported. Comment: probably not done |
| Blinding of outcome assessment (detection bias) | High risk | Self reported items |
| Incomplete outcome data (attrition bias) | Low risk | Drop‐out:
All participants: 7.9% (19/242) |
| Selective reporting (reporting bias) | Unclear risk | No study protocol published |
| Group similarity at baseline | Low risk | No significant differences |
| Adherence | High risk | Aerobic exercise group: 72% sessions; 95.6% met duration; 87.2% met intensity |
| Contamination | Unclear risk | Women were asked not to initiate an exercise programme. Otherwise not reported |
| Methods | RCT, 2 groups Study start and stop dates: not reported | |
| Participants | 22 breast cancer patients, stage I, II; after surgery, receiving adjuvant chemotherapy (doxorubicin (Adriamycin), cyclophosphamide (Cytoxan)), radiation therapy excluded | |
| Interventions | Intervention (n = 13): Aerobic training (walking) and resistance training (tubing), self directed, 60% of HRmax, 20 to 60 min per session, 3 to 5 d/week. Resistance training: 12 to 15 repetitions, approximately 20 minutes, 1 to 2 sets, 2 to 3 d/week, 13 weeks. Control (n = 9): Usual care, the same scheduled contact with the nurse researcher as the intervention group during weeks 1, 4, 7, 10, and 13, activity log | |
| Outcomes |
Outcomes were measured at:
Adverse events: lymphoedema in 1 participant | |
| Notes | Funding: Not reported Conflicts of interest: Not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A random numbers table was utilised prior to the initiation of the study to randomise participants to 1 of the 2 groups. Consecutive numbers on the table were used with numbers ending in an even integer assigned to the exercise group, and numbers ending in an odd integer assigned to the comparison group. Each number was placed in an envelope that was then sealed. The outside of the envelope was then numbered in consecutive order |
| Allocation concealment (selection bias) | Unclear risk | Sealed envelope. Comment: it was not mentioned if the envelope was opaque |
| Blinding of participants | High risk | Not done |
| Blinding of personnel/care providers | High risk | Not reported. Comment: probably not done |
| Blinding of outcome assessment (detection bias) | Low risk | "The study participant’s group assignment was blinded to the exercise physiologists performing the fitness testing at the week 13 appointment. Study participants were requested to not disclose which study group they had been randomized to." |
| Blinding of outcome assessment (detection bias) | High risk | Self reported items |
| Incomplete outcome data (attrition bias) | Low risk | Missing data were reported for 2 participants for strength assessment. "A second strength was the adherence by both groups in maintaining activity logs and completing all study measures." |
| Selective reporting (reporting bias) | Unclear risk | Detailed results only available for cardiorespiratory fitness |
| Group similarity at baseline | Low risk | "The groups were balanced in terms of demographic and disease characteristics." |
| Adherence | Unclear risk | The nurse researcher used the logs of both groups to assess adherence to the structured exercise program. Adherence defined as completion of 80% of the individualised targeted endurance and strength exercise, frequency, duration, and intensity. The intervention group walked a mean of 113 minutes per week, as |
| Contamination | Unclear risk | The intervention group walked a mean of 113 minutes per week, as compared to 53 minutes by the comparison group. Thus the intervention group was considered to be performing at a moderate level of activity per week, while the comparison group had a low level of activity over the study period. Study participants were asked to commit to not initiating participation in a formal exercise program during the study period. Continuation of an ongoing exercise regimen was acceptable |
| Methods | RCT, 3 groups Study start and stop dates: 1999 to 2006 | |
| Participants | 119 participants randomised, majority with breast cancer (n = 112), but people with ovarian and colorectal cancer also included, undergoing chemotherapy, Karnofsky score > = 60. Excluded if they were having concurrent radiation therapy, and if pain intensity score greater than 3 | |
| Interventions |
| |
| Outcomes | Primary outcomes:
Secondary outcomes:
Outcomes were measured at time point: baseline (T1: the week before the second chemotherapy treatment), at the end of cancer treatment (T2: 4 to 6 months after T1), and at the end of the study (T3: approximately 1 year after the start of T1). Adverse events: hip pain, sciatica (n = 16), arm discomfort (n = 4), knee discomfort (n = 10), ankle discomfort (n = 3), and foot discomfort (n = 8) | |
| Notes | Funding: National Cancer Institute (CA83316), and the Clinical Translational Research Institute, Clinical Research Center (CTSI‐CRC) (Dodd 2010) Conflicts of interest: The authors declared no potential conflicts of interest in 2 related publications | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Reported as randomised, but method not described |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants | High risk | Not done |
| Blinding of personnel/care providers | High risk | Not reported. Comment: probably not done |
| Blinding of outcome assessment (detection bias) | Low risk | Cardiopulmonary exercise testing was performed in the exercise physiology lab by laboratory staff blinded to the participant’s group assignment |
| Blinding of outcome assessment (detection bias) | High risk | Not reported for other outcomes. Comment: probably not done or self reported items |
| Incomplete outcome data (attrition bias) | Low risk | Drop‐out:
ITT, no imputation |
| Selective reporting (reporting bias) | High risk | Cardiopulmonary fitness was tested but not reported as an outcome. It was unclear if cardiorespiratory fitness was a predefined outcome or only measured to individualise the exercise prescription. In 1 of 5 related publications, a quality of life questionnaire was mentioned (MQOLS‐CA), but no results were reported in any publication. Outcomes were not reported completely, and could not be extracted for use in a meta‐analysis |
| Group similarity at baseline | Low risk | "The three groups of patients did not differ significantly in any of the demographic, disease, or treatment characteristics on entry into the study" |
| Adherence | High risk | Group 1 (exercise during and after adjuvant treatment) reported an adherence rate of 73% at T2 and 75.7% at T3 |
| Contamination | High risk | 44% of group 2 (exercise after adjuvant treatment) reported meeting ACSM 1998 guidelines (aerobic activities 3x/week, for 20‐minute duration, and at a moderate intensity) at T1, by T2 group 2 had decreased to 27%. 34% of group 3 (no exercise) reported meeting minimum criteria at T1, this decreased to 31% at T2. |
| Methods | RCT, 2 groups Study start and stop dates: not reported | |
| Participants | 23 breast cancer patients, stages 0 to III; after surgery, receiving radiotherapy, sedentary | |
| Interventions | Intervention (n = 13): aerobic training (walking), self directed, 50% to 70% HRmax, 20 to 45 min per session, 3 to 5/week Control (n = 10): stretching, 3 to 5/week | |
| Outcomes |
Outcomes were measured within 1 week prior to and within 1 week following a 7‐week radiation regimen. Adverse events: shoulder tendonitis and decreases in strength due to overtraining in 1 participant | |
| Notes | Funding: grants from the Elsa U. Pardee Foundation in Midland, MI, USA, and the Max and Victoria Dreyfus Foundation in White Plains, NY, USA Conflict of interest: None reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants | High risk | Not reported |
| Blinding of personnel/care providers | High risk | Not reported Comment: probably not done |
| Blinding of outcome assessment (detection bias) | High risk | Not reported Comment: probably not done |
| Blinding of outcome assessment (detection bias) | High risk | Self reported items |
| Incomplete outcome data (attrition bias) | Low risk | Drop‐out:
All participants: 2/23 (8.7%) |
| Selective reporting (reporting bias) | Unclear risk | No study protocol published |
| Group similarity at baseline | Low risk | Similarity for the most important prognostic indicators |
| Adherence | High risk | Adherence defined as 21 minimum sessions out of 35 possible sessions Adherence per group:
|
| Contamination | Unclear risk | Obese cohort (mean BMI > 30 kg/m2). Otherwise not reported |
| Methods | RCT, 2 groups Study start and stop dates: data were collected from April 2007 to April 2009 | |
| Participants | 142 non‐urban‐dwelling breast cancer patients, 111 underwent adjuvant therapy (chemotherapy, radiotherapy, or a combination) during the study intervention (unpublished data for these participants used in the analyses of this review) | |
| Interventions | Intervention (n = 58): home‐based, telephone‐delivered mixed (aerobic and resistance training) exercise Control (n = 53): usual care; did not receive any study exercise intervention‐related material until study completion | |
| Outcomes | Primary outcomes:
Secondary outcomes:
Outcomes measures at 6 and 12 months Adverse events: muscle soreness in 2 participants and musculoskeletal injury in 1 participant | |
| Notes | Funding: The National Breast Cancer Foundation (NBCF, Australia) and a Queensland Health Core Infrastructure grant funded the trial. EGE is supported by a National Health and Medical Research Council Senior Research Fellowship. SCH is supported by an Early Career Research Fellowship from the NBCF. Conflict of interest: The authors have no conflict of interest to disclose. Registered at: ACTRN12609000809235 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated, unblocked sequence of random numbers |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants | High risk | Not done |
| Blinding of personnel/care providers | High risk | Not reported. Comment: probably not done |
| Blinding of outcome assessment (detection bias) | High risk | Self reported items |
| Incomplete outcome data (attrition bias) | Low risk | Drop‐out all participants:
Retention was 97% at 6 months and 96% at 12 months. No imputation of missing data |
| Selective reporting (reporting bias) | Low risk | Registered prospectively |
| Group similarity at baseline | High risk | For 111 participants during adjuvant treatment: more with chemotherapy in the treatment group: 47/58 (81%) vs 36/53 (68%) For all participants: "There was no evidence of failure of randomization (i.e. all baseline group differences were P > 0.05), however there were some notable group differences (>=10%) in terms of income (<$52,000 per annum), receipt of radiotherapy at baseline, overall receipt of chemotherapy, surgery type and lymph node status." |
| Adherence | High risk | 41.2% at 6 months' postsurgery and 52.2% at 12 months' postsurgery met the criteria for aerobic activity. 45.6% at 6 months' postsurgery and 40.3% at 12 months' postsurgery met the criteria for strength. |
| Contamination | High risk | 30% to 40% of control group were active during the study period. They started out as being as active |
| Methods | RCT, 2 groups Study start and stop dates: not reported | |
| Participants | 50 sedentary (< 30 minutes of moderate‐intensity exercise 5 times a week) breast cancer patients (stage I to III) during adjuvant or neoadjuvant chemotherapy. Recruited from outpatient clinics | |
| Interventions | Intervention (n = 25): home‐based, moderate‐intensity walking intervention (defined as walking at brisk pace) after 2 cycles of chemotherapy. 30 minutes of moderate‐intensity walking 5 times a week, encouraged to gradually increase walking duration from 10‐ to 30‐minute bouts through the course of the intervention. Control group (n = 25): usual care | |
| Outcomes | Primary:
Secondary:
Outcomes measured at: midway through chemotherapy (pre‐intervention) and after the completion of chemotherapy (postintervention). All participants also completed outcome measures prior to receiving chemotherapy | |
| Notes | Funding: Loughborough University as part of a PhD project. Study registration: ISRCTN50709297 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Block randomisation using four blocks was used to allocate patients into one of two groups by the researcher. Within each group of four patients, two were allocated to the intervention group and two to the control group; the allocation of groups within each block was random." |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants | High risk | There is no masking of participants or the research team |
| Blinding of personnel/care providers | High risk | There is no masking of participants or the research team |
| Blinding of outcome assessment (detection bias) | High risk | There is no masking of participants or the research team |
| Incomplete outcome data (attrition bias) | Low risk | 5 participants in the exercise group (20%) discontinued the intervention (4 due to hospitalisation and 1 due to medical difficulties), but completed all follow‐up measures and were included in the analysis. ITT analysis, no imputation described |
| Selective reporting (reporting bias) | High risk | Measures of cognitive function (mentioned in the design paper and study registration) were not reported in the paper |
| Group similarity at baseline | Low risk | There were no significant differences between groups in sociodemographic or treatment‐related variables. Using ITT, there were no significant between‐group differences in baseline measures of anxiety, depression, fatigue, self esteem, mood, or subjective ratings of physical activity. There was a small difference between groups in 2 subscales of mood: vigour and confusion |
| Adherence | Low risk | 20 (80%) out of the 25 participants randomised to the physical activity group adhered to the intervention and completed walking diaries. |
| Contamination | Low risk | Sedentary patients at baseline. Self report of perceived physical activity for control group: 23/25 moderately inactive to inactive |
| Methods | RCT, 2 groups Study start and stop dates: recruitment between May 2006 and September 2007 | |
| Participants | 89 breast cancer patients undergoing adjuvant therapy: chemotherapy, radiation, or a combination of both (more than 90% radiation) | |
| Interventions | Intervention group (n = 46): home‐based strength, balance, shoulder mobility, and cardiovascular endurance program. 36 minutes, of which 20 minutes were walking. Frequency not reported. Control group (n = 43): Static stretching, supine relaxation program following the Feldenkrais method | |
| Outcomes | Primary outcome measure:
Secondary outcome measures:
Outcomes were measured at baseline, 3, 6, and 12 months Adverse events: 9 participants with musculoskeletal pain, 3 of which reported pain whilst performing exercises as a part of the intervention program and 1 as a part of the control program. 8 participants with 1 fall each, 1 of which was the result of an intervention group participant tripping on a tree stump whilst undertaking the walking program | |
| Notes | Funding: Project grant from the Princess Alexandra Hospital Cancer Collaborative Group, National Health and Medical Research Council Career Development Award (606732) Conflict of interest: No conflicts of interest declared | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomised to intervention or control groups using a computer‐generated randomisation sequence |
| Allocation concealment (selection bias) | Low risk | The randomisation sequence was entered into numbered, opaque, sealed envelopes by a study investigator and was held secure in an administration office separate from that of the investigators. Envelopes were only opened after completion of the initial assessment after which intervention or control programs were provided to participants according to the allocation sequence |
| Blinding of participants | High risk | Sham intervention control group provided with what looked like an exercise program with an equivalent amount of supporting material. The video material was of similar content to that in the intervention program (though the actual exercises described differed). Comment: Participants would still have been aware if they were in the exercise group or the stretching group |
| Blinding of personnel/care providers | High risk | Not reported. Comment: probably not done |
| Blinding of outcome assessment (detection bias) | Low risk | "blinded outcome assessment" |
| Blinding of outcome assessment (detection bias) | High risk | Self reported outcomes |
| Incomplete outcome data (attrition bias) | High risk | Intervention group: more than 30% of outcome data was missing at 6 months Control group: more than 30% of outcome data was missing at 6 months Handling of dropouts and missing data not reported |
| Selective reporting (reporting bias) | Low risk | The trial was registered prospectively with the Australia New Zealand Clinical Trials Registry (ACTRN12606000047594) |
| Group similarity at baseline | High risk | "Control group participants also appeared to be healthier at baseline." |
| Adherence | High risk | Participants were to document adherence in log books on a weekly basis and were asked about adherence in the last 2 weeks of 12 months. At the 12‐month review, 11 of 37 intervention group participants interviewed reported completing the strength/balance/shoulder mobility component of the program at least once in the past 2 weeks, whilst 7 reported completing it at least 3 times. The endurance component was completed at least once by 12, and at least 3 times by 7 |
| Contamination | High risk | In addition to the program provided, 25 out of 37 intervention group participants and 21 out of 36 control group participants had commenced other forms of exercise (e.g. walking, dancing, gymnasium, and aerobics) |
| Methods | RCT, 3 groups Study start and stop dates: recruitment between October 2006 and June 2008 | |
| Participants | 194 breast cancer patients, of which 142 underwent adjuvant therapy concurrently with the exercise intervention (unpublished data for these patients used in the analyses of this review) | |
| Interventions | Intervention group 1: 'Hayes 2013 Tel' Telephone (n = 50) ‐ incorporating both aerobic and strength‐based exercises Intervention group 2: 'Hayes 2013FtF' Face to face (n = 51) ‐ incorporating both aerobic and strength‐based exercises Control group (n = 41): usual care | |
| Outcomes | Primary outcome:
Secondary outcomes:
Outcomes measured at: 6 months, 12 months | |
| Notes | Funding: This research project was supported by the National Breast Cancer Foundation. The research positions of SH and EE are supported via an NBCF Early Career Research Fellowship and an NHMRC Senior Research Fellowship, respectively. Conflict of interest: Authors declare that they have no conflict of interest. Registered at ACTRN: 012606000233527 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Individually computer‐generated non‐blocked |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants | High risk | Not done |
| Blinding of personnel/care providers | High risk | Not reported Comment: probably not done |
| Blinding of outcome assessment (detection bias) | Low risk | "assessors blinded to group allocation" |
| Blinding of outcome assessment (detection bias) | High risk | All outcomes except outcomes with clinical assessment: self reported items |
| Incomplete outcome data (attrition bias) | Low risk | Drop‐out: all participants
All participants: 14/194 (7.2%) Comment: For the 142 participants undergoing adjuvant therapy: More than 20% drop‐out for 3‐min step test (cardiorespiratory fitness) High risk for cardiorespiratory fitness ITT analysis, no imputation of data |
