Probiotics for the prevention of pediatric antibiotic‐associated diarrhea
Referencias
References to studies included in this review
References to studies excluded from this review
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | Randomized, placebo‐controlled, double‐blinded. | |
| Participants | N = 167 enrolled | |
| Interventions | Probiotics: Lactobacillus GG (4 billion CFU/day orally over two weeks) | |
| Outcomes | ID (treatment 5% versus placebo 16%) | |
| Notes | Funding = Not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) | Low risk | Lactobacillus GG and placebo capsules were indistinguishable in appearance and taste. |
| Incomplete outcome data (attrition bias) | High risk | Withdrawals/loss to follow‐up: 48 participants (28.7%) |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
| Methods | Randomized, active‐controlled, double‐blinded. | |
| Participants | N = 779 enrolled | |
| Interventions | Probiotic: SB (4.5 billion CFU/day) | |
| Outcomes | ID (treatment 7.6%, diosmectite 5.5%) | |
| Notes | Funding = Not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Mentioned randomization, otherwise not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) | Unclear risk | Described as “double blind” without further details |
| Incomplete outcome data (attrition bias) | High risk | Withdrawals/loss to follow‐up: 163 participants (21%). The authors do not describe what happened to these patients |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported |
| Other bias | Unclear risk | No funding from industry or other sources mentioned |
| Methods | Randomized, placebo‐ controlled. | |
| Participants | N = 40 enrolled | |
| Interventions | Probiotics: LA, BB (3 billion CFU/day for 10 days) | |
| Outcomes | Mean number of stools (2 treatment versus 2.7 placebo (P < 0.0001)) | |
| Notes | Funding = Not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Manuscript stated, "suddivisi a randon [divided at random]", otherwise not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) | High risk | Blinding not used. |
| Incomplete outcome data (attrition bias) | Low risk | All participants completed the study |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
| Methods | Randomised, controlled trial (3 arms), double‐blind Withdrawals/ losses to follow‐up: 0 (data provided by authors) ITT: yes, but NA (obtained pediatric data from authors) Period of Follow‐up: 12 days | |
| Participants | N = 106 Diagnosis: NS Country: England Setting: rural general practice Age: 1 to 17 years inclusive | |
| Interventions | Probiotics: ST, LA, BA, LD (1 billion CFU bacteria/day). Antibiotics: NS | |
| Outcomes | ID (treatment 10.8% versus control 6.3%) Definition of diarrhea: 3 or more loose or liquid stools on at least 2 consecutive days | |
| Notes | Funding: Industry (medications) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table |
| Allocation concealment (selection bias) | Low risk | Sealed opaque envelopes |
| Blinding (performance bias and detection bias) | High risk | Blinding for the two groups allocated to yoghurts. Third group not blinded. To avoid unit of analysis errors, we combined the yogurt groups and compared against the third group (no treatment control). Given our analysis technique, will consider un‐blinded |
| Incomplete outcome data (attrition bias) | Unclear risk | Overall, 38 patients were LTFU from the adults and child data combined (n = 12, n = 9, n = 17). It is unclear how many children specifically were lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported |
| Other bias | Low risk | Acknowledged by authors: Imbalance for previous AAD might have distorted main outcome results |
| Methods | Randomized, formula‐controlled, double‐blinded. | |
| Participants | N = 169 enrolled | |
| Interventions | Probiotic: BL, ST (approximately 825 million CFU/day) | |
| Outcomes | ID (treatment 16.3% versus control 31.2%) | |
| Notes | Funding = Industry (Nestle, otherwise unclear re: medications versus operations) and independent | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) | Low risk | Double‐blind: The appearance and odour of the probiotic and nonsupplemented formulas were identical. |
| Incomplete outcome data (attrition bias) | Low risk | 12 patients dropped out (<10% and relatively even for each group). 7 from probiotic 5 from control. Reasons why are given. However the reasons given were not evenly distributed. Control lost 4 from loss to follow‐up while probiotic lost none for that reason. Probiotic lost 5 from insufficient ingestion and control lost none for that reason. However, the minimum amount needed for ingestion was described seemingly a priori. |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported |
| Other bias | Unclear risk | Nestle the maker of the probiotic intervention provided some funding. The report is not co‐authored by the company, however there is no clear mention of Nestle's involvement beyond that of providing the product |
| Methods | Randomized, no intervention controlled, open label trial Withdrawals/loss to follow‐up: 0 (data provided by authors) ITT: N/A Period of follow‐up: until end of antibiotic therapy (7 to 21 days) | |
| Participants | N = 323 Diagnosis: respiratory, genito‐urinary, skin and soft tissue infections Country: the Philippines Setting: hospital general care (inpatient and outpatient) Age: treatment 4.1 years and control 4 years (means) | |
| Interventions | Probiotics: BC (4 billion CFU bacteria/day) Antibiotics: Penicillins n = 151, cephalosporin n = 112, coamoxyclav/ampicillin‐sulbactam n = 25, other n = 35 | |
| Outcomes | ID: 1.85% treatment versus 4.35% control MDD: 4.00 (SD 3.46) treatment versus 3.86 (SD 2.26) control Definition of diarrhea: change in bowel habits with the passage of three or more liquid stools per day for at least 2 consecutive days 48 hours after initiation of antibiotic therapy | |
| Notes | Funding: Industry (otherwise unclear re: medications versus operations) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Complete blocks of varying sizes were randomly allocated by a "third party" through a central telephone randomization system |
| Allocation concealment (selection bias) | Unclear risk | “Complete blocks of varying sizes were randomly allocated by a "third party" through a central telephone randomization system.“ “Each patient was identified using a center number, a treatment number (provided by the treatment code found in the intervention drug label) and the patient's initials.” “ a research assistant assigned per center kept the randomization plan and only opened it when an eligible patient was entered in the study" |
| Blinding (performance bias and detection bias) | High risk | Blinding not used ‐ open label study |
| Incomplete outcome data (attrition bias) | Low risk | Two patients were lost to follow‐up (1 in each arm) after clinical outcomes were measured. So there was no missing outcome data. |
| Selective reporting (reporting bias) | Low risk | Protocol posted on clinicaltrials.gov and results as presented to us by authors match up |
| Other bias | Low risk | Study funded by industry. Not clear if author is employed by industry but assume so. Also no clear statement regarding industry involvement is trial design. The study appears to be free of other sources of bias |
| Methods | Randomized, no treatment controlled. | |
| Participants | N = 653 enrolled | |
| Interventions | Probiotic: SB (5 billion CFU/day) | |
| Outcomes | ID (treatment 5.7% versus control 18.9%) | |
| Notes | Funding = Not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization not described, however, contact with authors indicated that the trial was randomized |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) | Unclear risk | No mention is made of blinding |
| Incomplete outcome data (attrition bias) | High risk | Withdrawals/loss to follow‐up: 187 participants (28.6%). There is no mention of which proportion of drop outs were from each group. |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported |
| Other bias | Unclear risk | No mention of funding |
| Methods | Randomized, placebo‐controlled, double‐blinded | |
| Participants | N = 72 Diagnosis: otitis, pharyngitis, chest infections, other Country: Australia Setting: multisite general care Age: Mean age 6.8 years treatment group, 6.3 years control group | |
| Interventions | Probiotic: 2 x 100 gram tubs per day containing; LGG 5.2×109 CFU/day, Bb‐12 5.9×109 CFU/day, La‐5 8.3×109 CFU/day Antibiotics: Beta lactams n = 64 Macrolides n = 5 Tetracyclines n = 1 | |
