Contenido relacionado
Revisiones y protocolos relacionados
Alina Andras, Adriano Sala Tenna, Marlene Stewart | 24 julio 2017
Anne WS Rutjes, Ettore Porreca, Matteo Candeloro, Emanuele Valeriani, Marcello Di Nisio | 18 diciembre 2020
Aniek AG Zee, Kelly van Lieshout, Maaike van der Heide, Loes Janssen, Heinrich MJ Janzing | 6 agosto 2017
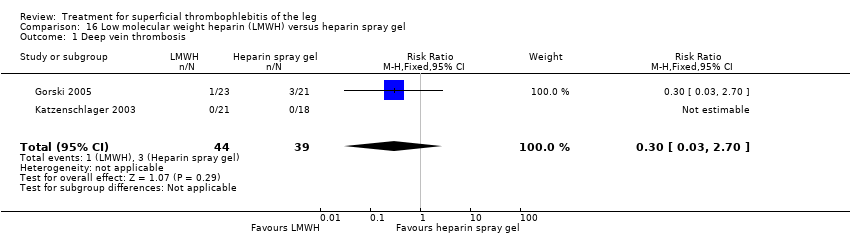
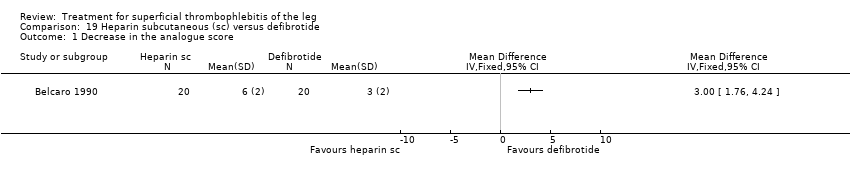
David K Cundiff, Juliet Manyemba, John C Pezzullo | 25 enero 2006
Meixuan Li, Jing Li, Xiaoqin Wang, Xu Hui, Qi Wang, Shitong Xie, Peijing Yan, Jinhui Tian, Jianfeng Li, Ping Xie, Kehu Yang, Liang Yao | 14 abril 2023
Sherab Bhutia, Peng F Wong | 16 julio 2013
Kezhou Dong, Yanzhi Song, Xiaodong Li, Jie Ding, Zhiyong Gao, Daopei Lu, Yimin Zhu | 31 octubre 2016
Carlos A Salazar, German Malaga, Giuliana Malasquez | 14 abril 2010
Marie-Claude Pelland-Marcotte, Nour Amiri, Maria L Avila, Leonardo R Brandão | 18 junio 2020
Raza Alikhan, Rachel Forster, Alexander T Cohen | 7 mayo 2014