| Selective reporting (reporting bias) | Low risk | Registered prospectively |
| Group similarity at baseline | High risk | Slight imbalance in numbers, place of treatment (public vs private hospital), and rates of mastectomy between groups following randomisation. Rate of mastectomies higher in telephone group. This group has the biggest difference in QoL |
| Adherence | High risk | 25% did not meet the intervention goal at mid‐ or postintervention and did not increase their total physical activity by 30+ min (a priori deemed clinically relevant) between baseline and mid‐ or postintervention |
| Contamination | High risk | The Active Australia Survey showed that the usual‐care group was more active (more minutes per week) than the FtF group and as active as the Tel group at 6 months. At 12 months, the usual‐care group was more active than the FtF group and less active than the Tel group |
| Methods | RCT, 3 groups Study start and stop dates: recruitment between October 2006 and June 2008 | |
| Participants | 194 breast cancer patients, of which 142 underwent adjuvant therapy concurrently with the exercise intervention (unpublished data for these patients used in the analyses of this review) | |
| Interventions | Intervention group 1: 'Hayes 2013Tel' Telephone (n = 50) ‐ incorporating both aerobic and strength‐based exercises Intervention group 2: 'Hayes 2013 FtF' Face to face (n = 51) ‐ incorporating both aerobic and strength‐based exercises Control group (n = 41): usual care | |
| Outcomes | Primary outcome:
Secondary outcomes:
Outcomes measured at: 6 months, 12 months | |
| Notes | Funding: This research project was supported by the National Breast Cancer Foundation. The research positions of SH and EE are supported via an NBCF Early Career Research Fellowship and an NHMRC Senior Research Fellowship, respectively. Conflict of interest: Authors declare that they have no conflict of interest. Registered at ACTRN: 012606000233527 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Individually computer‐generated non‐blocked |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants | High risk | Not done |
| Blinding of personnel/care providers | High risk | Not reported Comment: probably not done |
| Blinding of outcome assessment (detection bias) | Low risk | "assessors blinded to group allocation" |
| Blinding of outcome assessment (detection bias) | High risk | All outcomes except outcomes with clinical assessment: self reported items |
| Incomplete outcome data (attrition bias) | Low risk | Drop‐out: all participants
All participants: 14/194 (7.2%) Comment: For the 142 participants undergoing adjuvant therapy: More than 20% drop‐out for 3‐min step test (cardiorespiratory fitness) High risk for cardiorespiratory fitness ITT analysis, no imputation of data |
| Selective reporting (reporting bias) | Low risk | Registered prospectively |
| Group similarity at baseline | High risk | Slight imbalance in numbers, place of treatment (public vs private hospital), and rates of mastectomy between groups following randomisation. Rate of mastectomies higher in telephone group. This group has the biggest difference in QoL |
| Adherence | High risk | 25% did not meet the intervention goal at mid‐ or postintervention and did not increase their total physical activity by 30+ min (a priori deemed clinically relevant) between baseline and mid‐ or postintervention |
| Contamination | High risk | The Active Australia Survey showed that the usual‐care group was more active (more minutes per week) than the FtF group and as active as the Tel group at 6 months. At 12 months, the usual‐care group was more active than the FtF group and less active than the Tel group |
| Methods | RCT, 2 groups Study start and stop dates: recruitment between March 2007 and January 2010 | |
| Participants | 20 breast cancer patients, stage IIB to IIIC operable breast cancer, neoadjuvant chemotherapy consisted of 4 cycles of doxorubicin (60 mg/m2) and cyclophosphamide (600 mg/m2) every 3 weeks (i.e. 12 weeks in duration) Eligible if: Karnofsky performance status > 70 | |
| Interventions | Intervention (n = 10): Aerobic training consisted of 3 one‐on‐one supervised cycle ergometry sessions per week on non‐consecutive days for 12 weeks. Control (n = 10): Neoadjuvant therapy only, participants were instructed to maintain their usual exercise levels throughout the duration of the study | |
| Outcomes | Safety outcomes:
Efficacy outcomes:
Outcomes were measured at: CPET, echocardiogram, and self administered questionnaire were conducted at baseline and postintervention (12 weeks), whereas treatment‐related events were serially assessed across the study (i.e. baseline, 3, 6, 9, and 12 weeks). Exercise‐related events were monitored during CPET procedures and aerobic training sessions. Adverse event: unexplained leg pain in 1 participant | |
| Notes | Funding: United States Department of Defense Breast Cancer Research Program of the Office of the Congressionally Directed Medical Research Programs – Ideas Award and funds from George and Susan Beischer (1 author). 1 author is supported by research grants from the National Cancer Institute (CA143254, CA142566, CA138634, CA133895, CA164751). Conflict of interest: The authors report no conflicts of interest | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated program (n = 10/group) |
| Allocation concealment (selection bias) | Unclear risk | The allocation sequence was concealed from the study co‐ordinator who assigned participants to groups |
| Blinding of participants | High risk | It was not possible to blind participants or exercise staff to group assignment |
| Blinding of personnel/care providers | High risk | It was not possible to blind participants or exercise staff to group assignment |
| Blinding of outcome assessment (detection bias) | Low risk | Study exercise physiologists conducting the baseline and postintervention (12 weeks) assessments were blinded to group assignment |
| Blinding of outcome assessment (detection bias) | High risk | Self reported items |
| Incomplete outcome data (attrition bias) | Low risk | 19/20 (95%) completed all study procedures. ITT, handling of 1 dropout not described |
| Selective reporting (reporting bias) | Unclear risk | No study protocol published |
| Group similarity at baseline | Low risk | "The groups were balanced on all study outcomes at baseline." |
| Adherence | High risk | Overall attendance to planned exercise sessions was 82% (296 attended/360 prescribed; range 0 to 100%). Overall adherence to the planned exercise prescription was 66% (194 adhered sessions/296 attended). Adherence was calculated as the number of exercise sessions successfully completed (i.e. participant completed the exercise session at the planned duration and intensity) divided by the number of planned sessions attended |
| Contamination | Unclear risk | "There were no significant differences between groups for self‐reported exercise behavior." Otherwise not reported |
| Methods | RCT, 2 groups Study start and stop dates: 2010 to 2012 | |
| Participants | 67 breast cancer patients, stage I to III, surgically treated (mastectomy or lumpectomy), and allocated to adjuvant chemotherapy according to the national treatment guidelines of the Norwegian Breast Cancer Group | |
| Interventions | Intervention: Scheduled home‐based exercise intervention (n = 33), combined strength (resistance bands exercises 3 times a week) and aerobic (30 minutes of brisk walking daily) training Control group (n = 34): were advised to remain on their regular physical activity | |
| Outcomes |
The study sample completed questionnaires and physical tests after surgery prior to chemotherapy (baseline), 18 to 24 weeks after baseline, and at the end of chemotherapy (Post1), and approximately 6 months after completing the chemotherapy regimen (Post2). Adverse events: 1 participant with knee discomfort and 1 participant with a syncope related to a secondary chronic condition | |
| Notes | Funding: None reported. No conflict of interest declared | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "The random assignment of subjects to the intervention group or to the control group was carried out by the use of concealed envelopes, drawn by the research assistant prior to the first data collection." |
| Allocation concealment (selection bias) | Unclear risk | "concealed envelopes" Comment: it was not mentioned if they were opaque |
| Blinding of participants | High risk | Not reported |
| Blinding of personnel/care providers | High risk | Not reported. Comment: probably not done |
| Blinding of outcome assessment (detection bias) | High risk | Not reported. Comment: probably not done |
| Blinding of outcome assessment (detection bias) | High risk | Self reported items |
| Incomplete outcome data (attrition bias) | Low risk | Post1: immediately after chemo; Post2: 6 months' follow‐up 60 of 67 (89.6%) participants completed the data collection at time point 1. Comment: low risk. 52 of 67 (77.6%) participants completed the data collection at time point 2. Comment: high risk. ITT analysis for fatigue data. Per‐protocol analysis for physical activity (IPAQ) |
| Selective reporting (reporting bias) | Unclear risk | No study protocol published |
| Group similarity at baseline | Low risk | No significant differences in baseline values |
| Adherence | High risk | 17% adhered to the walking prescription of minimum 210 minutes/week of MVPA. 15% of the participants in the intervention group achieved the prescribed number of strength training (3/week) sessions. 58% met the general recommendations of 150 minutes/week of MVPA, and participants carried out approximately 2 sessions of resistance band exercises per week |
| Contamination | High risk | The control group had a mean exercise volume of 144 (SD 84) MVPA minutes per week, and 39% performed 150 minutes/week of MVPA or more. Data on exercise volume indicates that 48% of participants in both groups exercised according to the general recommended physical activity level or more. "there was a tendency of a significantly larger mean exercise volume in the intervention group compared to the control group (P = 0.051)" |
| Methods | RCT, 2 groups, parallel‐group design Study start and stop dates: not reported | |
| Participants | 13 breast cancer patients due to commence adjuvant chemotherapy | |
| Interventions | Intervention (n = 8): home‐based combined aerobic and resistance exercise program, 30 to 45 min of aerobic exercise, at least 4 times per week. 8 resistance exercises, which included the arms, legs, and trunk, 3 times per week with enough resistance so that she tired after 6 to 12 repetitions. Control group (n = 5): usual care | |
| Outcomes | Primary:
Secondary:
| |
| Notes | No outcomes reported as study was closed early. Funding: Canadian Breast Cancer Research Alliance Developmental and Explanatory Grant #16542 Conflict of interest: none declared | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants | High risk | Not done |
| Blinding of personnel/care providers | High risk | Not reported Comment: probably not done |
| Blinding of outcome assessment (detection bias) | High risk | Not reported Comment: probably not done |
| Blinding of outcome assessment (detection bias) | High risk | Not reported or self reported items Comment: probably not done |
| Incomplete outcome data (attrition bias) | Unclear risk | Study closed early |
| Selective reporting (reporting bias) | Unclear risk | No results reported because study closed early |
| Group similarity at baseline | Unclear risk | "No clinically meaningful or statistically significant differences between groups." |
| Adherence | High risk | Only 38% met all exercise targets |
| Contamination | High risk | "The women in the control group were unexpectedly quite active." Were asked not to begin a new exercise program |
| Methods | RCT, 3 groups, stratified by functional capacity Study start and stop dates: not reported | |
| Participants | 62 breast cancer patients, stage II; after surgery, receiving chemotherapy, entered the study; 45 patients were analysed for cardiorespiratory fitness; 24 patients (without placebo group and further patients excluded) were analysed for weight change and body composition | |
| Interventions | Intervention (n = 18): aerobic training (cycling, interval training), 60% to 85% HRmax, 20 to 30 min per session, 3/week Control group 1 (n = 11): flexibility and stretching exercises ("placebo group") Control group 2 (n = 16): no intervention | |
| Outcomes |
Outcomes measured at baseline and end of intervention | |
| Notes | Funding: National Institutes of Health Grants RO1 NR 01078, National Center for Nursing Research and P 3OCA 16058 14, National Cancer Institute Conflict of interest: Not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated to be randomised, but method not described |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants | High risk | Placebo group analysed for functional capacity did stretching exercises. Comment: participants would have been aware if they were in the exercise group or the stretching group |
| Blinding of personnel/care providers | High risk | Not reported. Comment: probably not done |
| Blinding of outcome assessment (detection bias) | High risk | Not reported. Comment: probably not done |
| Blinding of outcome assessment (detection bias) | Unclear risk | No other relevant outcomes |
| Incomplete outcome data (attrition bias) | High risk | Drop‐out rate for all participants: 17/62 (27.4%) 45 of 62 participants were analysed for functional capacity. Per‐protocol analysis (n = 45) of n = 62, who entered the study |
| Selective reporting (reporting bias) | High risk | It is unclear why data for only 2 groups was analysed for weight change and body composition |
| Group similarity at baseline | High risk | Educational status differed. Data for 17 women not shown for baseline |
| Adherence | Low risk | Adherence complete (missed sessions repeated) |
| Contamination | Unclear risk | "No subject participated in any other exercise or rehabilitation program during the 10 week data collection period." Comment: amount of physical activity outside of programs unclear |
| Methods | RCT, 2 groups Study start and stop dates: Potential participants were identified between 1998 and 2001 Length of follow‐up: end of intervention | |
| Participants | 119 breast cancer patients, stages 0 to III, after surgery, receiving chemotherapy or radiotherapy, sedentary | |
| Interventions | Intervention (n = 60): aerobic training (walking), self directed, 50% to 70% HRmax, 15 min per session, increased to 30 min as training progressed, 5 to 6/x week. Radiotherapy: 6 weeks exercise; chemotherapy: 3 to 6 months exercise. Control (n = 59): usual care | |
| Outcomes |
Outcomes measured at: baseline and end of the adjuvant therapy/intervention | |
| Notes | Funding: FIRE (Fatigue Initiative in Research and Education) multi‐institutional award from the Oncology Nursing Society Foundation Conflict of interest: Not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation |
| Allocation concealment (selection bias) | Low risk | Consecutively numbered, sealed, opaque envelopes, opened after baseline testing |
| Blinding of participants | High risk | Not done |
| Blinding of personnel/care providers | High risk | Not reported Comment: probably not done |
| Blinding of outcome assessment (detection bias) | High risk | Not reported Comment: probably not done |
| Blinding of outcome assessment (detection bias) | High risk | Self reported items |
| Incomplete outcome data (attrition bias) | Low risk | Drop‐out:
All participants 11/119 (9.2%) Reported both ITT and per‐protocol analysis |
| Selective reporting (reporting bias) | Unclear risk | No study protocol published |
| Group similarity at baseline | Low risk | No significant differences |
| Adherence | High risk | Not adherent: 15/54 (28%). 72% were adherent in the sense of the studies' definition (85% of minimum prescription) |
| Contamination | High risk | Contamination: 21/54 (39%) of the control group were exercising |
| Methods | RCT, 2 groups Study start and stop dates: not reported | |
| Participants | 22 breast cancer patients, chemotherapy, not exercising regularly | |
| Interventions | Intervention group (n = 11): “dynamic aerobic exercise” adapted individually, 60‐minute sessions at 60% to 70% of maximum heart rate, 3/week Control group (n = 11): usual care | |
| Outcomes |
Outcomes measured at time point: 10 to 15 days after end of intervention | |
| Notes | Funding: not reported Conflict of interest: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated as randomised, but method not described |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants | High risk | Not reported. Comment: probably not done |
| Blinding of personnel/care providers | High risk | Not reported. Comment: probably not done |
| Blinding of outcome assessment (detection bias) | High risk | Self reported items |
| Incomplete outcome data (attrition bias) | High risk | Drop‐out:
|
| Selective reporting (reporting bias) | Unclear risk | No study protocol published |
| Group similarity at baseline | High risk | There were more obese women in the control group |
| Adherence | Unclear risk | Not described |
| Contamination | Unclear risk | Not described |
| Methods | RCT; 2 groups, stratification for hospital and treatment Study start and stop dates: recruitment from January 2004 to January 2005 | |
| Participants | 203 breast cancer patients during treatment, chemo‐ or radiotherapy or both | |
| Interventions | Intervention (n = 101): supervised 12‐week group exercise 2 times/week, 45 minutes/session at moderate intensity (aerobic and strength). Participants encouraged to exercise 1x/week at home Control (n = 102): usual care | |
| Outcomes | Primary outcomes:
Secondary outcomes:
Outcomes measured at time point: baseline, 12 weeks (intervention: 82, control: 92), and 6‐month follow‐up (intervention: 82, control: 95), 18 months and 5 years after the intervention | |
| Notes | Funding: Cancer Research UK. One author was funded by the UK Medical Research Council. Conflict of interest: None declared | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised permuted blocks of length 4 and 6 |
| Allocation concealment (selection bias) | Low risk | “Randomisation was done by telephone to an interactive voice response system” |
| Blinding of participants | High risk | Not done |
| Blinding of personnel/care providers | High risk | Not reported. Comment: probably not done |
| Blinding of outcome assessment (detection bias) | Low risk | “We took steps to blind the evaluation of outcomes by having questionnaire responses in sealed envelopes and ensuring that outcome measures were taken by researchers who were not involved in exercise classes” |
| Blinding of outcome assessment (detection bias) | High risk | Self reported items |
| Incomplete outcome data (attrition bias) | High risk | Drop‐out: Intervention group:
Control group:
Comment: Differing rate between groups. Long term (18 months and 5 years >): 30% attrition ITT, no imputation of data |
| Selective reporting (reporting bias) | Low risk | Study protocol available |
| Group similarity at baseline | Low risk | "No obvious imbalances existed between study groups." Long term: Differences in baseline demographics between participants that did and did not return for follow‐up |
| Adherence | High risk | Participation in classes:
|
| Contamination | Unclear risk | Not reported |
| Methods | RCT, 2 groups Study start and stop dates: recruitment between April 2001 and July 2005 | |