| Outcomes | ID: 1/34 (2.9%) treatment group vs 21/36 (61.7%) control. P‐value = < 0.001 Various definitions of diarrhea. These included: (A) stool consistency ≥ 5 and stool frequency ≥ 2/day for more than 2 days; (B) stool consistency ≥ 5 and stool frequency ≥ 3/day for more than 2 days; (C) stool consistency ≥ 6 and stool frequency ≥ 2/day for more than 2 days; and (D) stool consistency ≥ 6 and stool frequency ≥ 3/day for more than 2 days | |
| Notes | Funding = Industry provided yogurt but had no input in study design Independent lab assessed the probiotics | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “A statistician generated independent allocation sequences and randomisation lists for each study site, using the random number generator in Microsoft Excel” |
| Allocation concealment (selection bias) | Low risk | “To ensure allocation concealment, an independent person oversaw packaging and labelling of trial treatments based on the randomisation schedule” |
| Blinding (performance bias and detection bias) | Low risk | “All investigators, participants, outcome assessors and data analysts were blinded to the assigned treatment throughout the study” Patients/parents recorded diarrhea events and AE in diary Participants and parents were blind |
| Incomplete outcome data (attrition bias) | Low risk | Two out of 72 randomized patients were lost to follow‐up It was not clear from which group they were. However the LTFU number was small and the event spread large LTFU would not significantly affect the composite AE outcome |
| Selective reporting (reporting bias) | Low risk | Trial was prospectively registered The outcomes listed of stool frequency and consistency are compatible with reported outcomes |
| Other bias | Low risk | The study was supported by Parmalat Australia who had no role in the formulation or conduct of the study or in the data analysis or interpretation |
| Methods | Randomized, double‐blind trial Withdrawals/Loss to follow‐up: 3 (3%) ITT: No Period of follow‐up: 21 days following end of antibiotic treatment | |
| Participants | N= 100 Diagnosis: Infections of respiratory, gastrointestinal, pancreas, eyes, ears nose, throat, urinary tracts or systems Country: Bulgaria Setting: hospital admitted patients Age: 3‐12 mean 8.85 | |
| Interventions | Probiotics: 100 million CFU per day Lactobacillus reuteri DSM 17938 Antibiotics: NS, 1‐3 | |
| Outcomes | ID: Control 1 versus Treatment 1 Definition of diarrhea: An episode of diarrhoea was defined as three or more (≥3) soft and unformed or watery bowel movements per day for at least 48 hours. | |
| Notes | Funding: NS | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Computer generated randomization list of case numbers” |
| Allocation concealment (selection bias) | Low risk | Participants entered consecutively starting with the lowest case number in each stratum Randomisation and labelling of the test‐samples were made by an independent physician |
| Blinding (performance bias and detection bias) | Low risk | Study described as double blind Diarrhea‐diary/ and Bristol scale filled out by parents/children both of whom were blind AE‐ It appears GSRS symptom score filled out by parents/children or study physicians both of whom were blind |
| Incomplete outcome data (attrition bias) | Unclear risk | Diarrhea – 3% missing outcome data It is unclear which group they were from. While the total number missing is low the total number of diarrhea events was also low. The missing outcome data could bias the results in a material way. |
| Selective reporting (reporting bias) | Low risk | Manuscript outcomes the same as a priori listed in clinicaltrials.gov |
| Other bias | Unclear risk | This is an unpublished trial. Funding was not reported and they did not respond to email inquiry |
| Methods | Randomized, placebo‐controlled, double‐blinded. | |
| Participants | N = 18 enrolled | |
| Interventions | Probiotics: LA, BI (1 capsule orally TID for 7 days, 6 billion CFU per day), | |
| Outcomes | ID (treatment 37.5% versus placebo 80%) | |
| Notes | Funding = Not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Based on a randomization list. Unclear how that was generated |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) | Unclear risk | Described as “double blind” without further details |
| Incomplete outcome data (attrition bias) | Unclear risk | There were no mentions of drop outs. There was mention of 3 cases of sepsis. There was also mention that cases where probiotics sepsis was possible would result in unblinding although it wasn't clear if those three were unblinded. There was no statistical analysis as well. |
| Selective reporting (reporting bias) | Unclear risk | There is no definition mentioned of diarrhoea. In the methods section they mentioned the “characteristics and frequency” of stools would be observed. In the results section the number of patients with diarrhoea and days of diarrhoea were noted. It is unclear what characteristics means and why they weren't reported. |
| Other bias | Unclear risk | No mention of funding source |
| Methods | Randomized, placebo‐contolled study, double‐blinded Withdrawals/Loss‐to‐follow‐up: 0 (0%) ITT: Not needed Period of follow‐up: 7 days (duration of antibiotics and probiotics) | |
| Participants | N = 66 Diagnosis: H.pylori Country: Iran Setting: multiple, children's medical center Age: range 3 to 14 years mean 9.09 years | |
| Interventions | Probiotics: 1 billion CFU/1 sachet per day of combination of following species: Lactobacillus acidophilus, Lactobacillus rhamnosus, Lactobacillus bulgaricus, Lactobacillus casei, Streptococcus thermophilus, Bifidobacterium infantis and Bifidobacterium breve Antibiotics: Oral amoxicillin 50 mg/kg/day twice daily; oral furazolidone 6 mg/kg/day twice daily, oral omeprazole 1 mg/kg/day (duration: 4 weeks) | |
| Outcomes | ID: Control 8 (24.24%) versus Treatment 2 (6.06%) Definition of diarrhea: NS | |
| Notes | Funding: NS | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “Randomized," however researchers did not explain further |
| Allocation concealment (selection bias) | Unclear risk | Not enough information provided |
| Blinding (performance bias and detection bias) | Low risk | Diarrhea and other AE were reported by parents and patients both of whom were blinded |
| Incomplete outcome data (attrition bias) | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | Outcomes reported in line with outcomes set a priori on register |
| Other bias | Low risk | Based on registry info it is sponsored by the university of Tehran |
| Methods | Randomized, placebo‐controlled, double‐blinded. | |
| Participants | N = 269 enrolled | |
| Interventions | Probiotic: SB (10 billion CFU/day for duration of antibiotic treatment [range 7 to 9 days] | |
| Outcomes | ID (treatment 7.5% versus placebo 23%) | |
| Notes | Funding = Not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated |
| Allocation concealment (selection bias) | Low risk | To ensure allocation concealment, an independent subject prepared the randomization schedule and oversaw the packaging and labelling of trial treatments |
| Blinding (performance bias and detection bias) | Low risk | Double‐blind: All investigators, participants, outcome assessors and data analysts were blinded to the assigned treatment throughout the study. |
| Incomplete outcome data (attrition bias) | Low risk | <10% dropout/lost to follow‐up. Dropouts balanced in numbers across intervention groups with similar reasons for missing data across groups. Additionally the authors conducted extreme case scenarios. |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported |
| Other bias | Unclear risk | No mention of funding |
| Methods | Randomized, placebo‐controlled, double‐blinded. | |
| Participants | N = 120 enrolled | |
| Interventions | Probiotic: LS (5.5 billion CFU/day) with Prebiotic: FOS (250 mg/d) for 10 days) | |
| Outcomes | ID (treatment 29% versus placebo 62%) | |
| Notes | Funding = Not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated |
| Allocation concealment (selection bias) | Low risk | Each patient was given a code. The treatment package corresponded with the code |
| Blinding (performance bias and detection bias) | Low risk | Double‐blind, identical placebo |
| Incomplete outcome data (attrition bias) | Low risk | Dropouts balanced in numbers across intervention groups with similar reasons for missing data across groups |
