Antibióticos para la tos productiva prolongada en niños
Referencias
Referencias de los estudios incluidos en esta revisión
Referencias de los estudios excluidos de esta revisión
Referencias de los estudios en curso
Referencias adicionales
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Open randomised study comparing erythromycin and no treatment (as control group). At baseline patients had a history and clinical examination performed. A nasopharyngeal swab was obtained at baseline and again 18‐36 hours after last antibiotic dose. A repeat doctors examination was performed on Day 8. Randomisation was open and done by a computer‐generated table. No other information given about randomisation. Allocation concealment was not done (grade C). Not blinded due to open design of study (quality assessment grade D). Compliance monitoring not described. Dropouts: n=1 (1.1% of patients randomised) from treatment group from adverse effects of antibiotics. No further description given and was not included in analysis as treatment failures. Quality assessment of reporting of participants and follow‐up of patients therefore were of a high quality (A). | |
| Participants | 88 children with cough >10 days duration were included. The number of participants with cough > 3 weeks duration was 50%. Approximately 75% of the participants had moist cough. Inclusion criteria: Children aged 0.5 ‐ 6 years attending one of 3 paediatric outpatient clinics with greater than 10 days of cough. Exclusion criteria: Children with allergy, asthma, cardiac disease, otitis media, tonsillitis, pneumonia or clinically suspected pertussis. | |
| Interventions | Treatment group received erythromycin ethylsuccinate suspension 50mg/kg/day in 2 divided doses for 7 days verus 'no treatment' control group. All children received nose drops (oxymetazoline chloride). Salbutamol mixture (0.1mg/kg/day) was allowed in both groups and was registered. | |
| Outcomes | 1. Clinical symptoms as recorded on a questionnaire by parents, including cough on 3 point scale (none,moderate or frequent), morning temperature and degree of activity of child (usual, reduced, bedridden). | |
| Notes | 4 children in each arm had received antibiotic treatment prior to enrolment for <30 days. Quality Score: CDAA | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Computer‐generated table |
| Allocation concealment? | High risk | Open |
| Blinding? | High risk | Open study |
| Incomplete outcome data addressed? | Low risk | Only one patient withdrew from the study. |
| Methods | Randomised double blind study comparing Amoxycillin/clavulanic acid vs placebo. At baseline clinical data was obtained and participants underwent nasopharyngeal aspirate and blood tests for B.pertussis and Mycoplasma pneumoniae. A cough scoring system which combined number of coughing attacks per 24 hours, coughing attacks associated with vomiting and wheeze or crackles on auscultation was obtained. At 2 weeks participants were followed‐up with repeat blood tests, nasopharyngeal aspirates and doctor assessment of clinical outcome. Parental assessment of treatment efficacy was also recorded. Number of coughing attacks per 24 hours was recorded for each day of treatment. Randomisation method was not described. Allocation method was not described. Compliance monitoring was not described. Although a double‐blinded study there was no mention of how this was achieved in the paper. Dropouts n=15 (26.3% of those recruited). 12 with pertussis, 2 failed to return to follow‐up visit, 1 refused medication. These were not further described and not included as treatment failures in the paper. | |
| Participants | 52 children with cough >10 days duration were included. The mean duration of cough was 3‐4 weeks. Number with moist cough was not reported. Inclusion criteria: Children aged 0.6‐7 years with > 10 days of cough and > 7 points on cough score system. Exclusion criteria: Children with any signs of pneumonia, acute otitis media or clinical suspicion of Bordetella pertussis infection. | |
| Interventions | Treatment group received amoxycillin/clavulanic acid 20mg/kg/day for 7 days verus placebo group. | |
| Outcomes | 1. Paediatrician's assessment of clinical recovery on day 12‐14. | |
| Notes | Due to lack of information in the paper and an inability to obtain data from authors about the dropouts with pertussis, it was assumed that there were equal numbers of patients in each treatment group analysed. (37 patients became 18 each group) Quality Score: BACD | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Not described |
| Allocation concealment? | Unclear risk | Not described |
| Blinding? | Low risk | Identical placebo |
| Incomplete outcome data addressed? | Unclear risk | 26% attrition not included in final analysis |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Review article on use of antibiotics for cough, pharyngitis and the common cold. | |
| Double blind RCT of ampicillin versus placebo in infants with cough and expiratory wheeze (bronchiolitis). Excluded from review as all patients had wheeze ‐ an exclusion criteria in our patients. | |
| RCT of Domiodol versus placebo in children. Excluded as domiodol is a mucolytic not antibiotic. | |
| Open randomised prospective trial of antibiotic versus control group in children with pneumonia. All had been unwell for < 1 week. Excluded as acute cough not chronic. | |
| Non RCT. Study of 129 children aged 2 ‐ 8 years who underwent endoscopy for investigation of chronic bronchitis. | |
| Review article on the investigation and treatment of (including use of antibiotics) in children with acute, subacute and chronic cough. | |
| Double blind RCT of bacterial lysate vs placebo in acute infections respiratory system therefore excluded as not antibiotic and not chronic infections. | |
| Non RCT. Review article on treatment of pneumonia. | |
| RCT of doxycycline versus placebo in adults with acute cough of <= 1 weeks duration. Excluded as adults and acute cough. | |
| Double blind RCT of amoxycillin, co‐trimoxazle and placebo in children with viral respiratory illnesses. Excluded as not chronic cough. | |
| Excluded as cough due to acute sinusitis (that is not non‐specific cough) and cough not chronic (<30 days). Double‐blind RCT of amoxycillin, amoxycillin‐clavulanate potassium and placebo in 171 children aged 2‐16 years with persistent nasal discharge and/or cough. Children receiving antibiotic more likely to be cured then those receiving placebo (p<0.05 at ten days). | |
| RCT of antibiotics for sinusitis in children presenting with chronic cough. Excluded because no placebo or "no treatment" control group. The non‐antibiotic group was given expectorants and decongestants which is not an appropriate control/placebo group. There was also no information given on nature of cough, moist or dry. It did however precisely defined "clinical cure" as no cough on day 14 of treatment. The results did suggest benefit with antibiotics in keeping with our results with the difference between the antibiotic( treatment event rate for cure 46%) and expectorant/decongestant group ( control cure rate 14%) which was statistically significant (p<0.001). |
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Randomised controlled double‐blinded trial of antibiotics in patients with chronic moist cough |
| Methods | |
| Participants | Children aged 6 months ‐ 14 years with chronic (>3 week duration) moist cough. |
| Interventions | Randomisation stratified by age <6 years or > 6 years. Allocated blindly to Augmentin Duo Suspension 400mg/5ml or placebo at dose 22.5mg/kg twice daily for 14 days. Enrolled children will complete cough diary cards for 5 days pre intervention and total of 4 weeks after intervention. Bronchoscopy and lavage will take place on day 0 in a number of children. |
| Outcomes | Primary Outcome: Assessment of cough scores ‐ pre treatment, immediately prior to treatment and at conclusion of treatment. Bronchoalveolar lavage results in responders and non‐responders will also be compared including cytology, microbiology and inflammatory markers. |
| Starting date | January 2004 |
| Contact information | Dr Julie M Marchant |
| Notes | Study continuing but recruitment slow. Results likely available late 2008. |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Children not cured or substantially improved at follow‐up (using 'intention to treat' analysis) Show forest plot | 2 | 140 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.13 [0.06, 0.31] |
| Analysis 1.1  Comparison 1 Clinical failure, Outcome 1 Children not cured or substantially improved at follow‐up (using 'intention to treat' analysis). | ||||
| 2 Children not cured or substantially improved at follow‐up (excluding those known to have B.Pertussis) Show forest plot | 2 | 128 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.13 [0.05, 0.30] |
| Analysis 1.2  Comparison 1 Clinical failure, Outcome 2 Children not cured or substantially improved at follow‐up (excluding those known to have B.Pertussis). | ||||
| 3 Children not cured or substantially improved at follow‐up (using available data only) Show forest plot | 2 | 124 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.12 [0.05, 0.29] |
| Analysis 1.3  Comparison 1 Clinical failure, Outcome 3 Children not cured or substantially improved at follow‐up (using available data only). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Participants with progression of disease resulting in additional medical therapy required Show forest plot | 2 | 125 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.10 [0.03, 0.34] |
| Analysis 2.1  Comparison 2 Illness progression, Outcome 1 Participants with progression of disease resulting in additional medical therapy required. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Reaction to medications (vomiting, diarrhoea, rash) Show forest plot | 2 | 128 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.38 [0.31, 6.08] |
| Analysis 3.1  Comparison 3 Adverse events (reaction to medications), Outcome 1 Reaction to medications (vomiting, diarrhoea, rash). | ||||