| Participants | 51 breast cancer patients, sedentary lifestyle (i.e. exercise less than 3/week for greater than 30 min/session in last 6 months); numbers in each group not reported. Many (44.1%) women received both radiation and chemotherapy, 26.5% received radiation only, 8.8% received chemotherapy only, and 20.6% received no adjuvant therapy | |
| Interventions | Intervention: individualised walking and resistance training program, 2 phases with a hospital‐based portion followed by a transition to home‐based exercise. Two 30‐min exercise adherence counselling sessions during the hospital‐based phase. Hospital based: 3 times per week for 4 weeks, aerobic: treadmill walking at 50% to 70% of GXT‐derived maximal heart rate (MHR). In subsequent weeks, duration was gradually increased by 5 min for a maximum of 40 min and a minimum of 30 min; intensity was increased to be within 70% to 85% of MHR according to participant comfort. Home based: instructed to walk 3 times per week, encouraged to walk every day for 30 minutes or more. Control: information control, 45 min session and informational brochure | |
| Outcomes |
Outcomes measured at baseline and 3 months | |
| Notes | Funding: National Cancer Institute (CAR0178801) and National Institutes of Health, General Clinical Research Grant (M01RR00533) Conflict of interest: Not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number sequence table |
| Allocation concealment (selection bias) | Low risk | Participant assignment to groups at enrolment was concealed from the project director |
| Blinding of participants | High risk | Not done |
| Blinding of personnel/care providers | High risk | Not reported. Comment: probably not done |
| Blinding of outcome assessment (detection bias) | Low risk | Physicians monitoring graded exercise tests were blinded to participant group assignment. Similarly, a physical therapist or an exercise physiologist, blinded to participant assignment, performed strength assessments. Comment: No fitness outcomes reported in this paper |
| Blinding of outcome assessment (detection bias) | High risk | Self reported items |
| Incomplete outcome data (attrition bias) | Unclear risk | Numbers randomised to each arm are as well as completion rates are unclear. "we used regression modeling to impute missing values to conduct our analyses." Amount of missing data was not reported |
| Selective reporting (reporting bias) | Unclear risk | Further outcomes to be reported in another paper |
| Group similarity at baseline | Low risk | "found no significant differences with respect to demographic, cancer stage, treatment, and exercise characteristics." |
| Adherence | Low risk | Completed an average of 83% of their scheduled hospital‐based exercise sessions (M = 9.9, SD = 3.3 sessions); 76.9% completed all 12 sessions |
| Contamination | Unclear risk | Contamination not reported/GLTEQ scores increased from baseline by 32.7% in the control group |
| Methods | RCT, 2 groups Study start and stop dates: all participants were diagnosed and treated between March 2009 and April 2011 Length of intervention: 4 to 6 months (duration of chemotherapy) | |
| Participants | Women undergoing neoadjuvant chemotherapy for locally advanced, non‐metastatic breast cancer. Women had to have oestrogen receptor‐positive breast cancer, a BMI greater than 25, and a Karnofsky score > 80% | |
| Interventions | Intervention (n = 5): home‐based exercise program, supervised, one on one, 3 times per week. Aerobic exercises and light weight lifting. Control (n = 5): usual care | |
| Outcomes |
Outcomes measured before and after neoadjuvant chemotherapy | |
| Notes | ClinicalTrials.gov (NCT01411787) Funding: Grant given by the Commercial Real Estate Women of Dallas Conflict of interest: None declared | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomization will occur by drawing cards entitled 'exercise' or 'control' from an envelope and then assigning the patient to this group." (ClinicalTrials.gov) |
| Allocation concealment (selection bias) | Unclear risk | An unlabeled envelope was opened by the research co‐ordinator to place the participant in either the control or boot camp arm of the study |
| Blinding of participants | High risk | Not done |
| Blinding of personnel/care providers | High risk | Not reported Comment: probably not done |
| Blinding of outcome assessment (detection bias) | High risk | Not reported Comment: probably not done |
| Incomplete outcome data (attrition bias) | Low risk | 10 women were randomised and completed all study parameters and were included in the analysis. All women were analysed in their respective group |
| Selective reporting (reporting bias) | High risk | "Improving fitness levels" not reported in results paper |
| Group similarity at baseline | Low risk | There were no statistically significant differences between groups with regard to tumour size, age, BMI, tumour grade, C‐peptide levels, or initial Ki‐67 |
| Adherence | Low risk | All 5 women in the exercise group completed > = 80% of the advised exercise sessions |
| Contamination | Unclear risk | Women were allowed to engage in their own exercise regimens and diet modifications. (ClinicalTrials.gov) BMI > 25, therefore "unlikely to have previously exercised" Comment: amount of physical activity not reported |
| Methods | RCT, 2 groups Study start and stop dates: November 2008 to January 2010 | |
| Participants | 41 breast cancer patients randomised, stage I to III, starting adjuvant radiotherapy. 26 participants (13 in each group) additionally received chemotherapy, 19 (10 in the exercise group and 9 in the control group) received hormone therapy, the latter "typically after chemotherapy and radiation therapy" | |
| Interventions | Intervention group (n = 22 randomised): Nia exercise ("cardiovascular and whole‐body conditioning program") 20 to 60 minutes 3 x per week for 12 weeks, and 3 meetings with principal investigator Control group (n = 19 randomised): usual care and 3 meetings with principal investigator, instructed to continue normal activities | |
| Outcomes |
Outcomes were measured at start of radiation therapy, the completion of radiation therapy, and 6 weeks after completion of radiation therapy. Some participants received more than 6 weeks of radiotherapy | |
| Notes | Funding: Not reported. Conflict of interest: "No financial relationships to disclose." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study described as "randomized", method not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants | High risk | Not done |
| Blinding of personnel/care providers | High risk | Not reported. Comment: probably not done |
| Blinding of outcome assessment (detection bias) | High risk | Not reported. Comment: probably not done |
| Blinding of outcome assessment (detection bias) | High risk | Self reported items |
| Incomplete outcome data (attrition bias) | High risk | Drop‐out (did not complete the 12‐week assessment):
Outcome data not reported for non‐adherent women in the exercise group. Per‐protocol analysis: The statistical analyses for fatigue, QoL, aerobic capacity, and shoulder flexibility compared the 12 women who practiced Nia to the 17 women randomised to the control group for whom data were collected at baseline, 6 weeks, and 12 weeks |
| Selective reporting (reporting bias) | Unclear risk | No study protocol published, study not registered prospectively |
| Group similarity at baseline | High risk | "The two groups did not differ statistically in their demographics, although clinical differences appear to exist in age and employment, with the Nia group aged, on average, five years younger and more likely to |
| Adherence | High risk | Assessed by reviewing participant logs. Logs were not uniformly maintained. Only 12 of 22 participants in the Nia group were adherent |
| Contamination | High risk | "66% of participants (n = 27) reported engaging in aerobic activity for at least three 20‐minute sessions per week prior to the cancer diagnosis. About 74% of those women (n = 20) continued to exercise during radiation therapy. No significant difference existed in the exercise history of the Nia group compared to the control group." Women in the control group reported engaging in aerobic exercise 0 to 41 times, or an average of almost 3 days per week (34 days in 6 weeks average) |
| Methods | RCT, 2 groups Study start and stop dates: April 2010 and August 2013 | |
| Participants | 101 randomised patients with breast cancer under adjuvant chemotherapy | |
| Interventions | Intervention (n = 52): 12‐week supervised machine‐based progressive resistance training program, 60 minutes 2x/week, 3 sets, 8 to 12 repetitions at 60% to 80% of 1 repetition maximum (1‐RM) Control group (n = 49): supervised group‐based progressive muscle relaxation training according to Jacobson, 60 minutes 2x/week | |
| Outcomes | Primary endpoint:
Secondary endpoints:
Outcomes measured during the first or second chemotherapy cycle pre‐intervention (baseline) and postintervention (week 13) | |
| Notes | BEATE study, ClinicalTrials.gov registration: NCT01106820 Funding: German Cancer Research Center (DKFZ), Division of Preventive Oncology. Foundations: “Stiftung Leben mit Krebs” and “Manfred‐Lautenschlaeger‐Stiftung”. Conflict of interest: Not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "randomly allocated 1:1": predetermined lists with random block size, stratified by age and baseline physical fatigue level |
| Allocation concealment (selection bias) | Low risk | Allocation was performed by a biostatistician uninvolved in recruitment, based on predetermined lists with random block size, stratified by age and baseline physical fatigue level. Other study personnel did not have access to the randomisation lists |
| Blinding of participants | High risk | Not done |
| Blinding of personnel/care providers | High risk | Not reported. Comment: probably not done |
| Blinding of outcome assessment (detection bias) | High risk | Not reported. Comment: probably not done |
| Blinding of outcome assessment (detection bias) | High risk | Self reported items |
| Incomplete outcome data (attrition bias) | Low risk | Pre‐ and postintervention assessment of the primary endpoint was available in a total of 95 of 101 (94%) participants, 49 in exercise group and 46 in relaxation control group. "intent‐to‐treat‐basis". "As very few fatigue values were missing (3%), we performed complete‐case analyses" |
| Selective reporting (reporting bias) | High risk | Registered at ClinicalTrials.gov (NCT01106820), study protocol published, not all outcomes reported in this publication |
| Group similarity at baseline | High risk | Baseline characteristics, fatigue, and QoL were similarly distributed between both intervention groups, except for depression, which was significantly more common in the relaxation control group than in the exercise group. 2 in control group with metastasised cancer |
| Adherence | High risk | Median attendance was similar in both groups, with 17 out of 24 scheduled sessions attended (71%; interquartile range 11 to 22 in the exercise group and 11 to 23 in the relaxation control group) |
| Contamination | Unclear risk | Patients already participating in systematic intensive resistance or aerobic training (at least 1 hr twice/week) were excluded. Otherwise not reported |
| Methods | RCT, parallel 3‐group design, stratified according to menopausal status (premenopausal or postmenopausal). Study start and stop dates: not reported | |
| Participants | 72 breast cancer patients, stages I to III (histologically confirmed); planning to begin chemotherapy with doxorubicin or methotrexate and receiving a glucocorticoid as part of the antiemetic regimen. Strenuous regular exercisers, that is women who exercised more than 250 minutes per week, were excluded | |
| Interventions | Intervention group 1 (n = 22): 'Schwartz AET' Aerobic exercise training (participant preferences); 77% weight‐bearing activities (walking/running), 15 to 30 minutes, 4 days per week Intervention group 2 (n = 21): 'Schwartz RET' Resistance exercise (Thera‐Band), 2 sets of 8 exercises (4 upper and 4 lower body) Control group (n = 23): usual care, women were instructed to continue usual activities, were not instructed to avoid exercise | |
| Outcomes |
Outcomes measured at baseline and 6 months | |
| Notes | Funding: Not reported Conflict of interest: Not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants | High risk | Not done |
| Blinding of personnel/care providers | High risk | Not reported Comment: probably not done |
| Blinding of outcome assessment (detection bias) | High risk | Not reported Comment: probably not done |
| Blinding of outcome assessment (detection bias) | Unclear risk | No other relevant outcomes |
| Incomplete outcome data (attrition bias) | Low risk | Drop‐out:
All participants: 8.3% (6/72) No data imputation |
| Selective reporting (reporting bias) | Unclear risk | No study protocol published |
| Group similarity at baseline | Low risk | No significant differences |
| Adherence | Unclear risk | No adherence data available |
| Contamination | Unclear risk | Not reported. Control group not sedentary before cancer diagnosis |
| Methods | RCT, parallel 3‐group design, stratified according to menopausal status (premenopausal or postmenopausal). Study start and stop dates: not reported | |
| Participants | 72 breast cancer patients, stages I to III (histologically confirmed); planning to begin chemotherapy with doxorubicin or methotrexate and receiving a glucocorticoid as part of the antiemetic regimen. Strenuous regular exercisers, that is women who exercised more than 250 minutes per week, were excluded | |
| Interventions | Intervention group 1 (n = 22): 'Schwartz AET' Aerobic exercise training (participant preferences); 77% weight‐bearing activities (walking/running), 15 to 30 minutes, 4 days per week Intervention group 2 (n = 21): 'Schwartz RET' Resistance exercise (Thera‐Band), 2 sets of 8 exercises (4 upper and 4 lower body) Control group (n = 23): usual care, women were instructed to continue usual activities, were not instructed to avoid exercise | |
| Outcomes |
Outcomes measured at baseline and 6 months | |
| Notes | Funding: Not reported Conflict of interest: Not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants | High risk | Not done |
| Blinding of personnel/care providers | High risk | Not reported Comment: probably not done |
| Blinding of outcome assessment (detection bias) | High risk | Not reported Comment: probably not done |
| Incomplete outcome data (attrition bias) | Low risk | Drop‐out:
All participants: 8.3% (6/72) No data imputation |
| Selective reporting (reporting bias) | Unclear risk | No study protocol published |
| Group similarity at baseline | Low risk | No significant differences |
| Adherence | Unclear risk | No adherence data available |
| Contamination | Unclear risk | Not reported. Control group not sedentary before cancer diagnosis |
| Methods | RCT, 3 groups Study start and stop dates: not reported Length of intervention: 26 weeks | |
| Participants | 123 patients within 2 weeks of the initiation of their prescribed adjuvant therapy (radiotherapy, hormonal therapy, or chemotherapy). Patients receiving only alternative or dose‐intensive chemotherapy regimens were excluded | |
| Interventions | Intervention group 1: 'Segal 2001 SD' self directed aerobic training (n = 40): progressive walking program at an exercise intensity of 50% to 60% of the predicted maximal oxygen uptake Intervention group 2: 'Segal 2001 SU' supervised training (n = 42): progressive walking program at an exercise intensity of 50% to 60% of the predicted maximal oxygen uptake Control group (n = 41): usual care | |
| Outcomes | Primary outcome:
Secondary outcomes:
Outcomes measured at baseline and 26 weeks | |
| Notes | Funding: National Cancer Institute of Canada with funds from the Canadian Cancer Society (Grant in Aid of Research No. 7191) Conflict of interest: Not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random numbers table |
| Allocation concealment (selection bias) | Unclear risk | Study co‐ordinator revealed group assignment after baseline testing. Method not described |
| Blinding of participants | High risk | Not done |
| Blinding of personnel/care providers | High risk | Not reported. Comment: probably not done |
| Blinding of outcome assessment (detection bias) | High risk | Not reported. Comment: probably not done |
| Blinding of outcome assessment (detection bias) | High risk | Self reported items |
| Incomplete outcome data (attrition bias) | Low risk |
End‐of‐intervention (26‐week) data were obtained for 99 participants (80.4%) ITT with imputation (most recent observed) |
| Selective reporting (reporting bias) | Unclear risk | No study protocol published prospectively. FACT‐G and FACT‐B: no detailed results, but stated that no significant differences |
| Group similarity at baseline | Low risk | "Baseline demographic, body weight, aerobic capacity, prior level of physical activity, and disease treatment characteristics of the subjects did not differ among the three groups. There were no baseline differences among groups for the eight SF‐36 scales" |
| Adherence | High risk | Adherence in intervention groups:
|
| Contamination | Unclear risk | Not reported |
| Methods | RCT, 3 groups Study start and stop dates: not reported Length of intervention: 26 weeks | |
| Participants | 123 patients within 2 weeks of the initiation of their prescribed adjuvant therapy (radiotherapy, hormonal therapy, or chemotherapy). Patients receiving only alternative or dose‐intensive chemotherapy regimens were excluded | |
| Interventions | Intervention group 1: 'Segal 2001 SD' self directed aerobic training (n = 40): progressive walking program at an exercise intensity of 50% to 60% of the predicted maximal oxygen uptake Intervention group 2: 'Segal 2001 SU' supervised training (n = 42): progressive walking program at an exercise intensity of 50% to 60% of the predicted maximal oxygen uptake | |
| Outcomes | Primary outcome:
Secondary outcomes:
Outcomes measured at baseline and 26 weeks | |
| Notes | Funding: National Cancer Institute of Canada with funds from the Canadian Cancer Society (Grant in Aid of Research No. 7191) Conflict of interest: Not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random numbers table |
| Allocation concealment (selection bias) | Unclear risk | Study co‐ordinator revealed group assignment after baseline testing. Method not described |
| Blinding of participants | High risk | Not done |
| Blinding of personnel/care providers | High risk | Not reported. Comment: probably not done |
| Blinding of outcome assessment (detection bias) | High risk | Not reported. Comment: probably not done |
| Blinding of outcome assessment (detection bias) | High risk | Self reported items |
| Incomplete outcome data (attrition bias) | Low risk |
End‐of‐intervention (26‐week) data were obtained for 99 participants (80.4%) ITT with imputation (most recent observed) |
| Selective reporting (reporting bias) | Unclear risk | No study protocol published prospectively. FACT‐G and FACT‐B: no detailed results, but stated that no significant differences |
| Group similarity at baseline | Low risk | "Baseline demographic, body weight, aerobic capacity, prior level of physical activity, and disease treatment characteristics of the subjects did not differ among the three groups. There were no baseline differences among groups for the eight SF‐36 scales" |
| Adherence | High risk | Adherence in intervention groups:
|
| Contamination | Unclear risk | Not reported |
| Methods | RCT, 2 groups Study start and stop dates: February 2011 and March 2013 | |