| Selective reporting (reporting bias) | High risk | Methods section indicate “condizioni generale” [general condition], but outcome not reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
| Methods | Randomized, placebo controlled, double‐blinded Withdrawal/loss to follow‐up: 8 participants (6.4%) ITT: no Period of follow‐up: 15 days | |
| Participants | N = 125 Diagnosis: URI Country: USA Setting: primary care office Age: 2.9 years treatment and 3.2 years control | |
| Interventions | Probiotics: LL, LP, LR, LC, LL subspecies diacetylactis, Leuconostoc cremoris, Bifidobacterium longum, BB, LA, SF (at least half of a 150 ml drink containing 7 to 10 billion CFU bacteria and yeast/day) Antibiotics: NS | |
| Outcomes | ID: 18.0% treatment versus 21.9% control Definition of diarrhea: NS | |
| Notes | Funding: Industry (medication and operations) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The randomization scheme was generated using permuted blocks with block size equal to 8 |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) | Low risk | Double blind: “All research personnel and statisticians were blinded throughout the study, including during initial review of data." A matching placebo was used |
| Incomplete outcome data (attrition bias) | Low risk | “Loss to follow‐up was exceptionally low. Only 4 participants in each group were unable to be contacted at the final follow‐up on day 15.” |
| Selective reporting (reporting bias) | Low risk | Outcomes identical to that reported in clinicaltrials.gov |
| Other bias | Low risk | Lifeway foods provided drink and funding although no author was associated with the company |
| Methods | Randomized, placebo‐controlled, double blind Withdrawals/loss to follow‐up: 0 ITT: yes Period of follow‐up: two weeks following end of antibiotic treatment | |
| Participants | N = 240 Diagnosis: Otitis, URT, LRT, UTI, other Country: Poland Setting: Two hospitals and one private practice Age: treatment 4.6 years and control 4.5 years | |
| Interventions | Probiotics: Lactobacillus Rhamosus (strains E ⁄ N, Oxy and Pen) (40 billion CFU bacteria/day) Antibiotics: penicillins = 15, broad spectrum penicillins = 119, cephalosporins = 89, macrolides = 15, clindamycin = 2 | |
| Outcomes | ID: (treatment 7.5% versus control 16.7%) Definition of diarrhea: greater than or equal to 3 loose stools per day for a minimum of 48 hours, occurring during and/or up to two weeks after the end of the antibiotic therapy | |
| Notes | Funding: Industry (otherwise unclear re: medications versus operations) and Independent (Medical University of Warsaw) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated: Permuted block of six (three received placebo and three, active treatment). Separate randomization lists were prepared for each site. |
| Allocation concealment (selection bias) | Low risk | To ensure allocation concealment, an independent subject prepared the randomization schedule and oversaw the packaging and labelling of trial treatments |
| Blinding (performance bias and detection bias) | Low risk | All investigators, participants, outcome assessors and data analysts were blinded to the assigned treatment throughout the study |
| Incomplete outcome data (attrition bias) | Low risk | Overall, three of the randomized children (one in the probiotic group and two in the placebo group) discontinued the study intervention and started to use one of the commercially available probiotics products. However, no patient was lost to follow‐up. |
| Selective reporting (reporting bias) | Low risk | All primary and secondary outcomes as reported in the methods section were reported on in the results section. |
| Other bias | Low risk | Biomed provided the intervention but they “had no role in the conception, design, or conduct of the study or in the analysis or interpretation of the data" |
| Methods | Randomized, placebo‐controlled, patient blinded Withdrawals/Loss to follow‐up: None ITT: None needed Period of follow‐up: NS | |
| Participants | N=50 Diagnosis: H.pylori Country: Iran Setting: Community healthcare Age: 4‐14 mean 8.2 treatment group, 9.5 control group | |
| Interventions | Probiotics: One sachet per day of 1 billion CFU combined of following species: Lactobasillus casei, Lactobacillus acidophilus, Lactobasillus reuteri, Lactobasillus bulgaricus, Streptococcus, Bifidobacterium bifidum, Bifidobacterium infantis Omeprazole 0.5 mg/kg BID (no max dose listed) | |
| Outcomes | ID: 13 Control versus 3 Treatment Definition of diarrhea: 3 times excretion per day or more, if it is loose or watery for at least 48 hours during the therapy or two weeks after the antibiotic therapy | |
| Notes | Funding: grant from university, other sources NS | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random sequence number table (random number generating) |
| Allocation concealment (selection bias) | Unclear risk | Nothing mentioned |
| Blinding (performance bias and detection bias) | Unclear risk | Sachets (of probiotic and placebo) look the same. Nothing else listed about blinding |
| Incomplete outcome data (attrition bias) | Low risk | All participants completed the study |
| Selective reporting (reporting bias) | Unclear risk | All outcomes listed in methods are reported in results. No registered protocol could be found |
| Other bias | Unclear risk | No funding from industry or other sources mentioned |
| Methods | Randomized open trial, nested observational Withdrawals/Loss to follow‐up: 50 ITT: No Period of follow‐up: 2 weeks following end of antibiotic treatment | |
| Participants | N= 333 Diagnosis: pneumonia, asthma, lower respiratory tract infection Country: China Setting: single site hospital Age: average 48 months | |
| Interventions | Probiotics: Saccharomyces boulardii 2×250 mg (10 billion CFU/day) Antibiotics: cefepime, cefoperazone, sulbactam, cefuroxime, amoxicillin, clavulanic acid, erthromycin | |
| Outcomes | ID: Conrol 42 (29.2%) versus treatment 11 (7.9%) Definition of diarrhea: ≥3 loose or watery stools (BSS type 5, 6 and 7) per day during at least 2 days, occurring during treatment and/ or up to 2 weeks after the antibiotic therapy had stopped. AAD was defined as diarrhoea caused by C. difficile or diarrhoea with negative stool cultures | |
| Notes | Funding: NS | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Randomisation was done according to a computer‐determined allocation to group A or B” |
| Allocation concealment (selection bias) | Low risk | “The [randomization] sequence was concealed in an envelope, and the next neutral envelope was opened each time the next patient was included in the study” |
| Blinding (performance bias and detection bias) | High risk | “This study was an open, randomised, controlled clinical trial” |
| Incomplete outcome data (attrition bias) | High risk | 15% missing outcome data |
| Selective reporting (reporting bias) | Low risk | Not registered. The outcomes in the methods section match the outcomes in the results section |
| Other bias | Unclear risk | Funding source unclear. One of the authors is a consultant for a probiotics company |
| Methods | Randomized, double‐blind study Withdrawals/Loss to follow‐up: 6 ITT: Yes Period of follow‐up: 4 weeks | |
| Participants | N= 86 Diagnosis: H.pylori Country: Czech Republic Setting: Hospital general care, 3 sites Age: average 12.6 treatment, average 12.9 control | |
| Interventions | Probiotics: Lactobacillus casei DN‐114 001, A dose of 100 mL of containing 10 billion CFU/day) Antibiotics: oral amoxicillin 25 mg/kg, oral clarithromycin 7.5 mg/ kg, omeprazole 10 mg (15–30 kg) or 20 mg (30 kg) | |
| Outcomes | ID: Control 5 versus Treatment 3 Definition of diarrhea: not defined; data in adverse events | |
| Notes | Funding: Danone, Ministry of Health of Czech Republic | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Randomization was performed using a computer generated randomization list” |
| Allocation concealment (selection bias) | Low risk | “All children received their patient number in ascending order corresponding to the order of inclusion. This number corresponded to a randomized medication scheme” |
| Blinding (performance bias and detection bias) | Low risk | Double blind Diarrhea and AE reported by patients, parents, and study personnel all of whom were blinded |
| Incomplete outcome data (attrition bias) | Low risk | The numbers and reasons for withdrawal/drop‐outs were described and comparable across groups (and ≤ approximately 10%) |