Children not cured or substantially improved at follow‐up

Comparison 1 Clinical failure, Outcome 1 Children not cured or substantially improved at follow‐up (using 'intention to treat' analysis).

Comparison 1 Clinical failure, Outcome 2 Children not cured or substantially improved at follow‐up (excluding those known to have B.Pertussis).
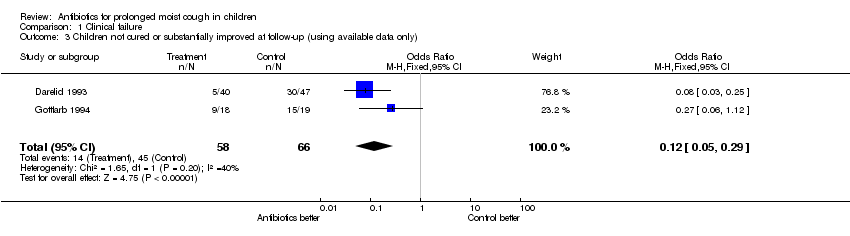
Comparison 1 Clinical failure, Outcome 3 Children not cured or substantially improved at follow‐up (using available data only).

Comparison 2 Illness progression, Outcome 1 Participants with progression of disease resulting in additional medical therapy required.

Comparison 3 Adverse events (reaction to medications), Outcome 1 Reaction to medications (vomiting, diarrhoea, rash).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Children not cured or substantially improved at follow‐up (using 'intention to treat' analysis) Show forest plot | 2 | 140 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.13 [0.06, 0.31] |
| 2 Children not cured or substantially improved at follow‐up (excluding those known to have B.Pertussis) Show forest plot | 2 | 128 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.13 [0.05, 0.30] |
| 3 Children not cured or substantially improved at follow‐up (using available data only) Show forest plot | 2 | 124 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.12 [0.05, 0.29] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Participants with progression of disease resulting in additional medical therapy required Show forest plot | 2 | 125 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.10 [0.03, 0.34] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Reaction to medications (vomiting, diarrhoea, rash) Show forest plot | 2 | 128 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.38 [0.31, 6.08] |