| Participants | 160 randomised participants with breast cancer (stage I to III after lumpectomy or mastectomy) undergoing adjuvant radiotherapy | |
| Interventions | Intervention (n = 80): supervised machine‐based progressive resistance training, 60 minutes, 2x/week, 3 sets, 8 to 12 repetitions at 60% to 80% of 1 repetition maximum Control (n = 80): supervised muscle relaxation training according to Jacobson, 60 minutes 2x/week | |
| Outcomes | Primary endpoint:
Secondary endpoints:
Outcomes measured before start of radiotherapy (baseline, T0), postradiotherapy (week 7, T1), and postintervention (week 13, T2) | |
| Notes | BEST study, registered at ClinicalTrials.gov (NCT01468766) Funding: Interdisciplinary Research Funding Program (intramural) of the National Center for Tumor Diseases (NCT), Heidelberg, Germany (grant number IFP project VI.1); foundations “Stiftung Leben mit Krebs” and the "Manfred‐Lautenschlaeger‐Stiftung” Conflict of interest: None declared | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Predetermined list generated with a blocked randomisation SAS procedure with a fixed block size, stratified by age and baseline physical fatigue level |
| Allocation concealment (selection bias) | Low risk | Allocation is done by the biometrician based on a predetermined list generated with a blocked randomisation SAS procedure with a fixed block size, stratified by age and baseline physical fatigue level. To prevent possible bias, study personnel involved in the recruitment and the baseline assessment do not have access to the randomisation lists and are not aware of the block size. Conversely, the biometrician does not have influence on the recruitment procedure |
| Blinding of participants | High risk | Not done |
| Blinding of personnel/care providers | High risk | Not reported Comment: probably not done |
| Blinding of outcome assessment (detection bias) | High risk | Not reported Comment: probably not done |
| Blinding of outcome assessment (detection bias) | High risk | Self reported items |
| Incomplete outcome data (attrition bias) | Low risk | Pre‐ and postintervention assessment of the primary endpoint was available for a total of 155 (97%) participants, 77 in the exercise group and 78 in the relaxation control group. Data were analysed on an ITT basis. As the number of missing fatigue values was very low (3%), we performed complete‐case analyses |
| Selective reporting (reporting bias) | High risk | Registered at ClinicalTrials.gov (NCT01468766), study protocol published. Not all outcomes reported in this publication |
| Group similarity at baseline | Low risk | Demographics and treatment characteristics did not differ significantly between both intervention groups. All primary and secondary outcome variables were equally distributed in exercise and relaxation control groups at baseline (all P > 0.05), except for the EORTC symptom dry mouth (P = 0.033) |
| Adherence | High risk | The median attended number out of 24 scheduled sessions was 19 in both groups (79%). (QR: 13 to 23, range 1 to 24) in exercise group and (QR: 12 to 22, range 0 to 24) in relaxation control group |
| Contamination | Unclear risk | Patients already participating in systematic intensive resistance or aerobic training (at least 1 hr twice/week) were excluded. Otherwise not reported |
| Methods | RCT, 2 groups Study start and stop dates: conducted between 2010 and 2013 Length of intervention: 18 weeks Length of follow‐up: 18 weeks | |
| Participants | Patients with breast or colon cancer undergoing cancer treatment (204 breast cancer patients of 237 patients in total), intervention started 6 weeks postdiagnosis. Unpublished data (final values) for breast cancer patients used in the analyses of this review | |
| Interventions | Intervention (n = 102): supervised group exercise (aerobic and resistance) program based on Bandura’s social cognitive theory Control (n = 102): asked to maintain their habitual physical activity pattern | |
| Outcomes | Primary outcome:
Secondary outcomes:
| |
| Notes | Trial registration: Current Controlled Trials (ISRCTN43801571), Dutch Trial Register (NTR2138) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Concealed computer‐generated randomisation, 1:1 ratio, stratified per age, adjuvant treatment, use of tissue expander, and hospital by sequential balancing |
| Allocation concealment (selection bias) | Low risk | "After the patient signed informed consent, the researcher (who was with the patient) called the data management department and provided the participants study number and the information necessary for stratification. The data manager then performed the randomization using a computer program and informed the researcher about the allocation (and also noted the allocation in the randomization log)." |
| Blinding of participants | High risk | "not possible" |
| Blinding of personnel/care providers | High risk | Not reported Comment: probably not done |
| Blinding of outcome assessment (detection bias) | Low risk | Outcome measures were assessed by researchers not involved with the participants |
| Blinding of outcome assessment (detection bias) | High risk | Self reported items |
| Incomplete outcome data (attrition bias) | Low risk | Drop‐out after 18 weeks:
Follow‐up: high risk Drop‐out after 36 weeks:
|
| Selective reporting (reporting bias) | High risk | Design paper published. EQ‐5D, IPA (Impact on Participation and Autonomy), self efficacy (items based on social cognitive theory) mentioned in the design paper, but not in the publication |
| Group similarity at baseline | High risk | "were comparable on most characteristics" Comment: More women in the intervention group were highly educated, had triple‐negative breast cancer, and were postmenopausal. Total physical activity levels tended to be higher in the control group |
| Adherence | Low risk | Participation in 83% (IQR 69% to 91%) of the classes. Reported to be physically active in 11 (IQR 6 to 14) of 18 weeks |
| Contamination | High risk | High level of physical activity reported by 56% of the controls at 18 weeks |
| Methods | RCT, 3 groups Study start and stop dates: recruitment between March 2010 and December 2012 Length of follow‐up: 6 months | |
| Participants | Patients with breast or colon cancer receiving adjuvant chemotherapy, 230 patients with breast cancer of 253 patients in total. Both intervention groups started exercising in the week of the first cycle of chemotherapy and continued until 3 weeks after the last cycle of chemotherapy. Mean length of chemotherapy 119.6 days (17 weeks) | |
| Interventions | 1. Intervention group (n = 77): Onco‐Move, a relatively low‐intensity, home‐based, individualised, self managed physical activity program 2. Intervention group (n = 76): OnTrack, a relatively high‐intensity exercise program supervised by a physical therapist in an outpatient or general physical therapy practice setting 3. Control group (n = 77): usual care | |
| Outcomes | Primary outcomes:
Secondary outcomes:
Outcomes assessed: before random assignment and start of chemotherapy (T0), at completion of chemotherapy (T1), and 6 months after completion of chemotherapy (T2) | |
| Notes | Published protocol. Trial registration: The Netherlands Trial Register (NTR 2159) Funding: Supported by Alpe d'Huzes/Dutch Cancer Society Grant No. ALPE‐2009‐4299, the CZ Fund, Zilveren Kruis Achmea, and the Comprehensive Cancer Centre of the Netherlands. Conflicts of interest: 2 authors disclosed research funding by pharmaceutical companies | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were randomly assigned to Onco‐Move, OnTrack, or UC using the minimization method, which balanced groups with respect to age, primary diagnosis, treating hospital, and use of trastuzumab." |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants | High risk | Not done |
| Blinding of personnel/care providers | High risk | Not reported. Comment: probably not done |
| Blinding of outcome assessment (detection bias) | High risk | Not reported. Comment: probably not done |
| Blinding of outcome assessment (detection bias) | High risk | Self reported items |
| Incomplete outcome data (attrition bias) | Low risk | Outcome data were available for 204 participants (89%) directly after chemotherapy, and for 196 (85%) at the 6‐month follow‐up. ITT without imputation |
| Selective reporting (reporting bias) | High risk | Differences between design paper and final publication (EQ‐5D, anthropometric measures and actigraph not mentioned in the final publication). Chemotherapy completion rate mentioned as outcome in the final publication, but not in the design paper |
| Group similarity at baseline | Low risk | Baseline characteristics were balanced across groups |
| Adherence | High risk | On average, participants in OnTrack attended 71% of the planned sessions. On the basis of the exercise diary, 48% of the OnTrack group and 55% of the Onco‐Move group followed the recommendations regarding daily activity levels at least 75% of the time |
| Contamination | Unclear risk | Not reported |
| Methods | RCT, 3 groups Study start and stop dates: recruitment between March 2010 and December 2012 Length of follow‐up: 6 months | |
| Participants | Patients with breast or colon cancer receiving adjuvant chemotherapy, 230 patients with breast cancer of 253 patients in total. Both intervention groups started exercising in the week of the first cycle of chemotherapy and continued until 3 weeks after the last cycle of chemotherapy. Mean length of chemotherapy 119.6 days (17 weeks) | |
| Interventions | 1. Intervention group (n = 77): Onco‐Move, a relatively low‐intensity, home‐based, individualised, self managed physical activity program 2. Intervention group (n = 76): OnTrack, a relatively high‐intensity exercise program supervised by a physical therapist in an outpatient or general physical therapy practice setting 3. Control group (n = 77): usual care | |
| Outcomes | Primary outcomes:
Secondary outcomes:
Outcomes assessed: before random assignment and start of chemotherapy (T0), at completion of chemotherapy (T1), and 6 months after completion of chemotherapy (T2) | |
| Notes | Published protocol. Trial registration: The Netherlands Trial Register (NTR 2159) Funding: Supported by Alpe d'Huzes/Dutch Cancer Society Grant No. ALPE‐2009‐4299, the CZ Fund, Zilveren Kruis Achmea, and the Comprehensive Cancer Centre of the Netherlands. Conflicts of interest: 2 authors disclosed research funding by pharmaceutical companies | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were randomly assigned to Onco‐Move, OnTrack, or UC using the minimization method, which balanced groups with respect to age, primary diagnosis, treating hospital, and use of trastuzumab." |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants | High risk | Not done |
| Blinding of personnel/care providers | High risk | Not reported. Comment: probably not done |
| Blinding of outcome assessment (detection bias) | High risk | Not reported. Comment: probably not done |
| Blinding of outcome assessment (detection bias) | High risk | Self reported items |
| Incomplete outcome data (attrition bias) | Low risk | Outcome data were available for 204 participants (89%) directly after chemotherapy, and for 196 (85%) at the 6‐month follow‐up. ITT without imputation |
| Selective reporting (reporting bias) | High risk | Differences between design paper and final publication (EQ‐5D, anthropometric measures and actigraph not mentioned in the final publication). Chemotherapy completion rate mentioned as outcome in the final publication, but not in the design paper |
| Group similarity at baseline | Low risk | Baseline characteristics were balanced across groups |
| Adherence | High risk | On average, participants in OnTrack attended 71% of the planned sessions. On the basis of the exercise diary, 48% of the OnTrack group and 55% of the Onco‐Move group followed the recommendations regarding daily activity levels at least 75% of the time |
| Contamination | Unclear risk | Not reported |
| Methods | RCT, 2 groups Study start and stop dates: final enrolment completed August 2010, otherwise not reported | |
| Participants | Breast cancer patients receiving weekly paclitaxel for 2 months, randomisation after 4 cycles of doxorubicin and cyclophosphamide were completed, and prior to the first paclitaxel infusion | |
| Interventions | Intervention group (n = 9): home‐based aerobic (walking and progressive interval training) and resistance exercises for upper and lower extremities using resistance power bands. Brisk walking 5 to 7 days per week for the first 4 weeks, weeks 4 to 12 interval‐based workout consisting of light‐ to moderate‐intensity exercises performed for 30 minutes. Control group (n = 10): breast cancer educational information, avoiding those topics related to exercise/physical activity in order to prevent contamination between groups. Educational sessions at the same intervals as intervention group | |
| Outcomes |
Outcomes were assessed at baseline, 4 weeks, 12 weeks, 24 weeks. Adverse events: No injuries or falls were reported | |
| Notes | Funding: This study was supported by a grant from the University of Nebraska Medical Center, Eppley Cancer Center, Dean’s Grant. Conflict of interest: None reported. Registered prospectively on ClinicalTrials.gov: NCT00869804 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation was generated using sealed envelopes that were numbered and selected sequentially |
| Allocation concealment (selection bias) | Unclear risk | Sealed envelopes. Comment: not mentioned if they were opaque |
| Blinding of participants | High risk | Not done |
| Blinding of personnel/care providers | High risk | The research nurses conducted all recruitment, data collection, and study interventions. The principal investigator provided ongoing supervision of the intervention |
| Blinding of outcome assessment (detection bias) | High risk | The research nurses conducted all recruitment, data collection, and study interventions. The principal investigator provided ongoing supervision of the intervention |
| Blinding of outcome assessment (detection bias) | High risk | Self reported items |
| Incomplete outcome data (attrition bias) | Unclear risk | No dropouts or missing data reported. All study participants were evaluated according to the randomisation schema regardless of completion of exercise sessions |
| Selective reporting (reporting bias) | High risk | Outcomes muscle strength, cold thermal sensation, and vibratory sensation, which were mentioned on ClinicalTrials.gov, were not reported. Results for symptom experience were not reported |
| Group similarity at baseline | Unclear risk | "No significant differences" |
| Adherence | High risk | Participants were given a diary to record both aerobic and resistance exercises. In order to objectively capture the aerobic exercise component, each exercise group participant was given a pedometer to be worn for each walking session throughout the entire 24‐week study. Mean walking time per week in minutes: 44.6 |
| Contamination | Unclear risk | Participants completed the Leisure Time Exercise (LTE) questionnaire at the baseline interview; "No significant differences" Participants assigned to the attention control group agreed not to begin a new exercise program or change their level of exercise during the course of the study. Level of exercise during study not reported |
| Methods | RCT, 3 groups Study start and stop dates: not reported | |
| Participants | 42 breast cancer patients, after surgery, receiving chemotherapy, within the first 6 months of chemotherapy, but having had at least 3 treatments prior to entering the study program, not on doxorubicin, Karnofsky 60% to 100%. None of the participants received antiemetic medication | |
| Interventions | Intervention (n = 16): aerobic training (cycling, interval training), 60% to 85% HRmax, 20 to 30 min per session, 3/week, 10 weeks, supervised Control group 1 (n = 12): no intervention Control group 2 (n = 14): "placebo" mild stretching, conversation, 1/week, supervised | |
| Outcomes |
Outcomes measured at baseline and end of intervention | |
| Notes | Funding: Not reported Conflict of interest: Not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated as randomised. The generation of the random sequence was not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants | High risk | Placebo group Comment: Patients in the placebo group would have been aware that they were not in the exercise group, but in the stretching group |
| Blinding of personnel/care providers | High risk | Not reported. Comment: probably not done |
| Blinding of outcome assessment (detection bias) | High risk | Self reported items |
| Incomplete outcome data (attrition bias) | Low risk | All participants were accounted for |
| Selective reporting (reporting bias) | High risk | Participants answered all 90 items of the SCL‐90‐R. Hereby, "the investigators hoped to avoid sensitizing patients to awareness of any one symptom". Results for other items apart from nausea and somatisation part were not reported |
| Group similarity at baseline | High risk | "similar in age, height and body weight". Participants in control group were higher educated than those in exercise and placebo groups. Placebo group had more married women than exercise and placebo groups. |
| Adherence | Unclear risk | Not reported |
| Contamination | Unclear risk | Contamination not reported. Inclusion criterion: "not in an exercise program". Control participants were advised to notify project personnel if they began exercising on a regular basis either as part of a group or on their own |
| Methods | RCT, 2 groups Study start and stop dates: 2008 to 2009 | |
| Participants | 44 sedentary breast cancer patients randomised, 40 completed the study | |
| Interventions | Intervention group (n = 19): home‐based walking program, developed using the American College of Sports Medicine guidelines. Walking starting 2 to 3 days after each chemotherapy session, included 5 minutes warm‐up, 30 minutes brisk walking (60% to 80% of age‐adjusted HRmax), 5 minutes cool‐down Control group (n = 21): maintenance of their previous lifestyle for 12 weeks | |
| Outcomes |
Outcomes measured at baseline, 6 and 12 weeks | |
| Notes | Funding: Taipei Medical University Hospital (95TMU‐TMUH‐19), Taipei, Taiwan, Republic of China. Conflict of interest: None declared | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants | High risk | Not done |
| Blinding of personnel/care providers | High risk | Not reported. Comment: probably not done |
| Blinding of outcome assessment (detection bias) | High risk | Self reported items |
| Incomplete outcome data (attrition bias) | Low risk | Drop‐out:
Total: 4/44 (9%) |
| Selective reporting (reporting bias) | Unclear risk | No study protocol published, study not registered prospectively |
| Group similarity at baseline | Low risk | The groups were generally balanced at baseline regarding demographic and disease‐related characteristics |
| Adherence | High risk | "About 20% did not completely adhere." Adherence was about 77% of the prescribed exercise sessions and 100% of the prescribed exercise intensity |
| Contamination | Unclear risk | Not reported |
6‐MWT: 6‐minute walk test
ACSM: American College of Sports Medicine
BDI: Beck Depression Inventory
BMI: body mass index
CES‐D: Center for Epidemiological Studies‐Depression Scale
DASH: Disabilities of the Arm, Shoulder and Hand Questionnaire
DEXA: dual‐energy X‐ray absorptiometry
EORTC QLQ‐BR23: European Organisation for Research and Treatment of Cancer Breast Cancer‐Specific Quality of Life Questionnaire
EORTC QLQ‐C30: European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire‐Core 36
FACIT‐F: Functional Assessment of Chronic Illness Therapy‐Fatigue Scale