| Selective reporting (reporting bias) | Low risk | Not registered. The primary outcome of interest was H pylori. However “patients and parents were asked to complete a standard questionnaire to assess the occurrence of prospectively defined adverse events.” AE which include our outcome diarrhea were identified a priori |
| Other bias | Low risk | Sponsor is acknowledged and no one from the sponsoring agency was an author |
| Methods | Randomized, placebo controlled, double blind Withdrawals/loss to follow‐up: 17 (20.9%) ITT: yes Period of follow‐up: 3 weeks (2 weeks after end of antibiotic treatment) | |
| Participants | N = 83 Diagnosis: H. pylori infection Country: Poland Setting: hospitalized/inpatients Age: 12.3 years treatment and 11.9 years control | |
| Interventions | Probiotics: Lactobacillus GG (1 billion CFU/day) Antibiotics: all patients received amoxicillin and clarithromycin (all patients also received omeprazole a proton pump inhibitor) | |
| Outcomes | ID: (6% treatment versus 20% control) Definition of diarrhea: 3 or more loose or watery stools per day for a minimum of 48 hours occurring during and/or up to 2 weeks after the end of antibiotic therapy | |
| Notes | Funding: Industry (medications) and Independent (Medical University of Warsaw) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated |
| Allocation concealment (selection bias) | Low risk | LGG and the control product were packed in identical forms. Randomization codes were secured until all of the data entry was complete |
| Blinding (performance bias and detection bias) | Low risk | All of the study personnel, patients, and personnel involved in the conduct of the study were unaware of treatment assignments throughout the study |
| Incomplete outcome data (attrition bias) | High risk | 10 drop outs versus 7 drop outs. Reasons why were given (no diary or UBT). Data was analyzed with opposite extremes of assumptions regarding those drop outs for H. Pylori but not for side effects |
| Selective reporting (reporting bias) | Low risk | All outcomes mentioned in methods section were reported on in results section |
| Other bias | Low risk | Baseline characteristics are very close. Dicofarm supplied study product but “had no role in the conception, design, or conduct of the study or in the analysis or interpretation of data” |
| Methods | Randomized, placebo controlled, double blind Withdrawal/loss to follow‐up: 0 ITT: yes Period of follow‐up: less than or equal to 4 weeks (2 weeks after end of antibiotic treatment) | |
| Participants | N = 78 Diagnosis: otitis media, respiratory tract infections, scarlet fever, other Country: Poland Setting: pediatric hospitals and outpatient clinics Age: median age 7 years (range 1 to 15 years) | |
| Interventions | Probiotics: Bifidobacterium longum PL03, LRKL53A, LP PL02 (200 million CFU bacteria/day) Antibiotics: amoxicillin w/ or w/o clavulanate = 34, cephalosporins = 20, penicillin = 5, macrolides = 18, aminoglycosides = 1 | |
| Outcomes | ID: (2.5% treatment versus 5.3% control) MSF: (1.0 +/‐ 0.4 treatment versus 1.3 +/‐ 0.6) Definition of diarrhea: 3 or more loose or watery stools per day for a minimum of 48 hrs, occurring during and/or up to 2 weeks after the end of the antibiotic therapy | |
| Notes | Funding: Industry (medications) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated |
| Allocation concealment (selection bias) | Low risk | To ensure allocation concealment, an independent person prepared the randomization schedule and oversaw the packaging and labelling of the trial treatments |
| Blinding (performance bias and detection bias) | Low risk | All study personnel and parents and guardians were unaware of the group assignments. Randomization codes were secured until all data entry was complete |
| Incomplete outcome data (attrition bias) | Low risk | The analysis was based on the intention‐to‐treat principle, with all patients included in their assigned group. No dropouts reported |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported |
| Other bias | Unclear risk | “The active treatment and placebo used in this study were prepared by IBSS Biomed S.A., Cracow, Poland.” No comment was offered with regards to IBSS Biomed's role in study design, analysis |
| Methods | Randomized, placebo‐controlled, double‐blinded. Withdrawals/loss to follow‐up: 22 participants (36.6%) | |
| Participants | N = 60 enrolled | |
| Interventions | Probiotics: LA, LB ((1 gram packets (500 million per packet) 4 times per day equalling approximately 2 billion CFUs/day) for 5 to 12 days | |
| Outcomes | ID (treatment 66% versus placebo 69.5%) | |
| Notes | Funding = supported in full by Hynson, Westcott & Dunning Products | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization provided by product manufacturer, otherwise unclear how randomization was generated |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) | High risk | Double‐blind, otherwise not described. Blinding codes were held by manufacturer. One reason mentioned for subjects not continuing the study was “taste.” There was an imbalance of drop outs from groups. Could taste be different for each intervention? Did this affect blinding on the side of the patient? It is unclear how many dropped out for taste reasons. |
| Incomplete outcome data (attrition bias) | High risk | There was a 37% drop‐out/ lost‐to‐follow‐up. The final number of subjects analyzed was not equal in magnitude (15 active, 23 placebo). The number of subjects who didn't finish the study was high when compared to observed outcomes (22 didn't finish, 26 cases of diarrhoea (10 in active, 16 in placebo)). |
| Selective reporting (reporting bias) | Low risk | Outcomes mentioned in Methods section were consistent to those mentioned in Results section |
| Other bias | High risk | Study was funded in full by manufacturer (i.e. provided product and placebo and also provided the randomization and held the codes) |
| Methods | Randomized, placebo‐controlled, double‐blinded. | |
| Participants | N = 202 enrolled | |
| Interventions | Probiotics: LGG (10 billion for children less than 12 kg; 20 billion for greater than or equal to 12 kg for duration of antibiotic treatment (7 to 14 days) | |
| Outcomes | ID (treatment 8% versus control 26%) | |
| Notes | Funding = Industry (operational funds from ConAgra Inc). Author also a consultant for ConAgra | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomized with a computer‐generated randomization table |
| Allocation concealment (selection bias) | Unclear risk | Product randomization by blinded numeric codes was performed by the supplier before the product was shipped to the investigation site. Codes were kept by the supplier until all data were collected |
| Blinding (performance bias and detection bias) | Low risk | The LGG and placebo were packed in identical bottles with identical capsule covers.” “Codes were kept by the supplier until all data were collected" |
| Incomplete outcome data (attrition bias) | Low risk | “The study was completed by 188 children (median age 4 years); 14 failed to complete the study, primarily because of antibiotic noncompliance or inability of the investigators to contact the primary caregiver at the assigned follow up time. None of the participants failed to complete the 10‐day course of antibiotics because of a change in stool consistency or frequency. There were no failures resulting from untoward effects of either LGG or placebo. Both active and placebo groups were similar for age distribution, sex, and type of antibiotics, and all who completed the study had no difficulty consuming the prescribed amount” |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported |
| Other bias | Unclear risk | Lead author is a consultant for CAG nutrition (division of ConAgra) which makes the product |
| Methods | Randomized, open‐label, no placebo‐control Withdrawals/Loss to follow‐up: 3 ITT: No Period of follow‐up: 7 days | |
| Participants | N= 372 Diagnosis: Pneumonia Country: China Setting: Hospital, in‐patient, 7 sites Age: average age in months: 13.99 | |
| Interventions | Probiotics: Clostridium Butyricum (50 million CFU), Bifidobacterium (500 million CFU) 4 packets a day 2.2 billion CFU/day Antibiotics: mixed pencillin, cephalosporin, macrolides | |
| Outcomes | ID: Control 30 (16.8%) versus Treatment 15 (7.8%) Definition of diarrhea: 2 or more BM over the pt amount (they has baseline BM # for each pt. And an increase of 2 or more over that baseline was considered diarrhea) | |