FACT‐An: Functional Assessment of Cancer Therapy‐Anaemia
FACT‐B: Functional Assessment of Cancer Therapy‐Breast
FACT‐ES: Functional Assessment of Cancer Therapy‐Endocrine Symptoms
FACT‐G: Functional Assessment of Cancer Therapy‐General
FACT‐Taxane: Functional Assessment of Cancer Therapy‐Taxane
FQL: Fatigue Quality List
GLTEQ: Godin Leisure‐Time Exercise Questionnaire
GXT: Graded Exercise Test
HADS: Hospital Anxiety and Depression Scale
HRmax: maximum heart rate
HRQoL: health‐related quality of life
IPAQ: International Physical Activity Questionnaire
IQR: interquartile range
ITT: intention‐to‐treat
L‐Dex: lymphoedema index
MDASI‐T: Taiwanese version of the MD Anderson Symptom Inventory
MET: metabolic equivalent of task
MFI: Multidimensional Fatigue Inventory
MOS SF‐36: Medical Outcomes Study 36‐Item Short Form Health Survey
MQOLS‐CA: Multidimensional Quality of Life Scale‐Cancer
MVPA: moderate to vigorous physical activity
QoL: quality of life
QR: Quartile Range
PAL: physical activity level
PANAS: Positive and Negative Affect Schedule
PFS: Piper Fatigue Scale
POMS: Profile of Mood States
RCT: randomised controlled trial
SD: standard deviation
SF‐36: 36‐Item Short Form Health Survey
SPAQ: Scottish Physical Activity Questionnaire
SWLS: Satisfaction With Life Scale
VO2 max: maximal oxygen uptake
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Post‐treatment | |
| Participants not predominantly breast cancer patients | |
| Duration of exercise intervention less than 6 weeks | |
| Participants not predominantly breast cancer patients | |
| Post‐treatment | |
| Yoga | |
| Post‐treatment | |
| Not an RCT | |
| Not an RCT | |
| Yoga | |
| Exercise intervention not concurrent with adjuvant cancer treatment | |
| Post‐treatment | |
| Post‐treatment | |
| Post‐treatment | |
| Not an exercise intervention | |
| Radiotherapy ongoing for maximum 3 weeks | |
| Participants not predominantly breast cancer patients (40%), exercise as part of a complex intervention (group psychotherapy plus exercise) | |
| Exercise intervention not concurrent with adjuvant cancer treatment (< 50% under current hormone therapy) | |
| Participants were post‐treatment. Exercise intervention not concurrent with adjuvant cancer treatment | |
| Post‐treatment | |
| Yoga | |
| No clinical trial, protocol status, exercise as part of a complex intervention (diet and exercise‐based counselling program) | |
| No clinical trial, design paper | |
| Complex intervention | |
| Complex intervention | |
| Complex intervention | |
| Participants not predominantly breast cancer patients | |
| Post‐treatment | |
| Post‐treatment | |
| Post‐treatment | |
| Post‐treatment | |
| Exercise intervention not concurrent with adjuvant cancer treatment | |
| Post‐treatment | |
| Post‐treatment | |
| Post‐treatment | |
| No exercise intervention | |
| Participants not predominantly breast cancer patients | |
| Not an exercise intervention | |
| Post‐treatment | |
| Participants not predominantly breast cancer patients | |
| Not an RCT | |
| Post‐treatment | |
| Post‐treatment | |
| No exercise intervention | |
| No health‐related outcome measure (adherence study) | |
| Post‐treatment | |
| Post‐treatment | |
| Post‐treatment | |
| Post‐treatment | |
| Complex intervention: exercise and stress management training | |
| Data were not analysed to be used for a full publication (personal communication September 2013) | |
| Not an RCT | |
| Yoga | |
| No clinical trial, review | |
| Post‐treatment | |
| Exercise restricted to shoulder | |
| Qigong | |
| Post‐treatment | |
| Post‐treatment | |
| Post‐treatment | |
| Not an RCT | |
| Duration of exercise intervention less than 6 weeks | |
| Post‐treatment | |
| Not an RCT | |
| Post‐treatment | |
| Intervention and adjuvant treatment not concurrent | |
| Radiotherapy ongoing for maximum 5 weeks | |
| Yoga | |
| Exercise as part of a complex intervention (walking plus support group) | |
| Not an RCT | |
| Trial does not compare 2 groups as assigned by investigator | |
| No exercise intervention | |
| Participants not predominantly breast cancer patients | |
| Post‐treatment | |
| Post‐treatment | |
| Post‐treatment | |
| No clinical trial, review | |
| Less than 6 weeks | |
| No health‐related outcome measure (adherence study) | |
| Exercise intervention not concurrent with adjuvant cancer treatment | |
| Post‐treatment | |
| Post‐treatment | |
| Post‐treatment | |
| Yoga | |
| Post‐treatment | |
| Yoga | |
| Yoga | |
| Post‐treatment | |
| Not an exercise intervention | |
| Post‐treatment | |
| Trial does not compare 2 groups as assigned by investigator | |
| Trial does not compare 2 groups as assigned by investigator | |
| Post‐treatment | |
| Exercise intervention not concurrent with adjuvant cancer treatment | |
| No clinical trial, protocol status, exercise as part of a complex intervention (calcium‐rich diet and exercise) | |
| Not an RCT | |
| Post‐treatment | |
| Commentary | |
| Complex intervention | |
| Complex intervention | |
| Post‐treatment | |
| Yoga | |
| Commentary | |
| Post‐treatment | |
| Post‐treatment | |
| Post‐treatment | |
| Not an RCT | |
| Post‐treatment | |
| Exercise intervention does not coincide with adjuvant therapy at least 6 weeks | |
| Participants not predominantly breast cancer patients (63% female), duration of intervention programme 4 weeks | |
| Qigong | |
| Post‐treatment |
RCT: randomised controlled trial
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | RCT, 2 groups |
| Participants | Women with stage I to III breast cancer during (neo‐)adjuvant therapy |
| Interventions | Weekly 60‐minute physical exercise session together with individual home‐based, self contained 20‐minute sessions twice a week. Control group: weekly 60‐minute Iyengar yoga session together with individual home‐based, self contained 20‐minute sessions twice a week |
| Outcomes |
|
| Notes |
| Methods | RCT, 2 groups Study start and stop dates: not reported |
| Participants | Women who were within 4 to 12 weeks of surgery for stage I to III breast cancer and undergoing adjuvant chemotherapy |
| Interventions | Structured exercise program (6 months), aerobic and resistance training Control group: usual oncology care |
| Outcomes |
Outcomes assessed at: baseline and 3‐month intervals through 12 months |
| Notes | Abstract |
EORTC QLQ‐C30: European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire‐Core 36
FACT‐B: Functional Assessment of Cancer Therapy‐Breast
RCT: randomised controlled trial
SF‐36: 36‐Item Short Form Health Survey
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Preventing ‘chemo‐brain’: Can exercise mitigate chemotherapy‐induced cognitive impairment in breast cancer patients? |
| Methods | RCT |
| Participants | Breast cancer patients scheduled to receive adjuvant chemotherapy with fluorouracil‐epirubicin‐cyclophosphamide‐docetaxel (FEC‐T) or docetaxel‐cyclophosphamide (TC) regimens, target sample size: 66 |
| Interventions | Combination of resistance (i.e. lifting weights) and aerobic exercise (e.g. walking, jogging, cycling), 2 times/week in an exercise clinic. moderate to high intensity (i.e. a perceived exertion of somewhat hard to hard) and will be relative to each participant's capabilities. Progressive exercise prescription, modified according to individual response. Approximately 60 min/session, small groups under the supervision of an accredited exercise physiologist. Length of program: will vary from 2.5 to 4 months according to the duration of the chemotherapy regimen Control group: usual care |
| Outcomes | Primary outcome:
Secondary outcomes:
|
| Starting date | June 2014 first participant enrolment |
| Contact information | Dr Prue Cormie ECU Health and Wellness Institute Edith Cowan University 270 Joondalup Drive Joondalup, WA 6027, Australia |
| Notes |
| Trial name or title | Aerobic training during or after adjuvant therapy |
| Methods | RCT, 4 arms |
| Participants | Sedentary breast cancer patients, estimated enrolment: 160 participants |
| Interventions |
|
| Outcomes | Primary:
Secondary:
|
| Starting date | January 2013 |
| Contact information | Lee W Jones Duke University Medical Center Durham, NC, USA, 27710 |
| Notes | Estimated study completion date: August 2017 |
| Trial name or title | Effects of a structured exercise program on cancer‐related fatigue in women receiving radiation therapy for breast cancer |
| Methods | RCT, estimated enrolment: 30 |
| Participants | African‐Americans undergoing radiation therapy for localised breast cancer. Sedentary, as defined as < 60 minutes of recreation or work requiring modest physical activity/week based on the 7‐day physical activity recall questionnaire. Having completed neo‐adjuvant or adjuvant chemotherapy |
| Interventions | 8‐week, moderate‐intensity aerobic exercise program Participants will be required to meet and maintain a goal of 75 min/week of aerobic exercise by using portable cycle ergometers, that is 15 min/day, 5 days/week |
| Outcomes | Fatigue: FACIT‐F Cancer‐specific quality of life: FACT‐G |
| Starting date | June 2013 |
| Contact information | |
| Notes | Estimated study completion date: December 2016 |
| Trial name or title | A randomized, controlled trial to determine the effects of an exercise intervention on physical activity during chemotherapy for patients with early stage breast cancer |
| Methods | RCT |
| Participants | Women or men with histologically confirmed breast cancer and no evidence of metastatic disease with a recommendation to begin chemotherapy within 4 weeks. Sedentary: participants must have a baseline activity level of < 150 minutes/wk of moderate to vigorous activity as calculated using the moderate to vigorous components of the Leisure‐Time Exercise Questionnaire for physical activity (completed during screening). Karnofsky performance status > or = to 80%. Estimated enrolment: 120 participants |
| Interventions | A physical therapist will design an exercise plan for each participant on the intervention arm. The participants randomised to the intervention arm will also receive phone calls to assist with tracking the study participant's exercise and motivating the study participant to adhere to the exercise prescription. Exercise prescription aimed at increasing physical activity by a minimum of 10 MET hours/week |
| Outcomes | Primary:
Secondary:
|
| Starting date | June 2014 |
| Contact information | Contact: Mary Chamberlin, MD |
| Notes | Estimated primary completion rate: June 2017 (Final data collection date for primary outcome measure) |
| Trial name or title | Energy Balance and Breast Cancer Aspects‐II (EBBA‐II) |
| Methods | RCT, 2 groups |
| Participants | Newly diagnosed breast cancer patients undergoing adjuvant therapy, DCIS grade 3, stage I + II breast cancer Estimated enrolment: 600 participants |
| Interventions | 12‐month exercise program comprised of strength and endurance training. Exercise groups supervised by experienced physiotherapists, the participants will attend the exercise groups for training 60 min, 2/week. Additionally, home‐based exercise for > = 120 minutes a week, aiming to perform a total of 240 minutes of exercise per week. The control group told to follow standard‐care regimen |
| Outcomes | Primary:
Secondary:
|
| Starting date | September 2014 |
| Contact information | Inger Thune |
| Notes | Estimated primary completion date: May 2016 Planned follow‐up: 10 years |
| Trial name or title | Adapted Physical Activity in Cancerology (APACAN) |
| Methods | RCT |
| Participants | Breast cancer patients receiving radio‐ or chemotherapy or both, estimated enrolment: 200 participants |
| Interventions | Physical activity, from 2 to 6 months Control group: physical activity after treatment |
| Outcomes | Fatigue: MFI QoL: EORTC QLQ‐C30 |
| Starting date | September 2014 |
| Contact information | Yves‐[email protected] |
| Notes | Estimated primary completion date: December 2016 (final data collection date for primary outcome measure) |
| Trial name or title | e‐CUIDACHEMO: Telerehabilitation During Chemotherapy in Breast Cancer |
| Methods | RCT |
| Participants | Breast cancer patients with internet access, estimated enrolment: 40 participants |
| Interventions | Resistance and endurance exercise via telerehabilitation |
| Outcomes | Primary:
Secondary:
|
| Starting date | April 2012 |
| Contact information | Manuel Arroyo‐Morales [email protected] |
| Notes | Estimated study completion date: June 2015 |
6‐MWT: 6‐minute walk test
DCIS: ductal carcinoma in situ
EORTC QLQ‐C30: European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire‐Core 36
FACIT‐F: Functional Assessment of Chronic Illness Therapy‐Fatigue Scale
FACT‐G: Functional Assessment of Cancer Therapy‐General
MET: metabolic equivalent of task
MFI: Multidimensional Fatigue Inventory
PFS: Piper Fatigue Scale
QoL: quality of life
RCT: randomised controlled trial
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Physical fitness Show forest plot | 20 | 1310 | Std. Mean Difference (IV, Random, 95% CI) | 0.42 [0.25, 0.59] |
| Analysis 1.1  Comparison 1 Exercise versus control, Outcome 1 Physical fitness. | ||||
| 2 Fatigue Show forest plot | 22 | 1698 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.28 [‐0.41, ‐0.16] |
| Analysis 1.2  Comparison 1 Exercise versus control, Outcome 2 Fatigue. | ||||
| 3 Cancer‐specific quality of life Show forest plot | 13 | 1012 | Std. Mean Difference (IV, Random, 95% CI) | 0.12 [‐0.00, 0.25] |
| Analysis 1.3  Comparison 1 Exercise versus control, Outcome 3 Cancer‐specific quality of life. | ||||
| 4 Health‐related quality of life Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 1.4  Comparison 1 Exercise versus control, Outcome 4 Health‐related quality of life. | ||||
| 5 Cancer site‐specific quality of life Show forest plot | 4 | 262 | Mean Difference (IV, Random, 95% CI) | 4.24 [‐1.81, 10.29] |
| Analysis 1.5  Comparison 1 Exercise versus control, Outcome 5 Cancer site‐specific quality of life. | ||||
| 6 Depression Show forest plot | 6 | 674 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.15 [‐0.30, 0.01] |
| Analysis 1.6  Comparison 1 Exercise versus control, Outcome 6 Depression. | ||||
| 7 Cognitive function Show forest plot | 2 | 213 | Mean Difference (IV, Random, 95% CI) | ‐11.55 [‐22.06, ‐1.05] |
| Analysis 1.7  Comparison 1 Exercise versus control, Outcome 7 Cognitive function. | ||||
| 8 Strength Show forest plot | 13 | 912 | Std. Mean Difference (IV, Random, 95% CI) | 0.27 [0.04, 0.50] |
| Analysis 1.8  Comparison 1 Exercise versus control, Outcome 8 Strength. | ||||
| 9 Subjective upper body function Show forest plot | 3 | 231 | Mean Difference (IV, Random, 95% CI) | ‐0.52 [‐4.45, 3.41] |
| Analysis 1.9  Comparison 1 Exercise versus control, Outcome 9 Subjective upper body function. | ||||
| 10 Shoulder mobility Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 1.10  Comparison 1 Exercise versus control, Outcome 10 Shoulder mobility. | ||||
| 11 Arm morbidity Show forest plot | 3 | 240 | Mean Difference (IV, Random, 95% CI) | 1.11 [‐4.07, 6.29] |
| Analysis 1.11  Comparison 1 Exercise versus control, Outcome 11 Arm morbidity. | ||||
| 12 Anxiety Show forest plot | 3 | 331 | Mean Difference (IV, Random, 95% CI) | ‐1.45 [‐4.36, 1.46] |
| Analysis 1.12  Comparison 1 Exercise versus control, Outcome 12 Anxiety. | ||||
| 13 Mood disturbances Show forest plot | 3 | 111 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.00 [‐1.40, ‐0.60] |
| Analysis 1.13  Comparison 1 Exercise versus control, Outcome 13 Mood disturbances. | ||||
| 14 Hospital Anxiety and Depression Scale Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 1.14  Comparison 1 Exercise versus control, Outcome 14 Hospital Anxiety and Depression Scale. | ||||
| 15 Self esteem Show forest plot | 4 | 323 | Mean Difference (IV, Random, 95% CI) | 1.69 [‐0.01, 3.39] |
| Analysis 1.15  Comparison 1 Exercise versus control, Outcome 15 Self esteem. | ||||
| 16 Physical activity Show forest plot | 8 | 549 | Std. Mean Difference (IV, Random, 95% CI) | 0.29 [0.12, 0.47] |
| Analysis 1.16  Comparison 1 Exercise versus control, Outcome 16 Physical activity. | ||||
| 17 Neuropathic pain Show forest plot | 2 | 130 | Mean Difference (IV, Random, 95% CI) | 3.64 [‐1.32, 8.60] |
| Analysis 1.17  Comparison 1 Exercise versus control, Outcome 17 Neuropathic pain. | ||||
| 18 Neuropathy symptoms Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 1.18  Comparison 1 Exercise versus control, Outcome 18 Neuropathy symptoms. | ||||
| 19 Endocrine symptoms Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 1.19  Comparison 1 Exercise versus control, Outcome 19 Endocrine symptoms. | ||||
| 20 Gait and balance Show forest plot | 3 | 122 | Std. Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.25, 0.46] |
| Analysis 1.20  Comparison 1 Exercise versus control, Outcome 20 Gait and balance. | ||||
| 21 Lymphoedema incidence Show forest plot | 4 | 436 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.35, 1.45] |
| Analysis 1.21  Comparison 1 Exercise versus control, Outcome 21 Lymphoedema incidence. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Physical fitness Show forest plot | 6 | 612 | Std. Mean Difference (IV, Random, 95% CI) | 0.26 [‐0.06, 0.57] |
| Analysis 2.1  Comparison 2 Exercise versus control follow‐up, Outcome 1 Physical fitness. | ||||
| 2 Fatigue Show forest plot | 8 | 814 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.21 [‐0.35, ‐0.07] |
| Analysis 2.2  Comparison 2 Exercise versus control follow‐up, Outcome 2 Fatigue. | ||||
| 3 Cancer‐specific quality of life Show forest plot | 6 | 583 | Std. Mean Difference (IV, Random, 95% CI) | 0.18 [0.01, 0.35] |
| Analysis 2.3  Comparison 2 Exercise versus control follow‐up, Outcome 3 Cancer‐specific quality of life. | ||||
| 4 Depression Show forest plot | 3 | 378 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.27 [‐0.48, ‐0.06] |
| Analysis 2.4  Comparison 2 Exercise versus control follow‐up, Outcome 4 Depression. | ||||
| 5 Strength Show forest plot | 4 | 386 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.00 [‐0.30, 0.30] |
| Analysis 2.5  Comparison 2 Exercise versus control follow‐up, Outcome 5 Strength. | ||||
| 6 Physical activity Show forest plot | 3 | 261 | Std. Mean Difference (IV, Random, 95% CI) | 0.28 [‐0.05, 0.61] |
| Analysis 2.6  Comparison 2 Exercise versus control follow‐up, Outcome 6 Physical activity. | ||||
| 7 Anxiety Show forest plot | 2 | 201 | Mean Difference (IV, Random, 95% CI) | ‐3.61 [‐7.24, 0.03] |
| Analysis 2.7  Comparison 2 Exercise versus control follow‐up, Outcome 7 Anxiety. | ||||
| 8 Self esteem Show forest plot | 2 | 201 | Mean Difference (IV, Random, 95% CI) | 1.20 [‐0.41, 2.81] |
| Analysis 2.8  Comparison 2 Exercise versus control follow‐up, Outcome 8 Self esteem. | ||||
| 9 Endocrine symptoms Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 2.9  Comparison 2 Exercise versus control follow‐up, Outcome 9 Endocrine symptoms. | ||||
| 10 Neuropathy symptoms Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 2.10  Comparison 2 Exercise versus control follow‐up, Outcome 10 Neuropathy symptoms. | ||||
| 11 Gait and balance Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 2.11  Comparison 2 Exercise versus control follow‐up, Outcome 11 Gait and balance. | ||||
| 12 Lymphoedema incidence Show forest plot | 2 | 194 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.37, 1.69] |
| Analysis 2.12  Comparison 2 Exercise versus control follow‐up, Outcome 12 Lymphoedema incidence. | ||||
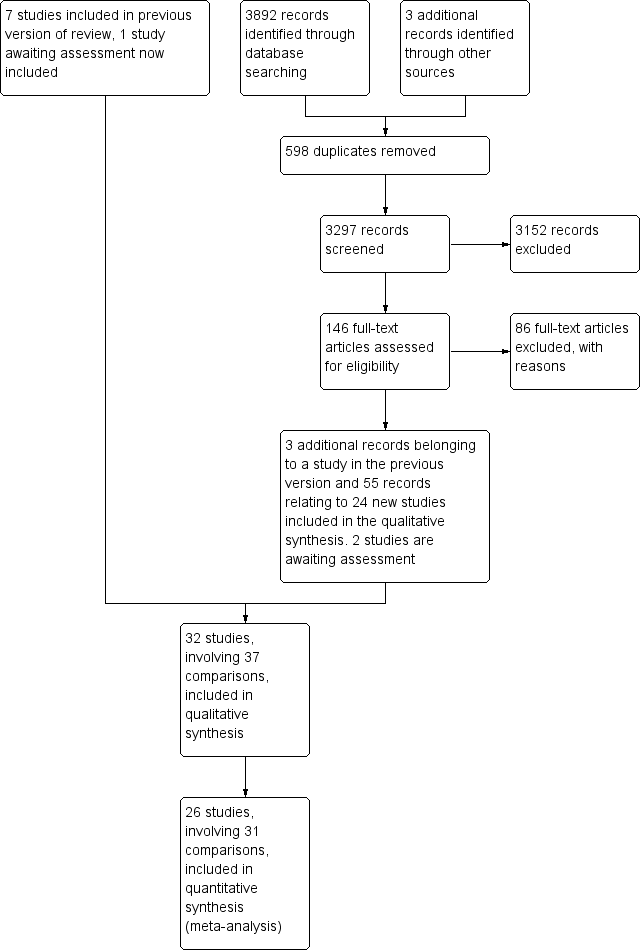
Study flow diagram.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
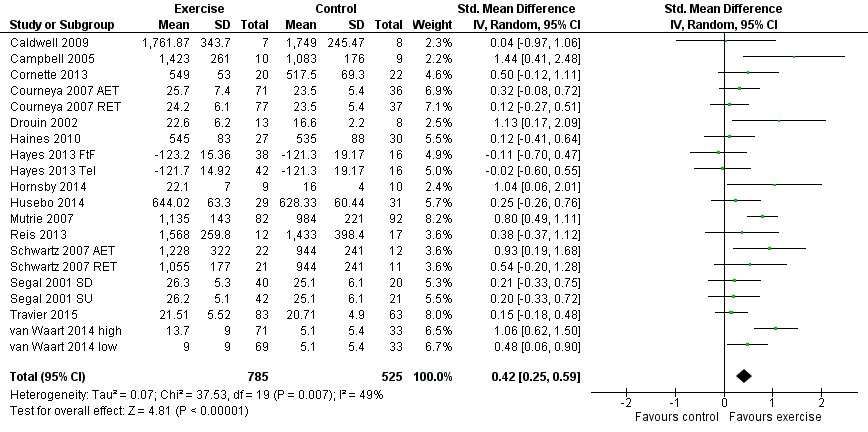
Forest plot of comparison: 1 Exercise versus control, outcome: 1.1 Physical fitness.