| Notes | Funding: NS | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomized block design. Use SAS software to generate 504 randomized number for the 7 hospital (72 numbers for each center) |
| Allocation concealment (selection bias) | High risk | Investigator appears to know the randomization schedule when assigning participants |
| Blinding (performance bias and detection bias) | High risk | No blinding procedure was described in the study. Seems to be an open label trial. No mention of blinding. No treatment comparison |
| Incomplete outcome data (attrition bias) | Low risk | 3 drop out of unknown reason & 5 exclusion (2 due to incomplete report, 3 due to rotavirus), from total of 380 (drop‐out rate 2.1%) |
| Selective reporting (reporting bias) | Low risk | Their outcome report is consistent with the study protocol. Study is registered at Chinese Ethics Committee of Registering Clinical Trials (http://www.chictrdb.org/) |
| Other bias | Unclear risk | The probiotic is provided by Shandong Kexing Bioproducts Co.,Ltd. (www.sdkexing.com) |
METHODS: Intention‐ to‐treat (ITT), Not specified (NS)
PARTICIPANTS: respiratory tract infection (RTI), upper respiratory tract infection (URTI), lower respiratory tract infection (LRTI), Not specified (NS)
INTERVENTIONS: Bifidobacteria anamalis subsp. lactus (BA), Bifidobacterium breve (BB), Bacillus clausii (BC), Bifidobacterium infantis (BI), Bifidobacterium lactis (BL), Lactobacillus acidophilus (LA), Lactobacillus bularicus (LB), Lactococcus casei (LC), Lactobacillus delbrueckii subsp. bulgaris (LD), Lactobacillus GG (LGG), Lactococcus lactis (LL),Lactococcus plantarum (LP),Lactococcus rhamnosus (LR), Lactobacillus sporogens (LS), Fructo‐Oligosaccaride (FOS), Saccharomyces boulardii (SB), Saccharomyces florentinus (SF), Streptococcus thermophilus (ST), Not available (NA)
OUTCOMES: Incidence of diarrhea (ID), Mean duration of diarhea (MDD), Mean stool consistency (MSC), Mean stool frequency (MSF)
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| Pediatric level data could not be ascertained | |
| Did not include children | |
| Did not include probiotics as intervention | |
| Did not include children | |
| Not randomized | |
| Not randomized | |
| Not randomized | |
| Pediatric level data could not be ascertained | |
| Not randomized | |
| Not a pediatric population | |
| Letter to the editor regarding pediatric AAD | |
| Article could not be found | |
| Did not administer probiotics concurrently with antibiotics | |
| AAD patient population excluded (studying nosocomial infections only) | |
| AAD outcome could not be obtained | |
| Not a pediatric population | |
| Did not include children | |
| Not randomized | |
| Did not include children | |
| Not associated with antibiotic use | |
| Participants were not taking antibiotics concurrently with probiotics, or this data could not be ascertained | |
| Used a gastro‐intestinal symptoms rating scale that, while inclusive of stool frequency and consistency, did not report data specific to those outcomes | |
| Letter to the editor regarding pediatric AAD | |
| Participants were not taking antibiotics concurrently with probiotics, or this data could not be ascertained | |
| A study of acute diarrhea not associated with antibiotic use | |
| Not a pediatric population | |
| Not a pediatric population | |
| Participants were not taking antibiotics concurrently with probiotics, or this data could not be ascertained | |
| Participants were not taking antibiotics concurrently with probiotics, or this data could not be ascertained | |
| Not a pediatric population | |
| Participants were not taking antibiotics concurrently with probiotics, or this data could not be ascertained | |
| Not randomized | |
| Participants were not taking antibiotics concurrently with probiotics, or this data could not be ascertained | |
| Involved Sacchromyces boulardii for pediatric infectious diarrhea (i.e., amebiasis‐associated diarrhea) not antibiotic associated diarrhea | |
| Did not report outcomes particular to AAD | |
| Not randomized | |
| Not a pediatric population | |
| Participants were not taking antibiotics concurrently with probiotics, or this data could not be ascertained | |
| Did not report outcomes particular to AAD | |
| Did not evaluate antibiotic use | |
| Not a pediatric population | |
| Had a high dose of prebiotics (>5 grams) | |
| No diarrhea outcome | |
| Probiotics not administered concurrently with antibiotics | |
| Not associated with antibiotic use | |
| Did not include children | |
| Not a pediatric population | |
| AAD outcome could not be obtained | |
| Primary outcome not diarrhea. A study of how antibiotics effect the gut flora |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incidence of diarrhea: Complete case Show forest plot | 22 | 3898 | Risk Ratio (M‐H, Random, 95% CI) | 0.46 [0.35, 0.61] |
| Analysis 1.1  Comparison 1 Probiotics versus control, Outcome 1 Incidence of diarrhea: Complete case. | ||||
| 1.1 Incidence of Diarrhea: Active controlled trials | 2 | 773 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.33, 2.21] |
| 1.2 Incidence of Diarrhea: Placebo controlled trials | 15 | 1575 | Risk Ratio (M‐H, Random, 95% CI) | 0.42 [0.29, 0.61] |
| 1.3 Incidence of Diarrhea: No treatment control | 5 | 1550 | Risk Ratio (M‐H, Random, 95% CI) | 0.39 [0.25, 0.60] |
| 2 Incidence of diarrhea: Probiotic dose Show forest plot | 22 | 3898 | Risk Ratio (M‐H, Random, 95% CI) | 0.46 [0.35, 0.61] |
| Analysis 1.2 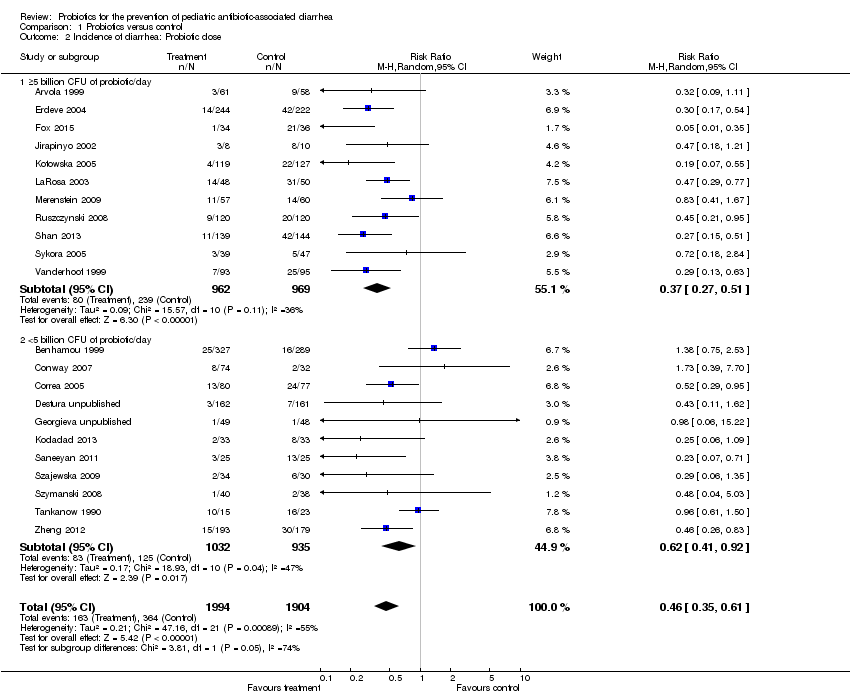 Comparison 1 Probiotics versus control, Outcome 2 Incidence of diarrhea: Probiotic dose. | ||||
| 2.1 ≥5 billion CFU of probiotic/day | 11 | 1931 | Risk Ratio (M‐H, Random, 95% CI) | 0.37 [0.27, 0.51] |
| 2.2 <5 billion CFU of probiotic/day | 11 | 1967 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.41, 0.92] |
| 3 Incidence of diarrhea: Probiotic species Show forest plot | 22 | 3898 | Risk Ratio (M‐H, Random, 95% CI) | 0.46 [0.35, 0.61] |
| Analysis 1.3  Comparison 1 Probiotics versus control, Outcome 3 Incidence of diarrhea: Probiotic species. | ||||
| 3.1 Lactobacillus rhamnosus (strains: GG and E/N, Oxy, Pen) | 4 | 611 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.22, 0.56] |
| 3.2 L. acidophilus & L. bulgaricus | 1 | 38 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.61, 1.50] |
| 3.3 L. acidophilus and Bifidobacterium infantis | 1 | 18 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.18, 1.21] |
| 3.4 L. sporogenes | 1 | 98 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.29, 0.77] |
| 3.5 Saccharomyces boulardii | 4 | 1611 | Risk Ratio (M‐H, Random, 95% CI) | 0.40 [0.17, 0.96] |
| 3.6 B. lactis & S. thermophilus | 1 | 157 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.29, 0.95] |
| 3.7 Bacillus clausii | 1 | 323 | Risk Ratio (M‐H, Random, 95% CI) | 0.43 [0.11, 1.62] |
| 3.8 Lactococcus lactis, L. plantarum, L. rhamnosus, L. casei, L. lactis subspecies diacetylactis, Leuconostoc cremoris, Bifidobacterium longum, B. breve, Lactobacillus acidophilus, and Saccharomyces florentinus | 1 | 117 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.41, 1.67] |
| 3.9 Bifidobacterium longum PL03, Lactobacillus rhamnosus KL53A, and Lactobacillus plantarum PL02 | 1 | 78 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.04, 5.03] |
| 3.10 Streptococcus thermophillus, L. acidophilus, B. anamalis subsp. lactus, L. delbrueckii subsp. bulgaris | 1 | 106 | Risk Ratio (M‐H, Random, 95% CI) | 1.73 [0.39, 7.70] |
| 3.11 Lactobacillus rhamnosus GG, Bifidobacterium animalis subsp. Lactis Bv‐12, L. acidophilus LA‐5 | 1 | 70 | Risk Ratio (M‐H, Random, 95% CI) | 0.05 [0.01, 0.35] |
| 3.12 Lactobasillus casei, Lactobacillus acidophilus, Lactobasillus reuteri, Lactobasillus bulgaricus, Streptococcus, Bifidobacterium bifidum, Bifidobacterium infantis | 1 | 50 | Risk Ratio (M‐H, Random, 95% CI) | 0.23 [0.07, 0.71] |
| 3.13 Lactobacillus reuteri DSM 17938 | 1 | 97 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.06, 15.22] |
| 3.14 Lactobacillus acidophilus, Lactobacillus rhamnosus, Lactobacillus bulgaricus, Lactobacillus casei, Streptococcus thermophilus, Bifidobacterium infantis and Bifidobacterium breve | 1 | 66 | Risk Ratio (M‐H, Random, 95% CI) | 0.25 [0.06, 1.09] |
| 3.15 L. casei DN‐114 001 | 1 | 86 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.18, 2.84] |
| 3.16 Clostridium Butyricum and Bifidobacterium | 1 | 372 | Risk Ratio (M‐H, Random, 95% CI) | 0.46 [0.26, 0.83] |
| 4 Incidence of diarrhea: Risk of bias Show forest plot | 22 | 3898 | Risk Ratio (M‐H, Random, 95% CI) | 0.46 [0.35, 0.61] |
| Analysis 1.4  Comparison 1 Probiotics versus control, Outcome 4 Incidence of diarrhea: Risk of bias. | ||||
| 4.1 Low Risk | 10 | 1344 | Risk Ratio (M‐H, Random, 95% CI) | 0.42 [0.30, 0.60] |
| 4.2 High Risk | 12 | 2554 | Risk Ratio (M‐H, Random, 95% CI) | 0.50 [0.33, 0.77] |
| 5 Incidence of diarrhea: Strictness of definition Show forest plot | 18 | 3611 | Risk Ratio (M‐H, Random, 95% CI) | 0.44 [0.32, 0.61] |
| Analysis 1.5 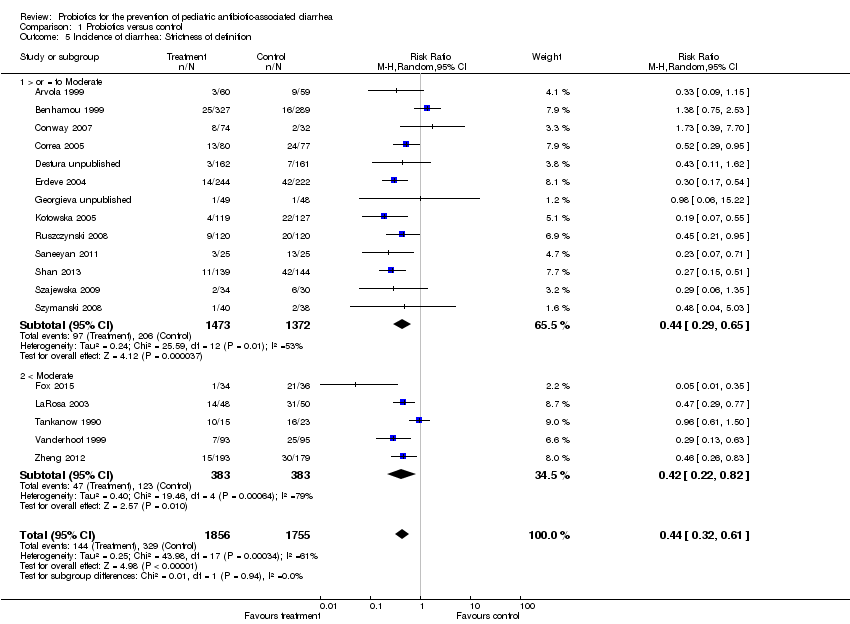 Comparison 1 Probiotics versus control, Outcome 5 Incidence of diarrhea: Strictness of definition. | ||||
| 5.1 > or = to Moderate | 13 | 2845 | Risk Ratio (M‐H, Random, 95% CI) | 0.44 [0.29, 0.65] |
| 5.2 < Moderate | 5 | 766 | Risk Ratio (M‐H, Random, 95% CI) | 0.42 [0.22, 0.82] |
| 6 Incidence of diarrhea: Definition of diarrhea Show forest plot | 18 | 3891 | Risk Ratio (M‐H, Random, 95% CI) | 0.38 [0.26, 0.54] |
| Analysis 1.6  Comparison 1 Probiotics versus control, Outcome 6 Incidence of diarrhea: Definition of diarrhea. | ||||
| 6.1 3 or more watery/liquid stools for more than 2 days | 1 | 70 | Risk Ratio (M‐H, Random, 95% CI) | 0.08 [0.00, 1.39] |
| 6.2 3 or more loose/watery/liquid stools per day for at least 2 consecutive days | 12 | 1833 | Risk Ratio (M‐H, Random, 95% CI) | 0.37 [0.26, 0.51] |
| 6.3 ≥3 watery/liquid stools per 24 hours | 2 | 1082 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.15, 2.85] |
| 6.4 ≥2 liquid stools per day on at least 2 occasions during study | 2 | 258 | Risk Ratio (M‐H, Random, 95% CI) | 0.24 [0.09, 0.65] |
| 6.5 >=2 loose/watery/liquid stools for more than 2 days | 1 | 70 | Risk Ratio (M‐H, Random, 95% CI) | 0.05 [0.01, 0.35] |
| 6.6 ≥2 liquid stools per 24 hr | 1 | 98 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.29, 0.77] |
| 6.7 ≥1 abnormally loose bowel movement per 24 hrs | 1 | 38 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.61, 1.50] |
| 6.8 2 or more BM over the patient's normal | 1 | 372 | Risk Ratio (M‐H, Random, 95% CI) | 0.46 [0.26, 0.83] |
| 6.9 "Any of Above (Fox)" | 1 | 70 | Risk Ratio (M‐H, Random, 95% CI) | 0.04 [0.01, 0.27] |
| 7 Incidence of diarrhea: Sensitivity analysis (complete case ‐ fixed effects) Show forest plot | 22 | 3898 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.44 [0.37, 0.53] |
| Analysis 1.7 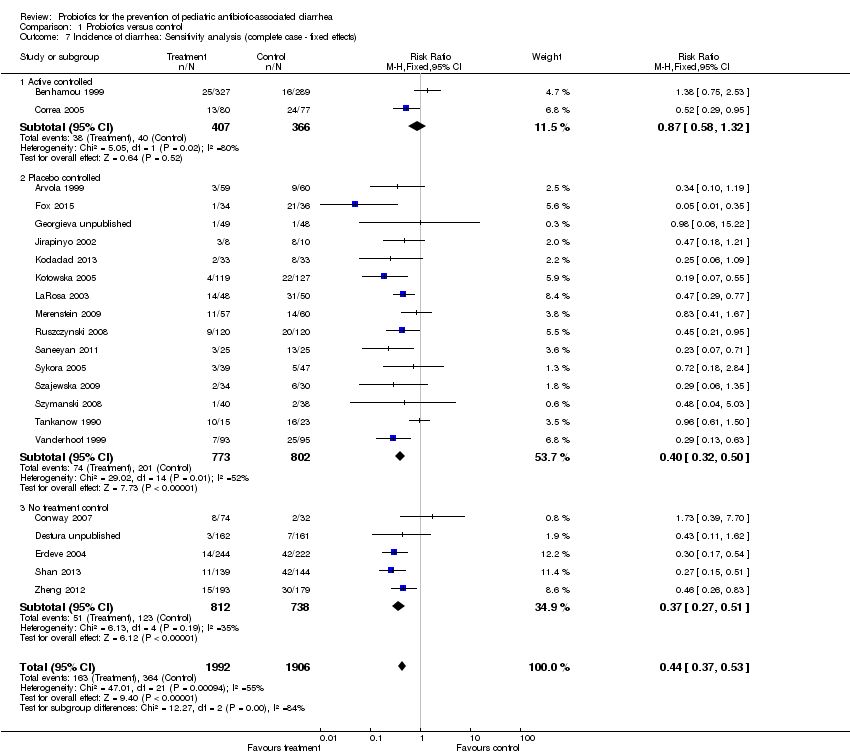 Comparison 1 Probiotics versus control, Outcome 7 Incidence of diarrhea: Sensitivity analysis (complete case ‐ fixed effects). | ||||
| 7.1 Active controlled | 2 | 773 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.58, 1.32] |
| 7.2 Placebo controlled | 15 | 1575 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.40 [0.32, 0.50] |
| 7.3 No treatment control | 5 | 1550 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.37 [0.27, 0.51] |
| 8 Incidence of diarrhea: Sensitivity analysis (extreme‐plausible analysis) Show forest plot | 22 | 4529 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.54, 0.89] |
| Analysis 1.8 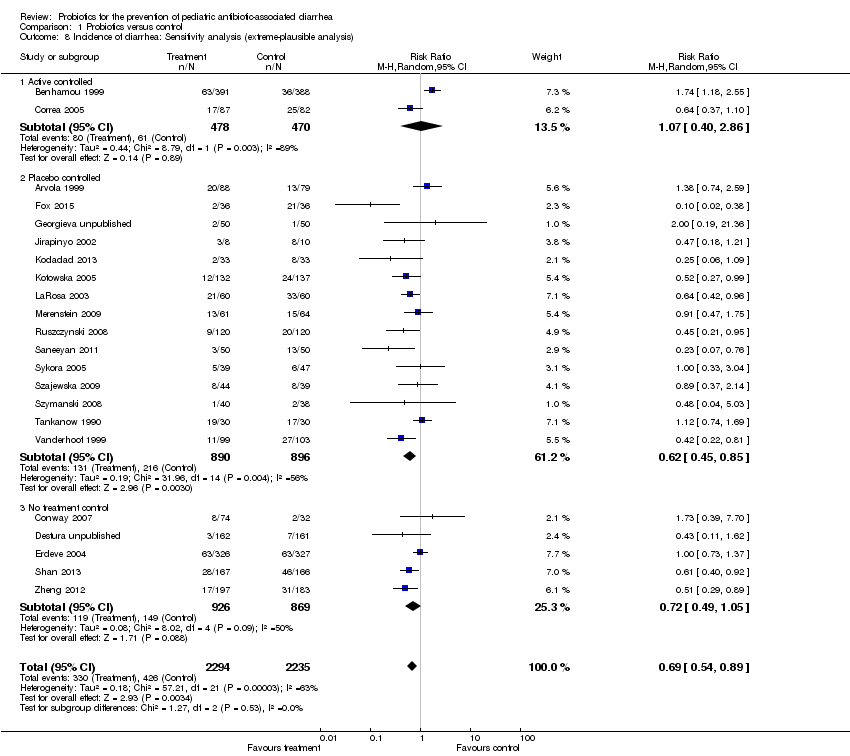 Comparison 1 Probiotics versus control, Outcome 8 Incidence of diarrhea: Sensitivity analysis (extreme‐plausible analysis). | ||||
| 8.1 Active controlled | 2 | 948 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.40, 2.86] |
| 8.2 Placebo controlled | 15 | 1786 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.45, 0.85] |
| 8.3 No treatment control | 5 | 1795 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.49, 1.05] |
| 9 Incidence of diarrhea: Probiotic dose (extreme‐plausible analysis) Show forest plot | 21 | 4511 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.55, 0.90] |
| Analysis 1.9  Comparison 1 Probiotics versus control, Outcome 9 Incidence of diarrhea: Probiotic dose (extreme‐plausible analysis). | ||||
| 9.1 ≥5 billion CFU of probiotic/day | 10 | 2267 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.49, 0.90] |
| 9.2 <5 billion CFU of probiotic/day | 11 | 2244 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.50, 1.16] |
| 10 Incidence of diarrhea: Diagnosis Show forest plot | 18 | 2553 | Risk Ratio (M‐H, Random, 95% CI) | 0.42 [0.31, 0.56] |
| Analysis 1.10 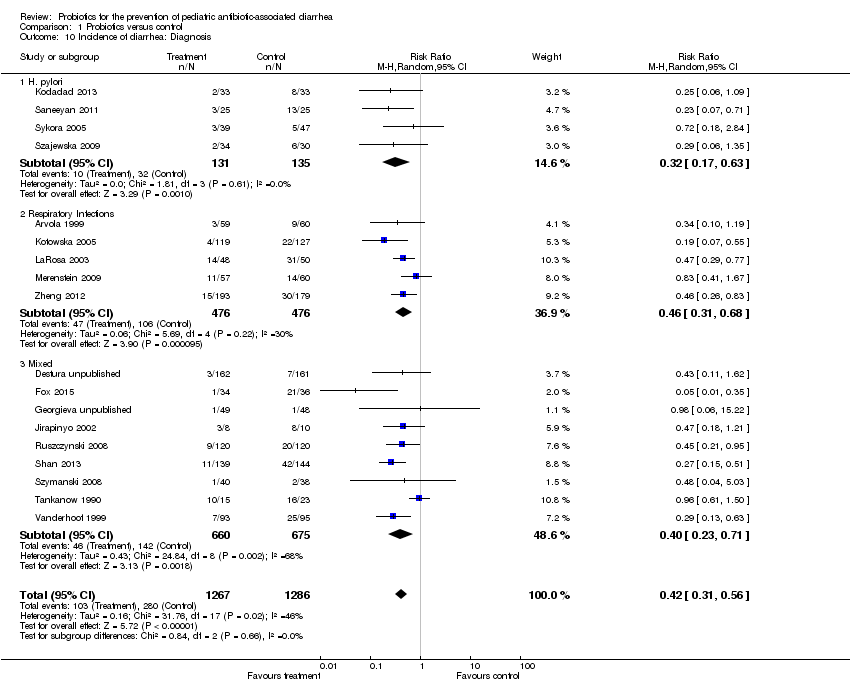 Comparison 1 Probiotics versus control, Outcome 10 Incidence of diarrhea: Diagnosis. | ||||
| 10.1 H. pylori | 4 | 266 | Risk Ratio (M‐H, Random, 95% CI) | 0.32 [0.17, 0.63] |
| 10.2 Respiratory Infections | 5 | 952 | Risk Ratio (M‐H, Random, 95% CI) | 0.46 [0.31, 0.68] |
| 10.3 Mixed | 9 | 1335 | Risk Ratio (M‐H, Random, 95% CI) | 0.40 [0.23, 0.71] |
| 11 Incidence of diarrhea: Industry sponsorship Show forest plot | 12 | 1517 | Risk Ratio (M‐H, Random, 95% CI) | 0.50 [0.33, 0.76] |
| Analysis 1.11  Comparison 1 Probiotics versus control, Outcome 11 Incidence of diarrhea: Industry sponsorship. | ||||
| 11.1 Industry Sponsored | 7 | 1149 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.40, 0.86] |
| 11.2 Non‐Industry | 5 | 368 | Risk Ratio (M‐H, Random, 95% CI) | 0.32 [0.11, 0.96] |
| 12 Incidence of diarrhea: Inpatient versus outpatient Show forest plot | 13 | 2176 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.34, 0.77] |
| Analysis 1.12  Comparison 1 Probiotics versus control, Outcome 12 Incidence of diarrhea: Inpatient versus outpatient. | ||||
| 12.1 Inpatient | 5 | 834 | Risk Ratio (M‐H, Random, 95% CI) | 0.38 [0.26, 0.55] |
| 12.2 Outpatient | 8 | 1342 | Risk Ratio (M‐H, Random, 95% CI) | 0.58 [0.34, 1.02] |
| 13 Incidence of diarrhea: Single strain versus multi strain Show forest plot | 22 | 3898 | Risk Ratio (M‐H, Random, 95% CI) | 0.46 [0.35, 0.61] |
| Analysis 1.13  Comparison 1 Probiotics versus control, Outcome 13 Incidence of diarrhea: Single strain versus multi strain. | ||||
| 13.1 Single Strain | 11 | 2586 | Risk Ratio (M‐H, Random, 95% CI) | 0.41 [0.28, 0.62] |
| 13.2 Multi Strain | 11 | 1312 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.35, 0.77] |
| 14 Adverse events: Complete case Show forest plot | 16 | 2455 | Risk Difference (M‐H, Random, 95% CI) | 0.00 [‐0.01, 0.01] |
| Analysis 1.14  Comparison 1 Probiotics versus control, Outcome 14 Adverse events: Complete case. | ||||
| 15 Adverse events: Same event rate assumptions analysis Show forest plot | 21 | 4369 | Risk Difference (M‐H, Random, 95% CI) | 0.00 [‐0.00, 0.01] |
| Analysis 1.15 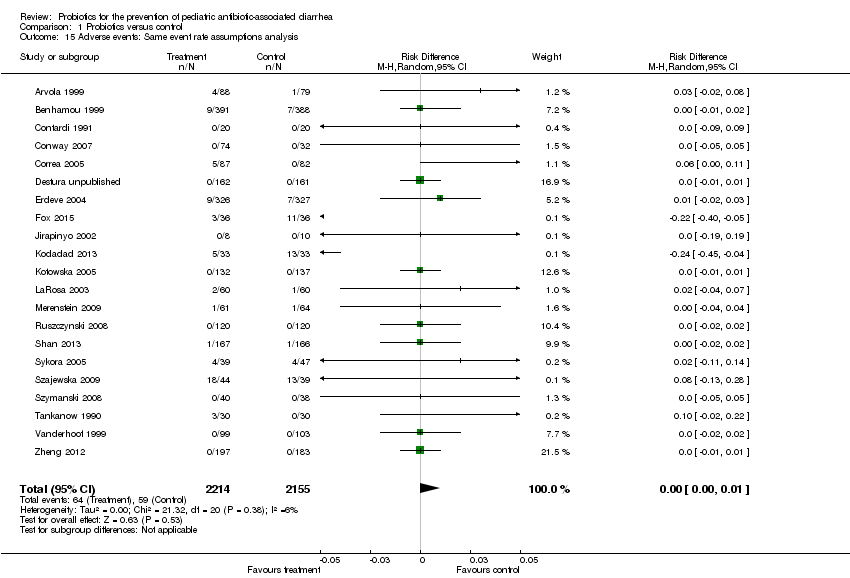 Comparison 1 Probiotics versus control, Outcome 15 Adverse events: Same event rate assumptions analysis. | ||||
| 16 Adverse events: Risk of bias Show forest plot | 16 | 2455 | Risk Difference (M‐H, Random, 95% CI) | 0.00 [‐0.01, 0.01] |
| Analysis 1.16  Comparison 1 Probiotics versus control, Outcome 16 Adverse events: Risk of bias. | ||||
| 16.1 Low RoB | 9 | 1249 | Risk Difference (M‐H, Random, 95% CI) | 0.00 [‐0.01, 0.02] |
| 16.2 High/Unclear | 7 | 1206 | Risk Difference (M‐H, Random, 95% CI) | ‐0.00 [‐0.01, 0.01] |
| 17 Mean duration of diarrhea: Complete case Show forest plot | 5 | 897 | Mean Difference (IV, Random, 95% CI) | ‐0.60 [‐1.18, ‐0.02] |
| Analysis 1.17  Comparison 1 Probiotics versus control, Outcome 17 Mean duration of diarrhea: Complete case. | ||||
| 18 Mean stool frequency: Complete case Show forest plot | 4 | 425 | Mean Difference (IV, Random, 95% CI) | ‐0.30 [‐0.60, ‐0.00] |
| Analysis 1.18 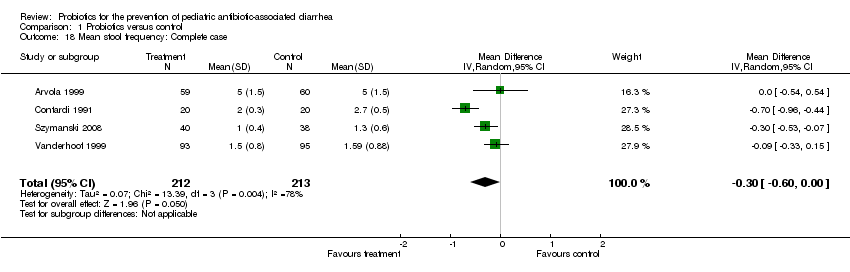 Comparison 1 Probiotics versus control, Outcome 18 Mean stool frequency: Complete case. | ||||

Study flow diagram.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
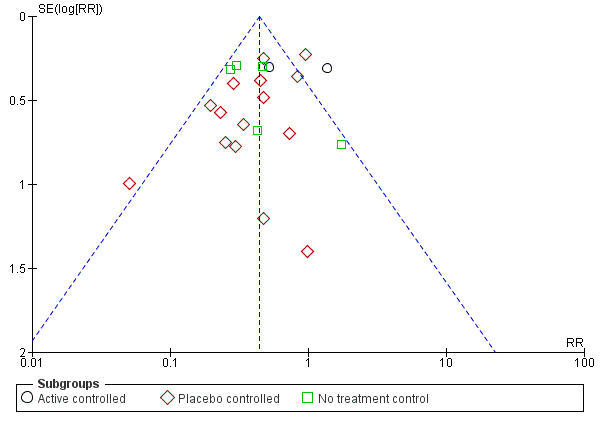
Funnel plot of comparison: 1 any specific probiotic versus control (placebo, active or no treatment), outcome: 1.6 Incidence of Diarrhea: Complete case ‐ fixed effects

Funnel plot of comparison: 1 Probiotics versus control, outcome: 1.1 Incidence of diarrhea: Complete case.

Comparison 1 Probiotics versus control, Outcome 1 Incidence of diarrhea: Complete case.

Comparison 1 Probiotics versus control, Outcome 2 Incidence of diarrhea: Probiotic dose.

Comparison 1 Probiotics versus control, Outcome 3 Incidence of diarrhea: Probiotic species.
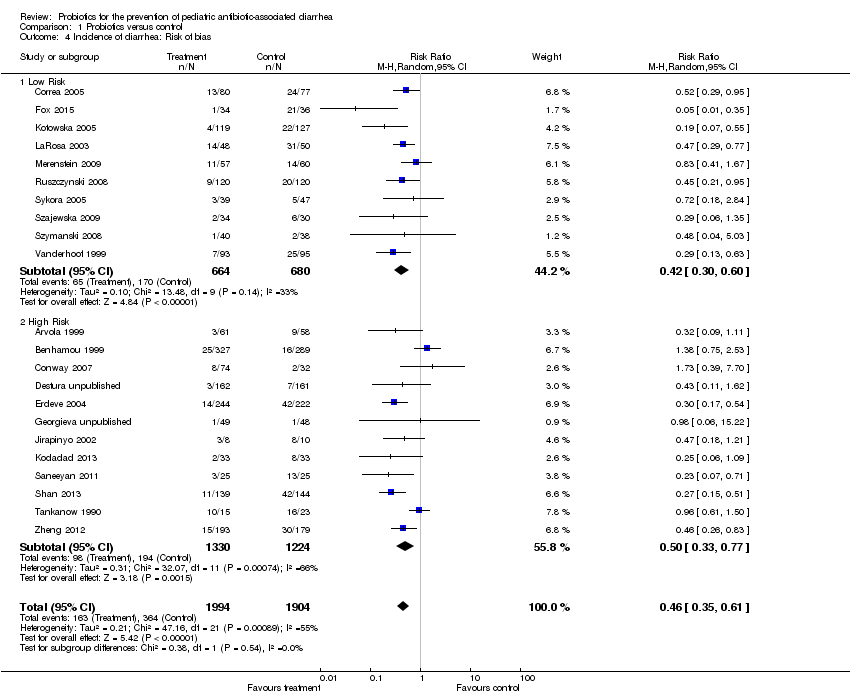
Comparison 1 Probiotics versus control, Outcome 4 Incidence of diarrhea: Risk of bias.

Comparison 1 Probiotics versus control, Outcome 5 Incidence of diarrhea: Strictness of definition.
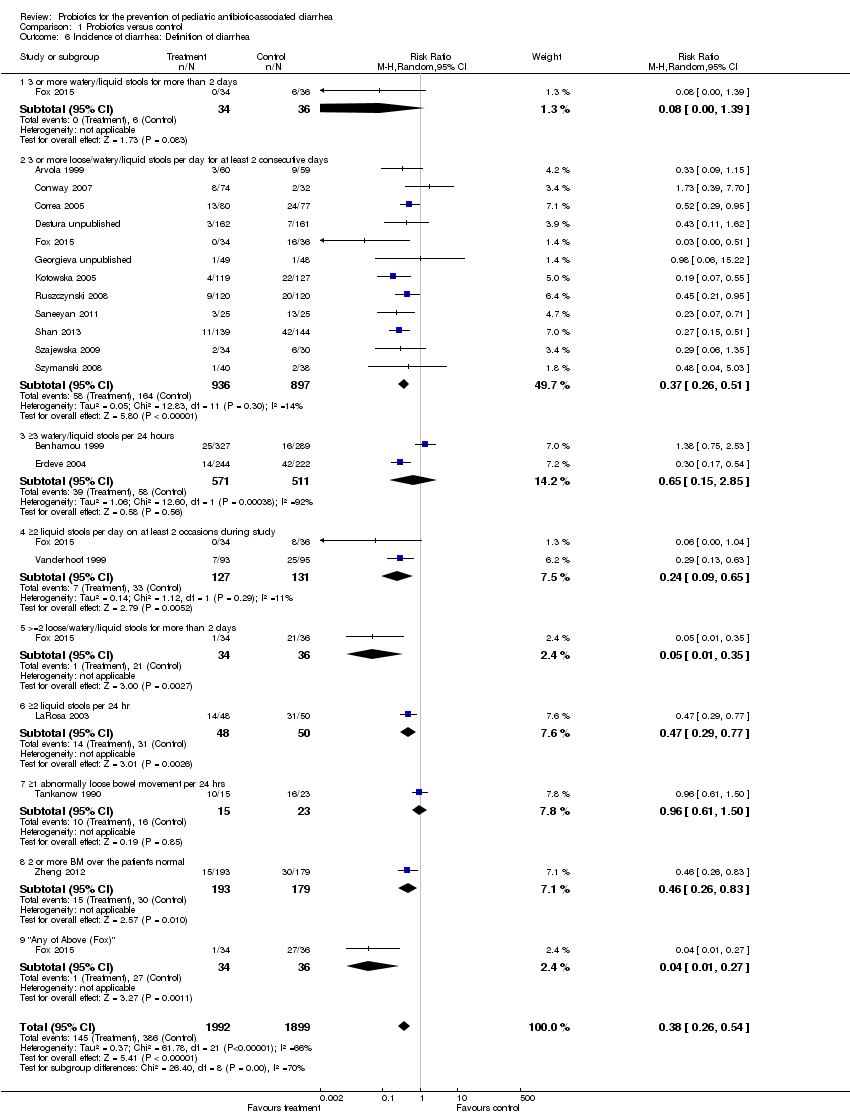
Comparison 1 Probiotics versus control, Outcome 6 Incidence of diarrhea: Definition of diarrhea.