Forest plot of comparison: 1 Exercise versus control, outcome: 1.2 Fatigue.

Forest plot of comparison: 1 Exercise versus control, outcome: 1.3 Cancer‐specific quality of life.

Forest plot of comparison: 1 Exercise versus control, outcome: 1.6 Depression.

Comparison 1 Exercise versus control, Outcome 1 Physical fitness.
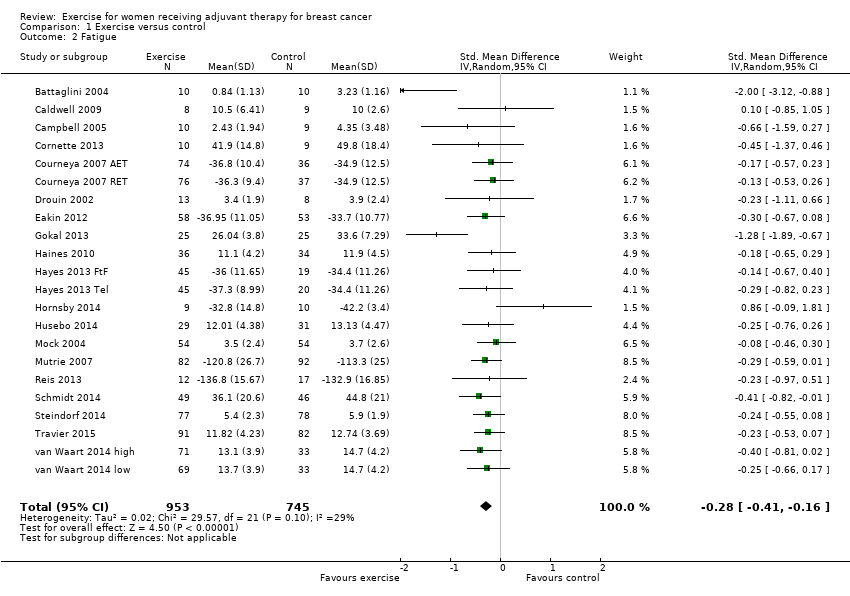
Comparison 1 Exercise versus control, Outcome 2 Fatigue.
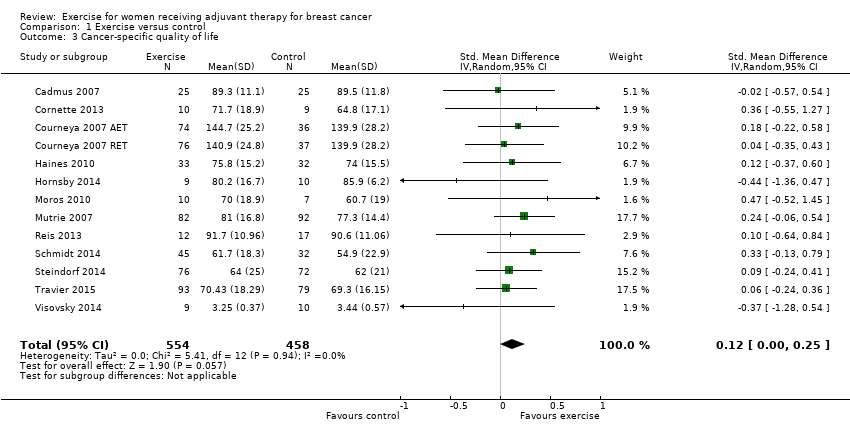
Comparison 1 Exercise versus control, Outcome 3 Cancer‐specific quality of life.
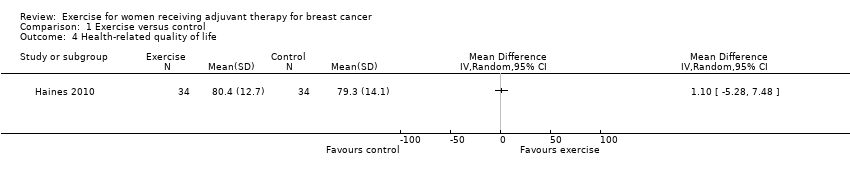
Comparison 1 Exercise versus control, Outcome 4 Health‐related quality of life.