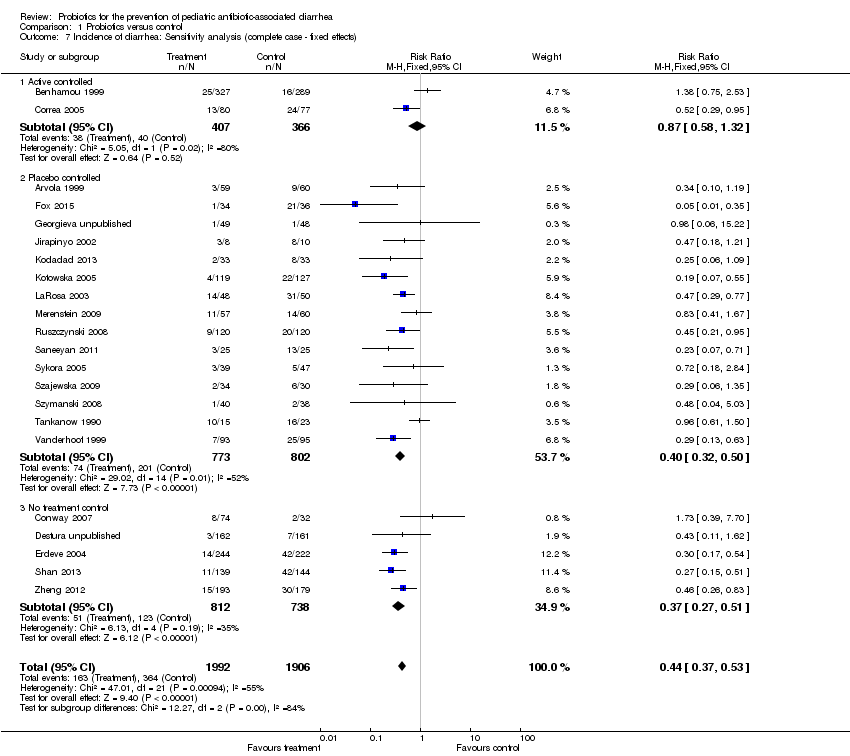
Comparison 1 Probiotics versus control, Outcome 7 Incidence of diarrhea: Sensitivity analysis (complete case ‐ fixed effects).
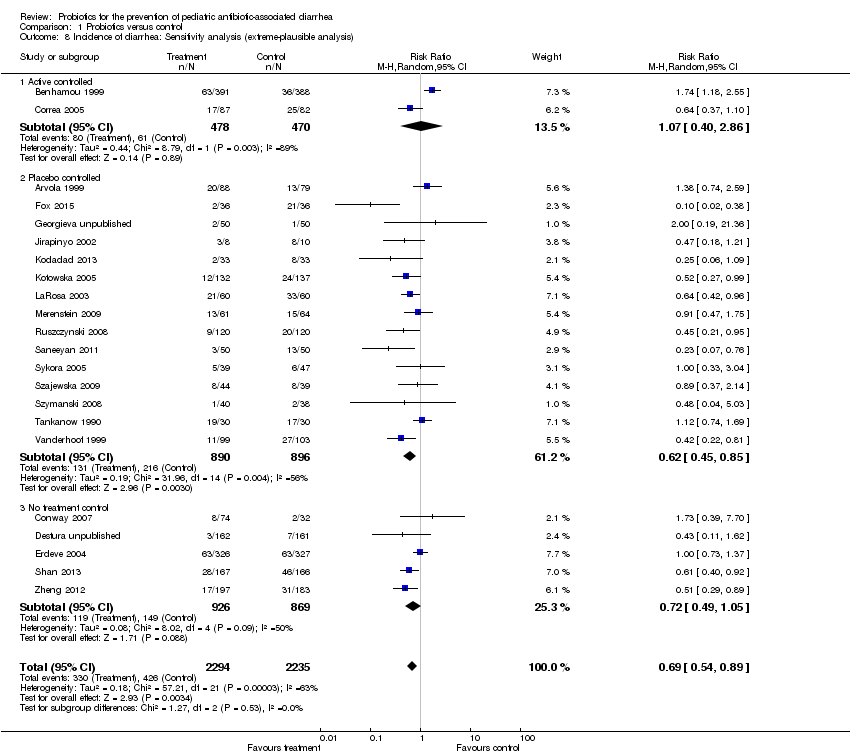
Comparison 1 Probiotics versus control, Outcome 8 Incidence of diarrhea: Sensitivity analysis (extreme‐plausible analysis).
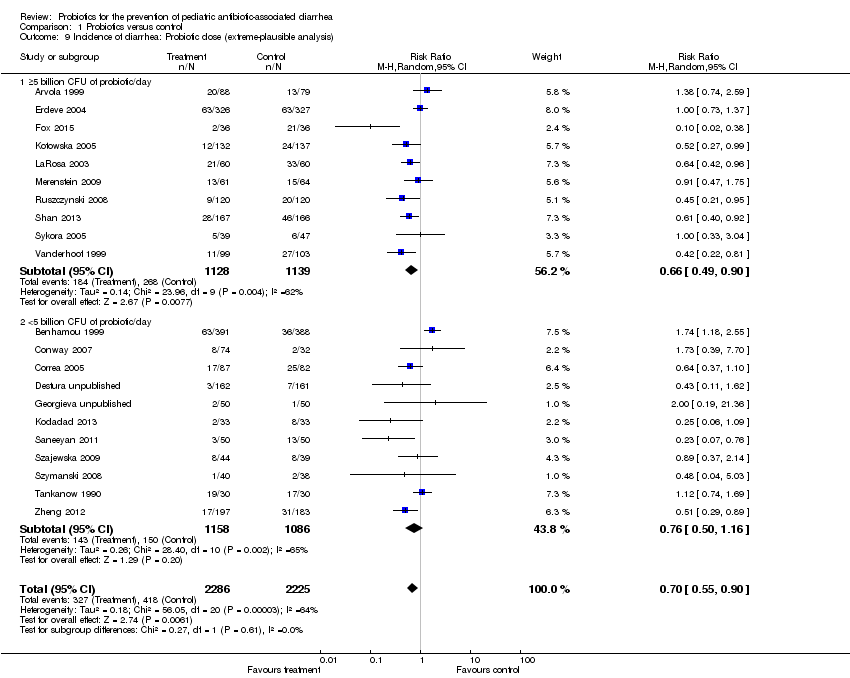
Comparison 1 Probiotics versus control, Outcome 9 Incidence of diarrhea: Probiotic dose (extreme‐plausible analysis).

Comparison 1 Probiotics versus control, Outcome 10 Incidence of diarrhea: Diagnosis.

Comparison 1 Probiotics versus control, Outcome 11 Incidence of diarrhea: Industry sponsorship.

Comparison 1 Probiotics versus control, Outcome 12 Incidence of diarrhea: Inpatient versus outpatient.

Comparison 1 Probiotics versus control, Outcome 13 Incidence of diarrhea: Single strain versus multi strain.
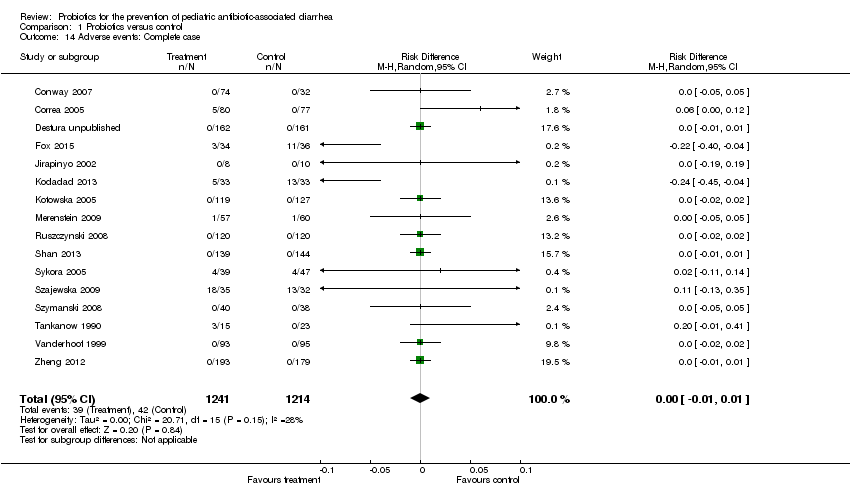
Comparison 1 Probiotics versus control, Outcome 14 Adverse events: Complete case.

Comparison 1 Probiotics versus control, Outcome 15 Adverse events: Same event rate assumptions analysis.
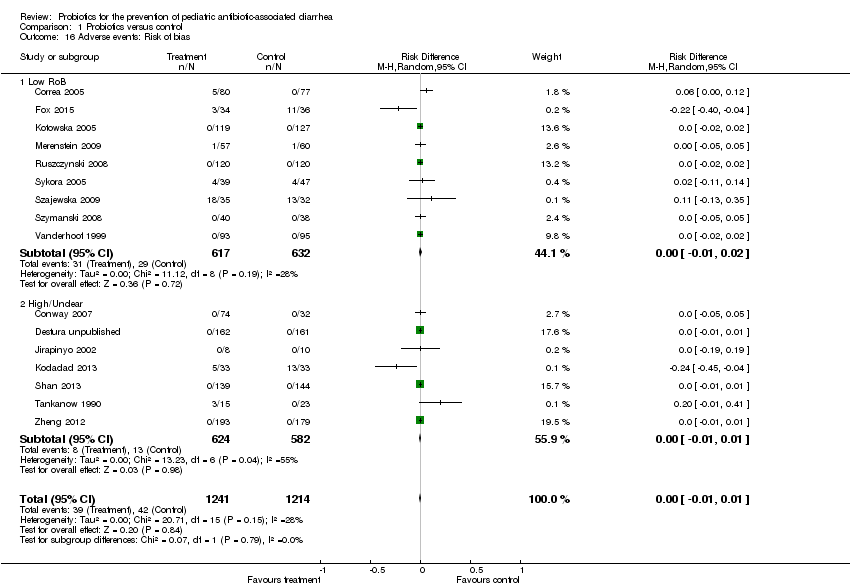
Comparison 1 Probiotics versus control, Outcome 16 Adverse events: Risk of bias.

Comparison 1 Probiotics versus control, Outcome 17 Mean duration of diarrhea: Complete case.

Comparison 1 Probiotics versus control, Outcome 18 Mean stool frequency: Complete case.