Comparison 1 Exercise versus control, Outcome 5 Cancer site‐specific quality of life.

Comparison 1 Exercise versus control, Outcome 6 Depression.

Comparison 1 Exercise versus control, Outcome 7 Cognitive function.

Comparison 1 Exercise versus control, Outcome 8 Strength.
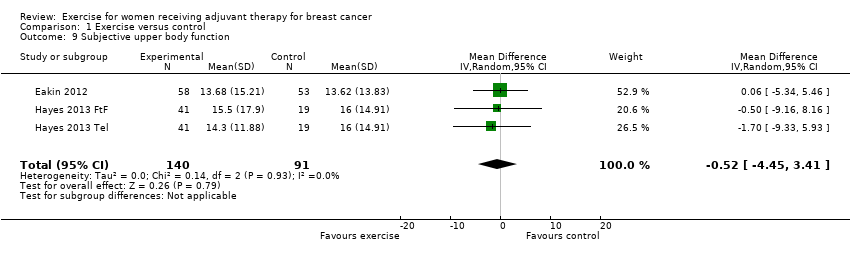
Comparison 1 Exercise versus control, Outcome 9 Subjective upper body function.

Comparison 1 Exercise versus control, Outcome 10 Shoulder mobility.

Comparison 1 Exercise versus control, Outcome 11 Arm morbidity.

Comparison 1 Exercise versus control, Outcome 12 Anxiety.

Comparison 1 Exercise versus control, Outcome 13 Mood disturbances.
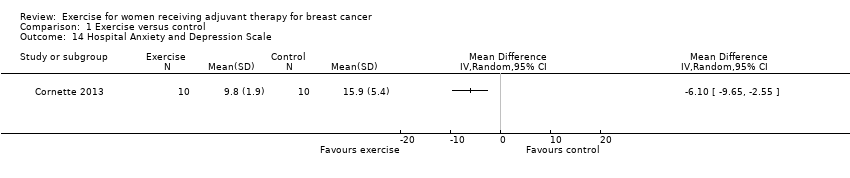
Comparison 1 Exercise versus control, Outcome 14 Hospital Anxiety and Depression Scale.
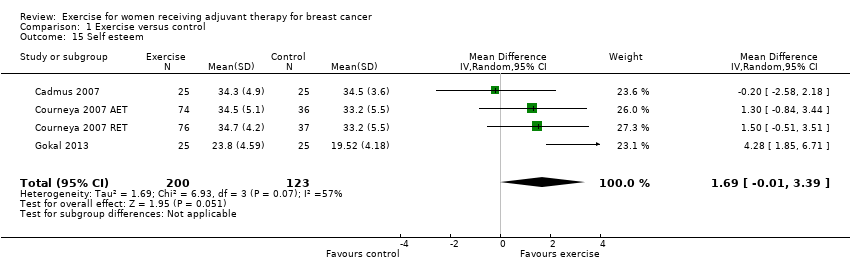
Comparison 1 Exercise versus control, Outcome 15 Self esteem.

Comparison 1 Exercise versus control, Outcome 16 Physical activity.

Comparison 1 Exercise versus control, Outcome 17 Neuropathic pain.

Comparison 1 Exercise versus control, Outcome 18 Neuropathy symptoms.
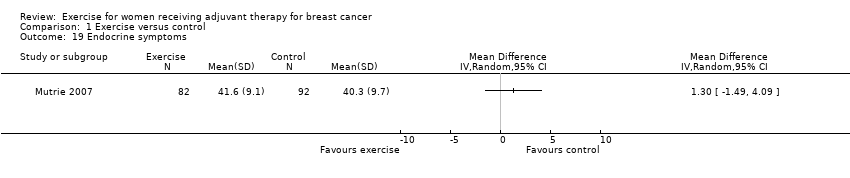
Comparison 1 Exercise versus control, Outcome 19 Endocrine symptoms.