| Probiotics as an adjunct to antibiotics for the prevention of antibiotic‐associated diarrhea in children | ||||||
| Patient or population: Children given antibiotics | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Effect size | Number of participants | Quality of the evidence | Comments | |
| Risk with control | Risk with Probiotics | |||||
| Incidence of diarrhea Follow up: range 1 week to 12 weeks | 191 per 1000 | 88 per 1000 | RR 0.46 | 3898 | ⊕⊕⊕⊝ | |
| Adverse events Follow up: range 1 week to 4 weeks | 35 per 1000 | 33 per 1000 | RD 0.00 (‐0.01 to 0.01) | 2455 | ⊕⊝⊝⊝ | |
| Duration of diarrhea Follow up: range 10 days to 12 weeks | The mean duration of diarrhea in the intervention group was 0.6 days fewer (1.18 fewer to 0.02 fewer) | 897 | ⊕⊕⊝⊝ | |||
| Stool frequency Follow up: range 10 days to 12 weeks | The mean stool frequency in the intervention group was 0.3 lower (0.6 lower to 0) | 425 | ⊕⊕⊝⊝ | |||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 A test for interaction between low risk of bias trials and high or unclear risk of bias trials was not statistically significant. Additionally, the low risk of bias trials actually showed a more favorable effect of intervention than the high or unclear risk of bias trials. 2 I2 is 55% with a p value of 0.0009 suggesting substantial heterogeneity. While we explored the heterogeneity we were unable to explain it completely with our a priori or post hoc analyses. 3 Because of widely varying definitions of adverse events there is considerable indirectness in terms of outcomes. 4 Only 16 or 22 trials reported adverse events, suggesting selective outcome reporting bias. 6 Inconsistency (large statistical heterogeneity with I2 of 79%, low P value [P = 0.04], point estimates and confidence intervals vary considerably). 7 The upper bound of 0.02 per day is not considered patient important. 8 Inconsistency (large statistical heterogeneity with I2 of 78%, low P value [P = 0.05], point estimates and confidence intervals vary considerably). 9 95% confidence interval includes no effect and lower bound of 0.60 per day is of questionable patient importance. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incidence of diarrhea: Complete case Show forest plot | 22 | 3898 | Risk Ratio (M‐H, Random, 95% CI) | 0.46 [0.35, 0.61] |
| 1.1 Incidence of Diarrhea: Active controlled trials | 2 | 773 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.33, 2.21] |
| 1.2 Incidence of Diarrhea: Placebo controlled trials | 15 | 1575 | Risk Ratio (M‐H, Random, 95% CI) | 0.42 [0.29, 0.61] |
| 1.3 Incidence of Diarrhea: No treatment control | 5 | 1550 | Risk Ratio (M‐H, Random, 95% CI) | 0.39 [0.25, 0.60] |
| 2 Incidence of diarrhea: Probiotic dose Show forest plot | 22 | 3898 | Risk Ratio (M‐H, Random, 95% CI) | 0.46 [0.35, 0.61] |
| 2.1 ≥5 billion CFU of probiotic/day | 11 | 1931 | Risk Ratio (M‐H, Random, 95% CI) | 0.37 [0.27, 0.51] |
| 2.2 <5 billion CFU of probiotic/day | 11 | 1967 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.41, 0.92] |
| 3 Incidence of diarrhea: Probiotic species Show forest plot | 22 | 3898 | Risk Ratio (M‐H, Random, 95% CI) | 0.46 [0.35, 0.61] |
| 3.1 Lactobacillus rhamnosus (strains: GG and E/N, Oxy, Pen) | 4 | 611 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.22, 0.56] |
| 3.2 L. acidophilus & L. bulgaricus | 1 | 38 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.61, 1.50] |
| 3.3 L. acidophilus and Bifidobacterium infantis | 1 | 18 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.18, 1.21] |
| 3.4 L. sporogenes | 1 | 98 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.29, 0.77] |
| 3.5 Saccharomyces boulardii | 4 | 1611 | Risk Ratio (M‐H, Random, 95% CI) | 0.40 [0.17, 0.96] |
| 3.6 B. lactis & S. thermophilus | 1 | 157 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.29, 0.95] |
| 3.7 Bacillus clausii | 1 | 323 | Risk Ratio (M‐H, Random, 95% CI) | 0.43 [0.11, 1.62] |
| 3.8 Lactococcus lactis, L. plantarum, L. rhamnosus, L. casei, L. lactis subspecies diacetylactis, Leuconostoc cremoris, Bifidobacterium longum, B. breve, Lactobacillus acidophilus, and Saccharomyces florentinus | 1 | 117 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.41, 1.67] |
| 3.9 Bifidobacterium longum PL03, Lactobacillus rhamnosus KL53A, and Lactobacillus plantarum PL02 | 1 | 78 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.04, 5.03] |
| 3.10 Streptococcus thermophillus, L. acidophilus, B. anamalis subsp. lactus, L. delbrueckii subsp. bulgaris | 1 | 106 | Risk Ratio (M‐H, Random, 95% CI) | 1.73 [0.39, 7.70] |
| 3.11 Lactobacillus rhamnosus GG, Bifidobacterium animalis subsp. Lactis Bv‐12, L. acidophilus LA‐5 | 1 | 70 | Risk Ratio (M‐H, Random, 95% CI) | 0.05 [0.01, 0.35] |
| 3.12 Lactobasillus casei, Lactobacillus acidophilus, Lactobasillus reuteri, Lactobasillus bulgaricus, Streptococcus, Bifidobacterium bifidum, Bifidobacterium infantis | 1 | 50 | Risk Ratio (M‐H, Random, 95% CI) | 0.23 [0.07, 0.71] |
| 3.13 Lactobacillus reuteri DSM 17938 | 1 | 97 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.06, 15.22] |
| 3.14 Lactobacillus acidophilus, Lactobacillus rhamnosus, Lactobacillus bulgaricus, Lactobacillus casei, Streptococcus thermophilus, Bifidobacterium infantis and Bifidobacterium breve | 1 | 66 | Risk Ratio (M‐H, Random, 95% CI) | 0.25 [0.06, 1.09] |
| 3.15 L. casei DN‐114 001 | 1 | 86 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.18, 2.84] |
| 3.16 Clostridium Butyricum and Bifidobacterium | 1 | 372 | Risk Ratio (M‐H, Random, 95% CI) | 0.46 [0.26, 0.83] |
| 4 Incidence of diarrhea: Risk of bias Show forest plot | 22 | 3898 | Risk Ratio (M‐H, Random, 95% CI) | 0.46 [0.35, 0.61] |
| 4.1 Low Risk | 10 | 1344 | Risk Ratio (M‐H, Random, 95% CI) | 0.42 [0.30, 0.60] |
| 4.2 High Risk | 12 | 2554 | Risk Ratio (M‐H, Random, 95% CI) | 0.50 [0.33, 0.77] |
| 5 Incidence of diarrhea: Strictness of definition Show forest plot | 18 | 3611 | Risk Ratio (M‐H, Random, 95% CI) | 0.44 [0.32, 0.61] |
| 5.1 > or = to Moderate | 13 | 2845 | Risk Ratio (M‐H, Random, 95% CI) | 0.44 [0.29, 0.65] |
| 5.2 < Moderate | 5 | 766 | Risk Ratio (M‐H, Random, 95% CI) | 0.42 [0.22, 0.82] |
| 6 Incidence of diarrhea: Definition of diarrhea Show forest plot | 18 | 3891 | Risk Ratio (M‐H, Random, 95% CI) | 0.38 [0.26, 0.54] |
| 6.1 3 or more watery/liquid stools for more than 2 days | 1 | 70 | Risk Ratio (M‐H, Random, 95% CI) | 0.08 [0.00, 1.39] |
| 6.2 3 or more loose/watery/liquid stools per day for at least 2 consecutive days | 12 | 1833 | Risk Ratio (M‐H, Random, 95% CI) | 0.37 [0.26, 0.51] |
| 6.3 ≥3 watery/liquid stools per 24 hours | 2 | 1082 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.15, 2.85] |
| 6.4 ≥2 liquid stools per day on at least 2 occasions during study | 2 | 258 | Risk Ratio (M‐H, Random, 95% CI) | 0.24 [0.09, 0.65] |
| 6.5 >=2 loose/watery/liquid stools for more than 2 days | 1 | 70 | Risk Ratio (M‐H, Random, 95% CI) | 0.05 [0.01, 0.35] |
| 6.6 ≥2 liquid stools per 24 hr | 1 | 98 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.29, 0.77] |
| 6.7 ≥1 abnormally loose bowel movement per 24 hrs | 1 | 38 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.61, 1.50] |
| 6.8 2 or more BM over the patient's normal | 1 | 372 | Risk Ratio (M‐H, Random, 95% CI) | 0.46 [0.26, 0.83] |
| 6.9 "Any of Above (Fox)" | 1 | 70 | Risk Ratio (M‐H, Random, 95% CI) | 0.04 [0.01, 0.27] |
| 7 Incidence of diarrhea: Sensitivity analysis (complete case ‐ fixed effects) Show forest plot | 22 | 3898 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.44 [0.37, 0.53] |
| 7.1 Active controlled | 2 | 773 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.58, 1.32] |
| 7.2 Placebo controlled | 15 | 1575 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.40 [0.32, 0.50] |
| 7.3 No treatment control | 5 | 1550 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.37 [0.27, 0.51] |
| 8 Incidence of diarrhea: Sensitivity analysis (extreme‐plausible analysis) Show forest plot | 22 | 4529 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.54, 0.89] |
| 8.1 Active controlled | 2 | 948 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.40, 2.86] |
| 8.2 Placebo controlled | 15 | 1786 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.45, 0.85] |
| 8.3 No treatment control | 5 | 1795 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.49, 1.05] |
| 9 Incidence of diarrhea: Probiotic dose (extreme‐plausible analysis) Show forest plot | 21 | 4511 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.55, 0.90] |
| 9.1 ≥5 billion CFU of probiotic/day | 10 | 2267 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.49, 0.90] |
| 9.2 <5 billion CFU of probiotic/day | 11 | 2244 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.50, 1.16] |
| 10 Incidence of diarrhea: Diagnosis Show forest plot | 18 | 2553 | Risk Ratio (M‐H, Random, 95% CI) | 0.42 [0.31, 0.56] |
| 10.1 H. pylori | 4 | 266 | Risk Ratio (M‐H, Random, 95% CI) | 0.32 [0.17, 0.63] |
| 10.2 Respiratory Infections | 5 | 952 | Risk Ratio (M‐H, Random, 95% CI) | 0.46 [0.31, 0.68] |
| 10.3 Mixed | 9 | 1335 | Risk Ratio (M‐H, Random, 95% CI) | 0.40 [0.23, 0.71] |
| 11 Incidence of diarrhea: Industry sponsorship Show forest plot | 12 | 1517 | Risk Ratio (M‐H, Random, 95% CI) | 0.50 [0.33, 0.76] |
| 11.1 Industry Sponsored | 7 | 1149 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.40, 0.86] |
| 11.2 Non‐Industry | 5 | 368 | Risk Ratio (M‐H, Random, 95% CI) | 0.32 [0.11, 0.96] |
| 12 Incidence of diarrhea: Inpatient versus outpatient Show forest plot | 13 | 2176 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.34, 0.77] |
| 12.1 Inpatient | 5 | 834 | Risk Ratio (M‐H, Random, 95% CI) | 0.38 [0.26, 0.55] |
| 12.2 Outpatient | 8 | 1342 | Risk Ratio (M‐H, Random, 95% CI) | 0.58 [0.34, 1.02] |
| 13 Incidence of diarrhea: Single strain versus multi strain Show forest plot | 22 | 3898 | Risk Ratio (M‐H, Random, 95% CI) | 0.46 [0.35, 0.61] |
| 13.1 Single Strain | 11 | 2586 | Risk Ratio (M‐H, Random, 95% CI) | 0.41 [0.28, 0.62] |
| 13.2 Multi Strain | 11 | 1312 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.35, 0.77] |
| 14 Adverse events: Complete case Show forest plot | 16 | 2455 | Risk Difference (M‐H, Random, 95% CI) | 0.00 [‐0.01, 0.01] |
| 15 Adverse events: Same event rate assumptions analysis Show forest plot | 21 | 4369 | Risk Difference (M‐H, Random, 95% CI) | 0.00 [‐0.00, 0.01] |
| 16 Adverse events: Risk of bias Show forest plot | 16 | 2455 | Risk Difference (M‐H, Random, 95% CI) | 0.00 [‐0.01, 0.01] |
| 16.1 Low RoB | 9 | 1249 | Risk Difference (M‐H, Random, 95% CI) | 0.00 [‐0.01, 0.02] |
| 16.2 High/Unclear | 7 | 1206 | Risk Difference (M‐H, Random, 95% CI) | ‐0.00 [‐0.01, 0.01] |
| 17 Mean duration of diarrhea: Complete case Show forest plot | 5 | 897 | Mean Difference (IV, Random, 95% CI) | ‐0.60 [‐1.18, ‐0.02] |
| 18 Mean stool frequency: Complete case Show forest plot | 4 | 425 | Mean Difference (IV, Random, 95% CI) | ‐0.30 [‐0.60, ‐0.00] |