Comparison 1 Exercise versus control, Outcome 20 Gait and balance.
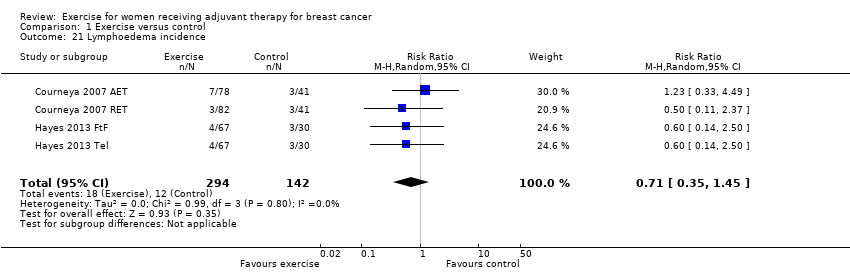
Comparison 1 Exercise versus control, Outcome 21 Lymphoedema incidence.

Comparison 2 Exercise versus control follow‐up, Outcome 1 Physical fitness.
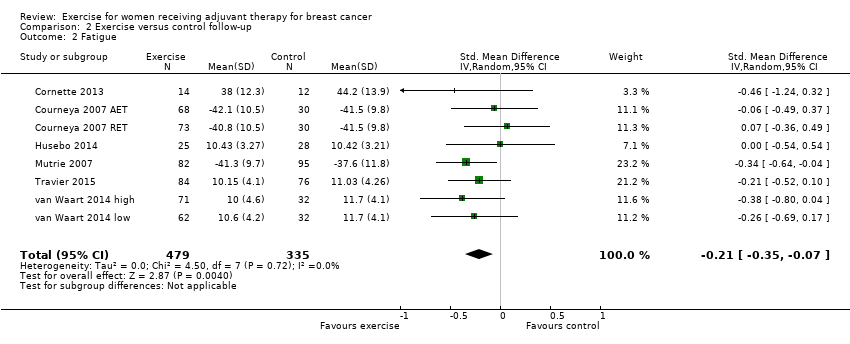
Comparison 2 Exercise versus control follow‐up, Outcome 2 Fatigue.

Comparison 2 Exercise versus control follow‐up, Outcome 3 Cancer‐specific quality of life.
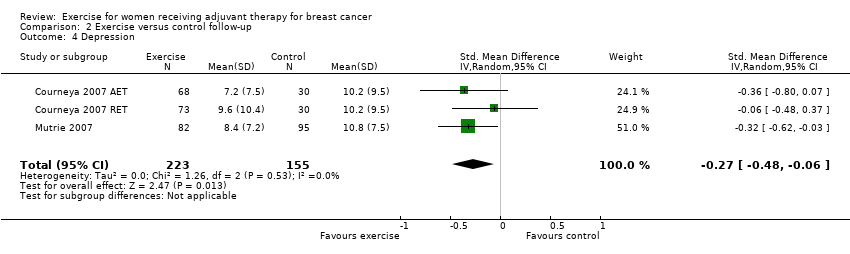
Comparison 2 Exercise versus control follow‐up, Outcome 4 Depression.
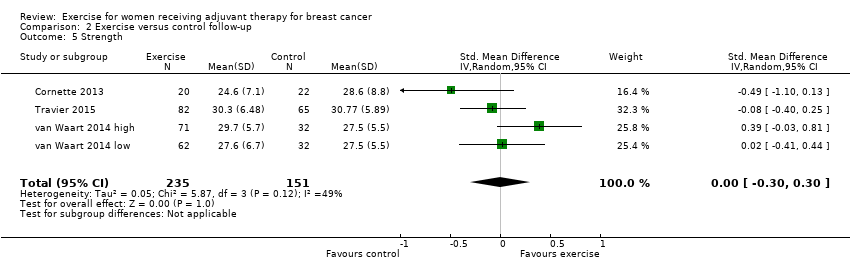
Comparison 2 Exercise versus control follow‐up, Outcome 5 Strength.
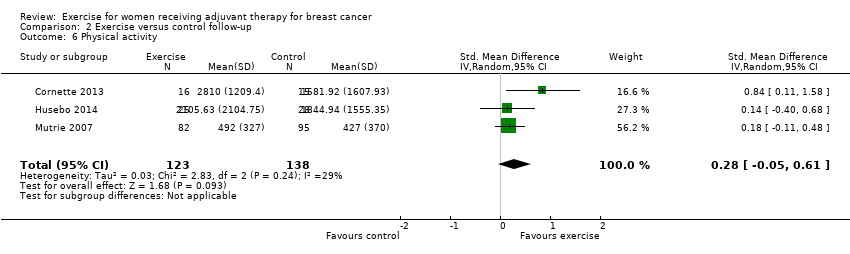
Comparison 2 Exercise versus control follow‐up, Outcome 6 Physical activity.

Comparison 2 Exercise versus control follow‐up, Outcome 7 Anxiety.

Comparison 2 Exercise versus control follow‐up, Outcome 8 Self esteem.

Comparison 2 Exercise versus control follow‐up, Outcome 9 Endocrine symptoms.

Comparison 2 Exercise versus control follow‐up, Outcome 10 Neuropathy symptoms.
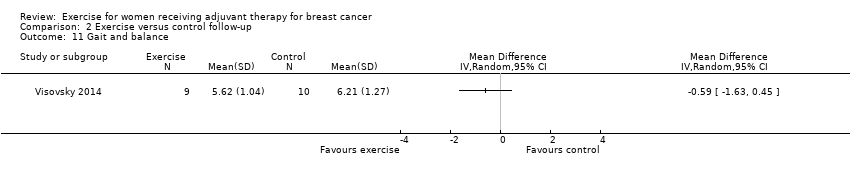
Comparison 2 Exercise versus control follow‐up, Outcome 11 Gait and balance.
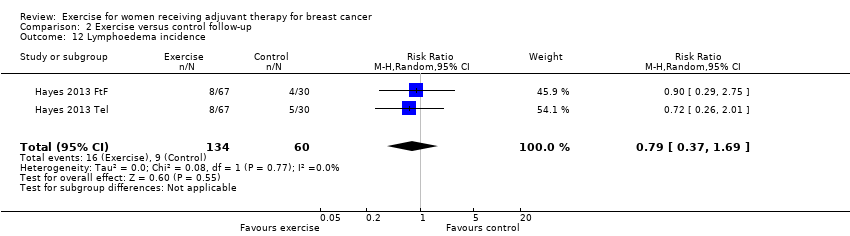
Comparison 2 Exercise versus control follow‐up, Outcome 12 Lymphoedema incidence.
| Exercise compared with control for women receiving adjuvant therapy for breast cancer | ||||
| Population: women receiving adjuvant therapy (chemo‐ or radiotherapy or both) for breast cancer Settings: supervised or home based Intervention: aerobic or resistance exercise or a combination of both Comparison: control intervention (usual care or intervention that was not exercise, such as stretching) | ||||
| Outcomes | Relative effects* (95% CI) | No of Participants | Quality of the evidence | Comments |
| Exercise vs control | ||||
| Physical fitness assessed with: 6‐ or 12‐minute walk test, peak oxygen uptake, and other scales (follow‐up: 18 weeks to 6 months) | The mean physical fitness in the intervention group was 0.42 standard deviations higher (0.25 to 0.59 higher) | 1310 (15 RCTs) | ⊕⊕⊕⊝ | SMD 0.42 (95% CI 0.25 to 0.59) |
| Fatigue assessed with: FACIT‐F scale, (revised) Piper Fatigue Scale, Multidimensional Fatigue Inventory and other scales (follow‐up: 18 weeks to 6 months) | The mean fatigue in the intervention group was 0.28 standard deviations lower (0.41 lower to 0.16 lower) | 1698 (19 RCTs) | ⊕⊕⊕⊝ | SMD ‐0.28 (95% CI ‐0.41 to ‐0.16) |
| Cancer‐specific quality of life assessed with: FACT‐G, EORTC QLQ‐C30 and other scales (follow‐up: 12 weeks to 6 months) | The mean cancer‐specific quality of life in the intervention group was 0.12 standard deviations higher (0.00 to 0.25 higher) | 1012 (12 RCTs) | ⊕⊕⊕⊝ | SMD 0.12 (95% CI 0.00 to 0.25) |
| Health‐related quality of life assessed with EQ‐5D visual analogue scale (higher scores indicate higher quality of life, score range from 0 to 100) MID: 7 points (follow‐up: end of intervention) | The mean health‐related quality of life in the intervention group was 1.10 points higher (5.28 lower to 7.48 higher) | 68 (1 RCT) | ⊕⊕⊝⊝ | MD 1.10 (95% CI ‐5.28 to 7.48) |
| Cancer site‐specific quality of life assessed with: FACT‐B (higher scores indicate better quality of life, score range from 0 to 144) MID: 7 to 8 points (follow‐up: end of intervention) | The mean cancer site‐specific quality of life in the intervention group was 4.24 points higher (1.81 lower to 10.29 points higher) | 262 (4 RCTs) | ⊕⊕⊝⊝ | MD 4.24 (95% CI ‐1.81 to 10.29) |
| Depression assessed with: BDI, CES‐D (follow‐up: 6 months) | The mean depression in the intervention group was 0.15 standard deviations lower (0.30 lower to 0.01 higher) | 674 (5 RCTs) | ⊕⊕⊕⊝ | SMD ‐0.15 (95% CI ‐0.30 to 0.01) |
| Cognitive function assessed with: Trail Making Test (less time in seconds needed for completing the test means less cognitive dysfunction) (follow‐up: end of intervention) | The mean time needed for completing the test in the intervention group was 11.55 seconds less (22.06 seconds less to 1.05 seconds less) | 213 (2 RCTs) | ⊕⊕⊝⊝ | MD ‐11.55 (95% CI ‐22.06 to ‐1.05) |
| Lymphoedema assessed with: volumetric arm measurements and bioimpedance spectroscopy (follow‐up: 8 weeks) | Assumed risk11: 60 per 1000 (30 to 123) | 436 (2 RCTs) | ⊕⊕⊝⊝ | RR 0.71 (95% CI 0.35 to 1.45) |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||
| GRADE Working Group grades of evidence | ||||
| 1Lack of blinding, low adherence and high or unclear contamination, several randomisation and many allocation concealment procedures were unclear, therefore we downgraded by one level. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Physical fitness Show forest plot | 20 | 1310 | Std. Mean Difference (IV, Random, 95% CI) | 0.42 [0.25, 0.59] |
| 2 Fatigue Show forest plot | 22 | 1698 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.28 [‐0.41, ‐0.16] |
| 3 Cancer‐specific quality of life Show forest plot | 13 | 1012 | Std. Mean Difference (IV, Random, 95% CI) | 0.12 [‐0.00, 0.25] |
| 4 Health‐related quality of life Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 5 Cancer site‐specific quality of life Show forest plot | 4 | 262 | Mean Difference (IV, Random, 95% CI) | 4.24 [‐1.81, 10.29] |
| 6 Depression Show forest plot | 6 | 674 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.15 [‐0.30, 0.01] |
| 7 Cognitive function Show forest plot | 2 | 213 | Mean Difference (IV, Random, 95% CI) | ‐11.55 [‐22.06, ‐1.05] |
| 8 Strength Show forest plot | 13 | 912 | Std. Mean Difference (IV, Random, 95% CI) | 0.27 [0.04, 0.50] |
| 9 Subjective upper body function Show forest plot | 3 | 231 | Mean Difference (IV, Random, 95% CI) | ‐0.52 [‐4.45, 3.41] |
| 10 Shoulder mobility Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 11 Arm morbidity Show forest plot | 3 | 240 | Mean Difference (IV, Random, 95% CI) | 1.11 [‐4.07, 6.29] |
| 12 Anxiety Show forest plot | 3 | 331 | Mean Difference (IV, Random, 95% CI) | ‐1.45 [‐4.36, 1.46] |
| 13 Mood disturbances Show forest plot | 3 | 111 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.00 [‐1.40, ‐0.60] |
| 14 Hospital Anxiety and Depression Scale Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 15 Self esteem Show forest plot | 4 | 323 | Mean Difference (IV, Random, 95% CI) | 1.69 [‐0.01, 3.39] |
| 16 Physical activity Show forest plot | 8 | 549 | Std. Mean Difference (IV, Random, 95% CI) | 0.29 [0.12, 0.47] |
| 17 Neuropathic pain Show forest plot | 2 | 130 | Mean Difference (IV, Random, 95% CI) | 3.64 [‐1.32, 8.60] |
| 18 Neuropathy symptoms Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 19 Endocrine symptoms Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 20 Gait and balance Show forest plot | 3 | 122 | Std. Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.25, 0.46] |
| 21 Lymphoedema incidence Show forest plot | 4 | 436 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.35, 1.45] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Physical fitness Show forest plot | 6 | 612 | Std. Mean Difference (IV, Random, 95% CI) | 0.26 [‐0.06, 0.57] |
| 2 Fatigue Show forest plot | 8 | 814 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.21 [‐0.35, ‐0.07] |
| 3 Cancer‐specific quality of life Show forest plot | 6 | 583 | Std. Mean Difference (IV, Random, 95% CI) | 0.18 [0.01, 0.35] |
| 4 Depression Show forest plot | 3 | 378 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.27 [‐0.48, ‐0.06] |
| 5 Strength Show forest plot | 4 | 386 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.00 [‐0.30, 0.30] |
| 6 Physical activity Show forest plot | 3 | 261 | Std. Mean Difference (IV, Random, 95% CI) | 0.28 [‐0.05, 0.61] |
| 7 Anxiety Show forest plot | 2 | 201 | Mean Difference (IV, Random, 95% CI) | ‐3.61 [‐7.24, 0.03] |
| 8 Self esteem Show forest plot | 2 | 201 | Mean Difference (IV, Random, 95% CI) | 1.20 [‐0.41, 2.81] |
| 9 Endocrine symptoms Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 10 Neuropathy symptoms Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 11 Gait and balance Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 12 Lymphoedema incidence Show forest plot | 2 | 194 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.37, 1.69] |

